Abstract
Background
Claudins are tight junction proteins expressed in epithelial tissues that play important roles in cell polarity and adhesion. Altered distribution of claudin-1(CLDN1) affects cell mobility and tumor invasiveness. Craniopharyngiomas (CPs) represent epithelial tumors of the sellar region, consisting of adamantinomatous (adaCP) and papillary (papCP) variants. Their tendency to infiltrate surrounding brain structures complicates successful surgery. Reliable markers are required to predict tumor behavior and to establish individualized treatment protocols.
Methods
We describe the distribution pattern of CLDN1 in a large cohort of 66 adaCPs, 21 papCPs, and 24 Rathke`s cleft cyst (RCC) cases using immunohistochemistry. CLDN1 mRNA levels were analyzed with qRT-PCR in 33 CP samples. The impact on the migration potential was studied in primary adaCP cell cultures (n = 11) treated with small interfering RNA (siRNA) for CLDN1. Furthermore, CLDN1 distribution patterns and expression levels were compared between invasive (n = 16) and noninvasive (n = 17) tumor groups.
Results
PapCPs and RCCs exhibited a distinct homogenous and membranous expression pattern, whereas CLDN1 immunoreactivity appeared weaker and more heterogeneous in adaCPs. In the latter cases, whirl-like cell clusters showed complete absence of CLDN1. mRNA analysis confirmed reduced CLDN1 levels in adaCPs versus papCPs. Interestingly, invasive tumors exhibited significantly lower CLDN1 expression compared with noninvasive counterparts regardless of CP subtype. Accordingly, siRNA treatment for CLDN1 altered tumor cell migration in vitro.
Conclusion
CLDN1 represents a novel marker in the differential diagnosis of CP variants and RCCs. Low CLDN1 expression levels correlate with an invasive CP growth pattern and may serve as a prognostic marker.
Keywords: claudin-1, craniopharyngioma, invasion, migration, tight junction
The differential diagnosis of space-occupying, nonadenomatous lesions of the sellar region is challenging and is of paramount importance with respect to distinct clinical manifestations and treatment strategies. Craniopharyngiomas (CPs) are the third most common pediatric brain tumors and account for ∼2%–5% of all primary intracranial neoplasms.1 They originate from the Rathke's pouch epithelium and can be divided into adamantinomatous (adaCP) and papillary (papCP) variants. Both subtypes are different not only histopathologically but also with respect to clinical manifestations and prognosis. Treatment of CP patients remains difficult due to the anatomical proximity of the lesion to important functional brain structures.1–4 Although they represent histologically benign (WHO grade 1) tumors, especially adaCPs, are often locally invasive within the perisellar neurovascular structures (eg, the hypothalamus, cavernous sinus, and visual system). Gross total resection can seldom be performed without the high risk of severe clinical and neuroendocrinological deficits such as obesity, diabetes insipidus, and pituitary hormone deficiencies.5–7 Subtotal resection requires adjuvant radiotherapy and is often accompanied by either tumor progression or treatment-associated side effects.1 Chemotherapeutical approaches, such as intracystic injection of bleomycin and interferon-alpha, are under investigation.8,9 Histologically, adaCPs are characterized by the presence of cells arranged in lobules with irregular trabeculae bordered by palisaded columnar epithelium. Further hallmarks are regressive elements like calcifications, inflammation, and wet keratin as well as cells with nuclear β-catenin accumulation (Fig. 1B). These cells are often arranged in whirls and represent an alternatively differentiated population compared with the nonaccumulating cells.10,11 In contrast, papCP is a homogenous lesion of well-differentiated squamous epithelium surrounding fibrotic and pseudocystic inclusions and always lacking nuclear β-catenin accumulation.12
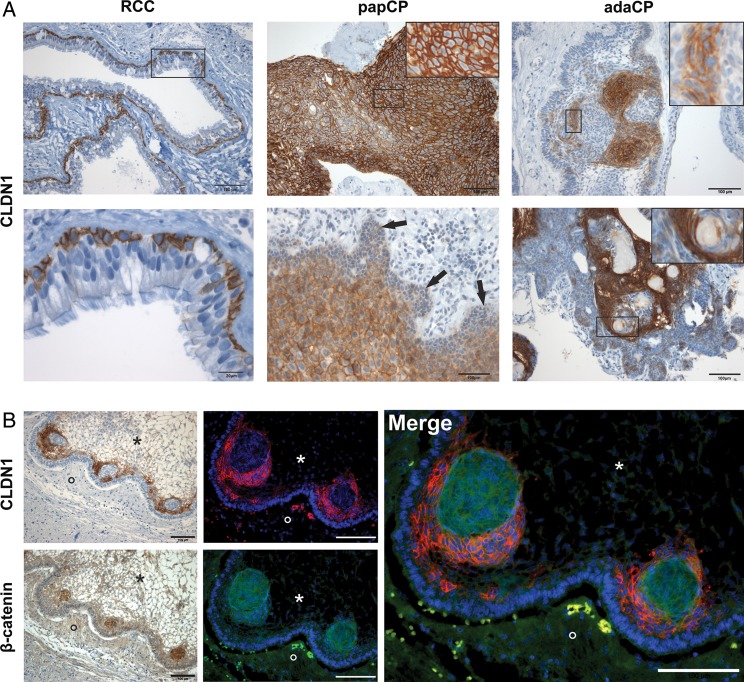
Fig. 1.
Immunohistochemical staining of CLDN1 and β-catenin in paraffin-embedded sections of CP variants and RCCs. RCCs (Fig. 1A, left) show distinct membranous and weak cytoplasmic CLDN1 expression exclusively in the basal cell layer of the cyst membrane (bottom left = enlargement of the marked area; scale bar 20 µm). The staining pattern appears homogenous and intense in papCPs (Fig. 1A, top middle). Invasive areas (↓) show diminished CLDN1 staining (bottom middle). In contrast, CLDN1 immunoreactivity is restricted to specific cellular elements in adaCPs (Fig. 1A, right). These cell groups are located next to whirl-like tumor cell clusters (top right) or adjacent to regressive changes (ie, calcifications, wet keratin and ghost cells) (bottom right). Serial sections as well as double immunofluorescence staining (Fig. 1B) using antibodies against CLDN1 (red) and β-catenin (green) proved the absence of CLDN1 in β-catenin-accumulating tumor cells located in finger-like tumor protrusions. Nuclei were counterstained with Hoechst 33342 (blue). Scale bars 100 µm; asterisk = tumor tissue, circle = brain tissue. Inserts = enlargement of the marked area. Abbreviations: adaCP, adamantinomatous craniopharyngioma; CLDN1, claudin-1; papCP, papillary craniopharyngioma; RCC, Rathke's cleft cyst.
