Abstract
Aim
β-adrenoceptor blockers have been used with caution in patients with obstructive lung diseases such as asthma or chronic obstructive pulmonary disease (COPD), due to the potentially increased airway reactivity and risk of bronchial obstruction. Cardioselective β-adrenoceptor blockers have a more beneficial profile than non-cardioselective β-adrenoceptor blockers and can be safely prescribed to patients with both cardiovascular disease and COPD. We hypothesized that cardioselective β-adrenoceptor blockers also affect pulmonary function.
Methods
This study was performed within the Rotterdam Study, a prospective population-based cohort study. Effects of cardioselective and non-cardioselective β-adrenoceptor blockers on pulmonary function were analysed using regression techniques with multivariable adjustment for potential confounders.
Results
Current use of non-cardioselective β-adrenoceptor blockers was significantly associated with a lower forced expiratory volume in 1 s (FEV1) of −198 ml (95% CI −301, −96), with a lower forced vital capacity (FVC) of −223 ml (95% CI −367, −79) and with a decreased FEV1 : FVC of −1.38% (95% CI −2.74, −0.13%). Current use of cardioselective β-adrenoceptor blockers was significantly associated with a lower FEV1 of −118 ml (95% CI −157, −78) and with a lower FVC of −167 ml (95% CI −222, −111), but did not affect FEV1 : FVC. After exclusion of patients with COPD, asthma and heart failure the effects of cardioselective β-adrenoceptor blockers remained significant for FEV1 (−142 ml [95% CI −189, −96]) and for FVC (−176 ml [95% CI −236, −117]).
Conclusion
In our study both non-cardioselective and cardioselective β-adrenoceptor blockers had a clinically relevant effect on both FEV1 and FVC. In contrast to cardioselective β-adrenoceptor blockers, use of non-cardioselective β-adrenoceptor blockers was associated with a significantly lower FEV1 : FVC.
Keywords: β-adrenoceptor blockers FEV1, epidemiology, FEV1 : FVC, FVC, population studies, spirometry
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT
Cardioselective β-arenoceptor blockers have a more beneficial risk profile than non-cardioselective β-adrenoceptor blockers. We hypothesized that cardioselective β-adrenoceptor blockers are not free of pulmonary effects.
WHAT THIS STUDY ADDS
Both cardioselective and non-cardioselective β-adrenoceptor blockers are associated with a lower FEV1 and FVC, but only non-cardioselective β-adrenoceptor blockers are associated with a lower FEV1 : FVC.
Introduction
Several highly prevalent cardiovascular diseases, including hypertension, ischaemic heart disease, heart failure and arrhythmia, are important indications for β-adrenoceptor blockers. Major adverse effects due to blockade of the β-adrenergic system are fatigue, decreased peripheral circulation and increased airway resistance. β-adrenoceptor blockers have been used with caution in patients with obstructive lung diseases such as asthma or chronic obstructive pulmonary disease (COPD), due to the potentially increased airway reactivity and subsequent risk of bronchial obstruction in asthmatics [1]. β-adrenoceptor blockade, especially β2-adrenoceptor blockade, can provoke bronchospasm in patients with airway hyper-reactivity.
These possible adverse effects of β-adrenoceptor blockers may complicate treatment of patients with concomitant COPD and cardiovascular disease (CVD), since both conditions often coexist [2–6]. Both diseases share common risk factors such as smoking and ageing. Moreover, both CVD (in the case of heart failure) and COPD may elicit similar symptoms such as dyspnoea, cough and wheezing, which can make it difficult to distinguish between both diseases. In the TORCH-study, 30% of mortality in patients with COPD was due to CVD [7]. The World Health Organization has estimated that CVD and COPD will be amongst the top four causes of death in 2030 [8]. This underlines that adequate treatment of both diseases is highly relevant for reducing mortality and morbidity in the general population.
β1-selective (cardioselective) β-adrenoceptor blockers are supposed to have a more beneficial profile than non-cardioselective β-adrenoceptor blockers and can be safely prescribed to patients with both CVD and COPD or asthma [9–11]. A systematic review of 22 randomized trials to evaluate the effects of cardioselective β-adrenoceptor blockers showed no significant change in respiratory symptoms or spirometry parameters of interest in participants with obstructive syndromes. The forced expiratory volume in 1 s (FEV1) did not change significantly nor did the response in FEV1 to β2-adrenoceptor agonist treatment [9]. Another meta-analysis analysed 19 randomized trials on single dose administration of cardioselective β-adrenoceptor blockers and 10 studies on continuous treatment [10]. In this meta-analysis the authors concluded that the first dose of a cardioselective β-adrenoceptor blocker produces a small decrease in FEV1 that is not associated with adverse respiratory effects compared with placebo [10]. Sirak et al. stated that cardioselective β-adrenoceptor blockers are routinely preferred to non-cardioselective β-adrenoceptor blockers in the treatment of congestive heart failure and can be safely prescribed [12]. Recently, a large observational study suggested that β-adrenoceptor blockers are well tolerated in patients with COPD and may reduce mortality [11].
It is common knowledge that randomized clinical trials predominantly include highly selected, relatively young and healthy patients and that those results cannot always be generalized to clinical care in real life circumstances. Therefore, the objective of our study was to assess the effects of non-cardioselective and cardioselective β-adrenoceptor blockers on pulmonary function in the general population.
Methods
Setting
This study was performed in the Rotterdam Study, a prospective population-based cohort study which started in 1990 in Ommoord, a suburb of Rotterdam. The Medical Ethics Committee of the Erasmus Medical Centre, Rotterdam, the Netherlands, approved the study. At baseline, all participants were visited for a standardized questionnaire, and were subsequently examined at the research centre. The first cohort (RS I) consists of 7983 participants, aged 55 years and over. Since the start of the initial cohort, two subsequent cohorts have been defined in the same area. The second cohort (RS II) started in 2000 and included 3011 participants aged 55 years and over. The third cohort (RS III) was enrolled in 2006 and included 3932 participants aged 45 years and over. As of 2009 the total of the combined cohorts encompasses 14 926 subjects aged 45 years or over. The main focus of the Rotterdam Study is on studying common diseases in the elderly. Every 3–5 years the participants undergo a round of interviews and physical examinations, and pulmonary function tests are routinely performed during every study round as of 2002. To monitor the cohort continuously for major diseases and mortality, the database is linked to the electronic records of the general practitioner and the municipality. All prescriptions dispensed to the participants are periodically collected by linking to the seven pharmacies covering the Ommoord region. The main objectives and methods of the Rotterdam Study have been described elsewhere [13, 14].
