Abstract
Aims
This study aimed to determine the association between potentially inappropriate prescribing (PIP) and health related outcomes [adverse drug events (ADEs), health related quality of life (HRQOL) and hospital accident and emergency (A&E) visits] in older community dwelling patients.
Methods
A retrospective cohort study of 931 community dwelling patients aged ≥70 years in 15 general practices in Ireland in 2010. PIP was defined by the Screening Tool of Older Person's Prescriptions (STOPP). ADEs were measured by patient self-report and medical record for the previous 6 months and reviewed by two independent clinicians. HRQOL was measured by the EQ-5D. A&E visits were measured by patients’ medical records and self-report. Multilevel logistic, linear and Poisson regression examined how ADEs, HRQOL and A&E visits varied by PIP after adjusting for patient and practice level covariates: socioeconomic status, co-morbidity, number of drug classes and adherence.
Results
The overall prevalence of PIP was 42% (n = 377). Patients with ≥2 PIP indicators were twice as likely to have an ADE (adjusted OR 2.21; 95% CI 1.02, 4.83, P < 0.05), have a significantly lower mean HRQOL utility (adjusted coefficient −0.09, SE 0.02, P < 0.001) and nearly a two-fold increased risk in the expected rate of A&E visits (adjusted IRR 1.85; 95% CI 1.32, 2.58, P < 0.001). The number of drug classes and adherence were also significantly associated with these same adverse health outcomes.
Conclusions
Reducing PIP in primary care may help lower the burden of ADEs, its associated health care use and costs and enhance quality of life in older patients.
Keywords: adverse drug events, health care use, HRQOL, older populations, potentially inappropriate prescribing, STOPP
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT
Previous studies have evaluated the prevalence and patterns of potentially inappropriate prescribing (PIP) in older populations but its effect on health outcomes (adverse drug events (ADEs), health related quality of life (HRQOL) and hospital visits) is still largely unknown.
Information on ADEs in older populations in hospitals and nursing home settings has grown substantially but there is limited information on ADEs in community dwelling patients.
Patient reported outcomes and adherence play an important role in assessing the efficacy of drug treatment in community dwelling older populations and few studies have considered such patient centred outcomes.
WHAT THIS STUDY ADDS
Almost 80% of the cohort of community dwelling older patients experienced at least one ADE in the previous 6 months.
PIP is independently associated with ADEs, reduced HRQOL and increased A&E visits in the older community dwelling population.
Reducing PIP in primary care may help lower the burden of ADEs, its associated health care use and costs and enhance quality of life in older patients.
Introduction
Medication related morbidity and mortality are a major health care concern in older populations and a significant burden on health care resources. Older people often have numerous co-morbidities, limited physiological reserve and are prescribed many medications, thereby increasing the risk of adverse drug events (ADEs), reduced health related quality of life (HRQOL) and hospitalization [1]. The prevalence of ADEs in community dwelling older patients in the US has been estimated as 50.1 per 1000 person-years, with 27.6% of events considered to be preventable [2]. An estimated 265 802 emergency department visits for ADEs occurred in older patients annually from 2007 to 2009 in the US with 37.5% requiring hospitalization [3]. In Europe nearly 10%–20% of acute older patient hospital admissions are drug related [4].
There is an increasing focus on potentially inappropriate medication use as a possible cause of adverse health outcomes in older populations and a number of criteria and screening tools have been developed to measure and assist prescribers in detecting potentially inappropriate prescribing (PIP). These measures consist of drugs to be avoided in older people independent of diagnosis or in the context of certain diagnoses [5]. The Screening Tool of Older Person's Prescription (STOPP) consists of 65 indicators of PIP associated with ADEs in older populations [6]. Prevalence rates of 22% have been reported in the US, 35%–77% in Europe and 24%–36% in Asia [7–10].
To date, there has been limited and conflicting evidence of an association between current measures of PIP and adverse patient outcomes restricting their value as indicators of clinical care in practice settings [1]. The focus has also largely been on older patients who are hospitalized, in nursing homes or attending outpatient clinics with few studies of primary care or community based patients [11]. Few studies of PIP and its association with adverse health outcomes involve patients directly and data are limited regarding patient reported adverse outcomes due to medication. The aims of the present study were to determine the association between PIP, as defined by the STOPP criteria, and patient reported adverse outcomes including ADEs, HRQOL and accident and emergency (A&E) department visits in an older community dwelling cohort in Ireland in 2010.
Methods
Study population
This is a retrospective cohort study examining the association between PIP defined by the STOPP criteria and patient-related health outcomes (ADEs, HRQOL, A&E visits) in a cohort of general practice (GP) patients aged ≥70 years in 15 practices in the Republic of Ireland in 2010. A random sample of practices affiliated with the Royal College of Surgeons and Trinity College Dublin were invited to take part in the study (response rate 81%). Patients aged ≥70 years, in the 15 participating practices, were assessed for eligibility to take part in the study by the research team and their GP (Figure 1). A random sample of eligible patients from each of the 15 participating practices was invited to take part in the study using proportionate stratified random sampling (Figure 1). Patients were recruited over a 5 month period from June to October 2010. Ethical approval was granted by the Royal College of Surgeons in Ireland. All participants gave informed consent before taking part in the study.
Figure 1.
