Abstract
Background
A phase II study of bevacizumab (BVZ) plus irinotecan (CPT-11) was conducted in children with recurrent low-grade glioma to measure sustained response and/or stable disease lasting ≥6 months and progression-free survival.
Methods
Thirty-five evaluable patients received 2 doses (10 mg/kg each) of single-agent BVZ intravenously 2 weeks apart and then BVZ + CPT-11 every 2 weeks until progressive disease, unacceptable toxicity, or a maximum of 2 years of therapy. Correlative studies included neuroimaging and expression of tumor angiogenic markers (vascular endothelial growth factor [VEGF], VEGF receptor 2, hypoxia-inducible factor 2α, and carbonic anhydrase 9).
Results
Thirty-five evaluable patients (median age 8.4 y [range, 0.6–17.6]) received a median of 12 courses of BVZ + CPT-11 (range, 2–26). Twenty-nine of 35 patients (83%) received treatment for at least 6 months. Eight patients progressed on treatment at a median time of 5.4 months (range, 1–17.8). Six patients (17.7%) still in follow-up have had stable disease without receiving additional treatment for a median of 40.1 months (range, 30.6–49.3) from initiating therapy. The 6-month and 2-year progression-free survivals were 85.4% (SE ± 5.96%) and 47.8% (SE ± 9.27%), respectively. The commonest toxicities related to BVZ included grades 1–2 hypertension in 24, grades 1–2 fatigue in 23, grades 1–2 epistaxis in 18, and grades 1–4 proteinuria in 15. The median volume of enhancement decreased significantly between baseline and day 15 (P < .0001) and over the duration of treatment (P < .037).
Conclusion
The combination of BVZ + CPT-11 appears to produce sustained disease control in some children with recurrent low-grade gliomas.
Keywords: bevacizumab, CPT-11, children, gliomas, recurrent
Pediatric low-grade glioma (LGG) is a heterogeneous group of tumors constituting the most frequent CNS neoplasia encountered in this population. Pilocytic astrocytoma (PA) is the commonest LGG in children.1 Initial treatment of children with LGG (excluding the optic pathway and intrinsic brainstem lesions) is surgical resection and is often curative.2 Patients with recurrent LGG usually receive chemotherapy and/or focal radiotherapy.3,4 Cytotoxic treatment, especially radiotherapy, has the potential for causing serious long-term toxicities, especially in young children with a disease that can be chronic and compatible with prolonged survival.5,6 Also, patients with recurrent LGG who fail standard chemotherapy regimens and/or radiotherapy have few available treatment options, and novel therapies are urgently needed.
Tumor angiogenesis is a process that is essential for tumor growth beyond a certain size and ultimately promotes tumor infiltration and metastasis.7 Vascular endothelial growth factor (VEGF) is a key angiogenesis mediator that is overexpressed in CNS tumors, including LGG.8–10 Bevacizumab (BVZ; Avastin, Genentech) is a humanized monoclonal antibody that is highly specific for all VEGF isoforms.11 In 2006, based on promising results from the use of BVZ + camptothecin (CPT)-11 in adults with recurrent malignant glioma, the Pediatric Brain Tumor Consortium (PBTC) initiated a phase II study in children with recurrent brain tumors, including malignant glioma, brainstem glioma, ependymoma, and medulloblastoma.12,13 In 2008, following encouraging results from a limited institution pilot study of the same combination in 10 patients with recurrent LGG, an additional stratum was added to assess the efficacy and toxicity of this regimen in this patient population.14,15 We now report on the efficacy of this combination in this stratum.
Patients and Methods
Study Objectives
The primary objective of the study was to estimate the rates of sustained (≥8 wk) objective response (OR) (complete response [CR] + partial response [PR]) to BVZ + CPT-11 in children with recurrent LGG over 4 courses of therapy and stable disease (SD) over 6 courses (measured at courses 2, 4, and 6). This endpoint will be denoted as OR/SD6 henceforth. Secondary objectives included estimating (i) treatment-related toxicities and progression-free survival (PFS), (ii) overall survival in patients who consented to follow-up for 5 years after initiation of treatment, (iii) changes in perfusion/diffusion on MRI and 18F-fluorodeoxyglucose (FDG) uptake using PET during treatment, (iv) BVZ plasma pharmacokinetics (results to be reported separately), and (v) expression of VEGF-A and -B, hypoxia-inducible factor (HIF)–2α, VEGF receptor (VEGFR)–2, and carbonic anhydrase (CA)–9 by tumor immunohistochemistry correlated with tumor response and PFS.16
Eligibility Criteria
Inclusion criteria
Patients <21 years old with recurrent or progressive histologically confirmed LGG and measurable disease were eligible for this study (those with visual pathway tumors or intrinsic brainstem tumors did not need histologic confirmation). Subjects were required to have a Karnofsky/Lansky score of at least 50, received at least standard chemotherapy and/or radiotherapy, be ≥3 weeks from prior myelosuppressive chemo- or biologic therapy, and be ≥6 weeks from prior major surgical resection and ≥3 months from local radiotherapy. Required evidence of adequate organ function included an absolute neutrophil count of ≥1500/µL (unsupported), platelets ≥100,000/µL (unsupported), hemoglobin >8 g/dL, serum creatinine less than or equal to the upper limit of institutional normal (ULN), blood urea nitrogen <25 mg/dL, bilirubin ≤1.5 × ULN, and serum glutamic oxaloacetic transaminase and serum glutamic pyruvic transaminase ≤3 × ULN. Eligibility also required absence of active systemic illness, stable neurologic function and corticosteroid dose (if any), and an agreement to use a medically acceptable form of birth control (in those of childbearing or fathering potential).
