Summary
Commensal bacteria impact host health and immunity through various mechanisms, including the production of immunomodulatory molecules. Bacteroides fragilis produces a capsular polysaccharide (PSA), which induces regulatory T cells and mucosal tolerance. However, unlike pathogens, which employ secretion systems, the mechanisms by which commensal bacteria deliver molecules to the host remain unknown. We reveal that Bacteroides fragilis releases PSA in outer membrane vesicles (OMVs) that induce immunomodulatory effects and prevent experimental colitis. Dendritic cells (DCs) sense OMV-associated PSA through TLR2, resulting in enhanced regulatory T cells and anti-inflammatory cytokine production. OMV-induced signaling in DCs requires Growth Arrest and DNA-Damage-Inducible protein (Gadd45α). DCs treated with PSA-containing OMVs prevent experimental colitis, whereas Gadd45α-/- DCs are unable to promote regulatory T cell responses or suppress pro-inflammatory cytokine production and host pathology. These findings demonstrate that OMV-mediated delivery of a commensal molecule prevents disease, uncovering a mechanism of inter-kingdom communication between the microbiota and mammals.
Introduction
Microbes have evolved complex adaptations to inhabit and replicate in numerous ecological niches on Earth. Animals are no exception, and provide an ideal environment for both transient and permanent microbial colonization. Many pathogens cause acute infections as a means to propagate and disseminate, and accordingly have developed myriad mechanisms to avoid, subvert and/or prevent host immune responses. Conversely to pathogens, symbiotic bacteria have taken a different evolutionary route to promote bacterial replication. The gastrointestinal tract of mammals is colonized for life with a diverse commensal microbiota that provides essential benefits to the host such as nutrient and caloric extraction from food, development of the immune system and protection from immunologic and metabolic diseases (Round and Mazmanian, 2009). The dichotomy between beneficial and harmful microbes is illustrated by studies showing that gut colonization by the microbiota inhibits infection by enteric pathogens (Chung et al., 2012). Therefore unlike most pathogens that acutely infect animals for days or weeks, symbiotic bacteria have evolved to improve host health, providing themselves a protected niche where they flourish for decades.
How do bacteria establish molecular communication with their hosts to promote microbial replication? In addition to the export of single proteins (such as toxins) by the general secretion pathway, Gram-negative bacteria have evolved intricate mechanisms that can deliver a concert of microbial molecules to the host. Well studied examples include type III secretion systems (T3SS), T4SS and T6SS, which assemble macromolecular surface appendages that translocate bacterial effectors following direct contact with host cells (Galan, 2009). In addition, outer membrane vesicles (OMVs) are released from the surface of bacteria and deliver a suite of molecular cargo to target cells. OMVs from pathogens transport molecules to animal, plant and other bacterial cells (Mashburn-Warren and Whiteley, 2006). Consequently, a fundamental difference between surface secretion systems and OMVs is the distance that microbial molecules can travel. As commensal bacteria generally do not make intimate contact with host cells, OMVs appear to provide a suitable mechanism for members of the microbiota to deliver molecules to distant targets in the host.
Of the numerous microbial species that inhabit the gastrointestinal tract of mammals, Bacteroidetes are the most abundant Gram-negative bacterial phylum (Ley et al., 2008). Bacteroides fragilis is a commensal that aids in host health by ameliorating inflammatory bowel disease (IBD) and multiple sclerosis (MS) in animal models (Mazmanian et al., 2008; Ochoa-Reparaz et al., 2010a; Ochoa-Reparaz et al., 2010b). Polysaccharide A (PSA) is an immunomodulatory molecule produced by B. fragilis that is required and sufficient for treatment of experimental disease. B. fragilis protects animals by inducing the development of interlekin-10 (IL-10)-producing CD4+Foxp3+regulatory T cells (TREGS) that suppress inflammation (Ochoa-Reparaz et al., 2010b; Round and Mazmanian, 2010). By promoting TREG development, PSA suppresses immune responses that drive inflammation, representing a potential candidate therapy for IBD and MS. Moreover, the production of PSA suppresses intestinal immunity directed toward B. fragilis during normal homeostatic colonization of animals (Round et al., 2011). Thus PSA promotes mutualism by providing benefits to both microbe and mammals during symbiosis. However, the mechanism by which B. fragilis delivers PSA to the immune system remains unknown.