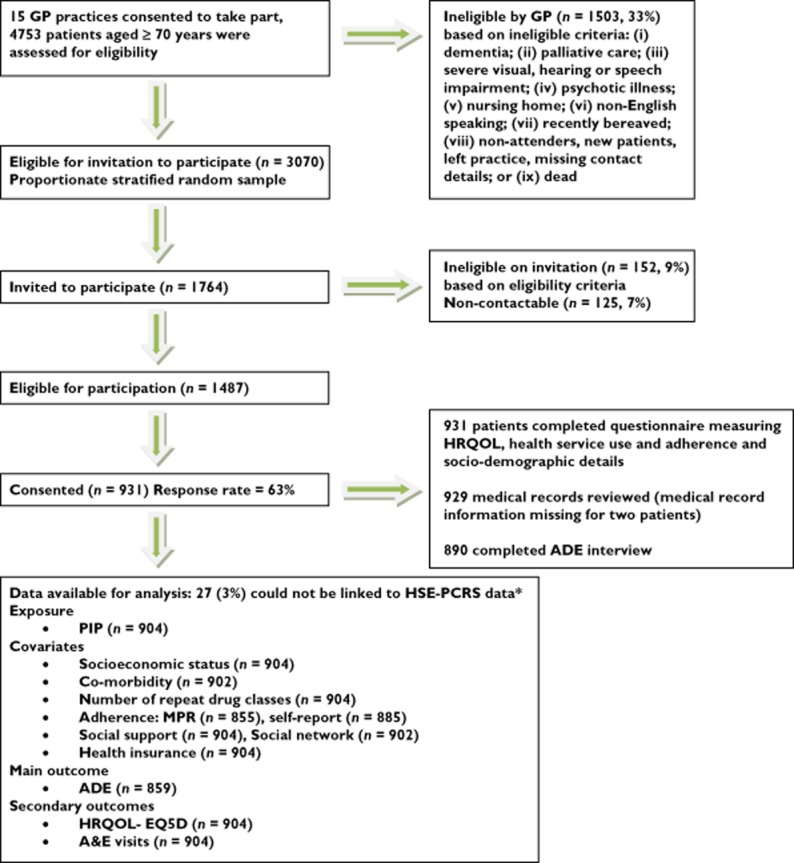
Number of patients at each stage of the study. *3% of patients could not be linked to the HSE-PCRS pharmacy claims database and were excluded. The socio-demographic characteristics of the HSE-PCRS and non-linked patients were compared and there were no significant differences (Chi Square: P > 0.05)
Exposure to PIP
Information on patient dispensed medications for the 6 months prior to each patient's date of interview for potential ADEs was extracted from the HSE-PCRS pharmacy claims database. The HSE-PCRS general medical services scheme is means tested and provides free health services, including medications, to eligible persons in Ireland. It is estimated that over 97% of older patients nationally avail of the scheme [12]. Prescriptions are coded using the World Health Organization Anatomical Therapeutic Chemical (ATC) classification system and prescriber information, defined daily doses, strength, quantity, method and unit of administration of each drug dispensed are available [13]. Consent was obtained from patients to link their prescription dispensing information with the information they reported and their GP medical record.
Fifty (77%) of the 65 STOPP criteria were applied to all patients’ dispensed medication for the study period. There was insufficient clinical information in some patients’ medical records to apply all of the criteria. All of the available STOPP criteria were included in a composite indicator which measured the total number of PIP indicators per patient classified into three levels: no indicators, 1 indicator and ≥2 indicators.
Main outcome
The main outcome measure was patient self-reported ADEs and their association with PIP. Consistent with other studies, an ADE was defined as ‘an event which results in unintended harm to the patient and is related to the care and/or services provided to the patient, rather than to the patient's underlying medical conditions’ [14]. Self-reported ADEs were low severity ADEs in the community that did not necessarily result in hospital admission.
Patients’ GP medical records were reviewed for repeat and acute prescriptions, drug allergies, ADEs, ongoing medical conditions, number of episodes of care and hospitalization for the 6 months prior to date of consent. This information formed the basis for a nurse led face-to-face or phone based interview about potential ADEs in the previous 6 months. Patients were asked if they had experienced a list of 74 specific symptoms (classified by physiological system) during the previous 6 months based on previously published work [15–17]. If the patient reported the symptom more structured questions followed including: (i) whether the patient believed the symptom to be caused by their medication; (ii) the name of the medication; (iii) approximate date symptom began; (iv) duration; (v) how much it had bothered them; (vi) had they discussed it with their GP; (vii) what action their GP had taken; and (viii) were they hospitalized because of the symptom. Patients who had a report of an ADE in their GP record were asked about the ADE if it had not already arisen as one of the 74 specific symptoms. Patients were also asked about over the counter (OTC) medication use.
Patient self-reported symptoms were reviewed by two independent academic clinicians who were blinded to the STOPP criteria. The reviewers determined the likelihood that the symptom was related to a medication and classified the symptom as an ADE if the likelihood exceeded 50%. This likelihood scale and cut off point were based on previous research using patient self-report of ADEs [17]. A random sample of 20% of patients was independently re-evaluated by a third clinician and the percentage of agreement and the kappa (k) statistic were calculated [18]. Any differences between the reviewers’ classification of ADEs were evaluated by a fourth clinician.
Secondary outcomes
HRQOL was measured using the EQ-5D, and converted into a single utility value for each patient based on the UK population value set which was derived using the time trade off valuation technique [19]. A questionnaire measuring HRQOL and other patient reported outcomes was sent to each patient with the option to self-complete, complete by phone or in person. The number of A&E visits for the 6 months prior to patients’ date of consent was measured by patient medical record review and self-report.
Covariates
Covariates included patient age, gender, socioeconomic status, private health insurance, co-morbidity, number of different repeat drug classes, social support and social network, adherence and practice level gender and deprivation. Patient socioeconomic status was established by social class and deprivation level [20]. Co-morbidity was measured using the Charlson co-morbidity index [21]. The number of different repeat drug classes was calculated using the HSE-PCRS pharmacy claims data for the 6 months previous to patients’ date of interview [10]. Social support was measured using the Medical Outcomes Social Support Survey (MOS) and the Lubbens Social Network Scale (LSNS) [22, 23]. The MOS is based on patients’ subjective assessment of affectionate, informational and physical support. The LSNS is an objective measure of family and friends networks, for use with older people, which asks patients how many people they have contact with and how often.
