Abstract
Background
Intramedullary ependymomas are rare and benign tumors in the adult. Little is known about their physiopathology, but the implication of the NF2 gene is suspected because of their presence in a third of patients with type 2 neurofibromatosis (NF2), a disorder caused by mutation of the NF2 gene.
Methods
We conducted a clinical and genetic study of a family in which 5 of 9 members suffered from intramedullary ependymoma. Karyotyping and CGH array analysis were performed on DNA from peripheral blood lymphocytes from affected participants. The NF2 gene sequences were then determined in DNA from 3 nonaffected and all 5 affected members of the family.
Results
Karyotype and CGH array findings were normal. Sequencing of NF2 revealed a heterozygous deletion, c.811-39_841del69bp, at the intron 8/exon 9 junction, in all affected members that was absent from all nonaffected members. RT-PCR analysis and sequencing revealed a novel NF2 transcript characterized by a skipping of exon 9 (75 bp). This deletion is predicted to result in a 25-amino acid deletion in the N-terminal FERM domain of neurofibromin 2. Modeling of this mutant domain suggests possible disorganization of the subdomain C.
Conclusion
We report the first family with an NF2 mutation associated with intramedullary ependymomas without other features of NF2 syndrome. This mutation, which has not been described previously, may particularly affect the function of neurofibromin 2 in ependymocytes leading to the development of intramedullary WHO grade II ependymomas. We propose that sporadic intramedullary ependymomas should also be analyzed for this region of NF2 gene.
Keywords: ependymoma, FERM domain, intramedullary tumor, merlin, NF2 mutation, type 2 neurofibromatosis
Ependymomas are glioneuronal tumors that arise from ependymal cells on the cerebral ventricular surface and the central medullary canal. They account for 3%–9% of brain tumors and about 60% of spinal cord tumors.1
In adults, the localization is more frequently spinal (60%) than intracranial (40%).2 Neurological symptoms appear very slowly. Thus, it is not rare that diagnosis is made several months or years after the initial onset.3 Magnetic resonance imaging (MRI) shows a centromedullary mass, usually heterogeneous, partially cystic, and enhanced after intravenous gadolinium infusion. The 2007 classification of the World Health Organization (WHO) recognizes 3 histopathological grades depending on cytonuclear atypia, endothelial capillary proliferation, necrosis, mitosis, and the immunohistochemical MIB1/Ki67 labeling index. Spinal cord ependymomas in the adult are mostly WHO grade II, and the best recovery rate without recurrence is obtained by surgical resection.4,5
Little is known about the physiopathology of ependymoma because it is a rare, clinically heterogeneous disease. However, there is imaging evidence of ependymomas in more than 33% of patients with type 2 neurofibromatosis (NF2),6,7 a rare autosomal-dominant inherited disorder caused by mutation of the NF2 gene.
The NF2 tumor suppressor gene is localized on 22q12.2.8,9 It encodes a 595-amino acid protein, neurofibromin 2,9 which is a membrane/cytoskeleton-associated protein mediating cell-to-cell contact inhibition of proliferation.10 Current models indicate that neurofibromin 2, when unphosphorylated, accumulates in the nucleus and binds the ubiquitin ligase CRL4 (DCAF1); it thereby suppresses CRL4 activity and consequently blocks mitogenic signals.11,12 According to the 2-hit model,13 tumorigenesis occurs when both NF2 alleles are inactivated. NF2 patients harbor a heterozygous germline mutation in the NF2 gene, so the loss or mutation of the second NF2 allele in a somatic cell is sufficient for a tumor to develop. The type of cells containing both inactivating events determines the histology of the tumor. The severity of the disease correlates with the extent of loss of function of the NF2 gene; the disease is severe in cases of truncating mutations (nonsense variant or frameshift insertions/deletions).14–16
Karyotyping abnormalities are frequent in ependymomas, particularly affecting chromosome 22.17,18 Several studies19–22 demonstrate that the NF2 gene is involved in the tumorigenesis of ependymomas, particularly in spinal cord forms. Consequently, some authors consider spinal cord ependymomas to be a distinct entity, characterized by a high frequency of loss of heterozygosity (LOH) at 22q and of NF2 gene mutations.19,20,23
In this study, we report a family with cervical intramedullary ependymomas without other features of the NF2 syndrome. A previously undescribed NF2 gene mutation was detected in all affected members.