Exclusion criteria
The general and BVZ-related exclusion criteria have been reported previously.12,13 The institutional review boards of each PBTC institution approved the protocol before initial patient enrollment; continuing approval was maintained throughout the study. Patients or their legal guardians gave written informed consent, and assent was obtained as appropriate at the time of enrollment.
Treatment plan, dose modifications, required observations prior to, during, and off study, and evaluation of response are available in the Appendix online.
Evaluation of permeability, perfusion, diffusion, and FDG-PET parameters was performed as described previously.12,13 In consenting patients, paraffin-embedded tumor sections were obtained from already available tumor tissue, stained for 4 protein markers (VEGF, VEGFR-2, HIF-2a, and CA-9), and scored by a neuropathologist (R.E.M.) who was unaware of the results of clinical and image analyses.16
Study Design and Statistical Analysis
The primary objective of the study was to determine the true sustained OR (CR + PR) or SD6 to BVZ + CPT-11 in children with recurrent LGG. In order to count as successes, the ORs had to occur within the first 4 courses of treatment and had to be sustained for 8 weeks. Using Simon's minimax 2-stage design with a ≤45% OR/SD6 rate as unacceptable versus a ≥75% OR/SD6 rate as desirable and 5% type I error and 10% type II error rates, 15 patients were enrolled during the first stage and at least 8 sustained ORs and/or SD6 were required to enroll an additional 20 patients in the second stage. The treatment regimen would be deemed ineffective if <21 responses and/or SD6 were observed in 35 patients. The analysis plan also specified models for exploring correlations between MR perfusion/diffusion imaging and FDG-PET uptake and responses (if any) in the same tumor site and with PFS. Wilcoxon signed-rank tests were used to explore changes in the MRI values from baseline to day 15. Linear mixed effects models were used to investigate trends in MRI values over time. To limit undue influence from patients who were able to stay on treatment longer, the longitudinal imaging data included in these models were restricted to those obtained within 1 year of the on-treatment date. To adjust for multiplicity, a Bonferroni adjusted P value threshold of .0017 was used. Results from this analysis were then classified into 3 categories: statistically significant (P ≤ .0017), suggestive (0.0017<P ≤ .05), and no evidence of association (P > .05). Kaplan–Meier estimates of the PFS distribution were obtained based on all eligible patients who received at least 1 dose of BVZ. PFS was measured from the date of initial treatment to the earliest date of disease progression, second malignancy, or death for patients who failed, and to the date of last contact or the date of initiation of alternative therapy, whichever was earlier, for patients who remained at risk for failure. Patients who initiated alternative therapies prior to progressive disease (PD) were censored at that time for the purposes of estimating PFS even if they experienced PD post-initiation of other therapies.
Results
Thirty-seven patients were enrolled on this stratum between November 2008 and June 2010. Two patients were inevaluable due to withdrawal from study before beginning protocol therapy. Hence, all results reported here are based on 35 patients who were eligible and received at least 1 dose of treatment.
Patient Characteristics
Patient characteristics are listed in Table 1. The median age at enrollment for eligible patients was 8.4 years (range, 0.6–17.6). Sixteen patients (46%) had PA. Five patients (14.2%) had neurofibromatosis type 1. The median number of prior recurrences was 1 (0–8; Table 1). Eight patients had at least 3 prior recurrences; all patients had received surgery and/or chemotherapy ± radiotherapy prior to entry (Table 1). The median performance status was 90 (range, 70–100). The median number of courses of BVZ + CPT-11 received was 12 (range, 2–26; Table 2).
Table 1.
Clinical characteristics in 35 evaluable patients with recurrent LGG treated with BVZ + CPT-11
| Clinical Characteristics | |
|---|---|
| Total N of evaluable patients | 35 |
| Median age, y, at enrollment (range) | 8.4 (0.6–17.6) |
| Histology | PA, 16 (46%) |
| Astrocytoma NOS, 5 (14%) | |
| Grade II astrocytoma, 1 (3%) | |
| Ganglioglioma, 1 (3%) | |
| OPG (not biopsied), 12 (34%) | |
| No. of patients with NF-1 | 5 (14.2%) |
| Median no. of prior recurrences | 1 (0–8) |
| Prior therapy | Chemotherapy ± biologic therapy only, 33 |
| Radiotherapy + chemotherapy, 2 | |
Abbreviations: NOS, not otherwise specified; OPG, optic pathway glioma; NF-1, neurofibromatosis type 1.