Adherence to medication was measured by: (i) the Medication Possession Ratio (MPR) and (ii) a self-report measure, Morisky Medication Adherence Scale (MMAS) [24, 25]. The MPR was calculated as the sum of the days supplied for all medications divided by the individual patient 6 month study period using the HSE-PCRS pharmacy claims data [24]. The average MPR across all drugs dispensed (ATC code) was calculated for each patient. At least two prescription fill dates were required for each drug [24]. MPR was converted to a percentage and categorized into three levels of adherence based on the percentage of days covered by dispensed medication, <50%, ≥50% <80% and ≥80%. Patients scoring ≥11 on the MMAS were classified as adherent, based on how patients theoretically would have completed the MMAS if they had taken at least 95% of prescribed doses [25].
Data analysis
The overall prevalence of PIP and the prevalence per individual STOPP criteria were calculated as a proportion of all eligible patients aged ≥70 years in the 15 practices in 2010. A retrospective national population study using the HSE-PCRS pharmacy claims data indicated that approximately 36% of those aged ≥70 years in Ireland received at least one potentially inappropriate indicator per STOPP criteria in 2007 [10]. An ADE rate of 10% was assumed for those not on any potentially inappropriate medications and 20% for those prescribed any potentially inappropriate medication [16, 26, 27]. For a significance level of 5% (two-sided) and power of 90%, a sample size of 800 was required. The number and percentage of patients with ADEs was calculated and the classes of drug most frequently associated with ADEs were identified. Multilevel logistic regression unadjusted and adjusted odds ratios with 95% confidence intervals (CIs) were estimated in a two level random intercept logistic model for: (i) patient level one exposure variable (PIP); (ii) patient level one covariates (age, gender, socioeconomic status, co-morbidity, number of different repeat drug classes, adherence); and (iii) practice level two covariates (gender, deprivation).
Multilevel linear regression investigated the association between PIP and HRQOL (EQ-5D). The model was additionally adjusted for patients’ perceived level of social support (MOS) and social network (LSNS). Multilevel Poisson regression investigated the association between PIP and the number of A&E visits. Incidence rate ratios (IRR) and 95% CIs were estimated [28]. The model was additionally adjusted for patients with private health insurance. Initial data analysis and application of the STOPP criteria to the data set was performed using SAS statistical software package version 9.1 (SAS Institute Inc. Cary, NC, USA). Multilevel modelling was performed in STATA Version 11.2 (StataCorp, Texas, USA). All of the variables and residuals were checked graphically for linearity, normality, heteroskedasticity and outliers.
Results
Study population
Nine hundred and thirty-one community-dwelling patients took part in the study of whom 504 (54%) were female and 584 (63%) were aged ≥75 years (mean age: 78, SD: 5.4, range: 70–98 years). Figure 1 outlines the number of participants at each stage of the study. The overall response rate was 63%.
Exposure to PIP
The prevalence of PIP in the older cohort, considering all fifty STOPP criteria, was 42% (n = 377). Two hundred and fifteen participants (almost 25% of the cohort), were prescribed one PIP indicator, 89 (10%) were prescribed two, 49 (5%) were prescribed three and 24 (3%) were prescribed four or more. Table 1 presents the most common PIP indicators.
Table 1.
The 10 most frequently prescribed PIP indicators as per STOPP criteria
| Criteria description | n | % (95% CI) |
|---|---|---|
| Cardiovascular system | ||
| β-adrenoceptor blocker with COPD (risk of increased bronchospasm) | 28 | 3.10 (2.90, 3.29) |
| Calcium channel blockers with chronic constipation* | 63 | 6.97 (6.55, 7.39) |
| Aspirin and warfarin without histamine H2-receptor antagonist (except cimetidine) or PPI (high risk of gastrointestinal bleeding) | 23 | 2.54 (2.38, 2.71) |
| Aspirin with a past history of peptic ulcer disease without histamine H2-receptor antagonist or PPI (risk of bleeding) | 58 | 6.42 (6.02, 6.81) |
| Central nervous system and psychotropic drugs | ||
| Long term (i.e. >1 month), long-acting benzodiazepines (risk of prolonged sedation, confusion, impaired balance, falls) | 23 | 2.54 (2.38, 2.71) |
| Gastrointestinal system | ||
| PPI for peptic ulcer disease at maximum therapeutic dosage for >8 weeks† (dose reduction or earlier discontinuation indicated) | 146 | 16.55 (15.27, 17.03) |
| Musculoskeletal system | ||
| NSAID with history of peptic ulcer disease or gastrointestinal bleeding, unless with concurrent histamine H2-receptor antagonist, PPI or misoprostol (risk of peptic ulcer relapse) | 26 | 2.88 (2.69, 3.06) |
| Long term use of NSAID (i.e. >3 months) for pain relief (simple analgesics preferable) | 62 | 6.86 (6.44, 7.27) |
| Analgesic drugs | ||
| Regular opiates for more than 2 weeks in those with chronic constipation without concurrent use of laxatives (risk of severe constipation) | 43 | 4.76 (4.46, 5.05) |
| Duplicate drug class prescription | ||
| All duplicates- two concurrent NSAIDs, SSRIs, loop diuretics and ACE inhibitors‡ (optimization of monotherapy within a single drug class) | 39 | 4.31 (4.05, 4.58) |
ACE inhibitors, angiotensin converting enzyme inhibitors; COPD, chronic obstructive pulmonary disease; NSAID, non-steroidal anti-inflammatory drug; PPI, proton pump inhibitor; SSRI, selective serotonin re-uptake inhibitor; TCA, tricyclic antidepressant.
Prevalence was assessed using patient report of chronic constipation and by GP record.
PPI at maximum therapeutic dose = 40 mg daily omeprazole, pantoprazole and esomeprazole, 30 mg daily lansoprazole and 20 mg daily rabeprazole.
Adjusted for those receiving more than one duplicate prescription.
