Abstract
Background
Proton craniospinal irradiation (p-CSI) has been proposed to reduce side effects associated with CSI. We evaluated acute toxicities and preliminary clinical outcomes in a series of adults treated with p-CSI.
Methods
We reviewed medical records for 50 patients (aged 16–63 y) with malignancies of varying histologies treated consecutively with vertebral body-sparing p-CSI at MD Anderson Cancer Center from 2007 to 2011. Median CSI and total boost doses were 30.6 and 54 Gy. Forty patients received chemotherapy, varying by histology. Median follow-up was 20.1 months (range, 0.3–59).
Results
Median doses to the thyroid gland, pituitary gland, hypothalamus, and cochleae were 0.003 Gy–relative biological effectiveness (RBE; range, 0.001–8.5), 36.1 Gy-RBE (22.5–53.0), 37.1 Gy-RBE (22.3–54.4), and 33.9 Gy-RBE (22.2–52.4), respectively. Median percent weight loss during CSI was 1.6% (range, 10% weight loss to 14% weight gain). Mild nausea/vomiting was common (grade 1 = 46%, grade 2 = 20%); however, only 5 patients experienced grade ≥2 anorexia (weight loss >5% baseline weight). Median percent baseline white blood cells, hemoglobin, and platelets at nadir were 52% (range, 13%–100%), 97% (65%–112%), and 61% (10%–270%), respectively. Four patients developed grade ≥3 cytopenias. Overall and progression-free survival rates were 96% and 82%, respectively, at 2 years and 84% and 68% at 5 years.
Conclusions
This large series of patients treated with p-CSI confirms low rates of acute toxicity, consistent with dosimetric models. Vertebral body-sparing p-CSI is feasible and should be considered as a way to reduce acute gastrointestinal and hematologic toxicity in adults requiring CSI.
Keywords: acute toxicity, craniospinal irradiation, proton therapy.
Craniospinal irradiation (CSI) plays an essential role in treating various tumors with predilection for leptomeningeal dissemination. Many patients have excellent clinical outcomes; however, conventional photon CSI (x-CSI) is associated with significant side effects.1–6 Exploring ways to minimize treatment-related acute and late morbidity is important. In comparison with x-CSI, proton beam CSI (p-CSI) reduces or eliminates unnecessary dose to the thyroid gland, larynx, heart, lung, liver, kidneys, gastrointestinal (GI) tract, and pelvic structures.7–10 In patients whose vertebrae have fully developed, the distal edge of the proton beam can be limited to just beyond the thecal sac, thereby minimizing dose to bone marrow within the vertebral bodies.11 Using proton beam radiation therapy (PBRT) for tumor bed boosts may also minimize CNS toxicity through reduced doses to uninvolved intracranial structures such as the nontarget brain, neuroendocrine structures, optic apparatus, auditory pathways, and temporal lobes, among others.
However, data supporting p-CSI have mostly been limited to dosimetric models7–9; while some clinical data are emerging for pediatric patients to confirm potential benefits of p-CSI, this technique has not been well studied in the adult population. We evaluated the feasibility, efficacy, and treatment-related morbidity of p-CSI in a population of adults treated at our institution.
Materials and Methods
Patient Characteristics
We reviewed medical records of 50 patients with various disease histologies treated consecutively with vertebral body-sparing p-CSI from 2007 to 2011 at The University of Texas MD Anderson Cancer Center. All patients who were treated with vertebral body-sparing p-CSI were included regardless of age at diagnosis, histology, stage at diagnosis, or prior treatment (Table 1). Vertebral body-sparing p-CSI was considered for patients who had reached skeletal maturity. Hand-wrist radiographs were obtained for younger patients, at the treating physicians' discretion, to determine skeletal maturity. Four patients received p-CSI as salvage therapy for local recurrence after receiving prior radiation therapy (RT). All patients were enrolled on institutional review board–approved protocols to follow toxicities of patients treated with PBRT. The tallest patient treated was 192.5 cm (6 feet 4 inches) and the heaviest patient weighed 139 kg (305 pounds).
Table 1.
