Abstract
Electrical impedance measurements of skeletal muscle may be sensitive to age-associated declines in muscle health. In an effort to evaluate this concept further, we performed electrical impedance myography (EIM) using a handheld array on 38 individuals aged 19–50 years and 41 individuals aged 60–85 years. Individuals either had 7 upper extremity or 7 lower extremity muscles measured. The 50 kHz reactance, resistance and phase were used as the major outcome variables. Although the phase values were similar in both groups, both reactance and resistance values were lower in the lower extremities of the older individuals as compared to the younger (−23 ± 6%, p = 0.001 for reactance and −27 ± 7%, p = 0.005 for resistance), whereas changes in upper extremity values were not significantly different (−9 ± 5%, p = 0.096 for reactance and +5 ± 9%, p = 0.55 for resistance). When analyzing the genders separately, it became clear that this reduction in lower extremity values was most pronounced in men and less consistently present in women. These findings suggest that age- and gender-associated differences in muscle condition are detectable using EIM. The relationship of these easily obtained parameters to standard functional, imaging, and pathological markers of sarcopenia deserves further study.
Keywords: Aging, electrical impedance, sarcopenia, gender
1. Introduction
The term sarcopenia is defined as the age-related loss of muscle quantity and quality (Thompson, 2009; Narici and de Boer, 2010). It is a major health concern ultimately resulting in loss of mobility and independence. Approximately 25% of individuals >70 years of age have established sarcopenia (i.e., loss of muscle mass < 2 standard deviations below young reference means) and this proportion increases to 30–50% for individuals >80 years of age (Baumgartner et al., 1998). The direct and indirect health costs associated with aging muscle loss are estimated to be over $300 billion in the United States alone (Booth et al., 2000). There is increasing recognition of the importance of preventing or reversing sarcopenia and maintaining muscle function. Indeed, much ongoing work seeks approaches to forestalling its onset, such as exercise and dietary/pharmacological interventions (Pillard et al., 2011). However, present methods of identifying the presence of sarcopenia and assessing the effects of such interventions rely largely on strength measurements or radiological imaging (Bijlsma et al., 2012). Strength measurements require active participation and introduce subjective elements, such as patient motivation, that extend well beyond the properties of the muscle tissue itself. Imaging methods, such as CT and MRI, while sensitive to sarcopenic change (Pahor et al., 2009), are costly and inconvenient thus rendering them an unfavorable choice to use in a clinical setting. A tool for the rapid, bedside evaluation of muscle condition and the presence of sarcopenia could be valuable both for individual patient care and clinical research.
Electrical impedance myography (EIM) is a localized tetrapolar bioimpedance-based technique that could serve in this role. EIM has been shown to be sensitive to disease-induced changes to muscle’s normal composition and architecture, including myocyte atrophy, hypertrophy and loss, edema, reinnervation, and the deposition of endomysial connective tissue and fat (Rutkove, 2009). Specifically, EIM phase values obtained at 50 kHz show substantial alterations in a variety of neuromuscular disease states, including amyotrophic lateral sclerosis (ALS), myopathy, and disuse atrophy (Rutkove et al., 2012; Tarulli et al., 2005; Tarulli et al., 2009). One study also suggested the 50 kHz EIM phase shows a consistent decline across individuals with increasing age (Aaron et al., 2006). In further support of EIM’s potential application to the evaluation of sarcopenia, standard whole-body bioimpedance analysis methods have shown large reductions in overall muscle mass with age (Janssen et al., 2002).
We recently studied the application of EIM in the diagnosis of spinal nerve root compression (radiculopathy) (Spieker et al., 2013). In that project, using a handheld electrode array, we evaluated individual muscles of bilateral upper or lower extremities in adults with radiculopathy, comparing the asymptomatic, non-radiculopathic side to the diseased side, as well as to values from healthy adults (Narayanaswami et al., 2012). While analyzing the data, we noticed an age-dependent decline in 50 kHz EIM values. In this study we formalize that analysis, comparing EIM data from a group of younger adults to that of a group of older.
2. Materials and Methods
2.1 Subjects
Data was obtained from the limbs of normal healthy volunteers with no history of significant neuromuscular disease affecting either the upper or lower extremities as well as patients with a history of radiculopathy affecting the contralateral limb. Individuals were recruited by advertisement, word of mouth and directly through the neurology clinic and electromyography laboratory. After signing the informed consent form, all underwent a detailed neurological history and examination to confirm the absence of appendicular edema and any underlying neuromuscular disease affecting the limb to be studied. All subjects had either upper extremities or lower extremities evaluated, but not both.