Main outcome: ADEs
In total, 674 (78%) participants were classified as having at least one ADE during the study period: 172 (20%) had one ADE, 152 (18%) had two, 118 (14%) had three and 232 (27%) had four or more. The median number of ADEs was 2 (IQR 1–4). Twenty-four percent of reported symptoms were established as an ADE per patient self-report and clinician review. There was 95% agreement between reviewers (k = 0.88, 95% CI 0.78, 0.98). Antithrombotic agents, mainly aspirin and warfarin, were the drugs most frequently associated with ADEs with 59% of participants reporting bruising, bleeding and indigestion or heartburn as the main adverse effects (Table 2). Anti-inflammatory and antirheumatic products (8% of participants) were also associated with the adverse effects of indigestion or heartburn and bruising. Analgesics (12% of participants), psychoanaleptics (8% of participants) and psycholeptics (6% of participants) were associated with the adverse effects of dizziness and lightheadness, unsteadiness on feet and constipation (Table 2). Table 3 shows the number and percentage of participants and the unadjusted and adjusted odds ratios for participants with at least one ADE by exposure to PIP and significant covariates in a two level random intercept logistic model. The likelihood of an ADE increased significantly with PIP: 94% of participants with ≥2 PIPs had an ADE compared with 71% for those with none. Participants with ≥2 PIPs were twice as likely to have an ADE, after adjusting for patient and practice level covariates. The number of different repeat drug classes and MPR were significantly associated with an ADE.
Table 2.
The therapeutic groups of drugs associated with ADEs (patient self-report and established as an ADE by independent clinician review)
| Therapeutic group (ATC) | n (%) | Main drug substances | n (%) of therapeutic group |
|---|---|---|---|
| Antithrombotic agents (B01) | 508 (59) | Aspirin | 395 (78) |
| Warfarin | 95 (19) | ||
| Diuretics (C03) | 337 (39) | Furosemide | 144 (43) |
| Bendroflumethiazide | 115 (34) | ||
| β-adrenoceptor blocking agents (C07) | 250 (29) | Bisoprolol | 155 (62) |
| Atenolol | 48 (19) | ||
| Calcium channel blockers (C08) | 132 (15) | Amlodipine | 86 (65) |
| Lercanidipine | 21 (16) | ||
| Agents acting on the renin-angiotensin system (C09) | 120 (14) | Ramipril | 42 (35) |
| Perindopril | 33 (28) | ||
| Serum lipid reducing agents (C10) | 112 (13) | Atorvastatin | 69 (62) |
| Pravastatin | 16 (14) | ||
| Analgesics (N02) | 102 (12) | Codeine combinations | 37 (36) |
| Tramadol | 36 (35) | ||
| Anti-inflammatory and antirheumatic products (M01) | 71 (8) | Diclofenac | 45 (63) |
| Ibuprofen | 13 (18) | ||
| Psychoanaleptics (N06) | 65 (8) | Amitriptyline | 35 (54) |
| Doxepin | 6 (9) | ||
| Psycholeptics (N05) | 48 (6) | Benzodiazepines | 27 (56) |
| Trifluoperazine | 7 (15) |
Table 3.
Number and percentage of participants and multilevel unadjusted and adjusted odds ratios (95% CI) for participants with at least one ADE by exposure to PIP and patient and practice level covariates*
| ADE | ||||
|---|---|---|---|---|
| Patient level fixed effects | Total (n) | n (%), median (IQR) with ≥1 ADE | Unadjusted OR (95% CI) (n = 859) | Adjusted OR (95% CI) (n = 806)† |
| Co-morbidity | n (%) | |||
| Charlson weights | ||||
| 0 | 512 | 373 (73) | 1 | 1 |
| ≥1 | 347 | 301 (87) | 2.46 (1.69, 3.59)‡ | 1.34 (0.87, 2.07) |
| Median (IQR) | ||||
| Number of drug classes | – | 6 (4, 9) | 1.42 (1.32, 1.52)‡ | 1.27 (1.16, 1.40)‡ |
| MPR§ | n (%) | |||
| MPR <50% | 67 | 41 (61) | 0.34 (0.20, 0.57) | - |
| MPR ≥50% <80% | 181 | 138 (76) | 0.72 (0.17, 0.95) | 1.17 (0.59, 2.30) |
| MPR ≥80% | 565 | 478 (85) | 2.08 (1.44, 2.99)‡ | 1.92 (1.03, 3.60)‡ |
| Self-report adherence§ | n (%) | |||
| Non-adherent | 317 | 256 (81) | 1 | 1 |
| Adherent | 527 | 413 (78) | 0.87 (0.61, 1.24) | 0.72 (0.47, 1.09) |
| PIP | n (%) | |||
| 0 | 495 | 350 (71) | 1 | 1 |
| 1 | 209 | 178 (85) | 2.43 (1.57, 3.76)‡ | 1.26 (0.77, 2.05) |
| ≥2 | 155 | 146 (94) | 7.20 (3.53, 14.66)‡ | 2.21 (1.02, 4.83)‡ |
Non-significant patient and practice level covariates are not reported (patient age, gender, social class, deprivation, GP gender, deprivation).
In the adjusted model, data were missing for 52 (6%) participants, MPR was missing for 45 (5%) participants and self-report adherence was missing for seven (0.8%) participants. These participants were excluded from the multivariable analysis (n = 806).
z score (P < 0.05).
In the unadjusted model, medication possession ratio (MPR) was missing for 46 (5%) participants and self-report adherence was missing for 15 (2%) participants.
Secondary outcome: HRQOL
The mean EQ-5D utility was 0.75 (SD 0.24). Table 4 shows the mean (SD) EQ-5D utility, the unadjusted and adjusted coefficients, and standard errors by exposure to PIP and significant covariates in a two level random intercept model. In the adjusted model, there was a statistically significant reduction in HRQOL utility of 0.09 for participants with two or more PIP indicators compared with no PIP. Age, gender, social class, co-morbidity, the number of different repeat drug classes and adherence were also still significantly associated with HRQOL utility. Participants who had a MPR ≥50% and/or reported being adherent to their medication had a higher mean HRQOL utility score than those who were non-adherent.