Study population
The study population consisted of all participants of the Rotterdam Study with a pulmonary function test during the fourth study round. These tests were analysed by two research physicians and validated by a specialist in pulmonary medicine. During the validation process the quality control was performed by two researchers, of whom at least one was a physician. Normal spirometry, airflow obstruction and spirometry suggestive of restrictive syndromes were classified.
Exposure
To assess exposure to β-adrenoceptor blockers, for participants with a pulmonary function test, prescription data were collected from the pharmacies. We collected prescription data on cardioselective and non-cardioselective β-adrenoceptor blockers using the Anatomical Therapeutic Chemical (ATC) classification system [15] for C07AB (cardioselective β-adrenoceptor blockers) and C07AA (non-cardioselective β-adrenoceptor blockers). For every participant, the prescription period was calculated and if the date of the pulmonary function test fell within the prescription period of a β-adrenoceptor blocker, the study participant was classified as exposed.
Outcome
Spirometry was performed by trained paramedical personnel using a SpiroPro® portable spirometer (Erich Jaeger, Hoechberg, Germany), according to the American Thoracic Society (ATS)/European Respiratory Society (ERS) guidelines [16]. FEV1, FVC and FEV1 : FVC ratio were measured. Spirometry procedures that yielded results that did not meet ATS/ERS criteria for acceptability and reproducibility were classified as not interpretable [17]. For practical reasons, reversibility testing could not be carried out. Participants were asked to refrain from using any prescribed pulmonary medication before the centre visit.
Covariables
Information on several potential confounders or effect modifiers such as age, gender, height, weight and body mass index (BMI) was gathered at the study round in which the pulmonary function test was performed. We collected data on medication prescribed for obstructive pulmonary diseases such as asthma and COPD. For our analyses, we included inhaled short acting β-adrenoceptor agonists (SABAs), long acting β-adrenoceptor agonists (LABAs), anticholinergic agents, inhaled glucocorticosteroids and combinations of the above mentioned substances. Furthermore, we collected information on use of oral glucocorticosteroids, xanthine derivatives and leukotriene receptor antagonists. Data on smoking were collected from the questionnaires and subjects were classified as current, former or never smokers. As a quantitative measure of tobacco exposure, we used the number of pack-years, with 1 pack-year defined as smoking 20 cigarettes day–1 for 1 year. We assessed COPD using spirometry and classified according to the GOLD criteria [18, 19]. Heart failure at baseline in the Rotterdam Study was assessed using a validated score based on the definition of heart failure of the European Society of Cardiology [20]. As described previously, cases of incident heart failure were obtained by continuously monitoring participants of the Rotterdam Study during follow-up [21]. For these analyses, we assessed if participants were cases at the time of the pulmonary function test and considered cases defined before the test as prevalent heart failure patients. Using echocardiography, fractional shortening and E : A ratio were calculated as measures of systolic and diastolic cardiac function, respectively [22]. Echocardiography was done on the same day as the spirometry. The methods for echocardiography in the Rotterdam Study have been described elsewhere [22]. Systolic and diastolic blood pressures were measured twice from the right upper arm with a random-zero sphygmomanometer with the patient in a sitting position. The mean of the two readings was used to determine blood pressure levels.
Data analysis
Differences in baseline characteristics between cardioselective and non-cardioselective β-adrenoceptor blocker users, and non-users were tested using a χ2-test for dichotomous variables and a t-test for continuous variables. The effect of cardioselective and non-cardioselective β-adrenoceptor blockers on pulmonary function measures was evaluated using linear regression analyses for FEV1 and for FVC, selecting covariables by assessing whether there was a higher than 10% change in point estimate. Furthermore, we tested for possible interaction by using multiplicative interaction terms. We adjusted for gender, age, height, systolic blood pressure, smoking status, pack-years, heart failure, obstructive and restrictive syndromes and concomitant respiratory medication, with pulmonary function in non-users of β-adrenoceptor blockers as a reference group. To avoid potential confounding by indication, we also performed analyses excluding patients with COPD and heart failure. We preferred this over adjustment because the occurrence of these conditions was considered as a contra-indication for β-adrenoceptor blockers for a substantial number of years within the study period.
In 784 participants, consecutive measurements were available, with a mean follow-up of 6.1 years (± 0.5) between consecutive centre visits. In this subanalysis, exposure categories were classified as ‘never exposed’ (no use of β-adrenoceptor blockers at the first as well as the second measurement), ‘starters’ (no use of β-adrenoceptor blockers at the first measurement but use at the second measurement), ‘continuous users’ (use of β-adrenoceptor blockers at the first as well as the second measurement) and ‘discontinued use’ (use of β-adrenoceptor blockers at the first measurement but no use at the second measurement).
All analyses above were repeated for non-cardioselective β-adrenoceptor blockers. All statistical analyses were performed with SPSS PASW software (IBM Corporation, Armonk, New York, USA).
P values <0.05 were considered statistically significant. All graphs were made using R 2.9.1 [23].
Results
Of the 14 926 subjects from the Rotterdam Study cohorts, a total of 6489 pulmonary function tests were performed and 4324 spirometry tests were of sufficient quality to be validated as interpretable and could be used for our analyses.
Subject characteristics
The population consisted of 1880 men (43.5%) and 2444 women (56.5%) (Table 1). The mean age at lung function examination was 66 years (range 46–97 years). In our cohort 651 persons were exposed to cardioselective β-adrenoceptor blockers at the time of the pulmonary function test. The mean FEV1 in the cohort was 3.23 l (± 0.9 l) for men and 2.34 l (± 0.6 l) for women. The mean FVC was 4.27 l (± 1.03 l) for men and 3.03 l (± 0.74 l) for women. We defined airflow limitation according to the GOLD definition (FEV1 : FVC ratio < 0.70). In our sample there were 308 subjects with COPD GOLD stage I (7.1%), 330 with COPD GOLD stage II (7.6%) and 63 with COPD GOLD stage III (1.5%). There were no subjects with COPD GOLD stage IV.
Table 1.