Since papCP almost always occurs as a solid and well-demarcated mass, this suprasellar tumor is generally treatable by radical surgery, and tumor relapse is less frequent as a result.13,14 The same applies for Rathke's cleft cysts (RCCs), which are benign intra- or suprasellar cystic lesions found in up to 30% of routine autopsies.15,16 It is thought that they also derive from residues of Rathke's pouch epithelium.17,18 RCCs are lined by single or pseudostratified cuboidal or columnar epithelium with or without cilia and goblet cells. Sometimes they show squamous metaplasia with prominent cholesterol clefts or groups of macrophages and lymphocytes, which may pose difficulties in the differential diagnosis from CP.19–21 Symptomatic cases are rare and are managed by surgery.15 Recurrence rates following resection are significantly lower in patients with RCCs compared with CPs, which often eliminates the need for postsurgical radiotherapy.15,22,23 Invasion into surrounding brain parenchyma is not seen, and proliferation rates are typically low.
Recently, tumor cell migration in adaCP has been linked to cells with activated canonical Wnt signaling pathway characterized by nuclear accumulation of its key player, β-catenin.10,24 Epithelial tumor cell mobility requires escaping from the united cell structure by dissolving cell-cell contacts, such as tight junctions [TJs] formed by cell adhesion molecules like occludin and claudins.25 Claudins are a family of TJ proteins expressed in endothelial and epithelial tissue. They play an important role in cell polarity and cell adhesion as well as in maintaining paracellular barrier functions.26,27 Claudin-1(CLDN1) is a 23 kDa integral membrane protein with 4 membrane-spanning regions. The extracellular loops interact each homotypic or heterotypic with another extracellular loop of CLDN family members of neighboring cells to build up connections, appearing as TJs.27 Deregulation, like pathological up- or downregulation, of various members of the claudin family has been previously described for different epithelial neoplasms.27,28 Data concerning CLDN1 expression in CPs and RCCs have not previously been reported. We analyzed the distribution pattern of CLDN1 in a representative cohort of CP variants and RCCs. Furthermore, we correlated CLDN1 mRNA expression levels with tumor cell migration and infiltrative growth pattern.
Materials and Methods
Patient Cohort
Surgical specimens from 111 patients with CPs and RCCs were retrieved from the archives of the Department of Neuropathology (University Hospital Erlangen). Each tumor sample was classified according to World Health Organization guidelines using hematoxylin and eosin as well as immunohistochemical staining (eg, pan-cytokeratin [KL-1] and β-catenin). A declaration of consent from each patient is available for all specimens for further scientific investigation, as approved by the local ethics committee of the University Erlangen-Nuremberg. Procedures were conducted in accordance with the Declaration of Helsinki. Clinical data of all patients are presented in Table 1.
Table 1.
Summary of clinical data of patients included in this study
| n | Female | Male | Age mean (years) | Age range (years) | |
|---|---|---|---|---|---|
| RCC | 24 | 17 | 7 | 43.13 | 10–83 |
| adaCP | 66 | 24 | 42 | 32.31 | 3–72 |
| papCP | 21 | 9 | 12 | 44.57 | 19–74 |
Abbreviations: adaCP, adamantinomatous craniopharyngioma; papCP, papillary craniopharyngioma, RCC, Rathke's cleft cyst.
Immunohistochemistry
Surgical samples were prepared as previously described.11 Immunohistochemical staining was performed using a staining machine (Benchmark XT) and the Ventana DAB staining system following the manufacturers' recommendations. CLDN1 was detected using a polyclonal rabbit-anti-claudin-1antibody (1:50; Cell Marque). For visualization of β-catenin, the monoclonal antibody mouse-anti-β-catenin (1:800; Clone 14; BD Biosciences) was applied. Double-immunofluorescence stainings were performed manually as described elsewhere.29 Cy2-anti-mouse and Cy3-anti-rabbit (1:100; Dianova) served as fluorescent secondary antibodies. Nuclei were counterstained with Hoechst 33342 (500 ng/mL; Sigma Aldrich).
Cell Culture
Eleven primary cell cultures of adaCP were established from representative surgical tumor samples as previously described.24 Instantaneous sections were prepared and microscopically reviewed to verify diagnosis and sufficient tumor content of each specimen prior to cultivation. We verified the epithelial differentiation of primary cell cultures immunohistochemically on cytospins using a monoclonal mouse-antibody against cytokeratin-large-spectrum (1:40; Clone Kl-1; Beckman Coulter).
CLDN1 Gene Silencing
CLDN1 downregulation was performed using CLDN1 validated Silencer Select Pre Designed siRNA (ID: s17315) and Lipofectamine 2000 (Ambion). Cells were cultured until reaching 40% confluence according to manufacturers's instructions in medium without antibiotics for 24 hours prior to transfection. Transfection was maintained using RNAi at a final concentration of 20 nM and 4.5 µL/mL Lipofectamine 2000 in serum-reduced medium. As reference control, cells were transfected in the same manner using Silencer Select Negative Control #1 siRNA (Ambion). Within 72 hours, sufficient mRNA and protein downregulation had been achieved as assessed by quantitative real-time (qRT) PCR analysis as well as immunoblotting. Cells treated by this procedure were used in further migration experiments.
Boyden Chamber Assay
Boyden chamber migration assay (QCM 24-well Colorimetric Cell Migration Assay; Millipore) was performed according to the manufacturer's protocol. Briefly, 0.5 × 105 cells, which were transfected with 20 nM siRNA (Ambion) for 72 hours, were transferred into each Boyden chamber and incubated with 10 ng/mL epidermal growth factor as chemoattractant, added to the lower chamber only. After 24 hours, cells remaining on the upper side of the membrane, which had not migrated through the 8.0 µm pores, were removed. Migrated cells were stained with staining solution. Subsequently, the optical density of the extracted staining could be measured at 560 nm; the optical density correlated with the amount of migrated cells. Each experiment was carried out in triplicate.