Table 2.
Treatment and outcome in 35 evaluable patients with recurrent LGG treated with BVZ + CPT-11
| Treatment and Outcome | |
|---|---|
| Median no. of courses received (range) | 12 (2–26) |
| Sustained responses | PR, 2 (at days 60 and 174) |
| n patients who stayed on treatment for at least 6 mo | 29/35 (82.86%) |
| n patients off treatment for toxicity or BVZ-related concerns | 15/35 (42.86%) |
| n patients who withdrew after beginning protocol therapy | 8/35 (22.86%) |
| Median time, mo, to PD on treatment (range) (n = 8) | 5.4 (1.6–17.8) |
| Median time, mo, to PD off treatment (range) (n = 12/27a) | 5.1 (2.9–16.6) |
| Median duration of sustained SD (range) for patients who remained progression free without alternative treatment at the time of this report (n = 6/27a) | 40.1 (30.6–49.3) |
aThese statistics are available in only 27 patients who consented to long-term follow-up.
Toxicity
The common grades 1–4 toxicities related to BVZ and CPT-11 are listed in Table 3. The most common toxicities related to BVZ were fatigue and grades 1–2 hypertension in 23 and 24 patients, respectively. One patient came off treatment for hypertension associated with proteinuria and another due to persistent fatigue. Eighteen patients had grades 1–2 epistaxis. Two patients came off treatment for persistent grade 2 epistaxis. Fifteen patients had grades 1–4 proteinuria. Three patients came off treatment for this toxicity. Proteinuria resolved over a period of weeks to months following cessation of BVZ and was not associated with deterioration of renal function. Two patients with grade 1 CNS hemorrhage (following courses 6 and 9, respectively) and 1 patient with CNS ischemia (after the second course) were taken off therapy. One patient had avascular necrosis of the lunate bone after 18 cycles of treatment. One patient came off study for hip pain after 18 cycles, which recurred predictably following BVZ administration without any changes on radiologic examination. Another patient was found to have metaphyseal sclerotic bands on routine X-ray of the knee following 7 cycles of BVZ + CPT-11 and was taken off treatment (Table 2). Neither of these 2 patients had an MRI scan of the affected bone to confirm findings. Bone marrow suppression, diarrhea, and mild transaminase elevation were found in association with the concomitant use of CPT-11 (Table 3). Fifteen patients stopped therapy due to toxicity related to BVZ (n = 13) or because they needed major surgery (n = 2), and continuing to give BVZ would have caused delayed wound healing (Table 2). There were no toxicity-related deaths.
Table 3.
Toxicity due to BVZ and CPT-11 in 35 children with recurrent LGG
| Toxicity | Grade | BVZ | Irinotecan |
|---|---|---|---|
| n = 35 | n = 35 | ||
| Hypertension | 1–2 | 24 | |
| Fatigue | 1–2 | 23 | |
| Epistaxis | 1–2 | 18 | |
| Proteinuria | 1–2/3–4 | 12/3 | |
| CNS hemorrhage | 1 | 2 | |
| Rectal bleeding | 1 | 2 | |
| Metaphyseal bands on knee X-ray | 2 | 1 | |
| Avascular necrosis of lunate bone | 3 | 1 | |
| Hip pain | 2 | 1 | |
| CNS ischemia | 2 | 1 | |
| Neutropenia | 1–2/3–4 | 11/4 | |
| Thrombocytopenia | 1 | 4 | |
| Diarrhea | 1–2/3 | 23/2 | |
| Elevated transaminases | 1 | 16 |
Responses and Progression-free Survival
Twenty-nine patients (83%) received protocol therapy for at least 6 months (Table 2). Sustained PR was observed in only 2 patients (5.7%) at days 60 and 174 of treatment, respectively. One patient with a recurrent thalamic PA had sustained this response for 17 months before stopping therapy for toxicity. He then suffered PD after coming off therapy. Another with a recurrent optic pathway glioma had sustained response for 7 courses, decided to stop protocol therapy, but remained on study for long-term follow-up. She subsequently had PD off treatment. One additional patient had a PR at 2 months following starting treatment but suffered PD at 4 months. Eight patients withdrew from study mostly due to the hardship of having to travel to a PBTC center to receive study drug (Table 2).