Baseline characteristics of the study population
| Characteristics | Total group | Unexposed | β1-selective | Non-selective | |
|---|---|---|---|---|---|
| Number of participants | 4324 | 3585 | 651 | 88 | |
| Gender n (%) | Male (%) | 1880 (43.5%) | 1533 (42.8%) | 303 (46.5%) | 44 (50%) |
| Female (%) | 2444 (56.5%) | 2052 (57.2%) | 348 (53.5%) | 44 (50%) | |
| Age (years) (SD) | 65.9 (9.5) | 65.2 (9.5) | 68.9 (9.0) | 71.8 (9.3)* | |
| Height (cm) (SD) | 168.9 (9.3) | 169.0 (9.3) | 168.1 (9.2)* | 169.5 (8.7) | |
| Smoking status*†n (%) | Never | 1418 (32.8%) | 1208 (32.9%) | 210 (32.2%) | 22 (25%) |
| Past | 2074 (48.0%) | 1734 (47.2%) | 340 (52.2%)* | 50 (56.8%) | |
| Current | 828 (19.1%) | 727 (19.8%) | 101 (15.5%)* | 16 (18.2%) | |
| Pack-years (SD) | 15.5 (21.1) | 14.9 (20.8) | 18.5 (22.7)* | 16.3 (19.1) | |
| Prevalent heart failure n (%) | 140 (3.2%) | 93 (2.5%) | 47 (7.3%)* | 7 (8%)* | |
| Blood pressure (mmHg) (SD) | Systolic | 142 (21) | 141 (21) | 150 (22)* | 143 (20) |
| Diastolic | 81 (11) | 81 (11) | 82 (12) | 78 (11)* | |
| BMI (kg m–1) (SD) | 27.6 (4.3) | 27.3 (4.2) | 29.0 (4.4)* | 28.0 (3.6) | |
| History of myocardial infarction (%) | 223 (5.2) | 111 (2.6) | 100 (15.4)* | 12 (13.6%)* | |
| Echocardiography§ | Fractional shortening % (SD) | 39.7 (6.7) | 40.2 (6.3) | 38.0 (7.8)* | 35.0 (7.7)* |
| E : A ratio | 1.0 (0.3) | 1.0 (0.3) | 1.0 (0.3) | 0.9 (0.3) | |
| Total cholesterol (mmol l−1) (SD) | 5.6 (1.0) | 5.6 (1.0) | 5.2 (1.0)* | 5.3 (1.1)* | |
| HDL cholesterol (mmol l−1) (SD) | 1.4 (0.4) | 1.5 (0.4) | 1.3 (0.3)* | 1.3 (0.3)* | |
| Chronic obstructive pulmonary disease GOLD classification n (%) | No obstruction‡ | 3623 (83.8%) | 3025 (84.4%) | 530 (81.5%) | 68 (77.3%) |
| I | 308 (7.1%) | 261 (7.3%) | 41 (6.3%) | 6 (6.8%) | |
| II | 330 (7.6%) | 248 (6.9%) | 69 (10.6%)* | 13 (14.8%)* | |
| III | 63 (1.5%) | 51 (1.4%) | 11 (1.7%) | 1 (1.1%) |
P value < 0.05 for difference, exposed vs. unexposed.
Smoking status missing: n = 4.
Spirometry suggestive for a restrictive syndrome: unexposed n = 215, exposed to β1-selective n = 72, exposed to non-selective n = 14. §Available in a random subset: n = 3629. BMI: body mass index, E : A ratio: ratio of early and late ventricular filling velocity, n: number of participants.
Furthermore, we identified 301 participants (7.1%) with lung function results suggestive of a restrictive syndrome. Of the participants with pulmonary function tests we had full data, including smoking history, for 4033 subjects. These were included in our analyses. Missing values were independent of β-adrenoceptor blocker use and multiple imputations followed by combined analyses did not change effect estimates.
Cardioselective β-adrenoceptorblockers
Exposure to cardioselective β-adrenoceptor blockers was significantly associated with a lower FEV1 in our population of −118 ml (95% CI −157; −78 ml). To investigate the effect of cardioselective β-adrenoceptor blockers on FVC we tested similar models. Current use of cardioselective β-adrenoceptor blockers was significantly associated with a lower FVC with a decrease of −167 ml (95% CI −222, −111 ml) (Table 2; Figures 1 and 2). Testing for interaction between gender, age or COPD and β-adrenoceptor blocker exposure was non-significant but with suspected pulmonary restrictive disease interaction seemed to be present. When we subsequently analysed the dataset stratified, the significant effect on FEV1 only persisted in the non-diseased group and was stronger (–145, 95% CI −188, −103).
Table 2.
Effect estimates
| Pulmonary function test | Effect, crude | 95% CI | Effect, adjusted‡ | 95% CI | P value, adjusted |
|---|---|---|---|---|---|
| FEV1 | |||||
| No use | Reference | Reference | |||
| Non-cardioselective* | −503 ml | −685, −321 | −198 ml | −301, −96 | <0.001 |
| Cardioselective† | −307 ml | −378, −236 | −118 ml | −157, −78 | <0.001 |
| FVC | |||||
| No use | Reference | Reference | |||
| Non-cardioselective | −524 ml | −752, −296 | −223 ml§ | −367, −79 | 0.001 |
| Cardioselective | −379 ml | −468, −290 | −167 ml | −222, −111 | <0.001 |
| FEV1/FVC | |||||
| No use | Reference | Reference | |||
| Non-cardioselective | −3.52% | −5.37, −1.66 | −1.38%¶ | −2.74, −0.13 | 0.048 |
| Cardioselective | −0.63% | −1.36, 1.01 | 0.12% | −0.41, 0.65 | 0.654 |
n exposed non-cardioselective = 8, n unexposed = 3585.
n exposed cardioselective = 651, n unexposed = 3585.
Adjusted for: gender, age, height, pack-years, smoking status, inhalation medication, oral glucocorticosteroids, heart failure, obstructive or restrictive syndromes and systolic blood pressure and cohort.
As above plus BMI.
Adjusted for: age, height, BMI, pack-years, smoking status, obstructive or restrictive lung diseases and inhalation medication and cohort.
Figure 1.
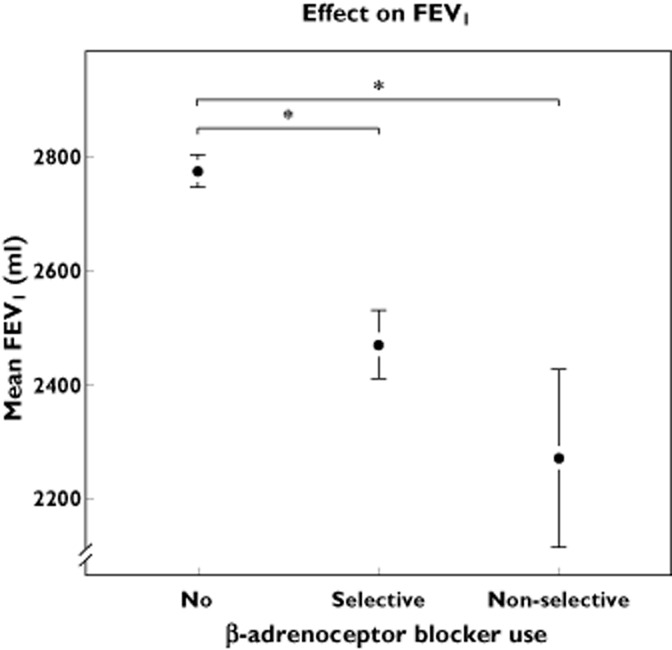
Effect of β-adrenoceptor blocker exposure on mean FEV1. Exposure is divided in three classes: no use, current use of cardioselective β-adrenoceptor blockers and current use of non-cardioselective β-adrenoceptor blockers. *P = 0.001. The whiskers represent the 95% CI
Figure 2.