Materials and Methods
Participants
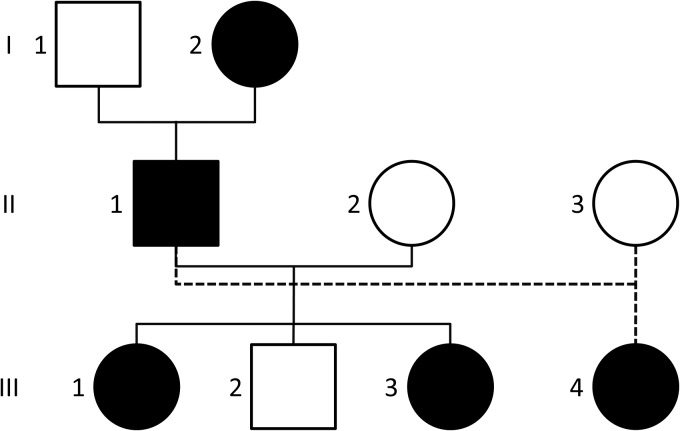
The family pedigree is shown in Fig. 1. Two of the family members underwent surgery, which allowed pathological investigations showing WHO grade II ependymomas in both cases. Spinal cord MRI was performed for all family members. Cerebral MRI of high resolution with series of sections of <1 mm was carried out for affected participants II-1, III-3 and III-4. Ophthalmologic and dermatologic examinations were performed in participants II-1 (symptomatic) and III-4 (asymptomatic).
Fig. 1.
Family pedigree. Individuals with ependymoma and NF2 mutation are shown in black. Participants II-1 and III-1 were operated for a cervical intramedullary ependymoma at ages 52 and 33 years, respectively. Participants I-2, III-3 and III-4 presented with asymptomatic cervical intramedullary ependymoma discovered after systematic MRI at ages 81, 27, and 23 years, respectively. MRI of the other members of the family revealed no cerebral or intramedullary tumor.
Chromosome, DNA and RNA Analysis
Written informed consent was obtained from all family members, and our project was approved by the institutional review board of the CHRU de Tours. High resolution R and G chromosome banding (RTBG and GTBG banding techniques and Giemsa staining) were performed with peripheral blood lymphocyte cultures (participant II-1).
DNA was extracted from peripheral blood leukocytes by automated purification protocol using QIAsymphony SP (Qiagen). DNA from participant II-1 was used for CGH array analysis (Array 105K, Agilent). DNA from participant I-2 was used for NF2 sequencing analysis. Primers specific for the 17 NF2 (NM_000268) coding exons and their intron boundaries were used to amplify the NF2 gene from genomic DNA (primer sequences and PCR conditions are available upon request). Mutations were identified by bidirectional DNA sequencing of the purified PCR products with the Big Dye terminator cycle sequencing kit and migration on an ABI 3130XL sequencer (Life Technologies). Sequences were aligned with Seqscape® v2.5 analysis software (Applied Biosystems).
Total RNA was extracted from the lymphoblastoid cell line of participant III-3 and nonaffected family member II-2 using Trizol (Invitrogen). Superscript II (Invitrogen) was used to produce cDNA from 1 μg of RNA, which was amplified by PCR with Taq polymerase Promega (60°C), a forward primer in exon 8 (5′-actgacccccaagtctcctt-3′), and reverse primers in exons 9 and 10 (Exon 9: 5′-aacacgaagctttgaggagtt-3′, product size: 128 bp; Exon 10: 5′-gggctttcatctgctgaact, product size: 220 bp). RT-PCR products were analyzed by electrophoresis on 1.5% agarose gel and sequencing with Applied 3130XL DNA sequencer.