Patient, tumor, and treatment characteristics
| Male |
n 33 |
% 66 |
| Female | 17 | 34 |
| Histology | ||
| Medulloblastoma | 19 | 38 |
| GCT | 9 | 18 |
| Nongerminomatous GCT | 6 | 12 |
| Pineoblastoma | 7 | 14 |
| Ependymoma | 2 | 4 |
| Atypical teratoid rhabdoid tumor | 1 | 2 |
| Glioma | 1 | 2 |
| Papillary tumor | 1 | 2 |
| Choroid plexus papilloma | 1 | 2 |
| Rhabdoid meningioma | 1 | 2 |
| Acute lymphoblastic leukemia | 2 | 4 |
| Modified Chang M stage | ||
| M0 | 30 | 60 |
| M1 | 1 | 2 |
| M2 | 8 | 16 |
| M3 | 9 | 18 |
| M4 | 0 | 0 |
| Chemotherapy | ||
| Any | 40 | 80 |
| Neoadjuvant | 20 | 40 |
| Concurrent | 15 | 30 |
| Adjuvant (n = 48) | 31 | 65 |
| Median | Range | |
| Age at radiation therapy, y | 26.7 | 16–63 |
| Height, cm | 173.4 | 153.6–192.5 |
| Weight, kg | 73 | 39.3–138.9 |
| Doses, Gy-RBE | ||
| Craniospinal dose | 30.6 | 15–39.6 |
| Total (CSI + boost) dose | 54 | 24–58.6 |
| Thyroid gland (n = 48) | 0.003 | 0.001–8.5 |
| Pituitary gland (n = 43) | 36 | 22.5–53 |
| Hypothalamus (n = 41) | 37 | 22.3–54.4 |
| Cochleae (n = 48) | 33.9 | 22.2–52.4 |
Surgery and Chemotherapy
Gross total or partial surgical resection was performed in patients prior to PBRT based on histology and neurosurgical feasibility. Forty patients (80%) received chemotherapy in addition to PBRT with regimens that varied broadly in timing and agents prescribed, according to histology, stage, and other clinical parameters. Five medulloblastoma patients received concurrent vincristine, with 1 also receiving carboplatin. Four medulloblastoma patients received chemotherapy before RT and 15 after RT with combinations of vincristine, platinum, etoposide, and classical alkylating agents. Four pineoblastoma patients received concurrent chemotherapy, 2 before RT, and 6 after RT with agents similar to medulloblastoma regimens. Six patients with germinomatous germ cell tumors (GCTs) received platinum, etoposide-based chemotherapy prior to RT, with 1 patient also receiving concurrent chemotherapy. Five patients with nongerminomatous GCTs received similar chemotherapy regimens before RT, 3 after RT, and 1 during RT.
Simulation and Treatment Techniques
Treatment plans were in accordance with the International Commission on Radiation Units and Measurements Report 78. A relative biological effectiveness (RBE) factor of 1.1 was used in dosimetric calculations, reported here as Gy-RBE. All treatment plans were generated by board-certified radiation oncologists and medical dosimetrists who specialize in CNS RT.
Patients were simulated in the supine position on a 10-cm-thick Styrofoam board used to provide sufficient clearance for posterior oblique cranial fields avoiding passage through the couch edge. Patients were immobilized using a lower extremity vac-loc cradle and custom thermoplastic mask with bite block incorporated into the mask to minimize daily head tilt variability. A noncontrast CT scan from the superior aspect of the immobilization device to the upper thighs was performed with a slice thickness of 2.5 mm, yielding 400 slices per patient. Patient alignment and immobilization were verified during simulation to ensure reproducibility.
The CSI clinical target volume (CTV) encompassed the entire CSF space, divided into the brain CTV and upper, middle, and lower spine CTVs. Posterior-oblique cranial fields were utilized, as described by Cochran et al,12 to minimize dose to the lens while allowing coverage of the cribriform plate. Three postero-anterior spinal fields were used to cover the thecal sac inferiorly to no higher than C1-C2 superiorly. The prescription line for junction gaps touched the spinal canal posteriorly with ∼107% overlap touching the spinal canal anteriorly. Spine fields were geometrically and dosimetrically matched to each other and to the cranial fields. Junctions were shifted by 1 cm every 5 fractions during treatment. Custom brass apertures were designed to create a 2-cm margin around the brain CTV and block the anterior orbits, posterior neck, trachea, and oral cavity. A 1-cm lateral margin was applied to the vertebral bodies for the spinal fields. Custom acrylic compensators were used to conform the distal beam edge along the axis of the spinal canal, minimizing the dose to the vertebral bodies anteriorly (Fig. 1). Posterior spine fields included a 7-mm planning target volume (PTV) anterior to the spinal canal to account for distal range uncertainty. In the cervical spine, at the treating physician's discretion, the distal PTV could be decreased by 1–2 mm in order to spare the thyroid gland. In addition, distal dose coverage extended slightly beyond the PTV due to the effects of compensator smearing. A conformal proton boost was used for most patients to treat the tumor bed and any residual disease within the brain or spine to higher doses. Treatment times varied but were generally 40–60 min.
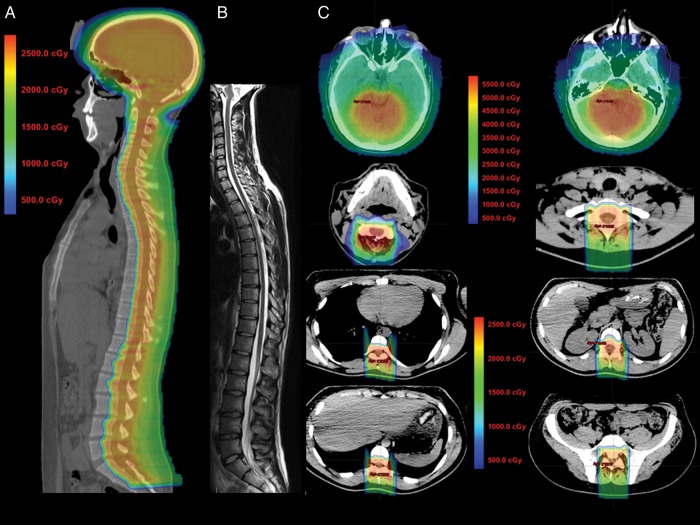
Fig. 1.
