Abstract
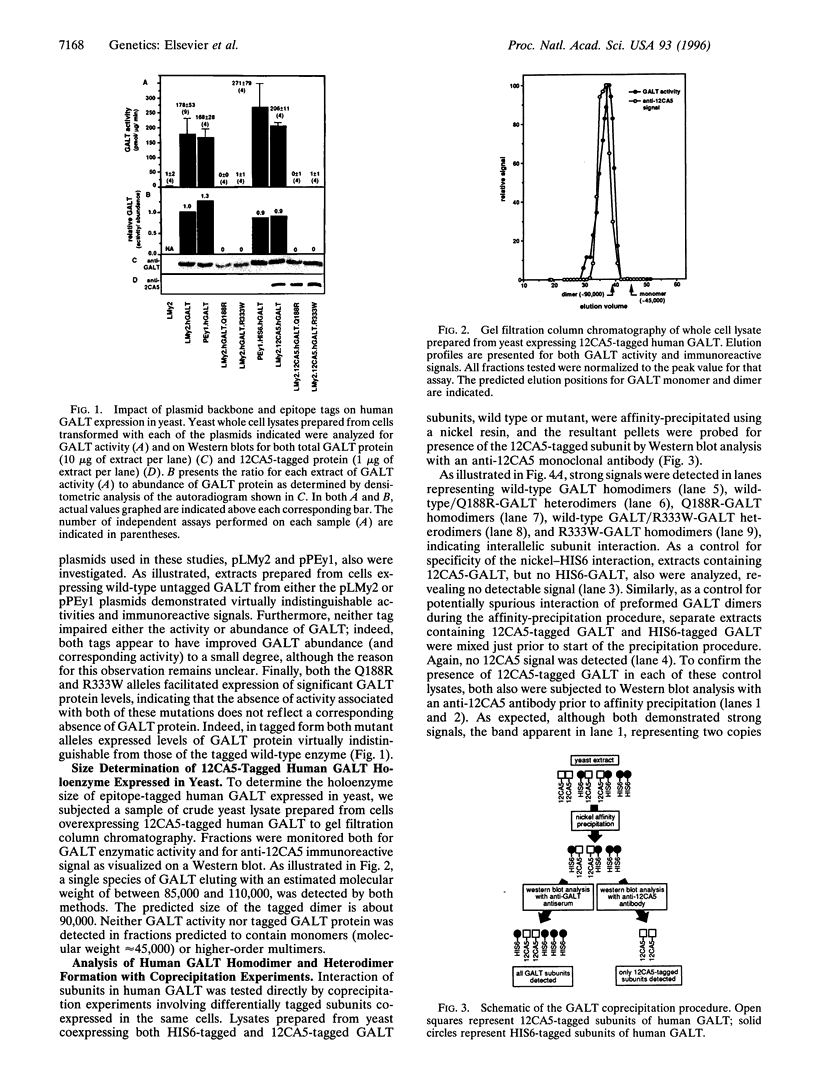
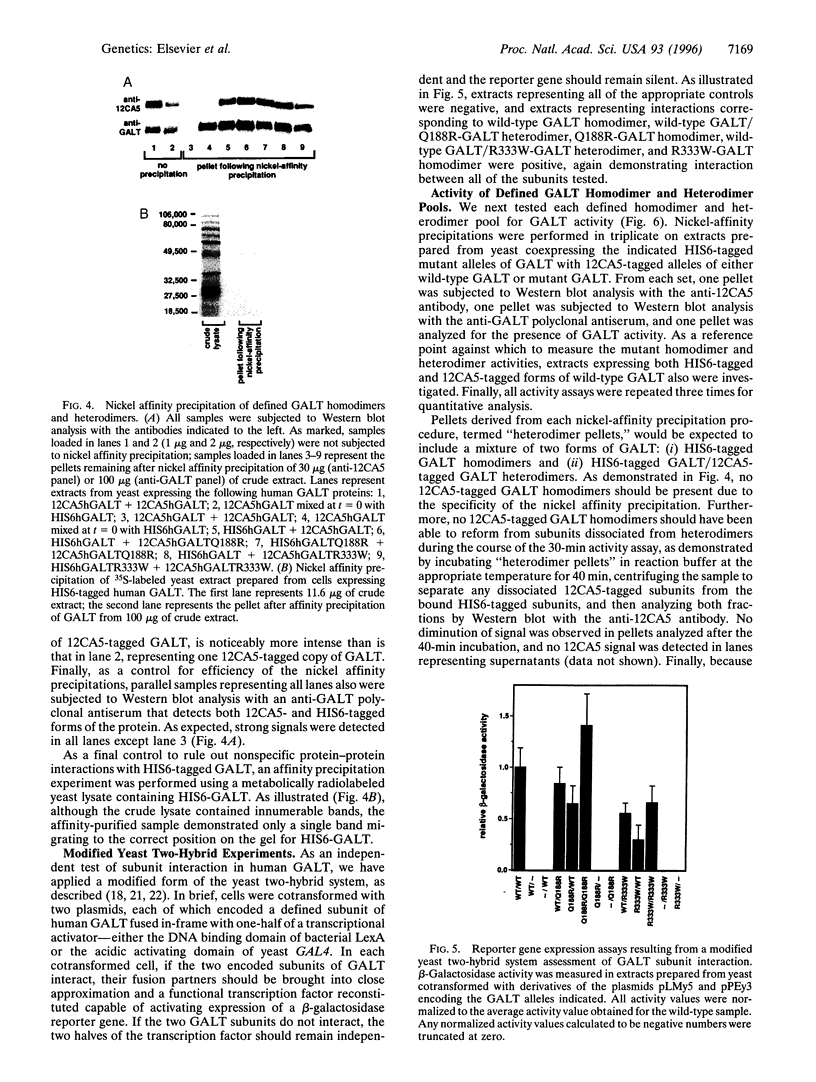
One of the fundamental questions concerning expression and function of dimeric enzymes involves the impact of naturally occurring mutations on subunit assembly and heterodimer activity. This question is of particular interest for the human enzyme galactose-l-phosphate uridylyl-transferase (GALT), impairment of which results in the inherited metabolic disorder galactosemia, because many if not most patients studied to date are compound heterozygotes rather than true molecular homozygotes. Furthermore, the broad range of phenotypic severity observed in these patients raises the possibility that allelic combination, not just allelic constitution, may play some role in determining outcome. In the work described herein, we have selected two distinct naturally occurring null mutations of GALT, Q188R and R333W, and asked the questions (i) what are the impacts of these mutations on subunit assembly, and (ii) if heterodimers do form, are they active? To answer these questions, we have established a yeast system for the coexpression of epitope-tagged alleles of human GALT and investigated both the extent of specific GALT subunit interactions and the activity of defined heterodimer pools. We have found that both homodimers and heterodimers do form involving each of the mutant subunits tested and that both heterodimer pools retain substantial enzymatic activity. These results are significant not only in terms of their implications for furthering our understanding of galactosemia and GALT holoenzyme structure-function relationships but also because the system described may serve as a model for similar studies of other complexes composed of multiple subunits.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banroques J., Gregori C., Dreyfus J. C. Purification of human liver uridylyl transferase and comparison with the erythrocyte enzyme. Biochimie. 1983 Jan;65(1):7–13. doi: 10.1016/s0300-9084(83)80023-6. [DOI] [PubMed] [Google Scholar]
- Banroques J., Gregori C., Schapira F. Purification and characterization of human erythrocyte uridylyl transferase. Biochim Biophys Acta. 1981 Feb 13;657(2):374–382. doi: 10.1016/0005-2744(81)90323-5. [DOI] [PubMed] [Google Scholar]
- Banroques J., Schapira F., Grégori C., Dreyfus J. C. Molecular studies on galactose 1 phosphate uridylyl transferase from normal and mutant subjects. An immunological approach. Ann Hum Genet. 1983 Jul;47(Pt 3):177–185. doi: 10.1111/j.1469-1809.1983.tb00986.x. [DOI] [PubMed] [Google Scholar]
- Chien C. T., Bartel P. L., Sternglanz R., Fields S. The two-hybrid system: a method to identify and clone genes for proteins that interact with a protein of interest. Proc Natl Acad Sci U S A. 1991 Nov 1;88(21):9578–9582. doi: 10.1073/pnas.88.21.9578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale G. L., Popják G. Purification of normal and inactive galactosemic galactose-1-phosphate uridylyltransferase from human red cells. J Biol Chem. 1976 Feb 25;251(4):1057–1063. [PubMed] [Google Scholar]