Since the genome of B. fragilis does not encode for known secretion system genes (Cerdeno-Tarraga et al., 2005) and PSA is a large capsular polysaccharide (Mazmanian et al., 2005), we reasoned that PSA may be delivered to the immune system by OMVs. We show herein that PSA is selectively packaged in OMVs that are released by B. fragilis. In vivo, OMVs containing PSA prevent experimental colitis by suppressing tumor necrosis factor (TNFα) production and T-helper 17 (Th17) cell development, unlike OMVs that do not contain PSA. OMVs are internalized into dendritic cells (DCs), and program DCs to induce the differentiation of IL-10-producing TREG cells in a PSA-dependent manner. In vitro, TREGS induced by OMVs are functionally suppressive and inhibit T cell proliferation. We recently showed that toll-like receptor 2 (TLR2) expression by T cells promotes TREG function in response to purified PSA (Round et al., 2011). Here, we reveal that TLR2 expression by DCs, and not T cells, is required for recognition of PSA within OMVs. Gene expression profiling led to identification of the Growth Arrest and DNA-Damage-Inducible protein (Gadd45α) as being required for PSA-mediated TLR2 signaling in DCs. The transfer of OMV-treated DCs to mice induced for colitis protects from disease, demonstrating a critical function for DCs in promoting PSA activity. Gadd45α deletion in DCs abrogates IL-10 induction by PSA, and Gadd45α-/- DCs are unable to provide protection from experimental colitis. Our findings show that delivery of PSA by OMVs underlies the probiotic properties of B. fragilis, revealing a unique mechanism by which the commensal microbiota communicates with the immune system during host-bacterial mutualism.
Results
PSA is Packaged into Outer Membrane Vesicles
Outer membrane vesicles (OMVs) have been observed in many Gram-negative bacteria. For Bacteroides fragilis, OMVs are produced by a subset of bacteria, known as the electron dense layer (EDL), which comprises <5% of the population in laboratory culture (Patrick et al., 1996). We imaged negatively stained EDL-enriched bacteria by transmission electron microscopy. OMVs were abundantly produced by B. fragilis (Figure 1A), and could be observed budding from the bacterial envelope (Figure 1A, higher magnification). Previous studies have shown that deletion of PSA abrogates the immunomodulatory capacity of B. fragilis (Mazmanian et al., 2005; Mazmanian et al., 2008). Electron micrographs of a PSA mutant strain (B. fragilisΔPSA) illustrated no defect in OMV production, and the size, shape and abundance of OMVs produced were indistinguishable from wild-type bacteria (Figure 1A and Figure S1A). To determine if PSA is associated with OMVs of B. fragilis, we purified vesicles and probed vesicle extracts with anti-sera specific for PSA. OMVs from wild-type bacteria displayed immunoreactivity to PSA, unlike OMVs from B. fragilisΔPSA, confirming antibody specificity (Figure 1B). In addition to PSA, B. fragilis produces at least 7 other capsular polysaccharides that coat the surface of bacterial cells (Krinos et al., 2001; Liu et al., 2008). While PSB was also detected in vesicle preparations, PSG was absent, possibly suggesting regulation of polysaccharide packaging into OMVs (Figure 1B). Immunogold labeling of purified vesicles confirmed that PSA is physically associated with OMVs (Figure 1C). To verify that the absence of PSA did not alter the molecular composition of vesicles, we performed proteomic analysis by mass spectrometry that showed negligible differences in the protein composition between vesicles from wild-type or PSA-mutant bacteria (Figure S1B). Together, these findings reveal that B. fragilis packages PSA into OMVs, potentially representing a mechanism to deliver immunomodulatory signals to its mammalian host.