Sagittal CT planning images with colorwash dose distribution for an adult patient with medulloblastoma treated with p-CSI (panel A) shows bone marrow–sparing technique with minimal to no dose reaching structures anterior to the vertebrae, but with coverage of the spinal canal with the prescription dose of 23.4 Gy. A midline sagittal, T2-weighted MRI (panel B) shows changes in the vertebral bodies with fatty bone marrow replacement that correspond with the dose distribution in the treatment plan. Axial images from the planning CT with colorwash dose distribution (panel C) show the treatment plan with boost. Note significant sparing of the lenses, oral cavity, esophagus, thyroid, heart, lungs, kidneys, stomach, liver, and GI tract, as well as partial sparing of the vertebral bodies and sparing of the cochlea from the high dose region.
Patient Workup and Follow-up
Initial evaluation included a clinical examination with baseline audiogram, ophthalmologic evaluation, neuropsychological evaluation, and endocrine studies. The modified Chang staging system was used, incorporating surgical, imaging, and CSF data. Patients were assessed weekly during treatment for acute toxicity, including weight measurements and basic laboratory studies, and approximately at 1 month after completion of PBRT. Tumor response was evaluated by MRI of the head and spine every 2–3 months for 2–3 years depending on the treatment regimen and physician preference. A majority of patients also received follow-up audiograms (n = 26). All toxicities were determined using the Radiation Therapy Oncology Group radiation morbidity scoring criteria. Anti-emetics were occasionally prescribed at the discretion of the attending physician; however, these patients were not scored as having nausea/vomiting unless they actually took the prescribed anti-emetics.
Statistics
Overall survival (OS) and progression-free survival (PFS) following completion of RT were evaluated using Kaplan–Meier estimates. Groups were compared using Fisher's exact test, and median values reported were compared using the Mann–Whitney–Wilcoxon test. Statistics were performed using the Stata Statistical Software Release 12 package.
Results
Patient, tumor, and treatment characteristics are reported in Table 1. A variety of histologies were treated, with medulloblastoma the most common (n = 19, 38%). The median age at CSI was 26.7 years (range, 16–63). Thirty patients (60%) had Chang M0 stage, with the remainder having Chang M1–M3 disease spread. Two patients with acute lymphoblastic leukemia had CSF involvement requiring CSI treatment.
Target and normal tissue doses are described in Table 1. The median craniospinal dose was 30.6 Gy-RBE (range, 15.0–39.6), and 47 of 50 patients received a conformal proton boost to a median total dose of 54 Gy-RBE (range, 24.0–58.6). The median doses to the thyroid, pituitary gland, hypothalamus, and cochleae were 0.003 Gy-RBE (0.001–8.5), 36.1 Gy-RBE (22.5–53.0), 37.1 Gy-RBE (22.3–54.4), and 33.9 Gy-RBE (22.2–52.4), respectively. Doses delivered to the lungs, heart, kidneys, bowel, and gonads were calculated for a subset of 10 randomly selected patients treated with high dose CSI (36 Gy; Table 2).
Table 2.
Normal tissue dosimetry for 10 patients treated with high dose (36 Gy-RBE) p-CSI
| Doses, Gy-RBE | Median | Range |
|---|---|---|
| Mean lung dose | 1.1 | 0.3–4 |
| Maximum lung dose | 35.9 | 29.4–37.5 |
| Mean heart dose | 0.002 | 0.002–0.1 |
| Maximum heart dose | 0.68 | 0.003–25.2 |
| Mean kidney dose | 0.04 | 0.002–1.4 |
| Maximum kidney dose | 22.1 | 0.7–33.4 |
| Mean bowel dose | 0.02 | 0.002–0.07 |
| Maximum bowel dose | 22.8 | 0.03–35.7 |
| Mean testicular dose (n = 7) | 0.002 | 0.002–0.002 |
| Maximum testicular dose (n = 7) | 0.003 | 0.003–0.004 |
| Mean ovarian dose (n = 3) | 0.002 | 0.002–0.002 |
| Maximum ovarian dose (n = 3) | 0.003 | 0.003–0.003 |
Toxicities are reported in Table 3. The median percent weight loss during CSI was 1.6% (range, 10% weight loss to 14% weight gain). The median percent weight loss 1 month after CSI remained 1.6% (range, 14% weight loss to 14% weight gain). Mild nausea and vomiting were common (grade 1 = 46% and grade 2 = 20%); however, only 5 patients experienced grade ≥2 anorexia, resulting in weight loss greater than 5% of baseline body weight. Weight loss and nausea/vomiting were not associated with CSI dose, chemotherapy, or age at diagnosis.
Table 3.