Protein Preparation and Immunoblotting
Cytoplasmic proteins of cultured cells were isolated according to manufacturer's instructions using the Nuclear/Cytosol Fractionation Kit (BioVision). The concentration of each fraction was evaluated by photometric measurement (Tecan, λ = 562 nm) using the BC Assay Kit (Uptima-Interchim). Protein extracts were separated in an SDS-Page (8% PAA-gel) by electrophoresis and transferred to a nitrocellulose membrane (0.2 µm pore size; Schleicher & Schuell). Equal protein loading (20 µg per lane) was estimated using monoclonal mouse-anti-β-Actin antibody (1:10000; Sigma-Aldrich) for cytoplasmic fraction. Membranes were incubated with polyclonal rabbit-anti-claudin-1(1:200) and thereafter with horseradish peroxidase-linked goat-anti-mouse and goat-anti-rabbit secondary antibodies (1:10000; Bio-Rad). Protein detection was performed by incubating the membrane with enhancing chemoluminescence solution.
cDNA Preparation
Total RNA of cultured cells was isolated with TRIzol Reagent (Invitrogen) according to manufacturer's protocol. The RNeasy Extraction Kit (Qiagen) was used for total tumor RNA isolation of snap-frozen tissue samples. From all specimens, frozen sections were microscopically reviewed to confirm tumor content. After digestion with RNase-free DNase I (Invitrogen), the total amount of RNA was determined by measuring probes on a NanoDrop (Thermo Fisher Scientific), followed by reverse transcription using SuperScript First-Strand Synthesis System (Invitrogen) with oligo (dT) primers. Due to limitations regarding tumor size and tumor content, the collectives of immunohistochemistry and mRNA are not absolutely congruent.
Quantitative Real-time PCR Analysis
Relative quantification by qRT-PCR with Sybr Green II (Applied Biosystems) was employed to assess the quantitative expression of CLDN1 in whole-tumor tissue of 14 papCPs and 19 adaCPs. To determine CLDN1 expression after RNAi treatment, relative quantification analyses were performed on cDNA from cultured adaCPs. All analyses were carried out with the Applied Biosystems 7500 Fast Real-Time-PCR. Glyceraldehyde 3-phosphate dehydrogenase was used as an endogenous control for cDNA amount. Sequences of mRNA-specific primer employed in qRT-PCR analyses are listed in Table 2. To exclude nonspecific amplification, no-template controls for each primer were arranged on every plate, and a melt curve analysis was performed. Analysis was conducted using the ΔΔCT-method according to manufacturer's instructions (Applied Biosystems). All analyses were carried out in quadruplicate and evaluated statistically.
Table 2.
Sequences of mRNA-specific primer employed in quantitative real-time PCR analyses
| Gene symbol | Forward primer 5′ - 3′ | Reverse Primer 3′ - 5′ | Fragment Length (bp) | Species |
|---|---|---|---|---|
| GAPDH | caacgaccactttgtcaagc | tcttcaaggggtctacatgg | 237 | human |
| CLDN1 | gaagtgcttggaagacgatg | gagcctgaccaaattcgtac | 180 | human |
Abbreviatons: CLDN1, claudin-1; GAPDH, glyceraldehyde 3-phosphate dehydrogenase.
Statistical Evaluation
Statistical analyses were performed with Graph Pad software Prism 4.02 (La Jolla). Data normality was tested with D'Agostino-Pearson-omnibus test (for data sets with n > 8). When the samples came from a normally distributed population, an unpaired Student t test was conducted to resolve hypothesized differences. Paired observations were statistically analyzed by a paired Student t test. Statistical procedures were computed using 2-tailed tests with an alpha error cutoff value of 0.05 for statistical significance.
Results
Differential Distribution Pattern of Tight Junction Protein Claudin-1 in Cystic Sellar Tumors
We examined the immunohistochemical distribution pattern of CLDN1 in a cohort of 66 adaCPs, 21 papCPs, and 24 RCCs. In RCC specimens, distinct CLDN1 immunoreactivity was observed in the basal cell layer with strong detection at the cell membrane (Fig. 1A, left). The cytoplasm showed a variable staining pattern, and immunoreactivity was always absent from the nuclei. The overlying cells displayed only weak expression in particular cases in which goblet cells and cilia appeared negative. In papCPs, the vast majority of tumor cells revealed a distinct CLDN1 expression pattern, predominantly at the cell membrane (Fig. 1A, top middle), which was consistent with its role in cell-cell adhesion. Interestingly, tumor cells bordering brain tissue and dura fragments demonstrated only pale staining and a switch from membranous to cytoplasmic CLDN1 compared with neighboring cell layers (Fig. 1A, bottom middle). Furthermore, areas with distinct squamous epithelial differentiation showed a weaker staining pattern compared with adjoining epithelial layers. Pseudocystic tumor areas with fibrotic degeneration and blood vessels were always negative. As in RCCs, CLDN1 was not detected in the nuclei. In contrast, overall CLDN1 staining was strikingly lower in adaCPs, where large tumor areas demonstrated absent or weak immunoreactivity (Fig. 1A, top right). Furthermore, we noticed strong CLDN1 immunoreactivity in regions harboring regressive changes (eg, ghost cells, wet keratin and calcifications) (bottom right). The staining pattern appears more diffuse within the cytoplasm (Fig. 1A, right inserts) compared with RCCs and papCPs. Sometimes nuclear staining in single tumor cells could not be excluded. None of the papCPs or RCCs showed such a nonhomogeneous staining pattern. Whirl-like cell clusters displaying nuclear β-catenin accumulation always lacked CLDN1, whereas its expression was obvious in adjoining cells as documented using serial sections and double immunofluorescence staining (Fig. 1B). Here it predominantly appeared at the cell membrane and in the cytoplasm. Central nervous tissue and normal pituitary gland adjacent to the lesions showed no expression of CLDN1 (Fig. 1A and B).