Two groups of people were included in this analysis to maximize sample size: a younger group of individuals aged 19–50 years (38 adults) and a group of older individuals aged 60–85 years (41 adults). Table 1 provides a detailed breakdown of the patient demographics by age and sex.
Table 1.
Demographics and limb distribution
| Upper Extremity | |||||||
|---|---|---|---|---|---|---|---|
| Younger | Older | Total Number |
|||||
| Number | Mean Age |
Range | Number | Mean Age |
Range | ||
| Men | 8 | 39 | 29–50 | 11 | 68 | 62–83 | 19 |
| Women | 8 | 32 | 21–44 | 13 | 74 | 62–85 | 21 |
|
Upper Extremity Totals |
16 | 38 | 21–50 | 24 | 71 | 62–85 | 40 |
|
Lower Extremity | |||||||
| Younger | Older |
Total Number |
|||||
| Number |
Mean Age |
Range | Number |
Mean Age |
Range | ||
| Men | 12 | 40 | 24–49 | 12 | 70 | 60–83 | 24 |
| Women | 10 | 25 | 19–38 | 5 | 70 | 63–78 | 15 |
|
Lower Extremity Totals |
22 | 33 | 19–49 | 17 | 70 | 60–83 | 39 |
|
(Upper + Lower) Totals |
38 | 41 | 79 | ||||
2.2 EIM measurements
EIM measurements were obtained using the Imp SFB7 bioimpedance spectroscopy device (Impedimed, San Diego, CA), measuring at frequencies from 3 kHz -1 MHz. This device provides an output of resistance (R) and reactance (X) values, from which phase is calculated via the relationship phase = arctan(X/R). In this analysis, however, only the 50 kHz data was utilized. The choice of using only the 50 kHz data is based on the fact that values obtained at this single frequency are most physiologically important as sensitive indicators of muscle status. Indeed, in most of our earlier studies referenced above, only 50 kHz data, rather than multifrequency data, was studied. The sensitivity stems from the fact reactance and phase values of healthy muscle peak at approximately 50 kHz, and in disease states, declines in impedance values at this frequency are most readily observed. The measurements were made with a handheld electrode array (HEA) as previously described (Narayanaswami et al., 2012). The HEA consisted of four stainless steel electrodes, the inner two serving as voltage-measuring electrodes and the outer two serving as current-emitting electrodes. Prior to applying the array, the skin overlying the various muscles studied was moistened with isotonic saline to ensure good electrical contact.
In the upper extremities, EIM testing was performed on deltoid, triceps, biceps, brachioradialis, wrist flexors, wrist extensors, and extensor indicis. In the lower extremities, EIM testing was performed on tensor fascia latae, vastus lateralis, vastus medialis, biceps femoris, medial gastrocnemius, tibialis anterior and extensor hallucis longus.
2.3 Subcutaneous fat thickness measurement
Since variations in subcutaneous fat may impact the measured impedance data, subcutaneous fat thickness was measured using a Terason 2000 ultrasound system (Teratech Corp, Burlington, MA). Measurements were made at the same location as the EIM measurements, using electronic calipers provided with the ultrasound software.
2.4 Data Analysis
The 50 kHz impedance values for each muscle were extracted from the multifrequency data and are the only data reported here. Since the data across individuals was parametric in form, comparisons between groups and subgroups were made by unpaired t-tests. Significance was assumed at p < 0.05, two-tailed. For the figures, the actual mean impedance values ± standard error were plotted. For the tables, differences between younger and older groups are reported as mean percent difference ± standard error in the older group as compared to the younger group ± standard error (e.g., a −10% ± 4% value would indicate that the mean older group values were 10% lower than that of the younger group with a standard error of the mean of ± 4%).