Treatment-related morbidity
| RTOG Acute Toxicity Score |
|||||
|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | |
| Nausea/vomiting (n = 50) | 17 (34%) | 23 (46%) | 10 (20%) | 0 | 0 |
| Dermatitis (n = 50) | 1 (2%) | 48 (96%) | 1 (2%) | 0 | 0 |
| Ototoxicity (n = 26) | 23 (92%) | 0 | 2 (8%) | 1 | 0 |
| Anemia (n = 46) | 33 (72%) | 6 (13%) | 7 (15%) | 0 | 0 |
| Leukopenia (n = 46) | 10 (22%) | 12 (26%) | 20 (43%) | 4 (9%) | 0 |
| Thrombocytopenia (n = 46) | 35 (76%) | 8 (17%) | 1 (2%) | 1 (2%) | 1 (2%) |
| Weight change, end of RT | Median | Range | |||
| Absolute, kg, n = 49 | −1.1 kg | −7.9 to +9.6 | |||
| % baseline, n = 49 | −1.6% | −10 to +14 | |||
| Weight change 1 month after RT | |||||
| Absolute, kg, n = 43 | −0.9 kg | −11 to +8.8 | |||
| % baseline, n = 43 | −1.6% | −14 to +14 | |||
| Blood counts at nadir | |||||
| % baseline WBC, n = 42 | 52% | 13%–100% | |||
| % baseline Hgb, n = 42 | 97% | 65%–112% | |||
| % baseline platelets, n = 42 | 61% | 10%–270% | |||
| Blood counts 1 mo after RT | |||||
| % baseline WBC, n = 30 | 73% | 21%–343% | |||
| % baseline Hgb, n = 31 | 103% | 79%–130% | |||
| % baseline platelets, n = 31 | 92% | 4%–360% | |||
| Percent weight loss | n | % | |||
| ≤2% | 30 | 60 | |||
| >2–5% | 15 | 30 | |||
| >5%–10% | 4 | 8 | |||
| >10% | 1 | 2 | |||
Abbreviations: RTOG, Radiation Therapy Oncology Group; Hgb, hemoglobin.
Grade 3 leukopenia occurred in 4 patients: all received concurrent chemoradiation and 3 received chemotherapy prior to CSI. Of those patients, one experienced grade 3 and another grade 4 thrombocytopenia. Three of 4 patients with grade 3 or 4 cytopenia began RT with cytopenias, with 2 of these patients receiving treatment for acute lymphoblastic leukemia. The median percents of baseline white blood cells (WBCs), hemoglobin, and platelets at nadir during RT were 52% (range, 13%–100%), 97% (65%–112%), and 61% (10%–270%), respectively. Four weeks after PBRT, WBCs, hemoglobin, and platelets had increased to 73% (21%–343%), 103% (79%–130%), and 92% (4%–360%), respectively (Table 3). Patients receiving chemotherapy before and/or during PBRT (n = 28) were more likely to develop grade ≥1 anemia (43% vs 6%; P = .007) and grade ≥2 leukopenia (68% vs 28%; P = .015), but not thrombocytopenia. Patients aged 25 years or younger at the time of RT (n = 23) were more likely to develop grade ≥1 anemia (52% vs 4%; P = .001) and grade ≥2 leukopenia (70% vs 35%; P = .04), but not thrombocytopenia. However, patients 25 years or younger were also significantly more likely to receive chemotherapy before or during RT (74% vs 44%; P = .05). Hematologic toxicity was not associated with CSI dose. Five patients required packed red blood cell transfusions; 4 also received granulocyte colony stimulating factor and 1 received platelet transfusion. All 5 patients requiring blood count support received concurrent chemotherapy, with 3 also receiving chemotherapy before PBRT.
Of 26 patients assessed for ototoxicity, 2 experienced grade 2 ototoxicity (hypoacusis detectable on audiogram only) and 1 experienced grade 3 ototoxicity (symptomatic hypoacusis). One patient with grade 2 ototoxicity received 23.4 Gy-RBE to the cochlea but also received CSI to a total dose of 24 Gy 4 years prior to re-irradiation. The second patient with grade 2 ototoxicity received 37.4 Gy-RBE to the involved cochlea, and the patient with grade 3 ototoxicity received 48.3 Gy-RBE. All 3 patients who experienced ototoxicity also received platinum-based chemotherapy.
With a median follow-up of 20.1 months (range, 0.3–59), the OS and PFS were 96% and 82%, respectively, at 2 years, and 84% and 68% at 5 years. Seven patients (14%) experienced disease progression; 5 with in-field local recurrences, including 4 within the high dose PTV and 1 with diffuse leptomeningeal disease, and 2 with extracranial metastasis. None of the recurrences occurred at field junction sites. Of the 5 patients with local recurrence, 1 with atypical teratoid rhabdoid tumor was disease free 39 months after salvage chemotherapy; 2 with ependymoma and pineoblastoma were alive with progression after salvage treatment 9 and 26 months, respectively, after recurrence; and 2 died of disease progression (one with ependymoma and the other with medulloblastoma). One patient with a nongerminomatous GCT developed peritoneal carcinomatosis from a ventriculoperitoneal shunt but was disease free 20 months after bone marrow transplant; the other patient was alive 13 months after a diagnosis of diffuse skeletal metastasis from medulloblastoma (Table 4).
Table 4.