Eight patients suffered PD on treatment at a median of 5.4 months from beginning therapy (range, 1.6–17.8; Table 2), 5 of whom did so within 6 months. In 27 patients who consented to long-term follow-up, 12 additional patients have had PD off therapy at a median of 5.1 months (range, 2.9–20.5) from stopping BVZ + CPT-11. Two additional patients experienced PD after initiating alternative therapy in the absence of progression. Six patients (17.1%) are still in follow-up and currently have had SD without receiving additional therapy for a median of 40.1 months (range, 30.6–49.3) since starting treatment (Table 2). The 6-month and 2-year PFS rates are 85.4% (SE ± 5.96) and 47.8% (SE ± 9.34), respectively.
Changes in Volume Enhancement, Cerebral Blood Volume 3D Max, Volume Fluid Attenuated Inversion Recovery, Kps 3D Max, and Diffusion Ratio Following 2 Doses of BVZ and During Treatment With BVZ + CPT-11
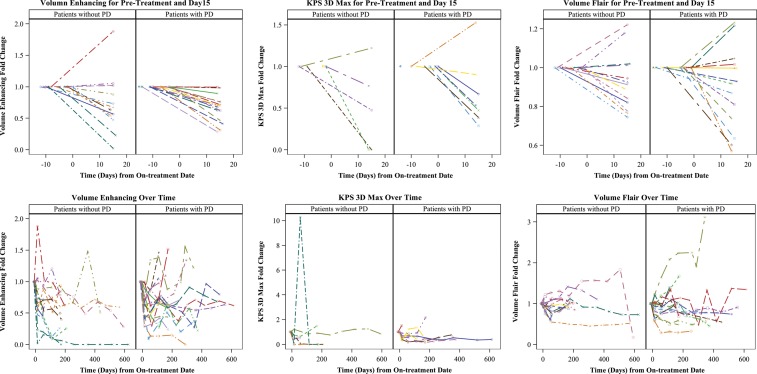
The numbers of patients with baseline, day 15, and at least 1 on-treatment MRI scan available for measurement of MRI parameters are listed in Table 4. The median fold change in volume enhancement showed a significant decrease from baseline to the day 15 scans (P < .0001; Table 4, Fig. 1). In a longitudinal model, there was suggestive evidence that the fold change in volume enhancement decreased on average over the course of treatment (P = .037; Table 4, Fig. 2). There was suggestive evidence that the median maximum permeability (Kps 3D Max) values decreased between baseline and day 15 (P = .016), but no such change was detected over time (P = .903). Similarly there was suggestive evidence that the median fold change in volume fluid attenuated inversion recovery (FLAIR) values decreased between baseline and day 15 (P = .015), but no significant changes were observed over time (Table 4). The median fold change values for diffusion ratio or perfusion ratio of maximum cerebral blood volume (CBV 3D Max) did not significantly change either at day 15 or over time. Figure 2 shows changes in enhancement, diffusion, and perfusion following treatment in 1 patient who demonstrated PR to treatment.
Table 4.
Fold change in neuroimaging parameters between baseline, day 15, and over 1 y of treatment
| Median Fold Change at Day 15 (range) | P | Median Maximum Fold Change Over 1 Year of Treatment (range) | P | |
|---|---|---|---|---|
| Volume enhancement | 0.69 (0.02, 1.88) (n= 28) | <.0001 | 0.73 (0.15, 1.88) (n = 33) | .037 |
| Volume FLAIR | 0.92 (0.57, 1.23) (n = 27) | .015 | 1.01 (0.57, 3.11) (n = 30) | .426 |
| Kps 3D Max | 0.51 (0.00, 1.53) (n = 12) | .016 | 0.78 (0.00, 10.28) (n = 14) | .903 |
| CBV 3D Max | 0.86 (0.00, 3.27) (n = 12) | .457 | 1.00 (0.00, 3.27) (n = 14) | .761 |
| Diffusion ratio | 0.94 (0.58, 1.47) (n = 30) | .156 | 1.06 (0.70, 2.22) (n = 33) | .111 |
| T/GM ratio* | 0.83 (0.68, 2.00) (n = 9) | .250 | – | – |
| T/WM ratio* | 0.83 (0.56, 1.23) (n = 9) | .164 | – | – |
P values are based on Wilcoxon signed-rank tests.
*Based on baseline and one on treatment scan.
Fig. 1.
Fold change in volume enhancement, Kps 3D Max, and FLAIR following 2 doses of BVZ (upper panel) and over the entire course of treatment (lower panel).
Fig. 2.