Figure 1.
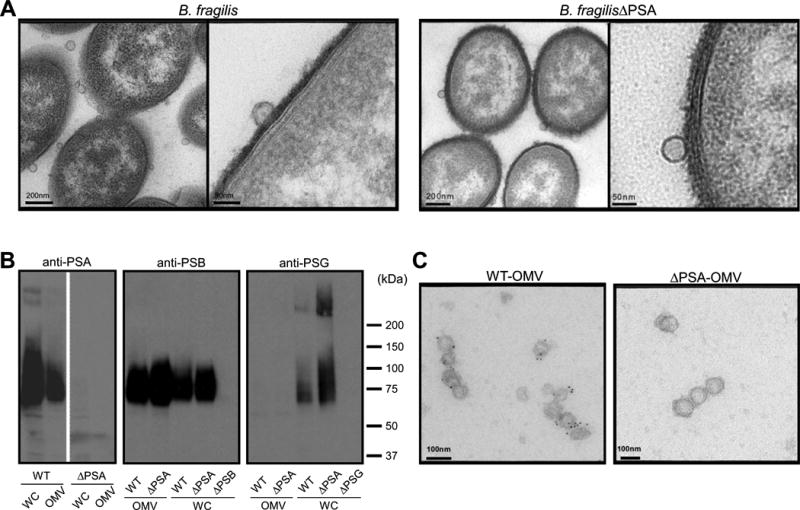
Outer membrane vesicles (OMVs) from Bacteroides fragilis contain PSA. (A) Transmission electron microscopy of electron dense layer (EDL) enriched B. fragilis (WT-OMV) and B. fragilisΔPSA (ΔPSA-OMV) reveals vesicles budding from the bacterial surface. (B) Immunoblot analysis of whole cell (WC) and OMV extracts ofbacteria show that PSA and PSB are associated with vesicles, while PSG is only found associated with the bacterial cell. Deletion mutants for PSA (ΔPSA), PSB (ΔPSB) and PSG (ΔPSG) confirm specificity of each anti-sera. (C) Immunogold labeling of purified OMVs, stained with anti-PSA and anti-IgG-colloidal gold conjugate (5nm), analyzed by electron microscopy shows specific staining for PSA only in OMVs from wild-type bacteria but not B. fragilisΔPSA. See also Figure S1.
OMVs Containing PSA Protect Animals from Experimental Colitis
Crohn's disease and ulcerative colitis (forms of IBD) are painful and medically incurable illnesses of the digestive system (Xavier and Podolsky, 2007). PSA has been shown to protect and treat colitis in animal models (Mazmanian et al., 2008; Round and Mazmanian, 2010), including intestinal inflammation induced by 2,4,6-trinitrobenzene sulfonic acid (TNBS) (Wirtz et al., 2007). To investigate if OMVs have therapeutic activity, we orally treated mice with vesicles containing PSA during experimental colitis. Control animals rapidly lost weight following rectal administration of TNBS (Figure 2A; PBS+TNBS), and did not recover compared to vehicle-treated mice that lost minimal weight (Figure 2A; PBS+EtOH). Remarkably, OMVs given orally to TNBS animals significantly protected from weight loss (Figure 2A; WT-OMV+TNBS). Importantly, when OMVs from B. fragilisΔPSA were administered, weight loss was indistinguishable from TNBS-treated animals (Figure 2A; ΔPSA-OMV+TNBS), demonstrating that PSA is responsible for preventing wasting disease. Reduction in colon length is a hallmark of TNBS colitis (Ishiguro et al., 2006). Measurements of intact colons (resected from cecum to rectum) showed normal intestinal lengths in animals treated with PSA-containing vesicles, but not OMVs lacking PSA (Figure 2B). These results show that oral feeding of OMVs corrects weight loss and gross pathology of the colon in a preclinical model of IBD.
Figure 2.