- Elsas L. J., Langley S., Steele E., Evinger J., Fridovich-Keil J. L., Brown A., Singh R., Fernhoff P., Hjelm L. N., Dembure P. P. Galactosemia: a strategy to identify new biochemical phenotypes and molecular genotypes. Am J Hum Genet. 1995 Mar;56(3):630–639. [PMC free article] [PubMed] [Google Scholar]
- Flach J. E., Reichardt J. K., Elsas L. J., 2nd Sequence of a cDNA encoding human galactose-1-phosphate uridyl transferase. Mol Biol Med. 1990 Aug;7(4):365–369. [PubMed] [Google Scholar]
- Frey P. A., Wong L. J., Sheu K. F., Yang S. L. Galactose-1-phosphate uridylyltransferase: detection, isolation, and characterization of the uridylyl enzyme. Methods Enzymol. 1982;87:20–36. doi: 10.1016/s0076-6879(82)87004-3. [DOI] [PubMed] [Google Scholar]
- Fridovich-Keil J. L., Jinks-Robertson S. A yeast expression system for human galactose-1-phosphate uridylyltransferase. Proc Natl Acad Sci U S A. 1993 Jan 15;90(2):398–402. doi: 10.1073/pnas.90.2.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridovich-Keil J. L., Langley S. D., Mazur L. A., Lennon J. C., Dembure P. P., Elsas J. L., 2nd Identification and functional analysis of three distinct mutations in the human galactose-1-phosphate uridyltransferase gene associated with galactosemia in a single family. Am J Hum Genet. 1995 Mar;56(3):640–646. [PMC free article] [PubMed] [Google Scholar]
- Fridovich-Keil J. L., Quimby B. B., Wells L., Mazur L. A., Elsevier J. P. Characterization of the N314D allele of human galactose-1-phosphate uridylyltransferase using a yeast expression system. Biochem Mol Med. 1995 Dec;56(2):121–130. doi: 10.1006/bmme.1995.1067. [DOI] [PubMed] [Google Scholar]
- Gentz R., Chen C. H., Rosen C. A. Bioassay for trans-activation using purified human immunodeficiency virus tat-encoded protein: trans-activation requires mRNA synthesis. Proc Natl Acad Sci U S A. 1989 Feb;86(3):821–824. doi: 10.1073/pnas.86.3.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyuris J., Golemis E., Chertkov H., Brent R. Cdi1, a human G1 and S phase protein phosphatase that associates with Cdk2. Cell. 1993 Nov 19;75(4):791–803. doi: 10.1016/0092-8674(93)90498-f. [DOI] [PubMed] [Google Scholar]
- Janknecht R., de Martynoff G., Lou J., Hipskind R. A., Nordheim A., Stunnenberg H. G. Rapid and efficient purification of native histidine-tagged protein expressed by recombinant vaccinia virus. Proc Natl Acad Sci U S A. 1991 Oct 15;88(20):8972–8976. doi: 10.1073/pnas.88.20.8972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leslie N. D., Immerman E. B., Flach J. E., Florez M., Fridovich-Keil J. L., Elsas L. J. The human galactose-1-phosphate uridyltransferase gene. Genomics. 1992 Oct;14(2):474–480. doi: 10.1016/s0888-7543(05)80244-7. [DOI] [PubMed] [Google Scholar]