Modeling of the Mutant
The 3D structure of the FERM domain of neurofibromin 2 (PDB 1H4R) has been determined by x-ray crystallography.24 We used the coordinates of this structure to model the mutant form of the FERM domain with SWISS-MODEL,25 a structure homology-modeling server.26 Ribbon representations of the wild-type and mutant forms of the FERM domain of neurofibromin 2 were obtained using the molecular graphics tool PyMOL v1.3.27
Results
Family Study
Participant II-1 was born in 1947. He presented at our institution (CHRU de Tours) in 1999 with cervical pain and paresthesia in the right upper limb of 3 months duration. Cerebral and medullary MRI showed a single spinal cord lesion from the myelencephalon to C7, enhanced after gadolinium infusion (Fig. 2B). Surgery in 1999 resulted in subtotal resection of the lesion. Pathological study showed a WHO grade II ependymoma (Supplementary material Fig. 1). Neurological status improved after surgery despite a persistent neuropathic pain in the right hand. Postoperative MRI at 6 months showed a tumor residue and subependymal dissemination along the spinal cord. No abnormal cells were found in the cerebrospinal fluid. Images remained stable on annual MRIs for 4 years. In 2004, the participant's neurological status worsened, with right C8 through T1 and right lower limb proprioceptive troubles, while MRI showed a cervical spinal cord recurrence at the upper part of the first surgical field. He underwent a second surgical intervention for total resection of this recurrence. Postoperatively, right spastic hemiparesis with complete C8 through T1 palsy and painful reduced mobility of the right shoulder appeared. Pathological study confirmed the diagnosis of WHO grade II ependymoma without any sign of malignancy. Spinal cord MRI in 2005 showed a new progression of the disease in the upper part of the surgical field. In 2005, he was treated with radiation therapy of the entire spinal cord (45 Gy by direct posterior beam: half-dose photon, half-dose electron, 5 fractions of 9 Gy per fraction in 1 week). Annual MRI and clinical follow-up indicated that there had been no evolution of the tumor residue and neurological status until 2011. In 2011, paresthesia of the right hand, bladder dysfunction, and progression of the tumor residue in the surgical cavity appeared. In 2012, MRI tumor residue continued to progress. Considering the neurological risks of a third surgery on a previously irradiated spinal cord, it was decided to try to treat with temozolomide despite the lack of evidence of efficacy of this treatment for that pathology. After the first temozolomide cure at 150 mg/m2, side effects (symptomatic dermographism and mild thrombopenia) imposed to maintain that posology. Since then, his neurological status has remained stable after 7 months of temozolomide . He walks with one cane and complains of difficulty with precise movements of both hands. Neurological examination reveals pyramidal spasticity in the right lower limb and right shoulder pain on mobilization. Ophthalmologic examination in 2012 revealed no posterior subcapsular lenticular opacity.
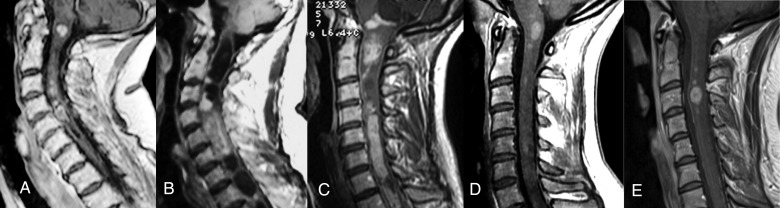
Fig. 2.
Magnetic Resonance Imaging of affected participants. Sagittal views of the cervical spinal cord of participants I-2 (A), II-1 (B), III-1 (C), III-3 (D), and III-4 (E), on T1 weighted MRI after gadolinium infusion. In each participant, the lesion involved the cervical spinal cord and/or the cervicomedullary junction was enhanced after gadolinium infusion and presented peripheral intramedullary cysts at their upper and lower extremities.
Participant III-1 was born in 1971. She presented at CHU Bicêtre (Assistance Publique des Hôpitaux de Paris) in 2004 with cervical pain and hypoesthesia with a T6 sensory level for the previous 2 years. Cerebral and medullary MRI revealed a single spinal cord lesion from the myelencephalon to the T2 level heterogeneously enhanced after gadolinium infusion (Fig. 2C). Total surgical resection of the lesion was performed in 2004. Pathological study showed a WHO grade II ependymoma. Postoperative neuropathic pain was treated medically. MRI follow-up showed dissemination along the spinal cord without any neurological sign. The participant did not receive any complementary treatment. Neurological and MRI status remain stable under antalgic medication.
MRI revealed a cervical spinal cord lesion in 5 of the 9 family members studied (Fig. 2). An asymptomatic cervical spinal cord lesion was found in each of participants I-2, III-3 and III-4 (Fig. 2A, D and E). The similarity between the 5 MRI lesions allowed strong suspicion for an ependymoma in all 5 family members. This high intrafamilial prevalence of cervical intramedullary ependymomas suggested a genetic origin compatible with autosomal dominant inheritance.
None of the family members suffered from hearing loss, and cerebral MRI with high resolution performed on 3 of the 5 affected members showed there was no vestibular schwannoma (Supplementary material Fig. 2).
Ophthalmologic and dermatologic examinations of participants II-1 and III-4 were normal with no posterior subcapsular lenticular opacity and no cutaneous abnormality. The other family members did not present any clinical signs of disease.
Genetic Study
Chromosome analysis revealed a normal karyotype, 46,XY, for participant II-1 (resolution, 850 bands). Array-CGH analysis of DNA from participant II-1 revealed 4 small copy-number polymorphisms described in the Database of Genomic Variants.28 No other copy-number variant was observed.