mRNA Expression of CLDN1 Is Altered in CP Variants and Correlates with Invasive Growth
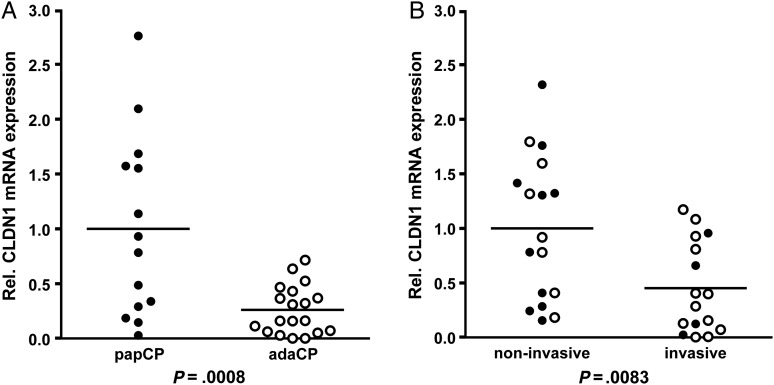
To examine CLDN1 mRNA expression levels in both CP subtypes, relative quantification by qRT-PCR with Sybr Green II was conducted using total RNA of 33 different tumor samples (14 papCPs and 19 adaCPs). The RNA collective only included primary tumor samples. Recurrent tumors and cases with tumor content lower than 50% were excluded. To compare CLDN1 mRNA levels of CP subtypes, we used the averaged CLDN1 value of all papCPs as the calibrator equalized to the value of one. All adaCP cases were compared herewith. In accordance to the immunohistochemical results, the overall relative CLDN1 expression level was significantly lower in adaCPs (n = 19) compared with papCPs (n = 14) (P = .0008; Student t test). Most of the adaCP samples exhibited extremely low CLDN1 levels compared with papCP samples (Fig. 2A). Interestingly, our collective included 4 papCP specimens with comparable amounts of low CLDN1 mRNA levels. Paraffin sections from 2 of these cases contained brain tissue in close association to the tumor, indicating infiltrative tumor growth. The marginal area of the tumor next to central nervous tissue showed diminished expression of CLDN1 by immunohistochemistry. To verify these results, we tried to investigate CLDN1 mRNA expression levels in correlation to the growth pattern of all tumors under study. There are presently no reliable clinical definitions for infiltrative growth for CPs, so we used 2 independent classification systems to generate a clinical and histological cohort of invasive tumors. Based on preoperative MR imaging and surgical reports, a clinically invasive group was determined. Tumors were defined and grouped as invasive if they impaired the integrity of brain parenchyma, hypothalamus, walls of the ventricles, cavernous sinus or optic chiasm, or if they exhibited glio-arachnoidal adhesion. Tumors that respected neuronal structures and did not demonstrate adhesion to any cranial structures were classified as noninvasive (M.B., S.-M.S., R.F., J.F.). A histologically invasive group was defined using the criteria already published by Weiner et al. in 1994, “… as the presence of an isolated nest of tumor cells surrounded on all sides … by brain tissue, which was clearly separate from the remainder of the tumor.”30 Small isolated nests of tumor cells are not visible in the operative microscope during surgery, however their existence does not absolutely exclude complete resectability of the tumor, as far as it is resected completely with the surrounding gliotic layer via transcranial surgery. Nevertheless isolated tumor cell nests can be an early predictor for recurrence. We evaluated all cases included in the mRNA study (n = 33) based on both criteria and established a clearly invasive cohort (n = 16: 4 clinically invasive papCPs plus 12 adaCPs, of which 10 were clinically invasive and 2 were histologically invasive) and a noninvasive group (n = 17: 10 papCPs and 7 adaCPs). To compare noninvasive against invasive CP cases, we used the averaged CLDN1 value of noninvasive CPs as the calibrator for each subtype separately. Statistical evaluation of both groups showed a significantly lower overall CLDN1 mRNA value in invasive CP cases regardless of CP subtype (Fig. 2B; P = .0083; Student t test). We also conducted separate analyses for adaCP and papCP cases. Therefore, adaCPs overall showed a significantly lower CLDN1 mRNA level in invasive tumors compared with noninvasive samples (P = .0339; Student t test). This observation was confirmed in papCPs where the CLDN1 level of each invasive case was below the average of noninvasive cases. Unfortunately, we could not perform statistical evaluation of CLDN1 expression in papCPs because invasive papCPs are extremely rare, and our collective comprised only 4 cases, which was not sufficient for reliable statistical evaluation (P = n.a.; n too small) (Supplemental Fig. S1). The sections of 2 invasive papCP cases revealed diminished CLDN1 staining in tumor protrusions into adjacent brain structures. The outermost cell layers of marginal tumor parts showed weak to absent CLDN1 expression with redistribution from the membrane to the cytoplasm. In fact the case number is too low to allow reliable evidence for this observation.
Fig. 2.
qRT-PCR analysis of CLDN1 mRNA expression in tumor samples of different CP patients. The arithmetic average of 14 analyzed papCP mRNA samples was set as the reference value. Subsequently, each of the 19 analyzed adaCP cases was compared with the normalized expression in papCPs. We revealed significantly lower overall CLDN1 mRNA expression levels in adaCPs compared with papCPs (P = .0008) (Fig. 2A). We measured a significant difference in CLDN1 mRNA expression upon investigating noninvasive versus invasive CPs (Fig. 2B; P = .0083). Filled circles = papCP, unfilled circles = adaCP. Abbreviations: adaCP, adamantinomatous craniopharyngioma); CLDN1,claudin-1; papCP, papillary craniopharyngioma.
CLDN1 Affects Migration in AdaCPs
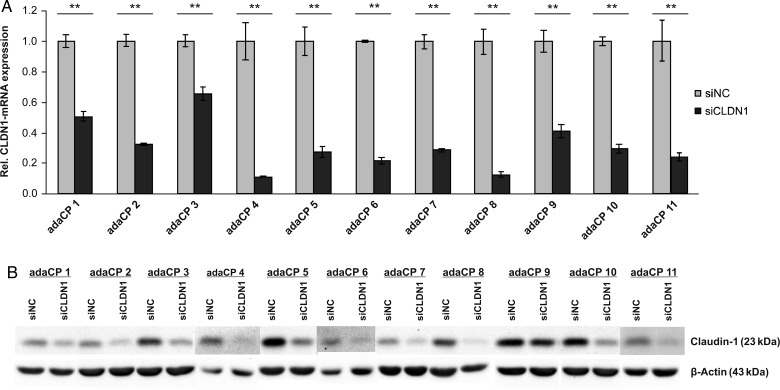
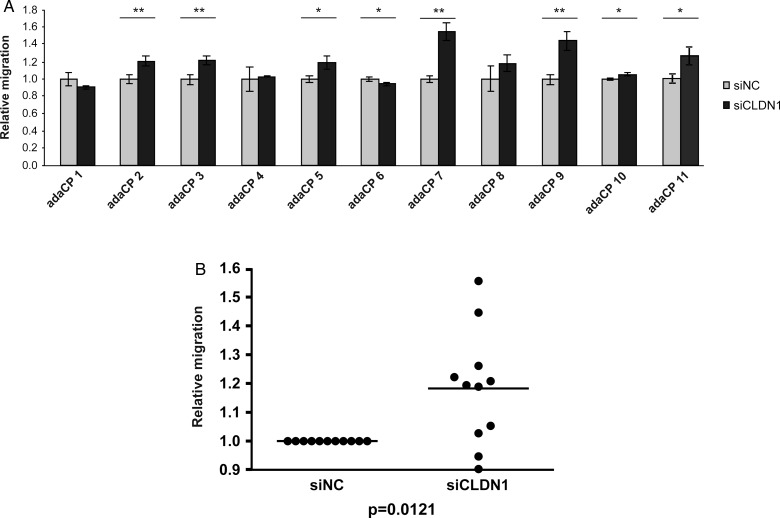
To analyze the influence of CLDN1 expression on cell migration, we performed siRNA experiments in primary adaCP tumor cell cultures (n = 11). The efficiency of CLDN1 downregulation reached up to 90%, illustrated by qRT-PCR (Fig. 3A) and immunoblotting (Fig. 3B). Subsequently, Boyden chamber assay was used to investigate the motility of CLDN1-RNAi transfected cells (Fig. 4). Overall, CLDN1 inhibition caused a significant increase of adaCP tumor cell migration (Fig. 4B; P = .0121; Student t test), pointing to a possible role in this aspect of invasion that deserves further study. However, single results were divergent. Sixty-four percent of cell cultures (n = 7) showed an elevated migratory potential, whereas one sample (adaCP 6) offered a significant reduction. Three specimens reached no statistical significance (Fig. 4A).