3. Results
3.1 Cumulative results
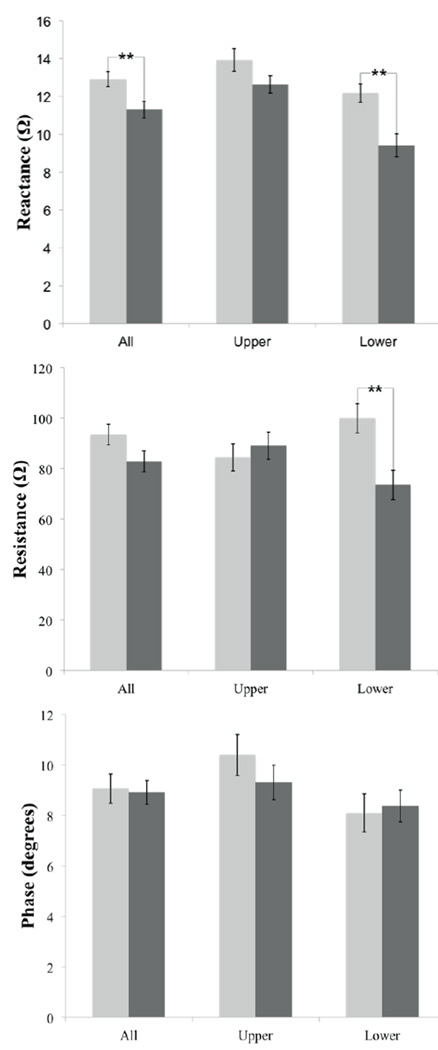
Figure 1 plots the actual impedance values ± standard error. Table 2 shows that the overall percentage difference in mean reactance (combined upper and lower extremity data) was lower in the older as compared to younger groups (−13 ± 4%, p = 0.009); however, neither resistance nor phase showed a significant reduction. When evaluating the lower extremities alone, the reactance was lower (−23 ± 6%, p = 0.001) in older individuals as compared to the younger; resistance was also lower (−27 ± 7%, p = 0.005); interestingly, this was true not only on average, but also for almost every individual lower extremity muscle. In contrast, there was no significant difference in upper extremity values for any measure, although there was a trend for reactance to be lower in the older group (−9 ± 5%, p = 0.096), whereas resistance was non-significantly higher in the older group (+5 ± 9%, p = 0.55).
Figure 1.
Summary of data; light gray, younger; dark gray, older. Impedance values ± standard error of the mean; **p < 0.01
Table 2.
Percent differences between younger and older adults (mean ± standard error)
| Younger | Older | Reactance (X) | Resistance (R) | Phase (P) | ||||
|---|---|---|---|---|---|---|---|---|
| Numbe r |
Numbe r |
% Diff | p value | % Diff | p value |
% Diff | p values | |
|
Mean Total Body |
38 | 41 | −13 ± 4 | 0.01 | −12 ± 6 | 0.09 | −2 ± 8 | 0.84 |
|
Mean Upper Extremity |
16 | 24 | −9 ± 5 | 0.10 | 5 ± 9 | 0.55 | −11 ± 10 | 0.32 |
| Deltoid | 16 | 24 | −7 ± 8 | 0.44 | −0 ± 8 | 0.97 | −6 ± 14 | 0.70 |
| Biceps brachii | 16 | 24 | −8 ± 6 | 0.20 | 10 ± 12 | 0.41 | −14 ± | 0.39 |
| Brachioradialis | 16 | 24 | −5 ± 6 | 0.46 | 7 ± 11 | 0.48 | −7 ± 11 | 0.59 |
| Triceps brachii | 16 | 24 | −20 ± 5 | 0.01 | −3 ± 13 | 0.83 | −15 ± | 0.24 |
| Forearm | 16 | 24 | −7 ± 6 | 0.28 | −5 ± 8 | 0.58 | −1 ± 10 | 0.95 |
| Forearm flexors | 16 | 24 | −18 ± 5 | 2E-03 | 11 ± 12 | 0.35 | −22 ± 9 | 0.05 |
| Extensor indicis | 16 | 24 | −12 ± 6 | 0.06 | −0 ± 6 | 0.95 | −9 ± 8 | 0.25 |
| Mean Lower Extremity |
22 | 17 | −23 ± 6 | 1E-03 | −27 ± 7 | 0.01 | 3 ± 12 | 0.79 |
| Tensor fascia | 22 | 17 | −33 ± 5 | 8E-06 | −20 ± 8 | 0.03 | −16 ± 9 | 0.12 |
| Biceps femoris | 22 | 17 | −16 ± 8 | 0.05 | −35 ± 6 | 5E-04 | 18 ± 19 | 0.32 |
| Vastus lateralis | 22 | 17 | −15 ± 8 | 0.10 | −32 ± 9 | 0.01 | 24 ± 22 | 0.24 |
| Vastus medialis | 22 | 17 | −21 ± 7 | 0.01 | −22 ± 8 | 0.03 | 0 ± 13 | 0.97 |
| Tibialis anterior | 22 | 17 | −20 ± 7 | 0.01 | −32 ± 7 | 3E-03 | 13 ± 14 | 0.30 |
| Medial gastroc. | 22 | 17 | −24 ± 7 | 2E-03 | −29 ± 9 | 0.02 | −5 ± 14 | 0.76 |
| Extensor | 22 | 17 | −30 ± 9 | 4E-03 | −34 ± 6 | 2E-04 | −2 ± 12 | 0.88 |
Significant differences shown in bold.