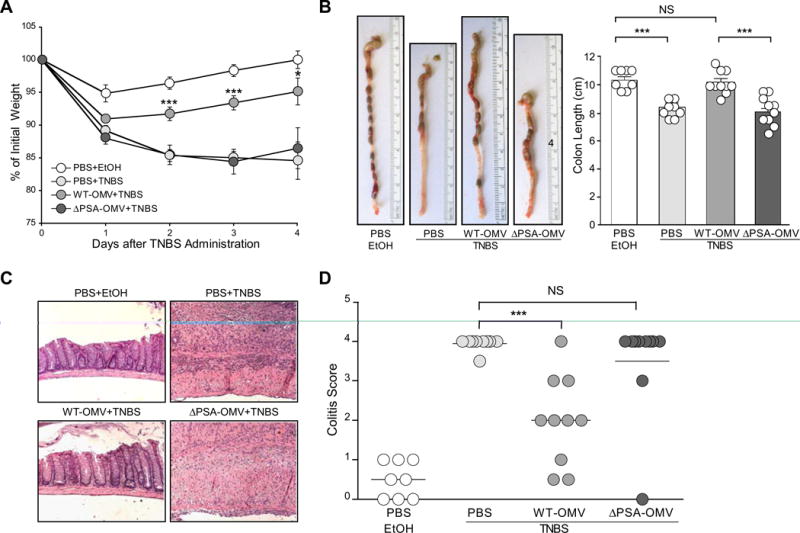
OMVs protect animals from experimental colitis and intestinal inflammation in a PSA-dependent manner. (A) Weight loss in animals following the induction of TNBS colitis (day 0) measured as reduction from initial weight until day of sacrifice (day 4). All groups contained at least 4 animals, with error bars indicating standard error (SEM). Results are representative of 3 independent trials. * p<0.05; *** p<0.001. (B) Images of colons immediately following resection and quantification of length (indicated in the bar graph) from vehicle treated (EtOH) and TNBS groups (n=4 animals/group). Error bars indicate SEM. Results are shown from 3 combined experiments where each was performed independently. *** p<0.001. NS: not significant. (C) Images from hematoxylin and eosin (H & E) stained colon sections representative of each treatment group. (D) Colitis scores from animals assigned by a blinded pathologist (G.W.L) according to a standard scoring system (see Experimental Procedures). Each symbol represents an individual animal. Results are shown from 3 combined experiments, each performed independently. *** p<0.001. NS: not significant. See also Figure S2.
IBD in humans, and colitis in animals, features inflammation driven by innate immune cells and CD4+ T cells, resulting in severe intestinal pathology (epithelial hyperplasia, sub-mucosal thickening, infiltration of leukocytes) (Xavier and Podolsky, 2007). Tumor necrosis factor (TNFα) causes damage to the epithelium and disrupts barrier function, and IL-17 promotes neutrophil migration into the gut mediating tissue damage. In contrast, IL-10 is protective in numerous animal models of colitis (Lindsay et al., 2002), and PSA requires IL-10 expression during protection from disease (Round and Mazmanian, 2010). TNFα and IL-17 levels were elevated in colonic tissues, and CD4+T cells from the mesenteric lymph nodes (MLNs) and colons of TNBS-treated animals; WT-OMVs suppressed pro-inflammatory cytokines and enhanced IL-10 production compared to ΔPSA-OMVs (Figure S2A-C), although these trends did not reach statistical significance. Upon histological analysis of colonic tissues, we observed significant disease in TNBS-treated animals that was ameliorated by oral administration of PSA-containing vesicles (Figure 2C). TNBS colitis manifests in focal lesions throughout the colon that mimic the pathology observed in Crohn's disease. Finally, clinical symptoms were evaluated by a blinded pathologist to assess disease (Wirtz et al., 2007). While all TNBS and ΔPSA-OMV treated animals were severely affected, oral administration of WT-OMVs significantly ameliorated disease (Figure 2D). Moreover, when OMVs were administered rectally (instead of orally) to the mouse colon, animals were protected from colitis (Figure S2D and S2E). We conclude that PSA packaging into OMVs protects animals from the pathological and immunological manifestations of experimental colitis, and that OMVs from B. fragilis represent a candidate therapy for IBD.