We analyzed the coding regions and intronic flanking regions of the NF2 gene in lymphocytes from participant I-2. Sequencing of NF2 revealed a heterozygous deletion, c.811-39_841del69 bp, at the intron 8/exon 9 junction. This same mutation was subsequently detected in all 5 family members with ependymoma but was absent from all nonaffected family members (II-2, II-3, III-2) from whom DNA was available.
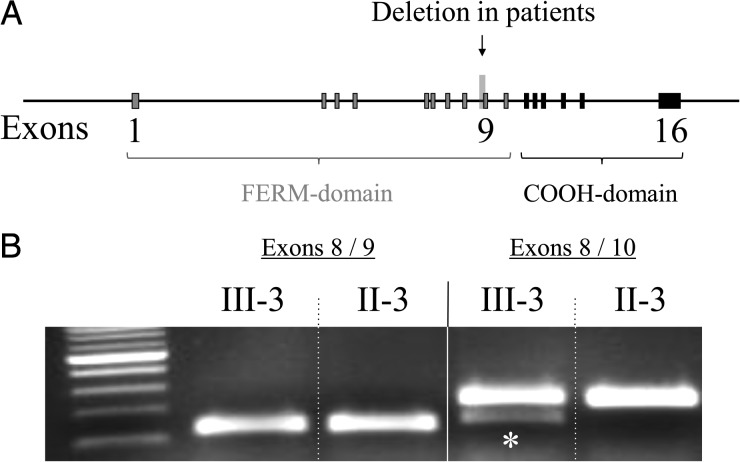
RNA extracted from lymphoblastoid cell lines from affected member III-3 yielded a novel, smaller RT-PCR product not found for nonaffected member II-2 (Fig. 3). Sequencing this altered cDNA fragment, purified from gel, revealed a deletion of the entire exon 9 that contains 75 bp and is therefore inframe.
Fig. 3.
NF2 intron 8/exon 9 deletion. A. Schematic structure of the NF2 gene with the location of the mutation c.811-39_841del69bp at the intron 8/exon 9 junction. B. Electrophoretic banding pattern of products of RT-PCR illustrating a new and smaller RT-PCR product for affected member III-3 (asterisk) not found for nonaffected member II-3.
Based on the 2-hit model of NF2, we planned to sequence the second allele of the NF2 gene in tumor tissue from affected members. Paraffin-embedded samples of ependymoma tissue from family member II-1 were used for DNA extraction for NF2 gene sequencing. However, 2 extractions failed to obtain DNA of sufficient quality for sequencing.
Modeling of the Mutant
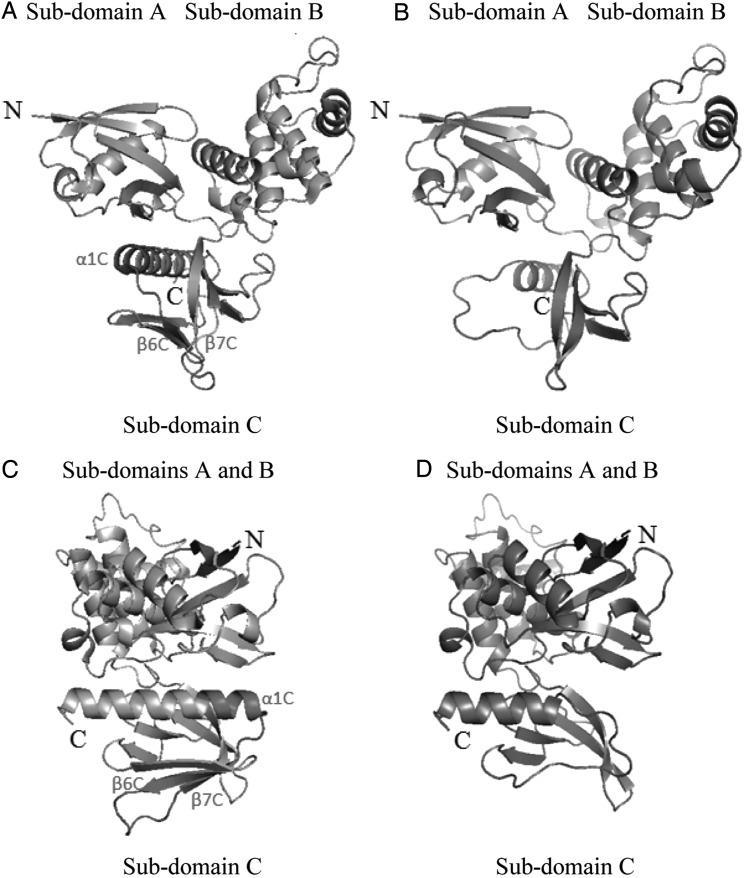
The mutated NF2 allele appeared to encode a neurofibromin 2 protein with a 25-amino acid deletion in its FERM domain in the N-terminal region. These deleted residues form the central region of the subdomain C (β-strand 6C, β-strand 7C, and beginning of α-helix 1C) in the wild-type FERM domain. We used the crystallographic structure of neurofibromin 2 to model the consequences of the deletion (Fig. 4). The model obtained for the mutant indicated a possible disorganization of the subdomain C of the FERM domain without consequences for the subdomains A and B.