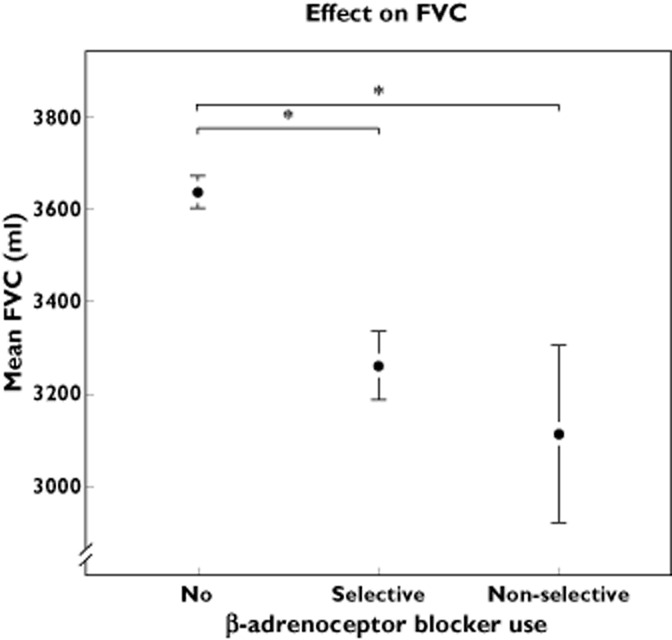
Effect of β-adrenoceptor blocker exposure on mean FVC. Exposure is divided in three classes; No use, current use of cardioselective β-adrenoceptor blockers and current use of non-cardioselective β-adrenoceptor blockers. *P < 0.001. The whiskers represent the 95% CI
To assess the effect in subjects without known heart failure and an obstructive or a restrictive syndrome, we separately analysed participants without these diseases. As asthma or COPD patients might be prone to a prescription of cardioselective β-adrenoceptor blockers because of the possible adverse effects of non-cardioselective β-adrenoceptor blockers, we also excluded participants with a prescription for inhalation medication and/or oral glucocorticosteroids.
In this sample (n = 3157), the associations of cardioselective β-adrenoceptor blocker exposure and a lower FEV1 and FVC are comparable with the complete sample. Exposure to cardioselective β-adrenoceptor blockers was associated with a lower FEV1 of −142 ml (95% CI −189, −96 ml) and a change of FVC of −176 ml (95% CI −236, −117 ml). To minimize the effect of misclassification of heart failure, we performed a sensitivity analysis in which we also adjusted for echocardiography, which was available in a random subset of 3629 participants. Fractional shortening and E : A ratio were used as measures of systolic and diastolic function, respectively. Of these measures only fractional shortening was of influence in our models. The effect estimate of cardioselective β-adrenoceptor blockers changed less than 5% and remained significant in this analysis.
Non-cardioselective β-adrenoceptor blockers
In our cohort, exposure to non-cardioselective β-adrenoceptor blockers was low and only 88 participants (2.2%) of our cohort of 4033 participants had a prescription for non-cardioselective β-adrenoceptor blockers. The majority of these users (75%) had a prescription for sotalol. Exposure of non-cardioselective β-adrenoceptor blockers was also associated with lower FEV1 and FVC (Table 2; Figures 1 and 2). The effect of non-cardioselective β-adrenoceptor blockers was −198 ml (95% CI −301, −96 ml) on FEV1 and −223 ml (95% CI −367, −79 ml) on FVC. We also analysed the effect in the subset of participants without obstructive and restrictive syndromes, heart failure and inhalation medication. For FEV1 the effect was −219 ml (95% CI −343, −94 ml) and for FVC the effect was −179 ml (95% CI −339, −19 ml).
FEV1 : FVC
No significant association was shown on the FEV1 : FVC ratio in participants exposed to cardioselective β-adrenoceptor blockers compared with unexposed participants, namely an effect of 0.12% (95% CI −0.41, 0.65). In the adjusted analysis, a significantly lower ratio of FEV1 : FVC of −1.38% (95% CI −2.74, −0.13%) was seen in participants exposed to non-cardioselective β-adrenoceptor blockers compared with unexposed subjects (Figure 3). Compared with users of cardioselective β-adrenoceptor blockers, the adjusted effect was −1.28% (95% CI −2.49, −0.76).
Figure 3.
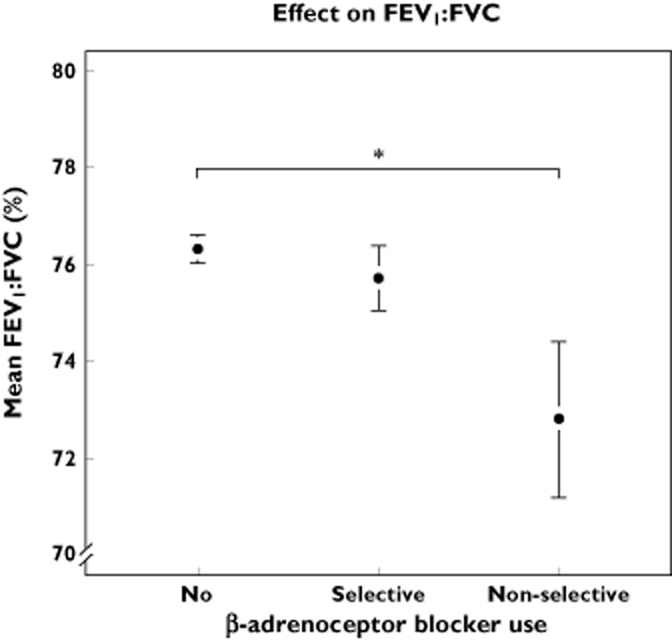
Effect of β-adrenoceptor blocker exposure on mean FEV : FVC. Exposure is divided in three classes; No use, current use of cardioselective β-adrenoceptor blockers and current use of non-cardioselective β-adrenoceptor blockers. *P < 0.05. The whiskers represent the 95% CI
Longitudinal effects
For one cohort (RS-I), consecutive spirometry measurements were available in 784 participants (Table 3). Participants starting cardioselective β-adrenoceptor blockers showed a significantly stronger percentual decrease in FVC than never users of β-adrenoceptor blockers, i.e. respectively 13.8% vs. 9.6% (Figure 3).