Fig. 3.
Significant reduction of CLDN1 expression in primary adaCP tumor cell cultures using siRNA. Cells were transfected with Lipofectamine and 20 nM siRNA directed against CLDN1 for 72 hours. Significant downregulation of CLDN1 was verified using qRT-PCR analysis (Fig. 3A; **P < .01) and immunobloting (Fig. 3B). Abbreviations: adaCP, adamantinomatous craniopharyngioma); CLDN1, claudin-1; papCP,papillary craniopharyngioma; siCLDN1, small interfering claudin-1RNA; siNC, small interfering negative control RNA.
Fig. 4.
Knockdown of CLDN1 (siCLDN1) induced increased migration potential. Boyden chamber assay revealed altered migration in 64% of siCLDN1-treated adaCP tumor cell cultures compared with control RNAi-transfected cells (siNC) (Fig. 4A; *P < .05; **P < .01). Overall significance was given (Fig. 4B; P = .0121), although one sample offered a significant reduction of cell migration. Abbreviations: adaCP, adamantinomatous craniopharyngioma; CLDN1, claudin-1; siCLDN1, small interfering claudin-1RNA; siNC, small interfering negative control RNA
Discussion
Migration and invasion of epithelial cells are prevented by the formation of TJs, which are responsible for cell polarity and cell-cell adhesion within epithelial structures.26,27 One of the key players in TJ formation are the claudins, a family of proteins whose expression is altered in several types of cancer.31 Claudin-1 (CLDN1) has been described as essential for TJ formation since CLDN1-deficient mice died within 1 day postnatally due to massive transepidermal dehydration.32 Accordingly, CLDN1 shows a homogenous membranous distribution pattern in nearly all epithelial tissues.33 Data concerning its relevance in cystic epithelial lesions of the sellar region (eg, CPs and RCCs) are not available so far. Therefore, we analyzed the expression pattern of CLDN1 in a representative cohort of 111 patients suffering from CPs or RCCs. Although all entities under study represent epithelial lesions being defined as subtypes of the same tumor entity (adaCPs and papCPs) or sharing the same embryologic ectodermal origin.34 (CPs and RCCs), adaCPs showed significantly lower CLDN1 mRNA levels compared with papCPs and RCCs. Using immunohistochemistry, we were able to demonstrate divergent expression patterns in CP variants and RCCs, suggesting CLDN1 as an immunohistochemical auxiliary in the differential diagnosis of these lesions. CLDN1 is already accepted as a diagnostic marker for meningioma35 and soft tissue perineurioma, distinguishing these from potential mimics.36,37 In this study, CLDN1 was always restricted to the cell membrane and appeared nearly exclusively in the basal cell layer of RCCs, whereas the distribution pattern in papCPs was almost always homogenous and widespread. Here, nearly all tumor cells showed a strong membranous staining that occurred sometimes parallel with a cytoplasmic expression. This staining pattern suggests an intact formation of TJs directed by CLDN1.
In contrast, large parts of the adaCP tumor samples exhibited only weak to absent CLDN1 expression. Immunoreactivity was restricted to cell groups located next to areas with extensive regressive changes (eg, calcifications) as well as surrounding whirl-like cell clusters. Both structures represent histomorphological hallmarks of adaCPs. Altered distribution of CLDN1, which is important for cell polarity and pattern formation, may contribute to the development of these peculiar, entropic structures. Staining was not always restricted to the cell membrane but rather was diffuse in the cytoplasm. Altered levels of claudins have already been shown to be associated with invasion and aggressiveness of several epithelial neoplasms (eg, colon cancer,38 oral squamous cell carcinoma,39 and melanoma), where elevated motility of tumor cells is accompanied with a higher level of cytoplasmic staining.40
In addition, we observed significantly diminished CLDN1 levels in invasive versus noninvasive CP cases. These findings suggest that the characteristic infiltrative growth pattern, especially observed in adaCPs, could be the result of lower CLDN1 expression levels resulting in detached TJs. In contrast to occludin, claudin family members are the essential components of functional TJ formation in epithelial cells.32,41,42 About 60% of CLDN1 molecules are fixed at the cell membrane during TJ formation, whereas zonula occludens proteins are permanently fluctuating between different cellular compartments43 and also occur in adherens junctions as well as other cell types besides epithelial and endothelial cells.44–46 Invasive CP showed protrusions into adjacent brain tissue, where the outermost cell layers displayed weaker or even absent membranous CLDN1 staining. AdaCP migration assays, using primary cell cultures, reinforced this hypothesis. Although occassionally showing divergent results, downregulation of CLDN1 was associated with a significantly higher migration potential of adaCP tumor cells overall. The significant increase of migration potential after RNAi treatment in 7 cases could be correlated with their high invasive potential either clinically or histologically. Cases that did not show a significant shift in their migration potential were classified as noninvasive by the neurosurgeons' classification. From the present results, we would postulate that CP cases with homogenous CLDN1 distribution and distinct mRNA levels do not represent patients who are at high risk for infiltrative tumor growth, whereas tumors showing low CLDN1 levels might represent patients at high risk who should be considered for stringent follow-up examination (eg, early postoperative radiotherapy or aggressive treatment strategies).
It is likely that impairment of TJ formation may have further consequences besides promotion of tumor cell migration and invasion. TJs provide a paracellular barrier function that maintains size-selective permeability of solutes and nutrients and regulates for example the leakiness of endothelia. Therefore, alterations of CLDN1 expression may influence the formation of characteristic CP morphology, (ie, enhanced inflammation and cyst formation caused by accumulation of fluid), an assumption that has to be established in further studies.
The role of CLDN1 in tumorigenesis and as a prognostic marker is incompletely understood at present. Whereas one study described loss of CLDN1 expression to be a strong predictor for disease recurrence and poor patient survival in stage II colon cancer,47 others were able to show elevated levels of CLDN1 in colorectal and pancreatic cancers.26,27,31 We intentionally excluded recurrent tumor samples from our study due to a small number of cases (5 patients). However, these samples always showed extremely low CLDN1 levels (data not shown). We were able to compare primary and recurrent tumor samples in 2 patients. Interestingly, both primary tumor cases could be classified as high-risk because of their extremely low CLDN1 mRNA levels in accordance with very weak immunohistochemical expression.