3.2 Results by gender
We next sought to determine if gender influenced these results. It is noteworthy that the average age of women in the study was slightly lower than that of men (50 vs. 56 years), mostly due to a difference in younger group, where the average age was 28 years as compared to 39 years.
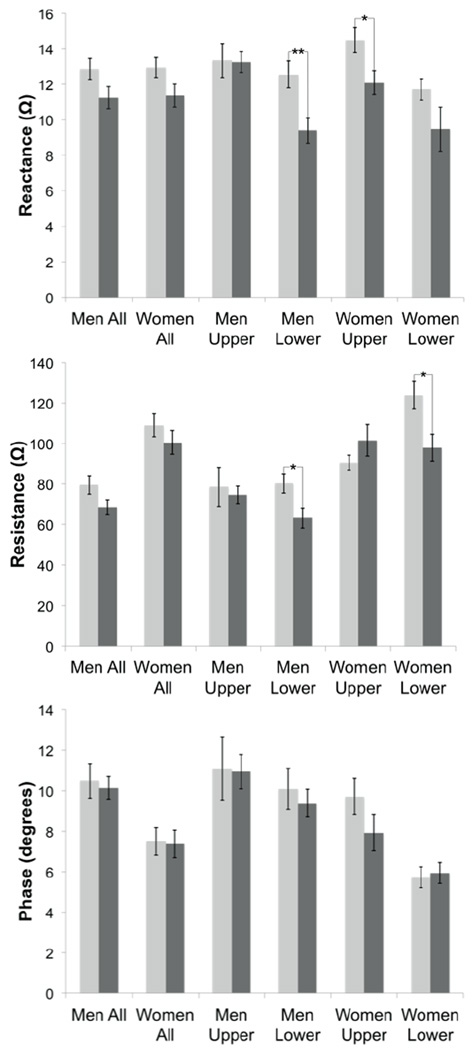
The gender-specific results are summarized in Figure 2 and Tables 3 and 4. Reactance values of combined upper and lower extremities showed small, near-significant differences between the younger and older groups for both men and women (−12 ± 6, p = 0.06 for men and −12 ± 6, p = 0.07 for women); resistance values were also near-significant for men but not for women; phase showed no difference for any of the measures. However, when we further separated the data into upper and lower extremities, and then evaluated muscles individually, the results were somewhat unexpected. In men (Table 3), no single muscle in the upper extremities was significantly different between the old and young group, whereas resistance and reactance values were consistently lower in the lower extremities of the older group (mean −25 ± 7%, p = 0.007 for reactance and −21 ± 8%, p = 0.019 for resistance). In women (Table 4), by contrast, a less consistent pattern was present. In the upper extremities, reactance was reduced on average (−17 ± 6, p = 0.03), whereas resistance was reduced in the lower extremities (−21 ± 7, p = 0.02); however, the difference across muscles was quite inconsistent.
Figure 2.
Summary of data broken down by gender; light gray, younger; dark gray, older. Impedance values ± standard error of the mean; *p < 0.05, **p < 0.01
Table 3.