Local recurrence, progression, and mortality
| Age, | Histology | Stage | Site of Recurrence | Months to Recurrence | Chemo | CSI Dose (Gy-RBE) | Boost (Gy-RBE) | Status and Treatment |
|---|---|---|---|---|---|---|---|---|
| 34 | Pineal ATRT | M0 | In-field recurrence | 9.8 | A | 30.6 | 23.4 | Alive; NED after chemo |
| 53 | Pineoblastoma | M0 | In-field recurrence | 23.1 | A | 30.6 | 23.4 | Alive; progression after chemo |
| 54 | Anaplastic ependymoma | M0 | In-field recurrence | 14.8 | C, A | 30.6 | 28 | Dead; progression after surgery and chemo |
| 63 | Anaplastic ependymoma, L spine | M3 | In-field recurrence, spine + brain | 14.7 | none | 39.6 | 14.4 | Alive; progression after re-irradiation and chemo |
| 29 | Medulloblastoma | M0 | Diffuse leptomeningeal disease | 6.4 | C, A | 23.4 | 30.6 | Dead; progression after chemo |
| 27 | Medulloblastoma | M0 | Diffuse skeletal metastasis | 44.6 | none | 23.4 | 30.6 | Alive; progression of metastases; primary controlled |
| 17 | Nongerminomatous GCT | M3 | Peritoneal carcinomatosis (related to VP shunt) | 2.3 | N, A | 36 | 18 | Alive; NED after BMT, primary controlled |
Abbreviations: ATRT, atypical teratoid rhabdoid tumor; L, lumbar; N, neoadjuvant; C, concurrent; A, adjuvant; NED, no evidence of disease; VP, ventriculoperitoneal; BMT, bone marrow transplant.
Discussion
To our knowledge this is the largest study of nonpediatric patients treated with p-CSI, representing a spectrum of patients with a variety of histologies who received different chemotherapy regimens and CSI doses. Because many of these patients have a favorable long-term prognosis, minimizing treatment-related morbidity has become an important treatment goal. Acute GI and hematologic toxicity can significantly impact quality of life as well as adversely affect outcomes if they result in treatment breaks during RT.3,13,14 A large portion of patients who require CSI also receive chemotherapy, compounding the risk of toxicity. In this study, p-CSI was well tolerated, with minimal weight loss, nausea/vomiting, and severe (grade 3 or 4) cytopenias. Of note, the majority of patients (80%) received chemotherapy as part of their treatment, which has been poorly tolerated historically with x-CSI.1,3,14–17
While the driving factor for hematologic toxicity in this study, and in others, seemed to be chemotherapy, vertebral column irradiation can also significantly reduce blood counts.1–3,6,15,18 In adults, ∼50% of bone marrow is in the spinal column, which also has the highest concentration of active hematopoietic cells.19,20 Thirty percent to 40% of active red marrow is irradiated during x-CSI in skeletally mature patients, compared with 25% in children.6 All of these patients receive doses >20 Gy, which can cause acute bone marrow aplasia. While bone marrow can compensate by increasing hematopoietic activity in previously quiescent areas,6,21 systemic chemotherapy may prevent adequate bone marrow recovery after irradiation.1,2,18,22 Vertebral body-sparing p-CSI in patients who have reached skeletal maturity has been suggested to reduce doses significantly to vertebral body bone marrow, thereby potentially reducing hematologic toxicity from CSI,23 confirmed by our analysis. The association of hematologic toxicity with younger age at RT observed in this study can likely be attributed to the greater percentage of young patients receiving chemotherapy before or during RT.
In this study, 21 patients began p-CSI with at least one low blood count, yet severe cytopenias occurred in only 4 patients. Proton beam CSI was not associated with reduced blood counts, even in patients who had received chemotherapy prior to CSI. In comparison, Jefferies et al1 analyzed 23 adult patients with a variety of malignant histologies who received chemotherapy and x-CSI. In our analysis, severe leukopenia and thrombocytopenia occurred in 13% and 6%, respectively, of patients who received any chemotherapy versus 30% and 13% found by Jefferies et al. Other studies have reported treatment breaks due to myelosuppression in 12% to 35% of patients, with worse disease outcomes in patients with treatment breaks.1,3,14–17 While patients in this study who received pre-CSI or concurrent chemotherapy were at a higher risk for myelosuppression, no treatment interruptions occurred.
Acute GI toxicity is another clinically relevant side effect of CSI. Anorexia, nausea, and vomiting can lead to weight loss, fatigue, and patient discomfort. In this study, GI toxicity was limited to mild nausea and vomiting. Prior studies using x-CSI have reported high-grade anorexia and nausea and vomiting.14,24,25 In one study of 66 adult and pediatric patients requiring CSI, 11% of patients receiving RT alone and 13% of patients receiving combined chemoradiation experienced treatment interruptions attributed to GI toxicity.14 Proton beam CSI can significantly reduce GI toxicity by eliminating the bowel dose associated with x-CSI, improving quality of life and possibly avoiding more serious effects of GI toxicity,23 which our results support.