Table 4.
Mean (SD) and multilevel linear regression unadjusted and adjusted coefficients (SE) for participant utility (EQ-5D) by exposure to PIP and patient and practice level covariates*
| Patient level fixed effects | Total (n) | Mean (SD) | EQ-5D utility† | |
|---|---|---|---|---|
| Unadjusted coefficient (SE) (n = 904) | Adjusted coefficient (SE) (n = 841)†,‡ | |||
| Age | 904 | – | −0.009 (0.001)§ | −0.006 (0.001)§ |
| Gender | ||||
| Male | 415 | 0.78 (0.22) | – | – |
| Female | 489 | 0.72 (0.25) | −0.06 (0.02)§ | −0.04 (0.02)§ |
| Social class | ||||
| Unskilled | 342 | 0.72 (0.24) | – | – |
| Skilled | 562 | 0.76 (0.23) | 0.05 (0.02)§ | 0.02 (0.01)§ |
| Co-morbidity¶ | ||||
| Charlson weights | ||||
| 0 | 537 | 0.78 (0.22) | – | – |
| ≥1 | 365 | 0.70 (0.24) | −0.07 (0.02)§ | −0.02 (0.02)§ |
| Number of drug classes | 904 | – | −0.03 (0.002)§ | −0.03 (0.004)§ |
| MPR¶ | ||||
| MPR <50% | 76 | 0.72 (0.21) | – | – |
| MPR ≥50% <80% | 187 | 0.76 (0.22) | 0.04 (0.03)** | 0.12 (0.04)§ |
| MPR ≥80% | 592 | 0.74 (0.25) | 0.01 (0.03)** | 0.11 (0.04)§ |
| Self-report adherence¶ | ||||
| Non-adherent | 332 | 0.70 (0.25) | – | – |
| Adherent | 553 | 0.77 (0.22) | 0.06 (0.02)§ | 0.05 (0.02)§ |
| PIP | ||||
| 0 | 527 | 0.80 (0.20) | – | – |
| 1 | 209 | 0.73 (0.23) | −0.06 (0.02)§ | −0.01 (0.02) |
| ≥2 | 155 | 0.60 (0.27) | −0.19 (0.02)§ | −0.09 (0.02)§ |
Non-significant patient and practice level covariates are not reported (patient deprivation, social support, social network, GP gender, deprivation). Age and number of drug classes were measured as continuous variables.
Robust standard errors and P values were calculated using the sandwich estimator.
In the adjusted model data were missing for 63 (7%) participants, co-morbidity was missing for 2 (0.22%) participants, medication possession ratio (MPR) was missing for 49 (5%) participants, self-report adherence was missing for nine (1%) participants, social support was missing for three (0.33%) participants. These participants were excluded from the multivariable analysis (n = 841).
z score: P < 0.05.
In the unadjusted model, co-morbodity was missing for two (0.22%) participants, medication possession ratio (MPR) was missing for 49 (5%) participants, self-report adherence was missing for 19 (2%) participants.
Adjusting the univariable MPR model to include the number of drug classes resulted in a coefficient of 0.15 for both MPR percentages of proportion of days covered (P < 0.0001).
Secondary outcome: A&E visits
Ninety-one (10%) participants had one A&E visit, 15 (2%) had two visits, 19 (2%) had three or more visits. The median number of A&E visits was 1 (IQR 0–2). There was nearly a two-fold increased risk in the expected rate of A&E visits (IRR 1.85, 95% CI 1.32, 2.58, P < 0.0001) for those with ≥2 PIP indicators after adjusting for covariates. The expected number of A&E visits significantly decreased for females (IRR 0.60, 95% CI 0.44, 0.81, P < 0.001), significantly increased (IRR 1.17, 95% CI 1.08, 1.26, P < 0.0001) for every 1 unit increase in the number of drug classes and decreased for participants who were adherent to their medication with a MPR >80% compared with those <50% (IRR 0.37, 95% CI 0.16, 0.82, P < 0.05).
Discussion
Principal findings
The overall prevalence of PIP, defined by the STOPP criteria, in the cohort of older community dwelling patients was 42%. This is higher than the national population PIP prevalence rate based on a subset of the STOPP criteria in 2007 [10]. Seventy-eight percent of the cohort experienced at least one ADE during the 6 month study period with aspirin being the most frequently implicated medication. Participants with ≥2 PIP indicators were twice as likely to have an ADE, had significantly reduced HRQOL, and an increased rate of A&E visits, after adjustment for a number of patient and practice level covariates. The number of repeat drug classes and adherence were also significantly associated with adverse health outcomes.
This study assessed the association between the STOPP criteria and ADEs in a community dwelling older population. A recent study of acutely ill older hospitalized patients reported similar results, with serious avoidable ADEs almost twice as likely in patients with PIP, after adjustment for covariates [11]. Aspirin, aspirin and warfarin, or NSAIDs with a past history of peptic ulcer disease without a H2-receptor antagonist or PPI, were the STOPP indicators with the highest prevalence rates. Low dose aspirin increases the risk of gastrointestinal bleeding and co-prescribing PPIs reduces the risk of gastric ulcer bleeding by nine-fold [29]. Aspirin was the drug most frequently implicated as a causal agent in 18% of hospital admissions in a UK prospective study of ADEs, with gastrointestional bleeding occurring in 72% of all aspirin related admissions [30]. Minor haemorrhages (e.g. bruising, bleeding from small cuts) are estimated to cause 60% of emergency department visits in the US for antithrombotic therapy and have been linked to early discontinuation of therapy [31].
NSAIDs have consistently been shown to be associated with preventable hospital admissions and ADEs, including gastrointestional bleeding and renal dysfunction, in older populations [32]. The prevalence of long term NSAID use was high in the current study (7%) and was associated with a number of gastrointestinal tract complaints. The average cost of hospital admissions per person due to NSAID gastropathy has been estimated as $14 000 in the US and up to 90% may be preventable [33].