Percent differences between younger and older men (mean ± standard error)
| Youngr | Older | Reactance (X) | Resistance (R) | Phase (P) | ||||
|---|---|---|---|---|---|---|---|---|
| Numbe r |
Numbe r |
% Diff | p value | % Diff | p value | % Diff | p values | |
| Mean Total | 20 | 23 | −13 ± 6 | 0.06 | −14 ± 7 | 0.07 | −3 ± 10 | 0.73 |
|
Mean Upper Extremity |
8 | 11 | −1 ± 8 | 0.94 | −5 ± 13 | 0.72 | −1 ± 16 | 0.94 |
| Deltoid | 8 | 11 | 6 ± 16 | 0.71 | −5 ± 13 | 0.73 | 4 ± 26 | 0.88 |
| Biceps brachii | 8 | 11 | −1 ± 9 | 0.92 | −4 ± 16 | 0.83 | −7 ± 21 | 0.77 |
| Brachioradialis | 8 | 11 | 6 ± 9 | 0.46 | 2 ± 13 | 0.87 | 2 ± 17 | 0.90 |
| Triceps brachii | 8 | 11 | −11 ± 9 | 0.37 | −18 ± | 0.58 | −1 ± 21 | 0.95 |
| Forearm | 8 | 11 | 2 ± 8 | 0.78 | 1 ± 12 | 0.91 | 2 ± 14 | 0.89 |
| Forearm flexors | 8 | 11 | −14 ± 8 | 0.15 | −7 ± 15 | 0.69 | −12 ± | 0.47 |
| Extensor indicis | 8 | 11 | −9 ± 9 | 0.32 | 0 ± 7 | 0.97 | −8 ± 9 | 0.38 |
|
Mean Lower Extremity |
12 | 12 | −25 ± 7 | 0.01 | −21 ± 8 | 0.02 | −7 ± 12 | 0.58 |
| Tensor fascia | 12 | 12 | −36 ± 6 | 3E-04 | −20 ± 6 | 0.01 | −22 ± | 0.10 |
| Biceps femoris | 12 | 12 | −17 ± | 0.13 | −30 ± 8 | 0.01 | 7 ± 20 | 0.73 |
| Vastus lateralis | 12 | 12 | −17 ± | 0.17 | −32 ± 6 | 4E-04 | 17 ± 21 | 0.38 |
| Vastus medialis | 12 | 12 | −20 ± 9 | 0.06 | −21 ± 7 | 0.01 | −2 ± 14 | 0.89 |
| Tibialis anterior | 12 | 12 | −26 ± 8 | 0.01 | −28 ± 8 | 0.01 | 0 ± 12 | 0.98 |
| Medial gastroc. | 12 | 12 | −27 ± 8 | 0.01 | −21 ± | 0.10 | −14 ± | 0.40 |
| Extensor | 12 | 12 | −36 ± | 4E-03 | −30 ± 6 | 2E-04 | −15 ± | 0.26 |
Significant differences shown in bold.
Table 4.
Percent differences between younger and older women (mean ± standard error)
| Younge r |
Older | Reactance (X) | Resistance (R) | Phase (P) | ||||
|---|---|---|---|---|---|---|---|---|
| Numbe r |
Numbe r |
% Diff | p value | % Diff | p value | % Diff |
p value | |
|
Mean Total Body |
18 | 18 | −12 ± 6 | 0.07 | −8 ± 7 | 0.31 | −2 ± 12 | 0.89 |
|
Mean Upper Extremity |
8 | 13 | −17 ± 6 | 0.03 | 12 ± 10 | 0.22 | −18 ± 11 | 0.12 |
| Deltoid | 8 | 13 | −16 ± 7 | 0.07 | 3 ± 9 | 0.74 | −13 ± 13 | 0.34 |
| Biceps brachii | 8 | 13 | −15 ± 8 | 0.11 | 17 ± 12 | 0.17 | −19 ± 17 | 0.30 |
| Brachioradialis | 8 | 13 | −13 ± 8 | 0.16 | 10 ± 14 | 0.49 | −13 ± 15 | 0.41 |
| Triceps brachii | 8 | 13 | −26 ± 6 | 0.01 | 7 ± 11 | 0.52 | −25 ± 9 | 0.02 |
| Forearm extensors |
8 | 13 | −14 ± 8 | 0.12 | −11 ± 10 | 0.32 | −2 ± 15 | 0.92 |
| Forearm flexors | 8 | 13 | −22 ± 6 | 0.01 | 23 ± 15 | 0.11 | −30 ± 11 | 0.02 |
| Extensor indicis | 8 | 13 | −15 ± 9 | 0.12 | −2 ± 8 | 0.84 | −10 ± 12 | 0.47 |
|
Mean Lower Extremity |
10 | 5 | −19 ± 11 | 0.16 | −21 ± 7 | 0.02 | 4 ± 12 | 0.76 |
| Tensor fascia lata |
10 | 5 | −26 ± 11 | 0.06 | −9 ± 13 | 0.54 | −17 ± 7 | 0.06 |
| Biceps femoris | 10 | 5 | −18 ± 13 | 0.24 | −30 ± 8 | 0.01 | 13 ± 18 | 0.47 |
| Vastus lateralis | 10 | 5 | −17 ± 14 | 0.28 | −19 ± 7 | 0.04 | −5 ± 15 | 0.77 |
| Vastus medialis | 10 | 5 | −26 ± 11 | 0.07 | −11 ± 8 | 0.26 | −15 ± 13 | 0.28 |
| Tibialis anterior | 10 | 5 | −12 ± 11 | 0.34 | −27 ± 8 | 0.02 | 21.6 ± 15 | 0.15 |
| Medial gastroc. | 10 | 5 | −20 ± 12 | 0.17 | −26 ± 8 | 0.02 | −3 ± 18 | 0.89 |
| Extensor hallucis |
10 | 5 | −16 ± 17 | 0.39 | −33 ± 6 | 5E-04 | 20 ± 19 | 0.30 |
Significant differences shown in bold.