- McClary J. A., Witney F., Geisselsoder J. Efficient site-directed in vitro mutagenesis using phagemid vectors. Biotechniques. 1989 Mar;7(3):282–289. [PubMed] [Google Scholar]
- Nadler H. L., Chacko C. M., Rachmeler M. Interallelic complementation in hybrid cells derived from human diploid strains deficient in galactose-1-phosphate uridyl transferase activity. Proc Natl Acad Sci U S A. 1970 Oct;67(2):976–982. doi: 10.1073/pnas.67.2.976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng W. G., Xu Y. K., Kaufman F. R., Donnell G. N., Wolff J., Allen R. J., Koritala S., Reichardt J. K. Biochemical and molecular studies of 132 patients with galactosemia. Hum Genet. 1994 Oct;94(4):359–363. doi: 10.1007/BF00201593. [DOI] [PubMed] [Google Scholar]
- Reichardt J. K., Berg P. Cloning and characterization of a cDNA encoding human galactose-1-phosphate uridyl transferase. Mol Biol Med. 1988 Apr;5(2):107–122. [PubMed] [Google Scholar]
- Reichardt J. K., Packman S., Woo S. L. Molecular characterization of two galactosemia mutations: correlation of mutations with highly conserved domains in galactose-1-phosphate uridyl transferase. Am J Hum Genet. 1991 Oct;49(4):860–867. [PMC free article] [PubMed] [Google Scholar]
- Saito S., Ozutsumi M., Kurahashi K. Galactose 1-phosphate uridylyltransferase of Escherichia coli. II. Further purification and characterization. J Biol Chem. 1967 May 25;242(10):2362–2368. [PubMed] [Google Scholar]
- Segawa T., Fukasawa T. The enzymes of the galactose cluster in Saccharomyces cerevisiae. Purification and characterization of galactose-1-phosphate uridylyltransferase. J Biol Chem. 1979 Nov 10;254(21):10707–10709. [PubMed] [Google Scholar]
- Wedekind J. E., Frey P. A., Rayment I. Three-dimensional structure of galactose-1-phosphate uridylyltransferase from Escherichia coli at 1.8 A resolution. Biochemistry. 1995 Sep 5;34(35):11049–11061. doi: 10.1021/bi00035a010. [DOI] [PubMed] [Google Scholar]
- Williams V. P., Helmer G. R., Jr, Fried C. Human galactose-1-phosphate uridylyltrsferase: purification and comparison of the red blood cell and placental enzymes. Arch Biochem Biophys. 1982 Jul;216(2):503–511. doi: 10.1016/0003-9861(82)90239-9. [DOI] [PubMed] [Google Scholar]
- Williams V. P. Purification and some properties of galactose 1-phosphate uridylyltransferase from human red cells. Arch Biochem Biophys. 1978 Nov;191(1):182–191. doi: 10.1016/0003-9861(78)90080-2. [DOI] [PubMed] [Google Scholar]
- Wilson I. A., Niman H. L., Houghten R. A., Cherenson A. R., Connolly M. L., Lerner R. A. The structure of an antigenic determinant in a protein. Cell. 1984 Jul;37(3):767–778. doi: 10.1016/0092-8674(84)90412-4. [DOI] [PubMed] [Google Scholar]