The proposed advantages of p-CSI include a reduction in late toxicities.7–9 Risk reduction estimates for p-CSI in pediatric patients have predicted improvements in hearing loss, pituitary and hypothalamic disorders, primary thyroid dysfunction, myocardial infarction and conduction arrhythmias, restrictive lung disease, primary gonadal dysfunction, neurocognitive deficits, and second primary neoplasms.4,26–28 In our study, median RT doses to the cochleae, pituitary, and hypothalamus were below standard constraints,29,30 with an extremely low dose to the thyroid, especially compared with x-CSI. In dosimetric models, PBRT was superior to intensity-modulated radiation therapy in reducing cochlear and pituitary doses.7 In our analysis of patients with sufficient follow-up audiograms, toxicity rates and the median cochlear doses were consistent with those of a recent study from our institution, which also reported minimal ototoxicity in children treated with cranial PBRT.31 Long-term assessment of endocrine toxicities requires additional follow-up.
Adult patients may have lower rates of late toxicities from dose reduction to organs at risk using p-CSI, similar to the proposed benefits to pediatric patients, especially following cases of curative treatment. In this study, nearly half of the patients were <25 years old at the time of RT, with many patients expected to be long-term survivors. We observed extremely low doses to the lung, heart, kidney, bowel, and gonads using p-CSI, all of which would presumably decrease the risk for associated late toxicities compared with x-CSI. Further follow-up is necessary to evaluate this hypothesis.
Long-term follow-up is also needed to confirm the efficacy of p-CSI, although the OS and PFS in this study are similar to those in other studies using x-CSI.32–38 Criticisms of the use of p-CSI include the possibility for worse local control because of the potential for underdosage of the target due to RBE differences in tumor cells and surrounding brain and spinal cord.39 Uncertainties at the distal edge of the proton beam when using a vertebral body-sparing technique could also potentially lead to underdosage of the spinal canal. The low rate of early local recurrences in this study, with no recurrences at junction sites, provides support that p-CSI was delivered as planned and that RT doses used were sufficient, although longer follow-up is necessary to fully evaluate disease outcomes after receiving p-CSI in this cohort.
Other limitations of this study include those associated with retrospective reviews, in particular selection bias of the patients receiving p-CSI. Also, patients with various histologies were analyzed here. While the large patient population provided opportunity to evaluate toxicity in a variety of clinical settings, this diversity causes difficulty in evaluating outcomes. Final conclusions regarding the efficacy of p-CSI cannot be reached at this point because of short follow-up, and future assessment is needed to determine long-term toxicity and disease-specific outcomes.
There are several other factors prohibitive to the implementation of p-CSI. In this study we have demonstrated that p-CSI is technically feasible, despite some of the complexities of treatment planning, including an evaluation of cost-effectiveness, which was beyond the scope of this study. One report in children suggested that a reduction in long-term sequelae would offset the increased upfront cost of p-CSI.40 Adults are likely to develop less long-term toxicities than children, so an evaluation of cost-effectiveness of p-CSI for adults is warranted as more long-term information becomes available.
This large series of p-CSI confirms low rates of acute toxicity, consistent with prior dosimetric models. Vertebral body-sparing p-CSI is feasible and should be considered as a way to reduce acute GI and hematologic toxicity in adults requiring CSI—especially in the setting of aggressive treatment with chemotherapy in addition to CSI, which may contribute to improved survival. Longer follow-up is needed to evaluate whether a reduction in toxicity translates into improvements in long-term treatment-related morbidity and overall disease outcomes. Further study is warranted to evaluate PBRT and other ways to reduce toxicity in adult patients requiring CSI.
Funding
The authors report no funding sources supporting this research.
Conflict of interest statement. None declared.
References
- 1.Jefferies S, Rajan B, Ashley S, et al. Haematological toxicity of cranio-spinal irradiation. Radiother Oncol. 1998;48:23–27. doi: 10.1016/s0167-8140(98)00024-3. [DOI] [PubMed] [Google Scholar]
- 2.Plowman PN. The effects of conventionally fractionated, extended portal radiotherapy on the human peripheral blood count. Int J Radiat Oncol Biol Phys. 1983;9:829–839. doi: 10.1016/0360-3016(83)90008-1. [DOI] [PubMed] [Google Scholar]