3.3 Analysis of subcutaneous fat thickness
In order to help confirm that the subcutaneous fat thickness was not confounding our results, we also analyzed the average subcutaneous fat thickness separately in each of the subgroups of subjects (Table 5); these data confirmed that there were no significant differences in subcutaneous fat thickness and thus the influence of tissue on the results was likely very small.
Table 5.
Subcutaneous fat thickness (in cm) as measured by ultrasound (mean ± standard error)
| Mean Upper Extremity |
Younger (Number) |
Older (Number) |
Upper Younger (cm) |
Upper Older (cm) |
P-value |
|---|---|---|---|---|---|
| Male | 8 | 11 | 0.43 ± 0.06 | 0.46 ± 0.03 | 0.61 |
| Female | 8 | 13 | 0.45 ± 0.06 | 0.60 ± 0.08 | 0.13 |
| All | 16 | 24 | 0.44 ± 0.04 | 0.54 ± 0.05 | 0.11 |
| Mean Lower Extremity |
Younger (Number) |
Older (Number) |
Lower Younger (cm) |
Lower Older (cm) |
P-value |
| Male | 12 | 12 | 0.56 ± 0.07 | 0.61 ± 0.07 | 0.62 |
| Female | 10 | 5 | 1.10 ± 0.13 | 0.90 ± 0.12 | 0.49 |
| All | 22 | 17 | 0.78 ± 0.09 | 0.70 ± 0.07 | 0.48 |
4. Discussion
The goal of this study was to perform a preliminary analysis of EIM sensitivity to age-associated change in muscle, or sarcopenia. Our earlier work in this area had already suggested that such a relationship existed (Aaron et al., 2006). However, the approach used during earlier studies involved inconvenient, adhesive electrodes placed on the distal extremities (Rutkove et al., 2002), evaluated only the phase angle, and did not evaluate muscles individually nor make any effort to assess differences between the sexes or between upper and lower extremities.
Through our work in ALS and in other neuromuscular diseases, we have identified a major change in the impedance parameters with progressive disease severity (Ahad and Rutkove, 2009; Wang et al., 2011). Changes in impedance values have also been reported in myotubes growing in cell culture (Rakhilin et al., 2011). Older individuals have fewer muscle fibers and smaller muscle fiber cross-sectional areas than younger individuals, and thus, a difference in the impedance characteristics of the muscle might be anticipated (Lexell, 1995). Therefore, in aging muscle, impedance values might be expected to alter in a consistent fashion.
When comparing young and old individuals, the upper extremity muscles show smaller and for the most part non-significant differences in impedance values; in contrast, lower extremity muscles show more consistent differences, especially in men. This finding is consistent with work that shows that sarcopenia generally impacts the lower extremities to a greater extent than the upper (Janssen et al., 2000). The unexpected result of this study, however, was the observed gender difference between upper and lower extremity alterations. In men the upper extremities showed no statistical difference for either reactance or resistance whereas nearly every muscle studied in the lower extremities showed large and highly significant differences for both measures. In women, in contrast, the picture was far less consistent with several upper extremity muscles showing lower reactance values and several lower extremity muscles showing lower resistance values. As far as is known, physical activity generally remains similar in both men and women of older age, which renders an activity-based explanation unlikely. Thus, the reason for the gender differences in upper extremity values is also not easily identified.