No previous research has reported the association between the STOPP criteria and HRQOL and hospitalization in community dwelling older patients. The difference in mean EQ-5D utility in the current study (0.09) exceeds the mean minimally important difference (MID) of 0.07 for the EQ-5D based on eight longitudinal studies and eleven patient groups [34]. The MID is the smallest change in a measure that is perceived by patients as beneficial or would result in a change in the patient's management [35]. A fall of 0.05 in EQ-5D utility has been associated with an increase in 5 years mortality in older populations [36]. A higher rate of A&E visits was reported for patients with PIP in this study. A prospective study of acutely ill older patients found that ADEs resulting from the STOPP criteria were causal or contributory to hospitalization [11].
The number of different repeat medications has consistently been shown to be an independent predictor for PIP and has also been associated with an increased risk of drug interactions, adherence problems and ADEs [17, 37]. Three hundred and eleven medications were discontinued in 64 community dwelling older patients with no significant adverse effects based on an algorithm that identified non-essential medications and those with a negative benefit : risk ratio [38]. Non-adherence to medication has received little attention in studies of PIP and adverse health outcomes. A systematic review identified one-third of drug related hospital admissions as being associated with patient adherence problems [32].
Strengths and limitations
The identification of ADEs was based on a self-report measure of symptoms with independent clinical review to determine the likelihood that the symptom was related to a medication. Patient self-report has inherent limitations due to its dependence on patients’ accurate recall of events. Interview questions were standardized and detailed to minimize recall bias. To measure ADEs accurately, a reliable assessment of the relationship between drug administration and the adverse clinical event is required both in terms of causality, avoidability and severity criteria [11]. Such criteria are difficult to interpret in the context of patient reported ADEs, multiple co-morbidities and medications and, in particular, in the community setting where there is limited availability of clinical and laboratory data. In general, the current study identified common, low severity ADEs in older patients that could reasonably have been predicted and are ameliorable but no formal classification system was applied [17]. The effectiveness and acceptability of a number of alternative pharmacological and non-pharmacological treatment options for the more prevalent STOPP indicators are currently being assessed in a randomized controlled trial in community dwelling patients [39].
This study was conducted across 15 practices in one region in Ireland and the results may not be generalizable to different regions or to the general older population. There were different practice manager software systems and differences in the quality and quantity of data recorded across the practices. In some practices there was not sufficient clinical information to apply the STOPP criteria and these criteria were excluded from the study. The association between PIP and A&E visits needs to be interpreted with caution. The current study controlled for a number of variables associated with health service use but results may be confounded by unknown risk factors.
Notwithstanding the limitations, this study is the first study to measure the association between PIP, defined by the STOPP criteria, and patient-centred adverse health outcomes in older people. The use of patient dispensing data from the national pharmacy claims database (HSE-PCRS) enabled an accurate application of the STOPP criteria, including assessment of drug duration and dosage. Review of patients’ GP medical records provided a clinical history and details of patient co-morbidities. There have been important limitations in the methods of previous studies, including no adjustment for important confounders (e.g. co-morbidity, number of drugs), duration and dose–response relation not considered, small and select samples and dependence on self-reported medication use and medical conditions on hospital admission [1]. The study also controlled for a number of covariates including adherence. OTC medications were also included in the data collection as well as previous history of drug allergies and ADEs.
Policy implications
There is evidence that the STOPP criteria as a process measure of PIP can be linked to adverse health outcomes in older people. This finding, alongside previous findings of an association in the acute care setting, strengthens the argument for the use of STOPP criteria in clinical practice as a means of reducing the risk of adverse health outcomes in older patients [11]. The STOPP criteria were also sensitive enough to detect differences in patient reported outcomes, such as HRQOL and self-reported ADEs, which helps validate their applicability to a primary care setting. Randomized controlled trials are needed to assess if routine application of PIP indicators in clinical practice substantially reduces PIP, improves health outcomes and reduces health care costs in older populations.
Not all PIP defined by the STOPP criteria are inappropriate, depending on individual patient circumstances. Some drug related adverse symptoms may be more tolerable than the severe symptoms associated with the untreated underlying condition. Patients might tolerate fatigue and constipation in order to manage chronic pain [40]. Reducing PIP and adverse health outcomes will require an enhancement in systems to regularly assess drug effectiveness, dosage, duration, interactions, adverse symptoms and adherence, while also balancing the risk of underuse of potentially beneficial drugs [41]. There is a need for patient centred measurement tools in the primary care setting that allow for early detection of ADEs, which may otherwise result in patient discontinuation of treatment or develop into more significant adverse effects requiring medical treatment or hospitalization. Health information technology and patient outreach programmes may provide an effective method of managing and tracking patient reported medication symptoms and engaging patients in monitoring their medication in the future [41].
In conclusion, PIP is a risk factor for ADEs, reduced HRQOL and increased A&E visits independent of the number of medications and other important covariates. This study provides important evidence of the association between the STOPP criteria and adverse health outcomes in older patients, confirming the STOPP criteria as important quality indicators for prescribing practice in community settings. Reducing PIP in primary care may help lower the burden of ADEs, its associated health care use and costs and enhance quality of life in older patients.
Acknowledgments
We wish to thank the Health Services Executive Primary Care Reimbursement Services (HSE-PCRS) for the use of the prescribing database and Dr Anthony Cummins, Dr Emma Wallace and Dr Patrick Redmond for evaluating patient interviews and records for potential ADEs. We are indebted also to all the study participants and the 15 general practices who kindly gave their time to take part in this study.
Competing Interests
Funding: This study was funded by the Health Research Board, HRC/1/2007.
All authors declare that they have no competing interests. All authors have completed the Unified Competing Interest form at http://www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare no support from any organization for the submitted work, no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years and no other relationships or activities that could appear to have influenced the submitted work.