Fig. 4.
Tridimensional structure of the FERM domain of neurofibromin 2. Crystal structure of the FERM domain of human protein neurofibromin 2 (A and C) with the region encoded by exon 9 shown in red. Model structure of the FERM domain of the mutant form of neurofibromin 2 protein (B and D). A-B, C-D: similar orientations of the FERM domain.
Discussion
We found a novel mutation of the NF2 gene causing deletion of the entire exon 9 associated with cervical intramedullary ependymomas in a family. The 5 affected participants were all adults, and specimens from 2 of the 5 cervical intramedullary tumors were available for analysis; both were WHO grade II ependymomas. The MRI characteristics of all 5 tumors were very similar, consistent with all 5 family members having the same pathology. Therefore, we considered this family to be a homogeneous family with cervical intramedullary ependymomas. Participants II-1 and III-4 underwent ophthalmologic and dermatologic examinations: there was no posterior subcapsular lenticular opacity nor any cutaneous lesion. The family does not satisfy the Manchester clinical criteria for NF2 syndrome.29
Two cases of familial ependymoma have been investigated previously. Nijssen et al30 described a Dutch family in which 4 male cousins developed intracranial WHO grade III ependymoma during childhood. Karyotyping on blood leukocytes of 2 patients did not reveal any abnormality. The tumor in 1 of the 4 patients was analyzed and showed LOH of chromosome 22 without any mutation of the NF2 gene. Hulsebos et al31 performed array-CGH on the same tumor and showed that the only unbalanced chromosomal abnormality was a monosomy 22. They also analyzed microsatellite markers covering the entirety of chromosome 22 in blood leukocyte DNA from 2 of the 4 patientss and for other members of the family covering 3 generations. The results suggested that there was a susceptibility gene in 22pter-22q11.2, proximal to the NF2 gene (22q12.2). The second family to be reported is a Japanese non-NF2 family32 in which 3 adult siblings (1 female and 2 males) developed cervicothoracic spinal cord tumors, 2 WHO grade III ependymomas and 1 schwannoma. The authors analyzed microsatellites covering the distal part of chromosome 22q, in blood leukocyte DNA from 2 healthy members of the family and from the tumors of each of the 3 patients. They obtained results for only 2 of the 3 patients; they found LOH for the distal part of 22q, with no mutation in the NF2 gene.
Other families have been reported in the literature but without any genetic analysis: they include families with intracranial ependymoma33 and subependymoma.34–36
We here describe a family in which a cervical intramedullary epdendymoma was observed in each of 5 members covering 3 consecutive generations, suggesting Mendelian autosomal dominant transmission. Sequencing of affected member I-2's DNA detected a heterozygous mutation, c.811-39_841 del 69 bp, in the NF2 gene. This was later confirmed in a second sample from the same participant and then from DNA from the 4 other affected members of the family. The 3 nonaffected members of the family did not carry this mutation. Perfect segregation with the pathology within the family strongly supports our hypothesis of Mendelian autosomal dominant transmission.