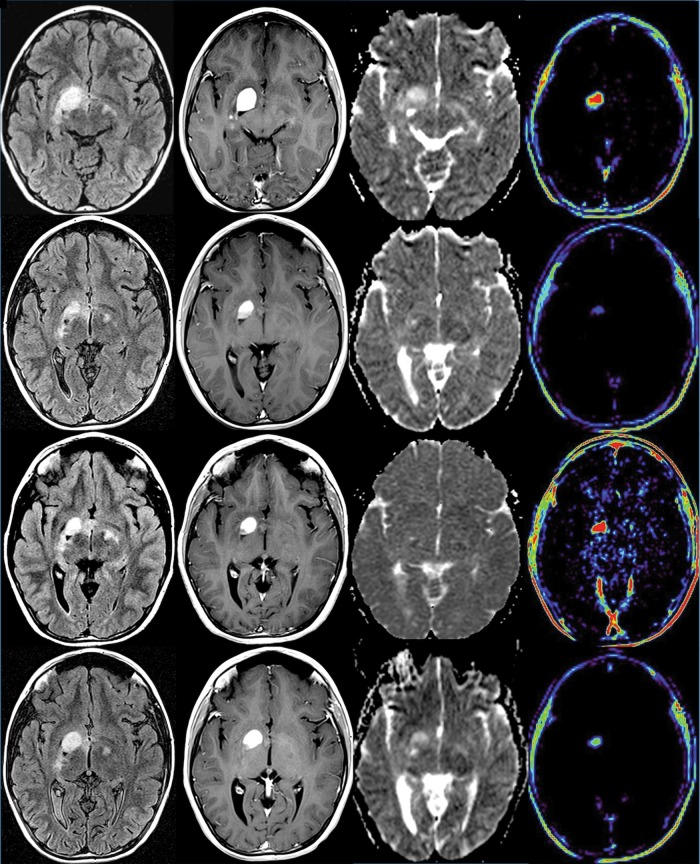
A 10-year-old girl (patient #30, Table 4) with primarily right optic pathway tumor extending into the right basal ganglia, with MRI at baseline (row 1), at 15 days (row 2), at 3 months (row 3), and off therapy at 9 months (row 4). Axial MRI from FLAIR (column 1), T1 images with gadolinium (column 2), apparent diffusion coefficient (ADC) maps (column 3), and T1 permeability maps (column 4). There is a decrease in FLAIR volume and enhancing volume over time. Permeability maps demonstrate a reduction in Kps values between baseline and day 15 and continuing to be present at 9 months. There is a transient increase in permeability at 3 months, without an increase in the volume of tumor. ADC maps demonstrate a reduction in the ADC values within the tumor from day 0 to day 15, likely due to edema reduction in the tumor that continues until 3 months after therapy and gradually increases at 9 months.
Changes in FDG-PET Parameters During Treatment With BVZ + CPT-11 Compared With Baseline
A baseline FDG-PET scan of brain was obtained in 21 patients. Fifteen patients had both a baseline and an on-treatment FDG-PET scan that were analyzable for FDG uptake. Mean tumor to gray matter (T/GM) and tumor to white matter (T/WM) ratios were available at baseline for 19 patients and paired scans for 9 patients. The baseline median values for mean T/GM and mean T/WM ratios were 0.58 (range, 0.35–1.31) and 1.79 (range, 0.87–2.87), respectively. Based on the subjective grading, 5 of 9 patients with increased FDG uptake (uptake more than WM) at baseline demonstrated decreased uptake following treatment. In 2 cases, uptake stayed the same, and in an additional 2 there was an increase in uptake upon treatment. The objective analysis yielded similar results, with uptake decreasing in 6 of 9 cases and increasing in 3 cases. However, there was no statistically significant change in FDG uptake or mean T/GM or T/WM ratios from baseline to those measured on treatment (Table 4).
Expression of VEGF, HIF-2α, VEGFR-2, and CA-9 by Tumor Immunohistochemistry
Immunohistochemistry was performed in available tumor samples from 8 patients (PA = 7, well-differentiated astrocytoma = 1). Median expressions for VEGF, HIF-2α, VEGFR-2, and CA-9 were 30% (range, 2%–80%), 1.5% (0%–5%), 35% (2%–70%), and 0% (0%–1%), respectively. Due to the very limited number of patients with immunohistochemical expression data, no analysis was attempted to explore associations with PFS.
Discussion
Bevacizumab, a humanized monoclonal antibody, was developed as a specific inhibitor of all VEGF-A isoforms and has been FDA approved in combination with chemotherapy for certain adult cancers, including recurrent glioblastoma multiforme (GBM).17 VEGF inhibition results in decreased vascular permeability and interstitial fluid pressure, more orderly blood flow due to pruning of unnecessary blood vessels, decrease in the number of tumor-initiating cells due to disruption of their perivascular niche, and increase in chemotherapy drug delivery into the tumor.18,19 PA is a vascular tumor that expresses VEGF predominantly in the neoplastic astrocytic foot processes at levels similar to that observed in GBM.8 On the other hand, expression of the phosphorylated form of VEGFR2 (kinase insert domain receptor) in PA, the cognate receptor for all VEGF-mediated effects, seems to be restricted to tumor endothelial cells.8 Similarly, VEGF expression has also been documented in grade II astrocytomas but at a lower level compared with higher-grade tumors.10 Due to robust target expression, inhibition of the VEGF-VEGFR2 angiogenic axis with BVZ is likely to be beneficial in controlling recurrent LGG.