References
- 1.Spinewine A, Schmader KE, Barber N, Hughes C, Lapane KL, Swine C, Hanlon JT. Appropriate prescribing in elderly people: how well can it be measured and optimised? Lancet. 2007;370:173–184. doi: 10.1016/S0140-6736(07)61091-5. [DOI] [PubMed] [Google Scholar]
- 2.Gurwitz JH, Field TS, Harrold LR, Rothschild J, Debellis K, Seger AC, Cadoret C, Fish LS, Garber L, Kelleher M, Bates DW. Incidence and preventability of adverse drug events among older persons in the ambulatory setting. JAMA. 2003;289:1107–1116. doi: 10.1001/jama.289.9.1107. [DOI] [PubMed] [Google Scholar]
- 3.Budnitz DS, Lovegrove MC, Shehab N, Richards CL. Emergency hospitalizations for adverse drug events in older Americans. N Engl J Med. 2011;365:2002–2012. doi: 10.1056/NEJMsa1103053. [DOI] [PubMed] [Google Scholar]
- 4.Routledge PA, Mahony MSO, Woodhouse KW. Adverse drug reactions in elderly patients. Br J Clin Pharmacol. 2004;57:121–126. doi: 10.1046/j.1365-2125.2003.01875.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fick DM, Cooper JW, Wade WE, Waller JL, Maclean JR, Beers MH. Updating the beers criteria for potentially inappropriate medication use in older adults: results of a US consensus panel of experts. Arch Intern Med. 2003;163:2716–2724. doi: 10.1001/archinte.163.22.2716. [DOI] [PubMed] [Google Scholar]
- 6.Gallagher P, Ryan C, Byrne S, Kennedy J, O'Mahony D. STOPP (Screening tool of older person's prescriptions) and START (screening tool to alert doctors to right treatment). Consensus validation. Int J Clin Pharmacol Ther. 2008;46:72–83. doi: 10.5414/cpp46072. [DOI] [PubMed] [Google Scholar]
- 7.Pyszka LL, Seys Ranola TM, Milhans SM. Identification of inappropriate prescribing in geriatrics at a veterans affairs hospital using STOPP/START screening tools. Consult Pharm. 2010;25:365–373. doi: 10.4140/TCP.n.2010.365. [DOI] [PubMed] [Google Scholar]
- 8.Gallagher P, Lang P, Cherubini A, Topinková E, Cruz-Jentoft A, Montero Errasquín B, et al. Prevalence of potentially inappropriate prescribing in an acutely ill population of older patients admitted to six European hospitals. Eur J Clin Pharmacol. 2011;67:1175–1188. doi: 10.1007/s00228-011-1061-0. [DOI] [PubMed] [Google Scholar]
- 9.Liu C-L, Peng L-N, Chen Y-T, Lin M-H, Liu L-K, Chen L-K. Potentially inappropriate prescribing (IP) for elderly medical inpatients in Taiwan: a hospital-based study. Arch Gerontol Geriatr. 2012;55:148–151. doi: 10.1016/j.archger.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 10.Cahir C, Fahey T, Teeling M, Teljeur C, Feely J, Bennett K. Potentially inappropriate prescribing and cost outcomes for older people: a national population study. Br J Clin Pharmacol. 2010;69:543–552. doi: 10.1111/j.1365-2125.2010.03628.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hamilton H, Gallagher P, Ryan C, Byrne S, O'Mahony D. Potentially inappropriate medications defined by STOPP criteria and the risk of adverse drug events in older hospitalized patients. Arch Intern Med. 2011;171:1013–1019. doi: 10.1001/archinternmed.2011.215. [DOI] [PubMed] [Google Scholar]
- 12.Naughton C, Bennett K, Feely J. Prevalence of chronic disease in the elderly based on a national pharmacy claims database. Age Ageing. 2006;35:633–636. doi: 10.1093/ageing/afl106. [DOI] [PubMed] [Google Scholar]
- 13.WHO Collaborating Centre for Drug Statistics Methodology. Anatomical Therapeutic Chemical (ATC) Classification Index. Oslo: WHO Collaborating Centre for Drug Statistics Methodology; 2010. [Google Scholar]
- 14.Parry G, Cline A, Goldman D. Deciphering harm measurement. JAMA. 2012;307:2155–2156. doi: 10.1001/jama.2012.3649. [DOI] [PubMed] [Google Scholar]
- 15.Jarernsiripornkul N, Krska J, Capps PA, Richards RM, Lee A. Patient reporting of potential adverse drug reactions: a methodological study. Br J Clin Pharmacol. 2002;53:318–325. doi: 10.1046/j.0306-5251.2001.01547.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chrischilles EA, VanGilder R, Wright K, Kelly M, Wallace RB. Inappropriate medication use as a risk factor for self-reported adverse drug effects in older adults. J Am Geriatr Soc. 2009;57:1000–1006. doi: 10.1111/j.1532-5415.2009.02269.x. [DOI] [PubMed] [Google Scholar]
- 17.Gandhi TK, Weingart SN, Borus J, Seger AC, Peterson J, Burdick E, Seger DL, Shu K, Federico F, Leape LL, Bates DW. Adverse drug events in ambulatory care. N Engl J Med. 2003;348:1556–1564. doi: 10.1056/NEJMsa020703. [DOI] [PubMed] [Google Scholar]
- 18.Landis J, Koch G. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–174. [PubMed] [Google Scholar]
- 19.The EuroQol Group. EuroQol- A new facility for the measurement of health-related quality of life. Health Policy. 1990;16:199–208. doi: 10.1016/0168-8510(90)90421-9. [DOI] [PubMed] [Google Scholar]
- 20.Grundy E, Holt G. The socioeconomic status of older adults: how should we measure it in studies of health inequalities? J Epidemiol Community Health. 2001;55:895–904. doi: 10.1136/jech.55.12.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Charlson M, Pompei P, Ales K, MacKenzie C. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 22.Sherbourne CD, Stewart AL. The MOS social support survey. Soc Sci Med. 1991;32:705–714. doi: 10.1016/0277-9536(91)90150-b. [DOI] [PubMed] [Google Scholar]
- 23.Lubben J, Blozik E, Gillmann G, Iliffe S, von Renteln Kruse W, Beck JC, Stuck AE. Performance of an abbreviated version of the lubben social network scale among three european community-dwelling older adult populations. Gerontologist. 2006;46:503–513. doi: 10.1093/geront/46.4.503. [DOI] [PubMed] [Google Scholar]
- 24.Peterson AM, Nau DP, Cramer JA, Benner J, Gwadry-Sridhar F, Nichol M. A checklist for medication compliance and persistence studies using retrospective databases. Value Health. 2007;10:3–12. doi: 10.1111/j.1524-4733.2006.00139.x. [DOI] [PubMed] [Google Scholar]
- 25.Morisky DE, Ward HJ, Liu KY. Self-reported medication taking behaviour: a valid indicator for assessing compliance. In 129th American Public Health Association Meeting. Atlanta, 2001.