No deletion causing the absence of exon 9 from the NF2 transcript has previously been reported both in patients and control individuals.37 The mutation we identified is a deletion of a part of the sequence of the intron 8/exon 9 junction that alters splicing, leading to an altered mature mRNA (demonstrated by RT-PCR experiments) and potentially to the loss of an anti-oncogene action of neurofibromin 2. Exon 9 of NF2 encodes residues 271–295 of the FERM domain, which is in the N-terminal region of neurofibromin 2. Most of the residues encoded by exon 9 in the FERM domain are exposed to solvent, with little interaction with the rest of the structure.24 The frequency of constitutional mutations in NF2 exon 9 is very low in NF2,38,39 and splice-site mutations are also rare in this region of the NF2 gene. FERM domains are found in a number of cytoskeletal-associated proteins that associate with various proteins at the interface between the plasma membrane and cytoskeleton. Neurofibromin 2 can exist in a “closed” state, where N- and C-terminal regions participate in an intramolecular interaction, masking the ligand-binding site.40 This mechanism seems to involve the B subdomain of the FERM domain but not the C subdomain affected by the deletion. The C subdomain does not appear to be required for NF2 growth inhibition activity, actin association, or activity of membrane protrusions.41 Further works will be necessary to determine whether or not this C subdomain of the FERM domain participates in the interaction between neurofibromin 2 and particular partners in ependymocytes and if it is necessary for suppressing spinal ependymomas. Neurofibromin 2 without the region encoded by exon 9 may have all functions of the normal NF2 except for suppressing spinal ependymomas. Severe phenotypes of NF2 are associated with nonsense/frameshift mutations or large deletions, but no particular type of mutation has been found to be associated with the presence of ependymoma in NF2 patients.42,43 Our observation is original in 2 ways: (i) this is the first time a particular type of NF2 mutation has been associated with the presence of ependymomas; and (ii) this is the first described NF2 familial mutation that is not associated with NF2 as defined by the Manchester criteria. Additional studies on NF2 gene, and particularly on exons 8-9, may help to determine whether individuals with germline alteration affecting the C subdomain of the FERM domain of neurofibromin 2 are at increased risk of developing ependymomas.
Supplementary Material
Funding
The authors declare no extra-institutional funding.
Supplementary Material
Acknowledgments
We thank the family for participating in this study. We are grateful to Professor Michel Jan (senior surgeon, CHU de Tours) for giving us access to clinical data for operated patients.
Conflict of interest. None declared.
References
- 1.Kleihues P, Cavenee WK. Pathology and genetics of tumours of the nervous system. Lyon: International Agency for Research on Cancer; 2000. World Health Organization of tumours ed. [Google Scholar]
- 2.Hamilton RL, Pollack IF. The molecular biology of ependymomas. Brain Pathol. 1997;7(2):807–822. doi: 10.1111/j.1750-3639.1997.tb01066.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martuza RL, Eldridge R. Neurofibromatosis 2 (bilateral acoustic neurofibromatosis) N Engl J Med. 1988;318(11):684–688. doi: 10.1056/NEJM198803173181106. [DOI] [PubMed] [Google Scholar]
- 4.Alkhani A, Blooshi M, Hassounah M. Outcome of surgery for intramedullary spinal ependymoma. Ann Saudi Med. 2008;28(2):109–113. doi: 10.5144/0256-4947.2008.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hoshimaru M, Koyama T, Hashimoto N, Kikuchi H. Results of microsurgical treatment for intramedullary spinal cord ependymomas: analysis of 36 cases. Neurosurgery. 1999;44(2):264–269. doi: 10.1097/00006123-199902000-00012. [DOI] [PubMed] [Google Scholar]
- 6.Mautner VF, Tatagiba M, Lindenau M, et al. Spinal tumors in patients with neurofibromatosis type 2: MR imaging study of frequency, multiplicity, and variety. AJR Am J Roentgenol. 1995;165(4):951–955. doi: 10.2214/ajr.165.4.7676998. [DOI] [PubMed] [Google Scholar]