Table 3.
Baseline characteristics of the subgroup with two spirometry tests
| Characteristics | Total | Unexposed | β1-selective | |
|---|---|---|---|---|
| Number of participants | 784 | 648 | 136 | |
| Gender n (%) | Male (%) | 337 (43.0%) | 278 (43.0%) | 59 (43.4%) |
| Female (%) | 447 (57.0%) | 370 (57.0%) | 77 (56.6%) | |
| Age baseline (years) (SD) | 72.3 | 72.3 (4.5) | 72.7 (4.1) | |
| Height (cm) (SD) | 167.6 (9.0) | 167.7 (8.9) | 166.9 (9.4) | |
| Smoking status n (%) | Never | 259 (33.0%) | 209 (32.3%) | 50 (36.8%) |
| Past | 453 (57.8%) | 376 (58.0%) | 77 (56.6%) | |
| Current | 72 (9.2%) | 63 (9.7%) | 9 (6.6%) | |
| Pack years (SD) | 15.5 (21.1) | 15.6 (21.5) | 14.8 (18.6) | |
| Prevalent heart failure n (%) | 23 (2.9%) | 15 (2.3%)* | 8 (5.9%)* | |
| Blood pressure mmHg (SD) | Systolic | 149 (20) | 148 (20)* | 156 (20)* |
| Diastolic | 80 (10) | 80 (10) | 81 (10) | |
| BMI kg m−2 (SD) | 27.3 (3.9) | 27 (3.8)* | 28.6 (3.8)* | |
| History of myocardial infarction (%) | 52 (6.6%) | 33 (5.1%)* | 19 (14.0%)* | |
| Chronic obstructive pulmonary disease GOLD classification, n (%) | No obstruction† | 579 (73.9%) | 483 (74.5%) | 96 (70.6%) |
| I | 65 (8.3%) | 54 (8.3%) | 11 (8.1%) | |
| II | 89 (11.4%) | 70 (10.8%) | 19 (14.0%) | |
| III | 8 (1.0%) | 8 (1.2%) | 0 | |
| Follow-up time (years) (SD) | 6.6 (0.4) |
P value < 0.05 for difference, exposed vs. unexposed.
Spirometry suggestive for a restrictive syndrome: n unexposed = 33, exposed = 10. BMI, body mass index; n, number of participants; SD, standard deviation.
For FEV1 a similar effect was seen with a decrease of 13.5% for starters of β-adrenoceptor blockers vs. 9.4% for never users.When prescriptions ended between the measurements, participants who had been using cardioselective β-adrenoceptor blockers showed less of a decrease in both FEV1 and FVC compared with starters (Figures 4 and 5). Linear regression with adjustment for age, gender, follow-up time, current smoking and pack-years, showed a statistically significant stronger percentage decrease for both FEV1 and FVC compared with never users (Figure 6).
Figure 4.
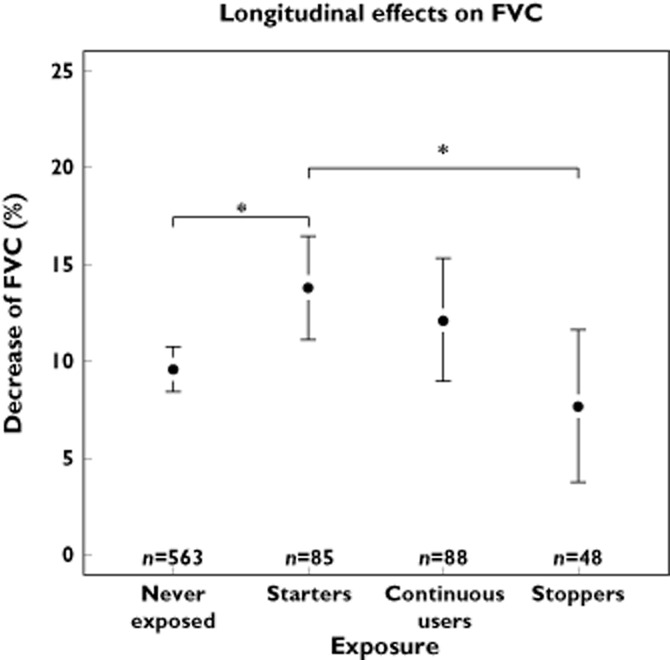
Longitudinal effects of cardioselective β-adrenoceptor blocker use on FVC. Divided in four exposure categories: never exposed, starters, continuous users, and discontinued use. *P < 0.05. The whiskers represent the 95% CI
Figure 5.
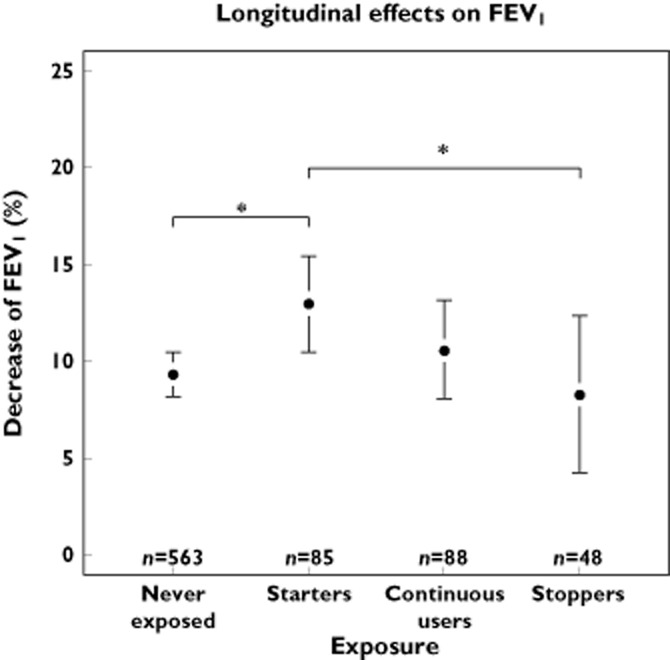
Longitudinal effects of cardioselective β-adrenoceptor blocker use on FEV1. Divided in four exposure categories; ‘never exposed’, ‘starters’, ‘continuous users’ and ‘discontinued use’. *P < 0.05. The whiskers represent the 95% CI
Figure 6.