Our trial is the largest study of children with recurrent LGG treated with BVZ + CPT-11. The combination was successful in producing disease stabilization in over 80% of patients who had previously failed standard radiotherapy and/or chemotherapy. PFS with this regimen is similar to what has been observed in comparable studies of salvage chemotherapies in children with recurrent LGG.6,20
Surprisingly, only 2 patients in our study had sustained PR with an OR rate of <6%. This is in contrast to the results published from a limited institution pilot study of 14 patients with recurrent LGG, in which 12 patients (86%) had centrally reviewed ORs at a median of 9 weeks following treatment, 9 of whom also experienced improvement in neurologic symptoms.14 Despite the impressive responses to BVZ in this report, all but 1 patient have suffered disease progression at a median of 5 months following cessation of therapy. Thus responses, although obviously desirable for better symptom control in the short term, do not necessarily predict for sustained disease stability. Interestingly, 4 patients in the same study were retreated with single-agent BVZ following initial PD and demonstrated both clinical and radiologic improvement for a median duration of 12.5 months (range, 9–17), suggesting that the tumor vasculature might still be responsive to BVZ on repeat exposure. The clinical benefit observed was probably due to an anti-edema effect resulting from a decrease in vascular permeability consequent to VEGF inhibition. The reasons for differences in the rate of ORs in the 2 studies are unknown. In another study of a putative anti-angiogenic approach using metronomic chemotherapy with weekly vinblastine in 50 evaluable children with recurrent LGG, Bouffet et al6 have similarly shown ORs in 36% of patients. However, the 5-year PFS in this study was only 38.5%, again demonstrating that ORs do not predict for improved PFS.
Although CPT-11 was utilized in our study due to the potential benefits of combining chemotherapy with a VEGF inhibitor, its individual role in contributing to either response or disease stabilization is unclear, especially in the context of lack of available data on the efficacy of single-agent CPT-11 in children with recurrent LGG. In general, CPT-11 has only limited efficacy in both adult and pediatric brain tumors.21,22 In a large phase II study of single-agent irinotecan (CPT-11) in children with recurrent CNS tumors, no ORs were observed in 12 patients with either astrocytoma (n = 8) or glioma (n = 4) (grade unknown).22
We assessed several correlative functional neuroimaging markers that were designed to measure changes in vasculature and tumor interstitium induced by BVZ. Predictably, significant reduction occurred in volume enhancement, perfusion (CBV 3D Max), Kps, and FLAIR that persisted over time. This confirms what has been repeatedly observed with the use of BVZ and other VEGF and VEGFR2 inhibitors, which rapidly reduce permeability/perfusion of tumor vasculature and peritumoral edema (reflected by FLAIR) that lasts for several weeks, even after discontinuation of treatment.23 However, in our study there was lack of appreciable decrease in apparent diffusion coefficient values (which reflect cell necrosis and death) despite significant decrease in volume enhancement and FLAIR. Since there were very few patients with PD on therapy, we could not perform meaningful correlations of these parameters with PFS. However, in a larger study of 84 adult patients with recurrent GBM treated with BVZ + CPT-11 using conventional MRI, Ellingson et al24 similarly demonstrated significant changes in volume enhancement and FLAIR following therapy with this combination that did not correlate with PFS or overall survival. Therefore, acute changes in perfusion, permeability, and FLAIR reflect just the effects of BVZ on the tumor vasculature and not cell death and hence do not seem to impact patient survival.
VEGF inhibition has been shown to decrease tumor metabolic activity, which can be measured by specific PET tracers (eg, FDG, fluorothymidine) and used as a surrogate marker of treatment response and survival.25 While LGGs are considered to be not as metabolically active as their malignant counterparts, recent studies have documented increased FDG uptake in a proportion of pediatric LGGs (PAs or fibrillary astrocytomas) that also seems to confer a worse prognosis.26 The decrease in glucose uptake following 2 courses of therapy in some patients in our study might indicate the negative impact of VEGF inhibition on tumor proliferation and metabolism.
Toxicity was fairly predictable and manageable in this study. BVZ-related toxicities occurred as a direct consequence of VEGF inhibition.27,28 Despite half of our patients receiving over a year of therapy, the commonest toxicities related to BVZ in our study were mild and similar to what has been previously described in adult and pediatric clinical trials of BVZ-containing regimens.29–31 More importantly, severe toxicities related to BVZ were quite rare and quickly reversible on drug withdrawal.
The results of this study indicate that BVZ + CPT-11 is a useful therapeutic modality for children with recurrent LGG who have failed standard chemotherapy regimens. It is also likely to produce tumor control for a reasonable period of time in young children to help delay or avoid radiotherapy. The toxicity due to VEGF inhibition seems to be tolerable, although based on an adverse effect of VEGF inhibition on the epiphyseal plate3 and ovarian function,32 and long-term follow-up is necessary to determine its impact on linear growth and fertility in children.