OMV-associated PSA Induces Foxp3 and IL-10 from CD4+ T cells
The mucosal immune system constitutively surveys the intestinal environment and dendritic cells (DCs) sample intestinal contents to coordinate T cell reactions (Rescigno et al., 2001). Purified PSA administered orally to animals is associated with CD11c+ DCs in the MLN (Mazmanian et al., 2005). To extend these studies to cell culture, we show that vesicles were taken up by bone marrow-derived DCs (BMDCs) regardless of PSA expression (Figure 3A and Figure S3A). Confocal microscopy revealed that OMVs (labeled with FITC) appeared as punctuate foci throughout the cytoplasm of cells (Figure S3A). DCs rapidly internalized vesicles in an actin-dependent manner, as treatment of cells with cytochalasin D significantly inhibited vesicle uptake (Figure S3B). Proportions of DC expressing activation markers (MHCII, CD86) increased following internalization of OMVs regardless of PSA expression (Figure 3B and Figure S3C), ruling out cell stimulation differences between the two different vesicle treatments.
Figure 3.
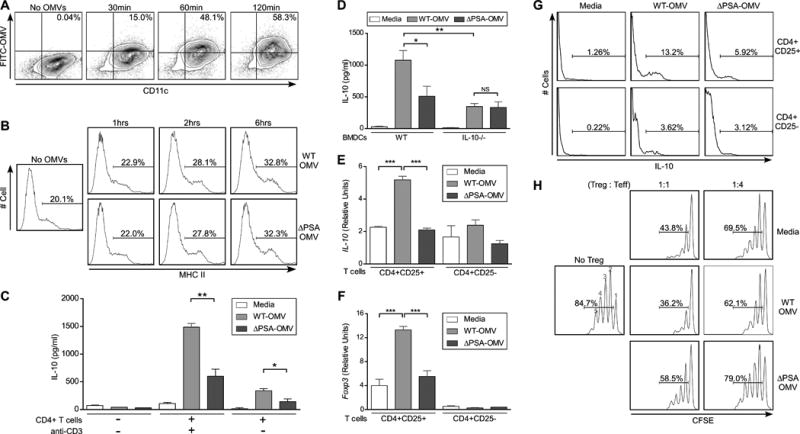
Treatment of DCs with PSA-containing OMVs induces IL-10 production and Foxp3 expression from T cells. (A) Flow cytometry (FC) analysis of OMV internalization by DCs. OMVs were labeled with FITC (Fluorescein isothiocyanate) and incubated with cultured DCs for various times. Cells were stained with anti-CD11c. Percentages show CD11c+OMV+ cell populations. (B) FC plots of DCs incubated with WT-OMVs and ΔPSA-OMVs for various times and stained with anti-CD11c and anti-MHCII (Major histocompatibility complex II). Percentages show MHCII+ populations among CD11c+ cells. (C) ELISA analysis for IL-10 production from culture supernatants of DCs or DC-T cell co-cultures, where DCs were pulsed with OMVs for 18 hours, washed and incubated with or without splenic CD4+ T cells. Supernatants were collected at day 4 of culture. Media samples indicate DCs that were not pulsed with OMVs, but otherwise treated identically. Anti-CD3 was added to some samples to demonstrate T cell-specific responses. Error bars indicate SEM from quadruplicate samples. Results are representative of over 5 independent trials. * p<0.05; ** p<0.01. (D) ELISA analysis of DC-T cell co-cultures similar to (C), with IL-10-/- DCs. Error bars indicate SEM from quadruplicate samples. Results are representative of 3 independent trials. * p<0.05. ** p<0.01. NS: not significant. (E, F) Transcript levels of IL-10 (E) and Foxp3 (F) of RNA recovered from purified CD4+ T cell subsets following in vitro culture with DCs. Co-cultures were set up as in (C); on day 4, CD4+CD25+ and CD4+CD25- T cells were purified by magnetic bead separation (>95% purity) and RNA extracted for qRT-PCR. Relative values were normalized to β-actin. Error bars indicate SEM. Results are shown from 3 combined experiments each performed independently. * p<0.05; *** p<0.001. NS: not significant. (G) FC histograms of IL-10 expression by CD4+ T cell subsets following 4 days of co-culture with DCs treated with OMVs. Splenic CD4+ T cell were purified from IL-10-GFP mice, stained