- 7.Patronas NJ, Courcoutsakis N, Bromley CM, Katzman GL, MacCollin M, Parry DM. Intramedullary and spinal canal tumors in patients with neurofibromatosis 2: MR imaging findings and correlation with genotype. Radiology. 2001;218(2):434–442. doi: 10.1148/radiology.218.2.r01fe40434. [DOI] [PubMed] [Google Scholar]
- 8.Rouleau GA, Merel P, Lutchman M, et al. Alteration in a new gene encoding a putative membrane-organizing protein causes neuro-fibromatosis type 2. Nature. 1993;363(6429):515–521. doi: 10.1038/363515a0. [DOI] [PubMed] [Google Scholar]
- 9.Trofatter JA, MacCollin MM, Rutter JL, et al. A novel moesin-, ezrin-, radixin-like gene is a candidate for the neurofibromatosis 2 tumor suppressor. Cell. 1993;72(5):791–800. doi: 10.1016/0092-8674(93)90406-g. [DOI] [PubMed] [Google Scholar]
- 10.McClatchey AI, Giovannini M. Membrane organization and tumorigenesis–the NF2 tumor suppressor, Merlin. Genes Dev. 2005;19(19):2265–2277. doi: 10.1101/gad.1335605. [DOI] [PubMed] [Google Scholar]
- 11.Cooper J, Li W, You L, et al. Merlin/NF2 functions upstream of the nuclear E3 ubiquitin ligase CRL4DCAF1 to suppress oncogenic gene expression. Sci Signal. 2011;4(188):pt6. doi: 10.1126/scisignal.2002314. [DOI] [PubMed] [Google Scholar]
- 12.Zhou L, Hanemann CO. Merlin, a multi-suppressor from cell membrane to the nucleus. FEBS Lett. 2012;586(10):1403–1408. doi: 10.1016/j.febslet.2012.03.016. [DOI] [PubMed] [Google Scholar]
- 13.Knudson AG., Jr. Mutation and cancer: statistical study of retinoblastoma. Proc Natl Acad Sci USA. 1971;68(4):820–823. doi: 10.1073/pnas.68.4.820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baser ME, Kuramoto L, Joe H, et al. Genotype-phenotype correlations for nervous system tumors in neurofibromatosis 2: a population-based study. Am J Hum Genet. 2004;75(2):231–239. doi: 10.1086/422700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ruttledge MH, Andermann AA, Phelan CM, et al. Type of mutation in the neurofibromatosis type 2 gene (NF2) frequently determines severity of disease. Am J Hum Genet. 1996;59(2):331–342. [PMC free article] [PubMed] [Google Scholar]
- 16.Sainio M, Jaaskelainen J, Pihlaja H, Carpen O. Mild familial neurofibromatosis 2 associates with expression of merlin with altered COOH-terminus. Neurology. 2000;54(5):1132–1138. doi: 10.1212/wnl.54.5.1132. [DOI] [PubMed] [Google Scholar]
- 17.Biernat W, Zawrocki A. Molecular alterations in ependymomas. Folia Neuropathol. 2007;45(4):155–163. [PubMed] [Google Scholar]
- 18.Mazewski C, Soukup S, Ballard E, Gotwals B, Lampkin B. Karyotype studies in 18 ependymomas with literature review of 107 cases. Cancer Genet Cytogenet. 1999;113(1):1–8. doi: 10.1016/s0165-4608(99)00046-1. [DOI] [PubMed] [Google Scholar]
- 19.Birch BD, Johnson JP, Parsa A, et al. Frequent type 2 neurofibromatosis gene transcript mutations in sporadic intramedullary spinal cord ependymomas. Neurosurgery. 1996;39(1):135–140. doi: 10.1097/00006123-199607000-00026. [DOI] [PubMed] [Google Scholar]
- 20.Ebert C, von Haken M, Meyer-Puttlitz B, et al. Molecular genetic analysis of ependymal tumors. NF2 mutations and chromosome 22q loss occur preferentially in intramedullary spinal ependymomas. Am J Pathol. 1999;155(2):627–632. doi: 10.1016/S0002-9440(10)65158-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rubio MP, Correa KM, Ramesh V, et al. Analysis of the neurofibromatosis 2 gene in human ependymomas and astrocytomas. Cancer Res. 1994;54(1):45–47. [PubMed] [Google Scholar]
- 22.von Haken MS, White EC, Daneshvar-Shyesther L, et al. Molecular genetic analysis of chromosome arm 17p and chromosome arm 22q DNA sequences in sporadic pediatric ependymomas. Genes Chromosomes Cancer. 1996;17(1):37–44. doi: 10.1002/(SICI)1098-2264(199609)17:1<37::AID-GCC6>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 23.Alonso ME, Bello MJ, Arjona D, et al. Analysis of the NF2 gene in oligodendrogliomas and ependymomas. Cancer Genet Cytogenet. 2002;134(1):1–5. doi: 10.1016/s0165-4608(01)00591-x. [DOI] [PubMed] [Google Scholar]
- 24.Kang BS, Cooper DR, Devedjiev Y, Derewenda U, Derewenda ZS. The structure of the FERM domain of merlin, the neurofibromatosis type 2 gene product. Acta Crystallogr D Biol Crystallogr. 2002;58(Pt 3):381–391. doi: 10.1107/s0907444901021175. [DOI] [PubMed] [Google Scholar]
- 25.SWISS-MODEL. Available at http://swissmodel.expasy.org .