The mechanism causing deregulation of CLDN1 expression in cancer is not fully understood. It is known that CLDN1 expression is affected by p63,48 CDX2,49 Smad4/TGF-β,50 Runx3,51 or mi-RNAs52, as well as epigenetically by HDAC2.53 Only one single mutation was identified in the CLDN1 gene, leading to progressive scaling of the skin and obstruction of the bile ducts known as neonatal sclerosing cholangitis and ichthyosis.54 The gene encoding for CLDN1 was sequenced in a single study of breast cancer, where no mutations in the coding sequence or putative promoter region could be detected.55 CLDN1 is being considered a target gene of β-catenin. This may be the mechanism of upregulation in colorectal cancer.56 A new genetically engineered animal model developed by Gaston-Massuet et al proved the impact of dysregulated Wnt/β-catenin signaling on the pathogenesis of CP.57 However, immunohistochemistry revealed absence of CLDN1 protein in nearly all β-catenin-accumulating cell clusters of adaCP. In addition, activation of the β-catenin target gene fascin, which is involved in cellular motility,58 is increased in the same adaCP cell clusters24 that show absence of CLDN1 expression (data not shown). Regulation of CLDN1 expression is a process that is important for several malignant epithelial tumors where TJ proteins are specified as potential tumor suppressors.55,59 Further studies are needed to test this hypothesis in CP.
In summary, the distinct expression pattern of CLDN1 can be helpful in the differential diagnosis of adaCPs, papCPs and RCCs. Furthermore, decreased CLDN1 expression, as well as diminished membranous and prominent cytoplasmic CLDN1 staining, correlates with the invasive potential of the certain cases. Further studies are required to understand the biological role of CLDN1 in the migratory and invasion capacity of CP tumor cells as well as its prognostic value for recurrence.
Supplementary Material
Acknowledgments
We thank Verena Schmidt for her excellent technical assistance. In addition, we are indebted to Tajana Jungbauer and Diana Maron for conducting immunohistochemical staining. We express our special thanks to Trevor Steve (M.D.) for revising the manuscript.
Conflict of interest statement. None declared.
References
- 1.Karavitaki N, Cudlip S, Adams CB, Wass JA. Craniopharyngiomas. Endocr Rev. 2006;27(4):371–397. doi: 10.1210/er.2006-0002. [DOI] [PubMed] [Google Scholar]
- 2.Crowley R, Hamnvik O, O'Sullivan E, et al. Morbidity and Mortality in Craniopharyngioma Patients after Surgery. Clin Endocrinol. 2010;73(4):516–521. doi: 10.1111/j.1365-2265.2010.03838.x. [DOI] [PubMed] [Google Scholar]
- 3.Fahlbusch R, Hofmann BM. Surgical management of giant craniopharyngiomas. Acta Neurochirurgica. 2008;150(12):1213–1226. doi: 10.1007/s00701-008-0137-9. [DOI] [PubMed] [Google Scholar]
- 4.Muller HL. Craniopharyngioma - a childhood and adult disease with challenging characteristics. Frontiers in Endocrinology. 2012;3:80. doi: 10.3389/fendo.2012.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sainte-Rose C, Puget S, Wray A, et al. Craniopharyngioma: the pendulum of surgical management. Child's Nervous System: ChNS: Official Journal of the International Society for Pediatric Neurosurgery. 2005;21(8–9):691–695. doi: 10.1007/s00381-005-1209-2. [DOI] [PubMed] [Google Scholar]
- 6.Pierre-Kahn A, Recassens C, Pinto G, et al. Social and psycho-intellectual outcome following radical removal of craniopharyngiomas in childhood. A prospective series. Child's Nervous System: ChNS: Official Journal of the International Society for Pediatric Neurosurgery. 2005;21(8–9):817–824. doi: 10.1007/s00381-005-1205-6. [DOI] [PubMed] [Google Scholar]
- 7.Reschke K, Busse S, Mohnike K, et al. [CranioNet – an interdisciplinary strategy for craniopharyngioma] Dtsch Med Wochenschr. 2006;131(15):821–824. doi: 10.1055/s-2006-939854. [DOI] [PubMed] [Google Scholar]
- 8.Steinbok P, Hukin J. Intracystic treatments for craniopharyngioma. Neurosurg Focus. 2010;28(4):E13. doi: 10.3171/2010.1.FOCUS09315. [DOI] [PubMed] [Google Scholar]
- 9.Cavalheiro S, Di Rocco C, Valenzuela S, et al. Craniopharyngiomas: intratumoral chemotherapy with interferon-alpha: a multicenter preliminary study with 60 cases. Neurosurg Focus. 2010;28(4):E12. doi: 10.3171/2010.1.FOCUS09310. [DOI] [PubMed] [Google Scholar]
- 10.Holsken A, Kreutzer J, Hofmann BM, et al. Target gene activation of the Wnt signaling pathway in nuclear beta-catenin accumulating cells of adamantinomatous craniopharyngiomas. Brain Pathol. 2009;19(3):357–364. doi: 10.1111/j.1750-3639.2008.00180.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buslei R, Holsken A, Hofmann B, et al. Nuclear beta-catenin accumulation associates with epithelial morphogenesis in craniopharyngiomas. Acta Neuropathologica. 2007;113(5):585–590. doi: 10.1007/s00401-006-0184-3. [DOI] [PubMed] [Google Scholar]
- 12.Buslei R, Nolde M, Hofmann B, et al. Common mutations of beta-catenin in adamantinomatous craniopharyngiomas but not in other tumours originating from the sellar region. Acta Neuropathologica. 2005;109(6):589–597. doi: 10.1007/s00401-005-1004-x. [DOI] [PubMed] [Google Scholar]
- 13.Adamson TE, Wiestler OD, Kleihues P, Yasargil MG. Correlation of clinical and pathological features in surgically treated craniopharyngiomas. Journal of Neurosurgery. 1990;73(1):12–17. doi: 10.3171/jns.1990.73.1.0012. [DOI] [PubMed] [Google Scholar]
- 14.Szeifert GT, Sipos L, Horvath M, et al. Pathological characteristics of surgically removed craniopharyngiomas: analysis of 131 cases. Acta Neurochirurgica. 1993;124(2–4):139–143. doi: 10.1007/BF01401137. [DOI] [PubMed] [Google Scholar]
- 15.Kim JE, Kim JH, Kim OL, et al. Surgical treatment of symptomatic Rathke cleft cysts: clinical features and results with special attention to recurrence. Journal of Neurosurgery. 2004;100(1):33–40. doi: 10.3171/jns.2004.100.1.0033. [DOI] [PubMed] [Google Scholar]