Funding
This work was supported by Pediatric Brain Tumor Consortium grant U01CA81457, National Center for Research Resources grant M01RR00188, and the American Lebanese Syrian Associated Charities.
Conflict of interest statement. H.S.F has served as a consultant and received honoraria and research funding from Genentech Corporation. J.M.B. has received research funding from Hoffman-LaRoche and Merck Corporations.
Supplementary Material
References
- 1.Rickert CH, Paulus W. Epidemiology of central nervous system tumors in childhood and adolescence based on the new WHO classification. Childs Nerv Syst. 2001;17(9):503–511. doi: 10.1007/s003810100496. [DOI] [PubMed] [Google Scholar]
- 2.CBTRUS. CBTRUS Statistical Report: Primary Brain and Central Nervous System Tumors Diagnosed in the United States in 2004–2005. Source: Central Brain Tumor Registry of the United States, 2009. www.cbtrus.org [Google Scholar]
- 3.Watson GA, Kadota RP, Wisoff JH. Multidisciplinary management of pediatric low-grade gliomas. Seminars in Radiation Oncol. 2001;11(2):152–162. doi: 10.1053/srao.2001.21421. [DOI] [PubMed] [Google Scholar]
- 4.Packer RJ, Ater J, Allen J, et al. Carboplatin and vincristine chemotherapy for children with newly diagnosed progressive low-grade gliomas. J Neurosurg. 1997;86(5):747–754. doi: 10.3171/jns.1997.86.5.0747. [DOI] [PubMed] [Google Scholar]
- 5.Perilongo G. Considerations on the role of chemotherapy and modern radiotherapy in the treatment of childhood low grade glioma. J Neurooncol. 2005;75(3):301–307. doi: 10.1007/s11060-005-6754-8. [DOI] [PubMed] [Google Scholar]
- 6.Bouffet E, Jakacki R, Goldman S, et al. Phase II study of weekly vinblastine in recurrent or refractory pediatric low-grade glioma. J Clin Oncol. 2012;30(12):1358–1363. doi: 10.1200/JCO.2011.34.5843. [DOI] [PubMed] [Google Scholar]
- 7.Folkman J. Angiogenesis: an organizing principle for drug discovery? Nat Rev Drug Discov. 2007;6(4):273–286. doi: 10.1038/nrd2115. [DOI] [PubMed] [Google Scholar]
- 8.Sikkema AH, de Bont ES, Molema G, et al. Vascular endothelial growth factor receptor 2 (VEGFR-2) signalling activity in paediatric pilocytic astrocytoma is restricted to tumour endothelial cells. Neuropathol Appl Neurobiol. 2011;37(5):538–548. doi: 10.1111/j.1365-2990.2011.01160.x. [DOI] [PubMed] [Google Scholar]
- 9.Sie M, de Bont ES, Scherpen FJ, Hoving EW, den Dunnen WF. Tumour vasculature and angiogenic profile of paediatric pilocytic astrocytoma: is it much different from glioblastoma? Neuropathol Appl Neurobiol. 2010;36(7):636–647. doi: 10.1111/j.1365-2990.2010.01113.x. [DOI] [PubMed] [Google Scholar]
- 10.Gesundheit B, Klement G, Senger C, et al. Differences in vasculature between pilocytic and anaplastic astrocytomas of childhood. Med Pediatr Oncol. 2003;41(6):516–526. doi: 10.1002/mpo.10308. [DOI] [PubMed] [Google Scholar]
- 11.Ellis LM. Bevacizumab. Nat Rev Drug Discov. 2005:S8–S9. doi: 10.1038/nrd1727. Suppl. [DOI] [PubMed] [Google Scholar]
- 12.Gururangan S, Chi SN, Young Poussaint T, et al. Lack of efficacy of bevacizumab plus irinotecan in children with recurrent malignant glioma and diffuse brainstem glioma: a Pediatric Brain Tumor Consortium study. J Clin Oncol. 2010;28(18):3069–3075. doi: 10.1200/JCO.2009.26.8789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gururangan S, Fangusaro J, Young Poussaint T, et al. Lack of efficacy of bevacizumab + irinotecan in cases of pediatric recurrent ependymoma—a Pediatric Brain Tumor Consortium study. Neuro Oncol. 2012;14(11):1404–1412. doi: 10.1093/neuonc/nos213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hwang EI, Jakacki RI, Fisher MJ, et al. Long-term efficacy and toxicity of bevacizumab-based therapy in children with recurrent low-grade gliomas. Pediatr Blood Cancer. 2012;60(5):776–782. doi: 10.1002/pbc.24297. [DOI] [PubMed] [Google Scholar]
- 15.Packer RJ, Jakacki R, Horn M, et al. Objective response of multiply recurrent low-grade gliomas to bevacizumab and irinotecan. Pediatr Blood Cancer. 2009;52(7):791–795. doi: 10.1002/pbc.21935. [DOI] [PubMed] [Google Scholar]