- 26.Hanlon JT, Schmader KE. What types of inappropriate prescribing predict adverse drug reactions in older adults? Ann Pharmacother. 2010;44:1110–1111. doi: 10.1345/aph.1P182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lund BC, Carnahan RM, Egge JA, Chrischilles EA, Kaboli PJ. Inappropriate prescribing predicts adverse drug events in older adults. Ann Pharmacother. 2010;44:957–963. doi: 10.1345/aph.1m657. [DOI] [PubMed] [Google Scholar]
- 28.Gelman A, Hall J. Data Analysis Using Regression and Multilevel/Hierarchial Models. London: Cambridge University Press; 2003. [Google Scholar]
- 29.Lai KC, Lam SK, Chu KM, Wong BCY, Hui WM, Hu WHC, Lau GKK, Wong WM, Yuen MF, Chan AOO, Lai CL, Wong J. Lansoprazole for the prevention of recurrences of ulcer complications from long-term low-dose aspirin use. N Engl J Med. 2002;346:2033–2038. doi: 10.1056/NEJMoa012877. [DOI] [PubMed] [Google Scholar]
- 30.Pirmohamed M, James S, Meakin S, Green C, Scott AK, Walley TJ, Farrar K, Park BK, Breckenridge AM. Adverse drug reactions as cause of admission to hospital: prospective analysis of 18 820 patients. BMJ. 2004;329:15–19. doi: 10.1136/bmj.329.7456.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shehab N, Sperling LS, Keqler SR, Budnitz DS. National estimates of emergency department visits for hemorrhage-related adverse events from clopidogrel plus aspirin and from warfarin. Arch Intern Med. 2010;170:1926–1933. doi: 10.1001/archinternmed.2010.407. [DOI] [PubMed] [Google Scholar]
- 32.Howard RL, Avery AJ, Slavenburg S, Royal S, Pipe G, Lucassen P, Pirmohamed M. Which drugs cause preventable admissions to hospital? A systematic review. Br J Clin Pharmacol. 2007;63:136–147. doi: 10.1111/j.1365-2125.2006.02698.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fick DM, Waller JL, Maclean JR, Heuvel RV, Tadlock JG, Gottlieb M, Cangialose CB. Potentially inappropriate medication use in a medicare managed care population: association with higher costs and utilization. J Manag Care Pharm. 2001;7:407–413. [Google Scholar]
- 34.Walters S, Brazier J. Comparison of the minimally important difference for two health state utility measures: EQ-5D and SF-6D. Qual Life Res. 2005;14:1523–1532. doi: 10.1007/s11136-004-7713-0. [DOI] [PubMed] [Google Scholar]
- 35.Revicki DA, Cella D, Hays RD, Sloan JA, Lenderking WR, Aaronson NK. Responsiveness and minimal important differences for patient reported outcomes. Health Qual Life Outcomes. 2006;4:70–75. doi: 10.1186/1477-7525-4-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Perera S, Studenski S, Chandler JM, Guralnik JM. Magnitude and patterns of decline in health and function in 1 year affect subsequent 5-year survival. J Gerontol A Biol Sci Med Sci. 2005;60:894–900. doi: 10.1093/gerona/60.7.894. [DOI] [PubMed] [Google Scholar]
- 37.Guthrie B, McCowan C, Davey P, Simpson CR, Dreischulte T, Barnett K. High risk prescribing in primary care patients particularly vulnerable to adverse drug events: cross sectional population database analysis in Scottish general practice. BMJ. 2011;342:d3514. doi: 10.1136/bmj.d3514. [DOI] [PubMed] [Google Scholar]
- 38.Garfinkel D, Mangin D. Feasibility study of a systematic approach for discontinuation of multiple medications in older adults: addressing polypharmacy. Arch Intern Med. 2010;170:1648–1654. doi: 10.1001/archinternmed.2010.355. [DOI] [PubMed] [Google Scholar]
- 39.Clyne B, Bradley M, Smith SM, Hughes CM, Motterlini N, Clear D, McDonnell R, Williams D, Fahey T OPTI-SCRIPT Study Team. Effectiveness of medicines review with web-based pharmaceutical treatment algorithms in reducing potentially inappropriate prescribing in older people in primary care: a cluster randomized trial (OPTI-SCRIPT study protocol) BMC Trials. 2013;14:72. doi: 10.1186/1745-6215-14-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weingart SN, Gandhi TK, Seger AC, Seger DL, Borus J, Burdick E, Leape LL, Bates DW. Patient-reported medication symptoms in primary care. Arch Intern Med. 2005;165:234–240. doi: 10.1001/archinte.165.2.234. [DOI] [PubMed] [Google Scholar]
- 41.Steinman MA, Handler SM, Gurwitz JH, Schiff GD, Covinsky KE. Beyond the prescription: medication monitoring and adverse drug events in older adults. J Am Geriatr Soc. 2011;59:1513–1520. doi: 10.1111/j.1532-5415.2011.03500.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