- 3.El-Aal HA, Mokhtar MM, Habib EE, et al. Medulloblastoma: conventional radiation therapy in comparison to chemo radiation therapy in the post-operative treatment of high-risk patients. J Egypt Nat Can Inst. 2005;17:301–307. [PubMed] [Google Scholar]
- 4.Fossati P, Ricardi U, Orecchia R. Pediatric medulloblastoma: toxicity of current treatment and potential role of proton therapy. Can Treat Rev. 2009;35:79–96. doi: 10.1016/j.ctrv.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 5.Brodin NP, Munck Af Rosenschöld P, Aznar MC, et al. Radiobiological risk estimates of adverse events and secondary cancer for proton and photon radiation therapy of pediatric medulloblastoma. Acta Oncol. 2011;50:806–816. doi: 10.3109/0284186X.2011.582514. [DOI] [PubMed] [Google Scholar]
- 6.Chang EL, Allen P, Wu C, et al. Acute toxicity and treatment interruption related to electron and photon craniospinal irradiation in pediatric patients treated at the University of Texas MD Anderson Cancer Center. Int J Radiat Oncol Biol Phys. 2002;52:1008–1016. doi: 10.1016/s0360-3016(01)02717-1. [DOI] [PubMed] [Google Scholar]
- 7.St. Clair WH, Adams JA, Bues M, et al. Advantage of protons compared to conventional X-ray or IMRT in the treatment of a pediatric patient with medulloblastoma. Int J Radiat Oncol Biol Phys. 2004;58:727–734. doi: 10.1016/S0360-3016(03)01574-8. [DOI] [PubMed] [Google Scholar]
- 8.Yoon M, Shin DH, Kim J, et al. Craniospinal irradiation techniques: a dosimetric comparison of proton beams with standard and advanced photon radiotherapy. Int J Radiat Oncol Biol Phys. 2011;81:637–646. doi: 10.1016/j.ijrobp.2010.06.039. [DOI] [PubMed] [Google Scholar]
- 9.Yuh GE, Loredo LN, Yonemoto LT, et al. Reducing toxicity from craniospinal irradiation: using proton beams to treat medulloblastoma in young children. Cancer J. 2004;10:386. doi: 10.1097/00130404-200411000-00009. [DOI] [PubMed] [Google Scholar]
- 10.Lee CT, Bilton SD, Famiglietti RM, et al. Treatment planning with protons for pediatric retinoblastoma, medulloblastoma, and pelvic sarcoma: how do protons compare with other conformal techniques? Int J Radiat Oncol Biol Phys. 2005;63:362–372. doi: 10.1016/j.ijrobp.2005.01.060. [DOI] [PubMed] [Google Scholar]
- 11.Krejcarek SC, Grant PE, Henson JW, et al. Physiologic and radiographic evidence of the distal edge of the proton beam in craniospinal irradiation. Int J Radiat Oncol Biol Phys. 2007;68:646–649. doi: 10.1016/j.ijrobp.2007.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cochran DM, Yock TI, Adams JA, et al. Radiation dose to the lens during craniospinal irradiation—an improvement in proton radiotherapy technique. Int J Radiat Oncol Biol Phys. 2008;70:1336–1342. doi: 10.1016/j.ijrobp.2007.08.027. [DOI] [PubMed] [Google Scholar]
- 13.Kuhlthau KA, Pulsifer MB, Yeap BY, et al. Prospective study of health-related quality of life for children with brain tumors treated with proton radiotherapy. J Clin Oncol. 2012;30:2079–2086. doi: 10.1200/JCO.2011.37.0577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rieken S, Mohr A, Habermehl D, et al. Outcome and prognostic factors of radiation therapy for medulloblastoma. Int J Radiat Oncol Biol Phys. 2011;81:e7–e13. doi: 10.1016/j.ijrobp.2010.12.042. [DOI] [PubMed] [Google Scholar]
- 15.Kortmann RD, Kühl J, Timmermann B, et al. Postoperative neoadjuvant chemotherapy before radiotherapy as compared to immediate radiotherapy followed by maintenance chemotherapy in the treatment of medulloblastoma in childhood: results of the German prospective randomized trial hit ‘91. Int J Radiat Oncol Biol Phys. 2000;46:269–279. doi: 10.1016/s0360-3016(99)00369-7. [DOI] [PubMed] [Google Scholar]
- 16.Malheiros SF, Franco CR, Stávale JN, et al. Medulloblastoma in adults: a series from Brazil. J Neurooncol. 2002;60:247–253. doi: 10.1023/a:1021178518361. [DOI] [PubMed] [Google Scholar]
- 17.Chan AW, Tarbell NJ, Black PML, et al. Adult medulloblastoma: prognostic factors and patterns of relapse. Neurosurgery. 2000;47:623–632. doi: 10.1097/00006123-200009000-00018. [DOI] [PubMed] [Google Scholar]
- 18.Marks LB, Cuthbertson D, Friedman HS. Hematologic toxicity during craniospinal irradiation: the impact of prior chemotherapy. Med Pediatr Oncol. 1995;25:45–51. doi: 10.1002/mpo.2950250110. [DOI] [PubMed] [Google Scholar]
- 19.Ellis RE. The distribution of active bone marrow in the adult. Phys Med Biol. 1961;5:255. doi: 10.1088/0031-9155/5/3/302. [DOI] [PubMed] [Google Scholar]
- 20.Bolch WE, Patton PW, Rajon DA, et al. Considerations of marrow cellularity in 3-dimensional dosimetric models of the trabecular skeleton. J Nucl Med. 2002;43:97–108. [PubMed] [Google Scholar]