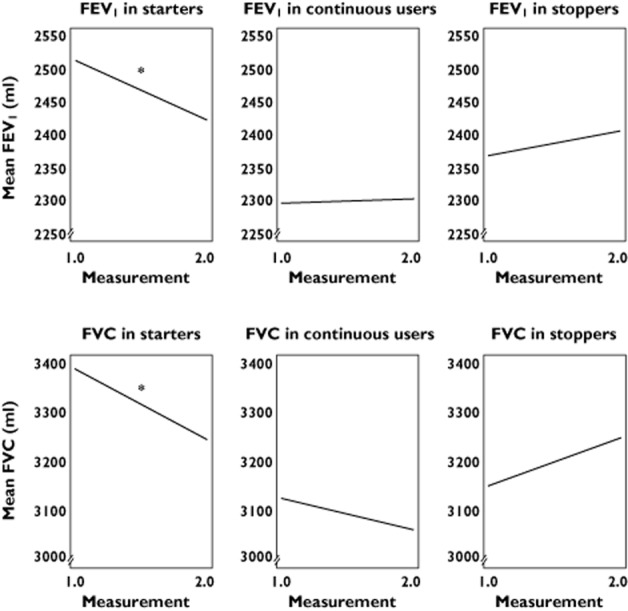
The decrease or increase in FEV1 and FVC for separate types of β-adrenoceptor blockers users compared with never users over the consecutive time points. The linear relation is corrected for age, gender, follow-up time, current smoking and pack-years. *P < 0.05 in never users
Discussion
In this population-based study, exposure to cardioselective β-adrenoceptor blockers was associated with a lower FEV1 of −118 ml and FVC of −167 ml, respectively. For non-cardioselective β-adrenoceptor blockers we found an association with a marginally lower FEV1 : FVC of −1.38% (95% CI −2.74, −0.13), a measure to assess airflow obstruction. For cardioselective β-adrenoceptor blockers, although the sample was much larger, no such association was found. When we compared the effect on FEV1 : FVC within users of non-cardioselective β-adrenoceptor blockers vs. cardioselective β-adrenoceptor blockers, the association remained significant. Longitudinal effects of exposure showed a stronger decrease of FVC over time in participants starting on β-adrenoceptor blockers vs. never users. For FEV1 a similar pattern was seen. When prescriptions ended between the two measurements, the decrease in FEV1 and FVC was less in quitters than in never users. However this was not significant. Although our results suggest negative effects of cardioselective β-adrenoceptor blockers on pulmonary function which can be defined as a clinically important difference [24, 25], several studies suggest beneficial effects on mortality in patients with concomitant CVD and COPD. Therefore, negative effects on pulmonary function may very well be outweighed by the positive effects of cardioselective β-adrenoceptor blockers on CVD with respect to morbidity and mortality. Apparently, cardioselective β-adrenoceptor blockers are also not completely free of pulmonary effects. Importantly, non-cardioselective β-adrenoceptor blockers seem to have greater association with an airflow obstruction than cardioselective β-adrenoceptor blockers. Only non-cardioselective β-adrenoceptor blockers had a significant association with a reduced FEV1 : FVC compared with non-exposed participants and compared to users of cardioselective β-adrenoceptor blockers.
To our knowledge, this is the first population-based cohort study solely investigating the effects of β-adrenoceptor blockers on pulmonary function. To deal with potential confounding by indication, we separately analysed the participants with heart failure and/or obstructive pulmonary disease. The results in this subset were consistent with the results in the complete set, suggesting that underlying obstructive pulmonary disease did not influence the magnitude of the effects. To minimize the possible misclassification of heart failure, we did a sensitivity analysis in which we adjusted for cardiac function, defined by fractional shortening and E : A ratio. These measures hardly influenced the effect estimate. The decreased spirometry measures can possibly be explained by increased airway resistance as has been stated in the past [1]. Another possible reason could be that some participants with a prescription for β-adrenoceptor blockers might have already developed heart failure, which was still unknown at the time of pulmonary function test. However as participants in The Rotterdam Study were also screened for heart failure this seems less likely [13, 14].
Our findings suggest that cardioselective β-adrenoceptor blockers also have an adverse effect on pulmonary function in the general elderly population, although this is less pronounced than with non-cardioselective β-adrenoceptor blockers and there is no effect on airflow obstruction. One, somewhat similar, study by Schnabel et al. showed a trend for a lower FEV1 in people taking β-adrenoceptor blockers and a significantly reduced FVC, but since the study focused on other objectives, the association was not extensively investigated [26]. Our study confirms this association with FVC and shows an association with FEV1.
Previous studies did not show a significant effect on FEV1 or respiratory symptoms in meta-analyses of randomized clinical trials [9, 10]. However, these meta-analyses included primarily small randomized placebo controlled studies. In these studies, 80% of the participants were male and COPD severity was mild to moderate and according to the authors this could be a limitation of the study [9]. In a crossover trial, Jabbour et al. showed a difference in FEV1 between patients with cardioselective and non-cardioselective β-adrenoceptor blockers for chronic heart failure (CHF) with COPD. The authors reported a 150 ml lower FEV1 in patients with CHF and COPD receiving non-cardioselective β-adrenoceptor blocker than in patients receiving cardioselective β-adrenoceptor blockers. No such difference could be detected for FVC [27]. This study in 51 males showed differences between patients receiving either of the β-adrenoceptor blockers, but did not compare pulmonary function with non-users. Another study by Çamsari et al. in 50 patients showed that metoprolol did not change pulmonary function according to symptoms, clinical findings and FEV1 changes [28]. Other studies focused on morbidity and mortality in patients with CVD and COPD. Rutten et al. performed a large observational study using general practitioners records. The authors assessed all cause mortality and exacerbations of COPD in the complete sample and in subsets. Rutten et al. showed a possible beneficial effect on survival and a reduced risk on COPD exacerbation in patients taking cardioselective β-adrenoceptor blockers [11]. Misclassification of patients could be an issue, according to the authors, and patients could have had concealed cardiac disease instead of COPD, which would show a positive effect of β-adrenoceptor blockers on survival [2, 11]. On the other hand, since heart failure and COPD exacerbations were suggested to be closely linked with regard to mortality [29, 30], treating patients with a multidrug regimen might provide disease-specific benefits and reduce mortality [31].
Chen et al. found that β-adrenoceptor blocker use after an acute myocardial infarction, in patients with COPD without β-adrenoceptor agonist use, significantly reduced 1 year mortality. The authors suggest that these patients may have a mild form of COPD and that the benefits of β-adrenoceptor blocker therapy outweigh the risk of bronchospasm [32].
In a recent paper by Short et al. [33], the investigators defined a cohort of COPD patients and compared mortality across different treatment regimens for COPD and CVD. The investigators found a 22% overall reduction of all cause mortality for patients with COPD if the treatment regimen included β-adrenoceptor blockers. Spirometry measurements were available in a subset of patients. The authors found no deleterious effect of β-adrenoceptor blockers on FEV1 and FVC. This observational study, like any observational study, is at risk for confounding by indication and post hoc treatment changes by physicians can influence the results [34]. Furthermore, the analysis of a subset with spirometry tests could be influenced by some form of selection bias in a retrospective database study.