Another finding that deserves further study was the virtual absence of a difference in phase values for any single muscle examined. This is in obvious contrast to our earlier study in which the 50 kHz phase value was the only measure reported (Aaron et al., 2006). The major difference between this study and that earlier study was that the current study utilized a handheld array and evaluated a number of upper and lower extremity muscles whereas in the previous study we evaluated only biceps and quadriceps using a very different electrode setup (with current-emitting electrodes placed on the palms of the hands for biceps measurement and the dorsum of the feet for quadriceps measurement). This difference in electrode set-up could potentially be responsible, since the earlier approach would be sensitive to overall muscle mass whereas our current approach, using a handheld array, is likely less sensitive since the current penetrates only the superficial aspects of the muscle (Jafarpoor et al., 2013).
Importantly, in this analysis we only evaluated EIM data obtained at 50 kHz. However, impedance values can be analyzed over a much larger frequency range, and such analyses may offer additional useful information and potentially sensitivity to age-dependent changes (Esper et al., 2006). However, due to the limitations of this retrospective analysis, and the current complexity of analysis already presented, such work is best left for future prospective studies specifically addressing this question.
This study has a number of clear limitations. Most importantly, the data analyzed here represent a retrospective analysis drawn from a larger data set from a study not focused on age effects but rather on the diagnosis of radiculopathy; moreover, we have integrated data from true healthy controls with that from unaffected limbs of radiculopathy subjects. Nonetheless, repeating this study with larger groups of truly healthy individuals would be ideal. Second, we arbitrarily chose our cut-off ages to attempt to maximize our group sizes, and a more precise analysis by decade with larger numbers would be useful. Third, having a larger number of older individuals would also be helpful such that trends within the older population could be identified. Finally, it is likely that subcutaneous fat thickness influences the results to some extent (Sung et al., 2012). Fortunately, the absence of a significant difference in values between the younger and older adults makes any contribution from this factor relatively small.
In summary, we have observed significant gender-specific differences in the 50 kHz reactance and resistance values between younger and older individuals obtained using a handheld array configured with a commercial bioimpedance measuring system. These observations suggest that EIM deserves further study as a method for assessing sarcopenia. However, more work is needed to better understand the relationship between these differences in impedance values and the functional and structural alterations in muscle associated with aging.
Acknowledgments
This work was funded by grant K24 NS060951 from the National Institutes of Health.
References
- Aaron R, Esper GJ, Shiffman CA, Bradonjic K, Lee KS, Rutkove SB. Effects of age on muscle as measured by electrical impedance myography. Physiol Meas. 2006;27:953–959. doi: 10.1088/0967-3334/27/10/002. [DOI] [PubMed] [Google Scholar]
- Ahad M, Rutkove SB. Electrical impedance myography at 50 kHz in the rat: technique, reproducibility, and the effects of sciatic injury and recovery Clinical Neurophysiology. Clinical Neurophys. 2009;120:1534–1538. doi: 10.1016/j.clinph.2009.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgartner RN, Koehler KM, Gallagher D, Romero L, Heymsfield SB, Ross RR, Garry PJ, Lindeman RD. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol. 1998;147:755–763. doi: 10.1093/oxfordjournals.aje.a009520. [DOI] [PubMed] [Google Scholar]
- Bijlsma AY, Meskers CG, Ling CH, Narici M, Kurrle SE, Cameron ID, Westendorp RG, Maier AB. Defining sarcopenia: the impact of different diagnostic criteria on the prevalence of sarcopenia in a large middle aged cohort. Age (Dordr) 2012 doi: 10.1007/s11357-012-9384-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth FW, Gordon SE, Carlson CJ, Hamilton MT. Waging war on modern chronic diseases: primary prevention through exercise biology. J Appl Physiol. 2000;88:774–787. doi: 10.1152/jappl.2000.88.2.774. [DOI] [PubMed] [Google Scholar]