with anti-CD4 and anti-CD25 following co-culture, and IL-10 expression measured by GFP expression. Percentages show IL-10+ populations amongCD4+CD25+ and CD4+CD25- subsets. Results are representative of 2 independent trials. (H)In vitro suppression of naïve responder cells by purified CD4+CD25+ T cells following co-culture with DCs treated with media (control), WT-OMVs and ΔPSA-OMVs. CD4+CD25-responder cells (effector cells; Teff) were pulsed with CFSE, incubated with TREGS and stimulated with anti-CD3 and APC (CD4+ T cell depleted splenocytes) for 3 days. Cell proliferation was measured by FC as a function of CFSE dilution. TREG:Teff ratios are indicated, and percentages show total proliferating cells. No TREG: CD4+CD25- cells only. Numbers above peaks represent number of cell divisions. Results are representative of 3 independent trials. See also Figure S3.
As IL-10 production is known to prevent colitis by suppressing unwanted inflammation (Maynard and Weaver, 2008), we examined if OMVs promote anti-inflammatory immune responses in cell culture. BMDCs were treated with OMVs, washed, and cultured with naïve CD4+ T cells. WT-OMVs induced the expression of IL-10 during in vitro DC-T cell co-culture, while minimal IL-10 was detected from DCs alone. Addition of anti-CD3 to the co-culture greatly increased IL-10 production, indicating the main source of IL-10 was from CD4+ T cells (Figure 3C). Vesicles purified from B. fragilisΔPSA induced significantly less IL-10 than WT-OMVs, although an increase was observed over media controls. IL-10 production by DCs is known to promote CD4+IL-10+ T cell development both in vivo and in vitro (O'Garra et al., 2004). Accordingly, we observed no increase in IL-10 production when wild-type T cells were co-cultured with IL-10-/- DCs following treatment with OMVs (Figure 3D), showing that IL-10 expression by DCs is required to induce IL-10 from CD4+ T cells.
CD4+CD25+ T cells that express the transcription factor Forkhead box P3 (Foxp3) are an important regulatory T cells (TREGS) subset shown to protect animals from inflammatory and autoimmune diseases (Izcue et al., 2009). Recent studies have shown that CD4+CD25+Foxp3+ TREGS can express IL-10, and IL-10 production from TREGS is required to prevent intestinal inflammation (Rubtsov et al., 2008). To determine the source of IL-10 induced by OMVs, we purified CD4+CD25+ and CD4+CD25- T cells following co-culture with DCs and measured the expression of Foxp3 and IL-10 by qRT-PCR. Remarkably, PSA containing OMVs significantly induced IL-10 expression in the CD4+CD25+ TREG population, but not from CD4+CD25- T cells (Figure 3E). Vesicles purified from B. fragilisΔPSA were unable to enhance IL-10 production from either T cell population compared to media controls. Expression of Foxp3 was increased exclusively in CD4+CD25+ T cells by OMVs in a PSA-dependent manner (Figure 3F). Furthermore, flow cytometry revealed that WT-OMVs stimulated increased IL-10 production from CD4+CD25+ T cells compared to ΔPSA-OMVs, whereas vesicles with or without PSA induced similar baseline levels of IL-10 from CD4+CD25- T cells (Figure 3G).