The strengths of our study are the prospective, population-based cohort design and the large number of study subjects with pulmonary function tests. The population-based character and high participation rate makes selection bias less likely. Due to the prospective nature of the data collection, information bias is also unlikely and information about several covariables was collected. Another strong point is that in the Rotterdam study we have both prescription and interview data to check adherence to therapy. An earlier analysis in the Rotterdam Study showed that there was a very high concordance between pharmacy dispensing data of β-adrenoceptor blockers and actual use during interview [35]. Although longitudinal data show interesting patterns, numbers of repeated measurements were limited. Due to the observational character of our data, residual confounding can not be ruled out completely. We adjusted for confounding by (contra-) indication by excluding participants with COPD, and as a proxy for asthma, we excluded patients with prescriptions for inhalation medication to study the effects in patients without obstructive pulmonary disease. Since spirometry was introduced in 2002, some form of survival bias could have occurred, but our expectation is that this would not explain the fact that we observed a difference between users and non-users of β-adrenoceptor blockers. Lastly we could not include disease-specific mortality data into these analyses to see if this reduction in FEV1 and FVC was associated with a higher mortality rate.
In conclusion, in this population-based study, we demonstrated that not only non-cardioselective, but also cardioselective β-adrenoceptor blockers had a clinically significant effect on both FEV1 and FVC in some patients. Unlike cardioselective β-adrenoceptor blockers, non-cardioselective β-adrenoceptor blockers were, beside FEV1 and FVC, significantly associated with a marginally decreased FEV : FVC.
Competing Interests
All authors have completed the Unified Competing Interest form at http://www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare no support from any organization for the submitted work, no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years and no other relationships or activities that could appear to have influenced the submitted work.
Study Design: A.H. Data collection: A.H., G.G.B and B.H.S. Data-analysis and writing: D.W.L, B.H.S, and G.G.B. Critical Review: G.G.B., L.L, A.H., H.G.M.L and B.H.S.
D.W.L and B.H.S. take responsibility for the integrity of this work.
The Rotterdam Study is funded by Erasmus Medical Center and Erasmus University, Rotterdam, Netherlands Organization for the Health Research and Development (ZonMw), the Research Institute for Diseases in the Elderly (RIDE), the Ministry of Education, Culture and Science, the Ministry for Health, Welfare and Sports, the European Commission (DG XII) and the Municipality of Rotterdam. The authors are grateful to the study participants, the staff from the Rotterdam Study and the participating general practitioners. Lies Lahousse is the recipient of a European Respiratory Society Fellowship (STRTF fellowship n°. 131–2011).
References
- 1.Benson MK, Berrill WT, Cruickshank JM, Sterling GS. A comparison of four beta-adrenoceptor antagonists in patients with asthma. Br J Clin Pharmacol. 1978;5:415–419. doi: 10.1111/j.1365-2125.1978.tb01647.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rutten FH, Cramer MJ, Lammers JW, Grobbee DE, Hoes AW. Heart failure and chronic obstructive pulmonary disease: an ignored combination? Eur J Heart Fail. 2006;8:706–711. doi: 10.1016/j.ejheart.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 3.Huiart L, Ernst P, Suissa S. Cardiovascular morbidity and mortality in COPD. Chest. 2005;128:2640–2646. doi: 10.1378/chest.128.4.2640. [DOI] [PubMed] [Google Scholar]
- 4.Mascarenhas J, Azevedo A, Bettencourt P. Coexisting chronic obstructive pulmonary disease and heart failure: implications for treatment, course and mortality. Curr Opin Pulm Med. 2010;16:106–111. doi: 10.1097/MCP.0b013e328335dc90. [DOI] [PubMed] [Google Scholar]
- 5.Johnston AK, Mannino DM, Hagan GW, Davis KJ, Kiri VA. Relationship between lung function impairment and incidence or recurrence of cardiovascular events in a middle-aged cohort. Thorax. 2008;63:599–605. doi: 10.1136/thx.2007.088112. [DOI] [PubMed] [Google Scholar]
- 6.Hannink JD, van Helvoort HA, Dekhuijzen PN, Heijdra YF. Heart failure and COPD: partners in crime? Respirology. 2010;15:895–901. doi: 10.1111/j.1440-1843.2010.01776.x. [DOI] [PubMed] [Google Scholar]
- 7.McGarvey LP, John M, Anderson JA, Zvarich M, Wise RA Torch Clinical Endpoint Committee. Ascertainment of cause-specific mortality in COPD: operations of the TORCH Clinical Endpoint Committee. Thorax. 2007;62:411–415. doi: 10.1136/thx.2006.072348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.WHO. Global burden of disease. 2004.