- 21.Mauch P, Constine L, Greenberger J, et al. Hematopoietic stem cell compartment: acute and late effects of radiation therapy and chemotherapy. Int J Radiat Oncol Biol Phys. 1995;31:1319–1339. doi: 10.1016/0360-3016(94)00430-S. [DOI] [PubMed] [Google Scholar]
- 22.Plowman PN. Haematologic toxicity during craniospinal irradiation—the impact of prior chemotherapy. Med Pediatr Oncol. 1997;28:238–239. doi: 10.1002/(sici)1096-911x(199703)28:3<238::aid-mpo17>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 23.Brown AP, Barney CL, Grosshans DR, et al. Proton beam craniospinal irradiation reduces acute toxicity for adults with medulloblastoma. Int J Radiat Oncol Biol Phys. 2013;86:277–284. doi: 10.1016/j.ijrobp.2013.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Herrlinger U, Steinbrecher A, Rieger J, et al. Adult medulloblastoma: prognostic factors and response to therapy at diagnosis and relapse. J Neurol. 2005;252:291–299. doi: 10.1007/s00415-005-0560-2. [DOI] [PubMed] [Google Scholar]
- 25.Ang C, Hauerstock D, Guiot MC, et al. Characteristics and outcomes of medulloblastoma in adults. Pediatr Blood Cancer. 2008;51:603–607. doi: 10.1002/pbc.21588. [DOI] [PubMed] [Google Scholar]
- 26.Miralbell R, Lomax A, Bortfeld T, et al. Potential role of proton therapy in the treatment of pediatric medulloblastoma/primitive neuroectodermal tumors: reduction of the supratentorial target volume. Int J Radiat Oncol Biol Phys. 1997;38:477–484. doi: 10.1016/s0360-3016(97)00004-7. [DOI] [PubMed] [Google Scholar]
- 27.Miralbell R, Lomax A, Russo M. Potential role of proton therapy in the treatment of pediatric medulloblastoma/primitive neuro-ectodermal tumors: spinal theca irradiation. Int J Radiat Oncol Biol Phys. 1997;38:805–811. doi: 10.1016/s0360-3016(97)00005-9. [DOI] [PubMed] [Google Scholar]
- 28.Newhauser WD, Fontenot JD, Mahajan A, et al. The risk of developing a second cancer after receiving craniospinal proton irradiation. Phys Med Biol. 2009;54:2277. doi: 10.1088/0031-9155/54/8/002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bhandare N, Jackson A, Eisbruch A, et al. Radiation therapy and hearing loss. Int J Radiat Oncol Biol Phys. 2010;76:S50–S57. doi: 10.1016/j.ijrobp.2009.04.096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lawrence YR, Li XA, el Naqa I, et al. Radiation dose–volume effects in the brain. Int J Radiat Oncol Biol Phys. 2010;76:S20–S27. doi: 10.1016/j.ijrobp.2009.02.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moeller BJ, Chintagumpala M, Philip JJ, et al. Low early ototoxicity rates for pediatric medulloblastoma patients treated with proton radiotherapy. Radiat Oncol. 2011;6:58. doi: 10.1186/1748-717X-6-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Balducci M, Chiesa S, Chieffo D, et al. The role of radiotherapy in adult medulloblastoma: long-term single-institution experience and a review of the literature. J Neurooncol. 2012;106:315–323. doi: 10.1007/s11060-011-0665-7. [DOI] [PubMed] [Google Scholar]
- 33.Selvanathan SK, Hammouche S, Smethurst W, et al. Outcome and prognostic features in adult pineoblastomas: analysis of cases from the SEER database. Acta Neurochir. 2012;154:863–869. doi: 10.1007/s00701-012-1330-4. [DOI] [PubMed] [Google Scholar]
- 34.Wolden SL, Wara WM, Larson DA, et al. Radiation therapy for primary intracranial germ-cell tumors. Int J Radiat Oncol Biol Phys. 1995;32:943–949. doi: 10.1016/0360-3016(95)00067-9. [DOI] [PubMed] [Google Scholar]
- 35.McCarthy BJ, Shibui S, Kayama T, et al. Primary CNS germ cell tumors in Japan and the United States: an analysis of 4 tumor registries. Neuro-Oncol. 2012;14:1194–1200. doi: 10.1093/neuonc/nos155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee JK, Wakabayashi T, Yoshida J. Management and survival of pineoblastoma: an analysis of 34 adults from the brain tumor registry of Japan. Neurol Med Chir (Tokyo) 2005;45:132–141. doi: 10.2176/nmc.45.132. discussion 141–142. [DOI] [PubMed] [Google Scholar]
- 37.Smith AA, Weng E, Handler M, et al. Intracranial germ cell tumors: a single institution experience and review of the literature. J Neurooncol. 2004;68:153–159. doi: 10.1023/b:neon.0000027670.96412.36. [DOI] [PubMed] [Google Scholar]
- 38.Chang SM, Lillis-Hearne PK, Larson DA, et al. Pineoblastoma in adults. Neurosurgery. 1995;37:383–390. doi: 10.1227/00006123-199509000-00003. discussion 390–391. [DOI] [PubMed] [Google Scholar]
- 39.Jones B, Wilson P, Nagano A, et al. Dilemmas concerning dose distribution and the influence of relative biological effect in proton beam therapy of medulloblastoma. Br J Radiol. 2012;85:e912–e918. doi: 10.1259/bjr/24498486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lundkvist J, Ekman M, Ericsson SR, et al. Cost-effectiveness of proton radiation in the treatment of childhood medulloblastoma. Cancer. 2005;103:793–801. doi: 10.1002/cncr.20844. [DOI] [PubMed] [Google Scholar]