- 16.Sathornsumetee S, Cao Y, Marcello JE, et al. Tumor angiogenic and hypoxic profiles predict radiographic response and survival in malignant astrocytoma patients treated with bevacizumab and irinotecan. J Clin Oncol. 2008;26(2):271–278. doi: 10.1200/JCO.2007.13.3652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cohen MH, Gootenberg J, Keegan P, Pazdur R. FDA drug approval summary: bevacizumab (Avastin) plus carboplatin and paclitaxel as first-line treatment of advanced/metastatic recurrent nonsquamous non-small cell lung cancer. Oncologist. 2007;12(6):713–718. doi: 10.1634/theoncologist.12-6-713. [DOI] [PubMed] [Google Scholar]
- 18.Jain RK. Lessons from multidisciplinary translational trials on anti-angiogenic therapy of cancer. Nat Rev Cancer. 2008;8(4):309–316. doi: 10.1038/nrc2346. [DOI] [PubMed] [Google Scholar]
- 19.Calabrese C, Poppleton H, Kocak M, et al. A perivascular niche for brain tumor stem cells. Cancer Cell. 2007;11(1):69–82. doi: 10.1016/j.ccr.2006.11.020. [DOI] [PubMed] [Google Scholar]
- 20.Gururangan S, Fisher MJ, Allen JC, et al. Temozolomide in children with progressive low-grade glioma. Neuro Oncol. 2007;9(2):161–168. doi: 10.1215/15228517-2006-030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Friedman HS, Petros WP, Friedman AH, et al. Irinotecan therapy in adults with recurrent or progressive malignant glioma. J Clin Oncol. 1999;17(5):1516–1525. doi: 10.1200/JCO.1999.17.5.1516. [DOI] [PubMed] [Google Scholar]
- 22.Bomgaars LR, Bernstein M, Krailo M, et al. Phase II trial of irinotecan in children with refractory solid tumors: a Children's Oncology Group Study. J Clin Oncol. 2007;25(29):4622–4627. doi: 10.1200/JCO.2007.11.6103. [DOI] [PubMed] [Google Scholar]
- 23.Batchelor TT, Sorensen AG, di Tomaso E, et al. AZD2171, a pan-VEGF receptor tyrosine kinase inhibitor, normalizes tumor vasculature and alleviates edema in glioblastoma patients. Cancer Cell. 2007;11(1):83–95. doi: 10.1016/j.ccr.2006.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ellingson BM, Cloughesy TF, Lai A, Nghiemphu PL, Mischel PS, Pope WB. Quantitative volumetric analysis of conventional MRI response in recurrent glioblastoma treated with bevacizumab. Neuro Oncol. 2011;13(4):401–409. doi: 10.1093/neuonc/noq206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen W, Delaloye S, Silverman DH, et al. Predicting treatment response of malignant gliomas to bevacizumab and irinotecan by imaging proliferation with [18F] fluorothymidine positron emission tomography: a pilot study. J Clin Oncol. 2007;25(30):4714–4721. doi: 10.1200/JCO.2006.10.5825. [DOI] [PubMed] [Google Scholar]
- 26.Kruer MC, Kaplan AM, Etzl MM, Jr., et al. The value of positron emission tomography and proliferation index in predicting progression in low-grade astrocytomas of childhood. J Neurooncol. 2009;95(2):239–245. doi: 10.1007/s11060-009-9922-4. [DOI] [PubMed] [Google Scholar]
- 27.Kamba T, McDonald DM. Mechanisms of adverse effects of anti-VEGF therapy for cancer. Br J Cancer. 2007;96(12):1788–1795. doi: 10.1038/sj.bjc.6603813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Izzedine H, Massard C, Spano JP, Goldwasser F, Khayat D, Soria JC. VEGF signalling inhibition-induced proteinuria: mechanisms, significance and management. Eur J Cancer. 2010;46(2):439–448. doi: 10.1016/j.ejca.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 29.Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350(23):2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 30.Glade Bender JL, Adamson PC, Reid JM, et al. Phase I trial and pharmacokinetic study of bevacizumab in pediatric patients with refractory solid tumors: a Children's Oncology Group Study. J Clin Oncol. 2008;26(3):399–405. doi: 10.1200/JCO.2007.11.9230. [DOI] [PubMed] [Google Scholar]
- 31.Chen HX, Cleck JN. Adverse effects of anticancer agents that target the VEGF pathway. Nat Rev Clin Oncol. 2009;6(8):465–477. doi: 10.1038/nrclinonc.2009.94. [DOI] [PubMed] [Google Scholar]
- 32.Hall AP, Westwood FR, Wadsworth PF. Review of the effects of anti-angiogenic compounds on the epiphyseal growth plate. Toxicologic pathology. 2006;34(2):131–147. doi: 10.1080/01926230600611836. [DOI] [PubMed] [Google Scholar]
- 33.GenenTech Corporation. Avastin™ Product safety information. 2011 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.