- 16.Osborn AG, Preece MT. Intracranial cysts: radiologic-pathologic correlation and imaging approach. Radiology. 2006;239(3):650–664. doi: 10.1148/radiol.2393050823. [DOI] [PubMed] [Google Scholar]
- 17.Shanklin WM. The histogenesis and histology of an integumentary type of epithelium in the human hypophysis. The Anatomical Record. 1951;109(2):217–231. doi: 10.1002/ar.1091090206. [DOI] [PubMed] [Google Scholar]
- 18.Fager CA, Carter H. Intrasellar epithelial cysts. Journal of Neurosurgery. 1966;24(1):77–81. doi: 10.3171/jns.1966.24.1.0077. [DOI] [PubMed] [Google Scholar]
- 19.Billeci D, Marton E, Tripodi M, Orvieto E, Longatti P. Symptomatic Rathke's cleft cysts: a radiological, surgical and pathological review. Pituitary. 2004;7(3):131–137. doi: 10.1007/s11102-005-1755-3. [DOI] [PubMed] [Google Scholar]
- 20.Steinberg GK, Koenig GH, Golden JB. Symptomatic Rathke's cleft cysts. Report of two cases. Journal of Neurosurgery. 1982;56(2):290–295. doi: 10.3171/jns.1982.56.2.0290. [DOI] [PubMed] [Google Scholar]
- 21.Harrison MJ, Morgello S, Post KD. Epithelial cystic lesions of the sellar and parasellar region: a continuum of ectodermal derivatives? Journal of Neurosurgery. 1994;80(6):1018–1025. doi: 10.3171/jns.1994.80.6.1018. [DOI] [PubMed] [Google Scholar]
- 22.Hofmann BM, Kreutzer J, Saeger W, et al. Nuclear beta-catenin accumulation as reliable marker for the differentiation between cystic craniopharyngiomas and rathke cleft cysts: a clinico-pathologic approach. The American Journal of Surgical Pathology. 2006;30(12):1595–1603. doi: 10.1097/01.pas.0000213328.64121.12. [DOI] [PubMed] [Google Scholar]
- 23.Mukherjee JJ, Islam N, Kaltsas G, et al. Clinical, radiological and pathological features of patients with Rathke's cleft cysts: tumors that may recur. The Journal of Clinical Endocrinology and Metabolism. 1997;82(7):2357–2362. doi: 10.1210/jcem.82.7.4043. [DOI] [PubMed] [Google Scholar]
- 24.Holsken A, Buchfelder M, Fahlbusch R, Blumcke I, Buslei R. Tumour cell migration in adamantinomatous craniopharyngiomas is promoted by activated Wnt-signalling. Acta Neuropathologica. 2010;119(5):631–639. doi: 10.1007/s00401-010-0642-9. [DOI] [PubMed] [Google Scholar]
- 25.Gonzalez-Mariscal L, Betanzos A, Nava P, Jaramillo BE. Tight junction proteins. Progress in Biophysics and Molecular Biology. 2003;81(1):1–44. doi: 10.1016/s0079-6107(02)00037-8. [DOI] [PubMed] [Google Scholar]
- 26.Swisshelm K, Macek R, Kubbies M. Role of claudins in tumorigenesis. Adv Drug Deliv Rev. 2005;57(6):919–928. doi: 10.1016/j.addr.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 27.Oliveira SS, Morgado-Diaz JA. Claudins: multifunctional players in epithelial tight junctions and their role in cancer. Cellular and Molecular Life Sciences: CMLS. 2007;64(1):17–28. doi: 10.1007/s00018-006-6314-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morin PJ. Claudin proteins in human cancer: promising new targets for diagnosis and therapy. Cancer Research. 2005;65(21):9603–9606. doi: 10.1158/0008-5472.CAN-05-2782. [DOI] [PubMed] [Google Scholar]
- 29.Holsken A, Gebhardt M, Buchfelder M, Fahlbusch R, Blumcke I, Buslei R. EGFR signaling regulates tumor cell migration in craniopharyngiomas. Clinical Cancer Research: An Official Journal of the American Association for Cancer Research. 2011;17(13):4367–4377. doi: 10.1158/1078-0432.CCR-10-2811. [DOI] [PubMed] [Google Scholar]
- 30.Weiner HL, Wisoff JH, Rosenberg ME, et al. Craniopharyngiomas: a clinicopathological analysis of factors predictive of recurrence and functional outcome. Neurosurgery. 1994;35(6):1001–1010. doi: 10.1227/00006123-199412000-00001. discussion 1010–1001. [DOI] [PubMed] [Google Scholar]
- 31.Turksen K, Troy TC. Junctions gone bad: claudins and loss of the barrier in cancer. Biochimica et biophysica acta. 2011;1816(1):73–79. doi: 10.1016/j.bbcan.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 32.Furuse M, Hata M, Furuse K, et al. Claudin-based tight junctions are crucial for the mammalian epidermal barrier: a lesson from claudin-1-deficient mice. J Cell Biol. 2002;156(6):1099–1111. doi: 10.1083/jcb.200110122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hewitt KJ, Agarwal R, Morin PJ. The claudin gene family: expression in normal and neoplastic tissues. BMC Cancer. 2006;6:186. doi: 10.1186/1471-2407-6-186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sartoretti-Schefer S, Wichmann W, Aguzzi A, Valavanis A. MR differentiation of adamantinous and squamous-papillary craniopharyngiomas. AJNR. American Journal of Neuroradiology. 1997;18(1):77–87. [PMC free article] [PubMed] [Google Scholar]
- 35.Hahn HP, Bundock EA, Hornick JL. Immunohistochemical staining for claudin-1 can help distinguish meningiomas from histologic mimics. American Journal of Clinical Pathology. 2006;125(2):203–208. doi: 10.1309/G659-FVVB-MG7U-4RPQ. [DOI] [PubMed] [Google Scholar]
- 36.Folpe AL, Billings SD, McKenney JK, Walsh SV, Nusrat A, Weiss SW. Expression of claudin-1, a recently described tight junction-associated protein, distinguishes soft tissue perineurioma from potential mimics. The American Journal of Surgical Pathology. 2002;26(12):1620–1626. doi: 10.1097/00000478-200212000-00010. [DOI] [PubMed] [Google Scholar]
- 37.Hornick JL, Fletcher CD. Soft tissue perineurioma: clinicopathologic analysis of 81 cases including those with atypical histologic features. The American Journal of Surgical Pathology. 2005;29(7):845–858. doi: 10.1097/01.pas.0000155166.86409.d2. [DOI] [PubMed] [Google Scholar]