- 26.Arnold K, Bordoli L, Kopp J, Schwede T. The SWISS-MODEL workspace: a web-based environment for protein structure homology modelling. Bioinformatics. 2006;22(2):195–201. doi: 10.1093/bioinformatics/bti770. [DOI] [PubMed] [Google Scholar]
- 27.PyMOL. Available at http://pymol.org .
- 28.Database of Genomic Variants. Available at http://projects.tcag.ca/variation .
- 29.Evans DG, Huson SM, Donnai D, et al. A genetic study of type 2 neurofibromatosis in the United Kingdom. I.: Prevalence, mutation rate, fitness, and confirmation of maternal transmission effect on severity . J Med Genet. 1992;29(12):841–846. doi: 10.1136/jmg.29.12.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nijssen PC, Deprez RH, Tijssen CC, et al. Familial anaplastic ependymoma: evidence of loss of chromosome 22 in tumour cells. J Neurol Neurosurg Psychiatry. 1994;57(10):1245–1248. doi: 10.1136/jnnp.57.10.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hulsebos TJ, Oskam NT, Bijleveld EH, et al. Evidence for an ependymoma tumour suppressor gene in chromosome region 22pter-22q11.2. Br J Cancer. 1999;81(7):1150–1154. doi: 10.1038/sj.bjc.6690822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yokota T, Tachizawa T, Fukino K, et al. A family with spinal anaplastic ependymoma: evidence of loss of chromosome 22q in tumor. J Hum Genet. 2003;48(11):598–602. doi: 10.1007/s10038-003-0078-3. [DOI] [PubMed] [Google Scholar]
- 33.Dimopoulos VG, Fountas KN, Robinson JS. Familial intracranial ependymomas. Report of three cases in a family and review of the literature. Neurosurg Focus. 2006;20(1):E8. doi: 10.3171/foc.2006.20.1.9. [DOI] [PubMed] [Google Scholar]
- 34.Clarenbach P, Kleihues P, Metzel E, Dichgans J. Simultaneous clinical manifestation of subependymoma of the fourth ventricle in identical twins. Case report. J Neurosurg. 1979;50(5):655–659. doi: 10.3171/jns.1979.50.5.0655. [DOI] [PubMed] [Google Scholar]
- 35.Honan WP, Anderson M, Carey MP, Williams B. Familial subependymomas. Br J Neurosurg. 1987;1(3):317–321. doi: 10.3109/02688698709023773. [DOI] [PubMed] [Google Scholar]
- 36.Ryken TC, Robinson RA, VanGilder JC. Familial occurrence of subependymoma. Report of two cases. J Neurosurg. 1994;80(6):1108–1111. doi: 10.3171/jns.1994.80.6.1108. [DOI] [PubMed] [Google Scholar]
- 37.1000 Genomes Project. Available at http://www.1000genomes.org .
- 38.Ahronowitz I, Xin W, Kiely R, Sims K, MacCollin M, Nunes FP. Mutational spectrum of the NF2 gene: a meta-analysis of 12 years of research and diagnostic laboratory findings. Hum Mutat. 2007;28(1):1–12. doi: 10.1002/humu.20393. [DOI] [PubMed] [Google Scholar]
- 39.Baser ME. The distribution of constitutional and somatic mutations in the neurofibromatosis 2 gene. Hum Mutat. 2006;27(4):297–306. doi: 10.1002/humu.20317. [DOI] [PubMed] [Google Scholar]
- 40.Yogesha SD, Sharff AJ, Giovannini M, Bricogne G, Izard T. Unfurling of the band 4.1, ezrin, radixin, moesin (FERM) domain of the merlin tumor suppressor. Protein Sci. 2011;20(12):2113–2120. doi: 10.1002/pro.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lallemand D, Saint-Amaux AL, Giovannini M. Tumor-suppression functions of merlin are independent of its role as an organizer of the actin cytoskeleton in Schwann cells. J Cell Sci. 2009;122(Pt 22):4141–4149. doi: 10.1242/jcs.045914. [DOI] [PubMed] [Google Scholar]
- 42.Plotkin SR, O'Donnell CC, Curry WT, Bove CM, MacCollin M, Nunes FP. Spinal ependymomas in neurofibromatosis Type 2: a retrospective analysis of 55 patients. J Neurosurg Spine. 2011;14(4):543–547. doi: 10.3171/2010.11.SPINE10350. [DOI] [PubMed] [Google Scholar]
- 43.Selvanathan SK, Shenton A, Ferner R, et al. Further genotype–phenotype correlations in neurofibromatosis 2. Clin Genet. 2010;77(2):163–170. doi: 10.1111/j.1399-0004.2009.01315.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.