- 9.Salpeter SS, Ormiston T, Salpeter E, Poole P, Cates C. Cardioselective beta-blockers for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2002;(2) doi: 10.1002/14651858.CD003566. CD003566. [DOI] [PubMed] [Google Scholar]
- 10.Salpeter SR, Ormiston TM, Salpeter EE. Cardioselective beta-blockers in patients with reactive airway disease: a meta-analysis. Ann Intern Med. 2002;137:715–725. doi: 10.7326/0003-4819-137-9-200211050-00035. [DOI] [PubMed] [Google Scholar]
- 11.Rutten FH, Zuithoff NP, Hak E, Grobbee DE, Hoes AW. Beta-blockers may reduce mortality and risk of exacerbations in patients with chronic obstructive pulmonary disease. Arch Intern Med. 2010;170:880–887. doi: 10.1001/archinternmed.2010.112. [DOI] [PubMed] [Google Scholar]
- 12.Sirak TE, Jelic S, Le Jemtel TH. Therapeutic update: non-selective beta- and alpha-adrenergic blockade in patients with coexistent chronic obstructive pulmonary disease and chronic heart failure. J Am Coll Cardiol. 2004;44:497–502. doi: 10.1016/j.jacc.2004.03.063. [DOI] [PubMed] [Google Scholar]
- 13.Hofman A, Grobbee DE, de Jong PT, van den Ouweland FA. Determinants of disease and disability in the elderly: the Rotterdam Elderly Study. Eur J Epidemiol. 1991;7:403–422. doi: 10.1007/BF00145007. [DOI] [PubMed] [Google Scholar]
- 14.Hofman A, van Duijn CM, Franco OH, Ikram MA, Janssen HL, Klaver CC, Kuipers EJ, Nijsten TE, Stricker BH, Tiemeier H, Uitterlinden AG, Vernooij MW, Witteman JC. The Rotterdam Study: 2012 objectives and design update. Eur J Epidemiol. 2011;26:657–686. doi: 10.1007/s10654-011-9610-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.WHO Collaborating Centre for Drug Statistics Methodology. ATC classification index with DDDs. 2009. Available at: http://www.whocc.no/atc_ddd_index/ (last accessed December 2010)
- 16.Celli BR, MacNee W Ats Ers Task Force. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J. 2004;23:932–946. doi: 10.1183/09031936.04.00014304. [DOI] [PubMed] [Google Scholar]
- 17.van Durme YM, Verhamme KM, Stijnen T, van Rooij FJ, Van Pottelberge GR, Hofman A, Joos GF, Stricker BH, Brusselle GG. Prevalence, incidence, and lifetime risk for the development of COPD in the elderly: the Rotterdam study. Chest. 2009;135:368–377. doi: 10.1378/chest.08-0684. [DOI] [PubMed] [Google Scholar]
- 18. From the Global Strategy for the Diagnosis, Management and Prevention of COPD, Global Initiative for Chronic Obstructive Lung Disease (GOLD). 1997. Available at: http://www.goldcopd.org (last accessed December 2010)
- 19.Pauwels RA, Buist AS, Calverley PM, Jenkins CR, Hurd SS Gold Scientific Committee. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. NHLBI/WHO Global Initiative for Chronic Obstructive Lung Disease (GOLD) Workshop summary. Am J Respir Crit Care Med. 2001;163:1256–1276. doi: 10.1164/ajrccm.163.5.2101039. [DOI] [PubMed] [Google Scholar]
- 20.Remme WJ, Swedberg K Task Force for the Diagnosis and Treatment of Chronic Heart Failure European Society of Cardiology. Guidelines for the diagnosis and treatment of chronic heart failure. Eur Heart J. 2001;22:1527–1560. doi: 10.1053/euhj.2001.2783. [DOI] [PubMed] [Google Scholar]
- 21.Bleumink GS, Knetsch AM, Sturkenboom MC, Straus SM, Hofman A, Deckers JW, Witteman JC, Stricker BH. Quantifying the heart failure epidemic: prevalence, incidence rate, lifetime risk and prognosis of heart failure The Rotterdam Study. Eur Heart J. 2004;25:1614–1619. doi: 10.1016/j.ehj.2004.06.038. [DOI] [PubMed] [Google Scholar]
- 22.Kardys I, Deckers JW, Stricker BH, Vletter WB, Hofman A, Witteman J. Distribution of echocardiographic parameters and their associations with cardiovascular risk factors in the Rotterdam Study. Eur J Epidemiol. 2010;25:481–490. doi: 10.1007/s10654-010-9453-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.R Development Core Team. R: a language and environment for statistical computing. 2008. Available at: http://www.R-project.org (last accessed December 2010)
- 24.Martinez FJ, Donohue JF, Rennard SI. The future of chronic obstructive pulmonary disease treatment – difficulties of and barriers to drug development. Lancet. 2011;378:1027–1037. doi: 10.1016/S0140-6736(11)61047-7. [DOI] [PubMed] [Google Scholar]
- 25.Donohue JF. Minimal clinically important differences in COPD lung function. COPD. 2005;2:111–124. doi: 10.1081/copd-200053377. [DOI] [PubMed] [Google Scholar]
- 26.Schnabel E, Nowak D, Brasche S, Wichmann HE, Heinrich J. Association between lung function, hypertension and blood pressure medication. Respir Med. 2011;105:727–733. doi: 10.1016/j.rmed.2010.12.023. [DOI] [PubMed] [Google Scholar]
- 27.Jabbour A, Macdonald PS, Keogh AM, Kotlyar E, Mellemkjaer S, Coleman CF, Elsik M, Krum H, Hayward CS. Differences between beta-blockers in patients with chronic heart failure and chronic obstructive pulmonary disease: a randomized crossover trial. J Am Coll Cardiol. 2010;55:1780–1787. doi: 10.1016/j.jacc.2010.01.024. [DOI] [PubMed] [Google Scholar]
- 28.Çamsari A, Arikan S, Avan C, Kaya D, Pekdemir H, Cicek D, Kiykim A, Sezer K, Akkus N, Alkan M, Aydogdu S. Metoprolol, a beta-1 selective blocker, can be used safely in coronary artery disease patients with chronic obstructive pulmonary disease. Heart Vessels. 2003;18:188–192. doi: 10.1007/s00380-003-0706-z. [DOI] [PubMed] [Google Scholar]
- 29.Chang CL, Robinson SC, Mills GD, Sullivan GD, Karalus NC, McLachlan JD, Hancox RJ. Biochemical markers of cardiac dysfunction predict mortality in acute exacerbations of COPD. Thorax. 2011;66:764–768. doi: 10.1136/thx.2010.155333. [DOI] [PubMed] [Google Scholar]
- 30.Zvezdin B, Milutinov S, Kojicic M, Hadnadjev M, Hromis S, Markovic M, Gajic O. A postmortem analysis of major causes of early death in patients hospitalized with COPD exacerbation. Chest. 2009;136:376–380. doi: 10.1378/chest.08-2918. [DOI] [PubMed] [Google Scholar]
- 31.Rabe KF, Wedzicha JA. Controversies in treatment of chronic obstructive pulmonary disease. Lancet. 2011;378:1038–1047. doi: 10.1016/S0140-6736(11)61295-6. [DOI] [PubMed] [Google Scholar]
- 32.Chen J, Radford MJ, Wang Y, Marciniak TA, Krumholz HM. Effectiveness of beta-blocker therapy after acute myocardial infarction in elderly patients with chronic obstructive pulmonary disease or asthma. J Am Coll Cardiol. 2001;37:1950–1956. doi: 10.1016/s0735-1097(01)01225-6. [DOI] [PubMed] [Google Scholar]
- 33.Short PM, Lipworth SI, Elder DH, Schembri S, Lipworth BJ. Effect of {beta} blockers in treatment of chronic obstructive pulmonary disease: a retrospective cohort study. BMJ. 2011;342:d2549. doi: 10.1136/bmj.d2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kazani S, Israel E. Treatment with beta blockers in people with COPD. BMJ. 2011;342:d2655. doi: 10.1136/bmj.d2655. [DOI] [PubMed] [Google Scholar]
- 35.Sjahid SI, van der Linden PD, Stricker BH. Agreement between the pharmacy medication history and patient interview for cardiovascular drugs: the Rotterdam elderly study. Br J Clin Pharmacol. 1998;45:591–595. doi: 10.1046/j.1365-2125.1998.00716.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