- 38.Dhawan P, Singh AB, Deane NG, et al. Claudin-1 regulates cellular transformation and metastatic behavior in colon cancer. The Journal of Clinical Investigation. 2005;115(7):1765–1776. doi: 10.1172/JCI24543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Oku N, Sasabe E, Ueta E, Yamamoto T, Osaki T. Tight junction protein claudin-1 enhances the invasive activity of oral squamous cell carcinoma cells by promoting cleavage of laminin-5 gamma2 chain via matrix metalloproteinase (MMP)-2 and membrane-type MMP-1. Cancer Research. 2006;66(10):5251–5257. doi: 10.1158/0008-5472.CAN-05-4478. [DOI] [PubMed] [Google Scholar]
- 40.Leotlela PD, Wade MS, Duray PH, et al. Claudin-1 overexpression in melanoma is regulated by PKC and contributes to melanoma cell motility. Oncogene. 2007;26(26):3846–3856. doi: 10.1038/sj.onc.1210155. [DOI] [PubMed] [Google Scholar]
- 41.Saitou M, Furuse M, Sasaki H, et al. Complex phenotype of mice lacking occludin, a component of tight junction strands. Molecular Biology of the Cell. 2000;11(12):4131–4142. doi: 10.1091/mbc.11.12.4131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schulzke JD, Gitter AH, Mankertz J, et al. Epithelial transport and barrier function in occludin-deficient mice. Biochimica et biophysica acta. 2005;1669(1):34–42. doi: 10.1016/j.bbamem.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 43.Shen L. Tight junctions on the move: molecular mechanisms for epithelial barrier regulation. Annals of the New York Academy of Sciences. 2012;1258:9–18. doi: 10.1111/j.1749-6632.2012.06613.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Howarth AG, Hughes MR, Stevenson BR. Detection of the tight junction-associated protein ZO-1 in astrocytes and other nonepithelial cell types. The American Journal of Physiology. 1992;262(2 Pt 1):C461–C469. doi: 10.1152/ajpcell.1992.262.2.C461. [DOI] [PubMed] [Google Scholar]
- 45.Inoko A, Itoh M, Tamura A, Matsuda M, Furuse M, Tsukita S. Expression and distribution of ZO-3, a tight junction MAGUK protein, in mouse tissues. Genes Cells. 2003;8(11):837–845. doi: 10.1046/j.1365-2443.2003.00681.x. [DOI] [PubMed] [Google Scholar]
- 46.Itoh M, Nagafuchi A, Yonemura S, Kitani-Yasuda T, Tsukita S. The 220-kD protein colocalizing with cadherins in non-epithelial cells is identical to ZO-1, a tight junction-associated protein in epithelial cells: cDNA cloning and immunoelectron microscopy. J Cell Biol. 1993;121(3):491–502. doi: 10.1083/jcb.121.3.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Resnick MB, Konkin T, Routhier J, Sabo E, Pricolo VE. Claudin-1 is a strong prognostic indicator in stage II colonic cancer: a tissue microarray study. Modern Pathology: An Official Journal of the United States and Canadian Academy of Pathology, Inc. 2005;18(4):511–518. doi: 10.1038/modpathol.3800301. [DOI] [PubMed] [Google Scholar]
- 48.Lopardo T, Lo Iacono N, Marinari B, et al. Claudin-1 is a p63 target gene with a crucial role in epithelial development. PloS one. 2008;3(7):e2715. doi: 10.1371/journal.pone.0002715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bhat AA, Sharma A, Pope J, et al. Caudal homeobox protein Cdx-2 cooperates with Wnt pathway to regulate claudin-1 expression in colon cancer cells. PloS one. 2012;7(6):e37174. doi: 10.1371/journal.pone.0037174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tian X, Du H, Fu X, Li K, Li A, Zhang Y. Smad4 restoration leads to a suppression of Wnt/beta-catenin signaling activity and migration capacity in human colon carcinoma cells. Biochem Biophys Res Commun. 2009;380(3):478–483. doi: 10.1016/j.bbrc.2009.01.124. [DOI] [PubMed] [Google Scholar]
- 51.Chang TL, Ito K, Ko TK, et al. Claudin-1 has tumor suppressive activity and is a direct target of RUNX3 in gastric epithelial cells. Gastroenterology. 2010;138(1):255–265 e251–253. doi: 10.1053/j.gastro.2009.08.044. [DOI] [PubMed] [Google Scholar]
- 52.Zhang GJ, Xiao HX, Tian HP, Liu ZL, Xia SS, Zhou T. Upregulation of microRNA-155 promotes the migration and invasion of colorectal cancer cells through the regulation of claudin-1 expression. International Journal of Molecular Medicine. 2013;31(6):1375–1380. doi: 10.3892/ijmm.2013.1348. [DOI] [PubMed] [Google Scholar]
- 53.Krishnan M, Singh AB, Smith JJ, et al. HDAC inhibitors regulate claudin-1 expression in colon cancer cells through modulation of mRNA stability. Oncogene. 2010;29(2):305–312. doi: 10.1038/onc.2009.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hadj-Rabia S, Baala L, Vabres P, et al. Claudin-1 gene mutations in neonatal sclerosing cholangitis associated with ichthyosis: a tight junction disease. Gastroenterology. 2004;127(5):1386–1390. doi: 10.1053/j.gastro.2004.07.022. [DOI] [PubMed] [Google Scholar]
- 55.Kramer F, White K, Kubbies M, Swisshelm K, Weber BH. Genomic organization of claudin-1 and its assessment in hereditary and sporadic breast cancer. Human Genetics. 2000;107(3):249–256. doi: 10.1007/s004390000375. [DOI] [PubMed] [Google Scholar]
- 56.Miwa N, Furuse M, Tsukita S, Niikawa N, Nakamura Y, Furukawa Y. Involvement of claudin-1 in the beta-catenin/Tcf signaling pathway and its frequent upregulation in human colorectal cancers. Oncol Res. 2001;12(11–12):469–476. doi: 10.3727/096504001108747477. [DOI] [PubMed] [Google Scholar]
- 57.Gaston-Massuet C, Andoniadou CL, Signore M, et al. Increased Wingless (Wnt) signaling in pituitary progenitor/stem cells gives rise to pituitary tumors in mice and humans. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(28):11482–11487. doi: 10.1073/pnas.1101553108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jayo A, Parsons M. Fascin: a key regulator of cytoskeletal dynamics. The International Journal of Biochemistry and Cell Biology. 2010;42(10):1614–1617. doi: 10.1016/j.biocel.2010.06.019. [DOI] [PubMed] [Google Scholar]
- 59.Tokes AM, Kulka J, Paku S, et al. Claudin-1, -3 and -4 proteins and mRNA expression in benign and malignant breast lesions: a research study. Breast Cancer Research: BCR. 2005;7(2):R296–R305. doi: 10.1186/bcr983. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.