- Esper GJ, Shiffman CA, Aaron R, Lee KS, Rutkove SB. Assessing neuromuscular disease with multifrequency electrical impedance myography. Muscle Nerve. 2006;34:595–602. doi: 10.1002/mus.20626. [DOI] [PubMed] [Google Scholar]
- Jafarpoor M, Li J, White JK, Rutkove SB. Optimizing Electrode Configuration for Electrical Impedance Measurements of Muscle via the Finite Element Method. IEEE Trans Biomed Eng. 2013;60:1446–1452. doi: 10.1109/TBME.2012.2237030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen I, Heymsfield SB, Ross R. Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. J Am Geriatr Soc. 2002;50:889–896. doi: 10.1046/j.1532-5415.2002.50216.x. [DOI] [PubMed] [Google Scholar]
- Janssen I, Heymsfield SB, Wang ZM, Ross R. Skeletal muscle mass and distribution in 468 men and women aged 18–88 yr. J Appl Physiol. 2000;89:81–88. doi: 10.1152/jappl.2000.89.1.81. [DOI] [PubMed] [Google Scholar]
- Lexell J. Human aging, muscle mass, and fiber type composition. J Gerontol A Biol Sci Med Sci. 1995;50(Spec No 11-6) doi: 10.1093/gerona/50a.special_issue.11. [DOI] [PubMed] [Google Scholar]
- Narayanaswami P, Spieker AJ, Mongiovi P, Keel JC, Muzin SC, Rutkove SB. Utilizing a handheld electrode array for localized muscle impedance measurements. Muscle Nerve. 2012;46:257–263. doi: 10.1002/mus.23307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narici MV, de Boer MD. Disuse of the musculo-skeletal system in space and on earth. Eur J Appl Physiol. 2010;111:403–420. doi: 10.1007/s00421-010-1556-x. [DOI] [PubMed] [Google Scholar]
- Pahor M, Manini T, Cesari M. Sarcopenia: clinical evaluation, biological markers and other evaluation tools. J Nutr Health Aging. 2009;13:724–728. doi: 10.1007/s12603-009-0204-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillard F, Laoudj-Chenivesse D, Carnac G, Mercier J, Rami J, Riviere D, Rolland Y. Physical activity and sarcopenia. Clin Geriatr Med. 2011;27:449–470. doi: 10.1016/j.cger.2011.03.009. [DOI] [PubMed] [Google Scholar]
- Rakhilin S, Turner G, Katz M, Warden R, Irelan J, Abassi YA, Glass DJ. Electrical impedance as a novel biomarker of myotube atrophy and hypertrophy. J Biomol Screen. 2011;16:565–574. doi: 10.1177/1087057111401392. [DOI] [PubMed] [Google Scholar]
- Rutkove SB. Electrical Impedance Myography: Background, Current State, and Future Directions. Muscle Nerve. 2009;40:936–946. doi: 10.1002/mus.21362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutkove SB, Aaron R, Shiffman CA. Localized bioimpedance analysis in the evaluation of neuromuscular disease. Muscle Nerve. 2002;25:390–397. doi: 10.1002/mus.10048. [DOI] [PubMed] [Google Scholar]
- Rutkove SB, Caress JB, Cartwright MS, Burns TM, Warder J, David WS, Goyal N, Maragakis NJ, Clawson L, Benatar M, Usher S, Sharma KR, Gautam S, Narayanaswami P, Raynor EM, Watson ML, Shefner JM. Electrical impedance myography as a biomarker to assess ALS progression. Amyotrophic Lateral Sclerosis. 2012 doi: 10.3109/17482968.2012.688837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spieker AJ, Narayanaswami P, Fleming L, Keel JC, Muzin SC, Rutkove SB. Electrical Impedance Myography in the Diagnosis of Radiculopathy. Muscle Nerve. 2013 doi: 10.1002/mus.23833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung M, Spieker AJ, Narayanaswami P, Rutkove SB. The effect of subcutaneous fat on electrical impedance myography when using a handheld electrode array: The case for measuring reactance. Clin Neurophysiol. 2012 doi: 10.1016/j.clinph.2012.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarulli A, Esper G, Lee K, Aaron R, Shiffman C, Rutkove S. Electrical impedance myography in the bedside assessment of inflammatory myopathy. Neurology. 2005;65:451–452. doi: 10.1212/01.wnl.0000172338.95064.cb. [DOI] [PubMed] [Google Scholar]
- Tarulli AW, Duggal N, Esper GJ, Garmirian LP, Fogerson PM, Lin CH, Rutkove SB. Electrical impedance myography in the assessment of disuse atrophy. Arch Phys Med Rehabil. 2009;90:1806–1810. doi: 10.1016/j.apmr.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson LV. Age-related muscle dysfunction. Exp Gerontol. 2009;44:106–111. doi: 10.1016/j.exger.2008.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang LL, Spieker AJ, Li J, Rutkove SB. Electrical impedance myography for monitoring motor neuron loss in the SOD1 G93A amyotrophic lateral sclerosis rat. Clin Neurophysiol. 2011;122:2505–2511. doi: 10.1016/j.clinph.2011.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]