We sought to determine if PSA could enhance the anti-inflammatory capacity of TREGS by measuring their function in cell culture suppression assays. CD4+CD25- responder cells were labeled with the intracellular dye CFSE (Carboxyfluoresceinsuccinimidyl ester), the fluorescence of which decreases proportionally as cells proliferate (Figure 3H; No TREGS). Addition of CD4+CD25+ TREGS suppresses cell division (Figure 3H; Media). DCs were pulsed with OMVs, washed and cultured with naïve CD4+ T cells; subsequently, CD4+CD25+ TREGS were purified after co-culture and added to CFSE-labeled responder CD4+CD25- T cells; proliferation was measured by flow cytometry. TREGS recovered from conditions with WT-OMV-treated DCs displayed significantly enhanced suppressive capacity compared to CD4+CD25+ T cells treated with ΔPSA-OMVs (Figure 3H and Figure S3D). Incubation of in vitro suppression cultures with a neutralizing anti-IL-10 receptor antibody partially abrogated TREG function induced by WT-OMVs, but had no effect on ΔPSA-OMV stimulated TREGS (Figure S3E). Collectively, we conclude that OMVs specifically induce functional TREGS in cell culture, and that the absence of PSA from OMVs abrogates the ability of B. fragilis vesicles to promote TREG activity by dendritic cells.
Toll-like Receptor 2 on DCs is Required to Sense OMV-associated PSA
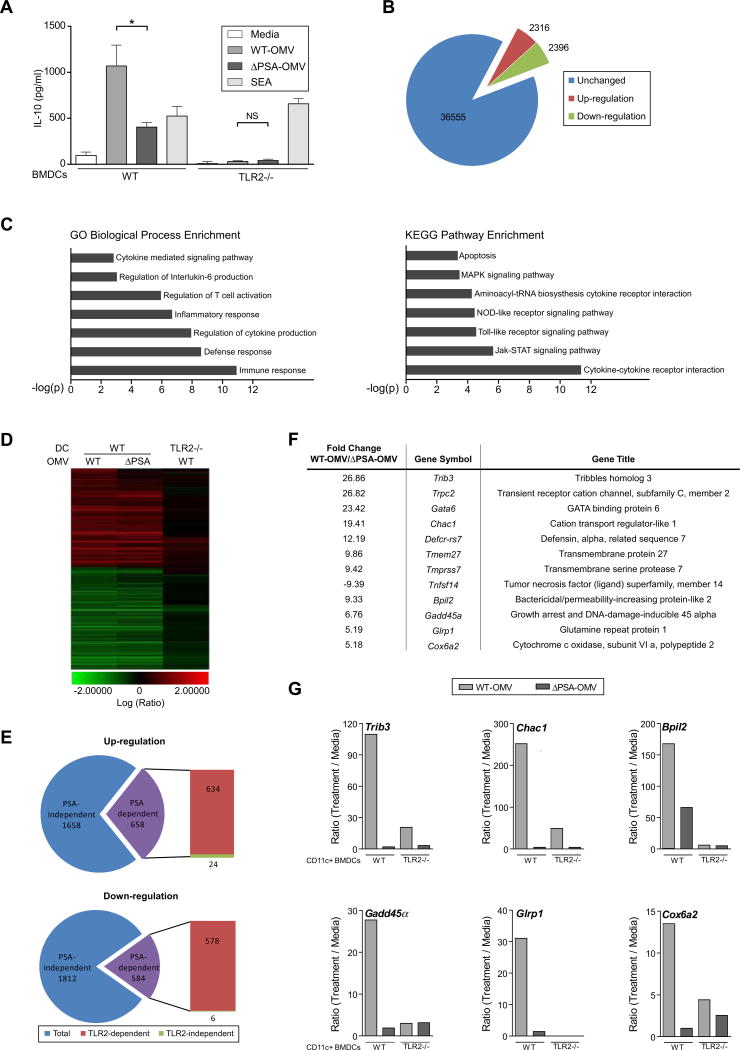
Microbial ligands are detected by pattern recognition receptors (Medzhitov, 2007), and PSA is sensed by toll-like receptor 2 (TLR2) (Wang et al., 2006). Unlike other microbial molecules, PSA promotes an anti-inflammatory response upon TLR2 signaling (Round et al., 2011). Innate immune cells (such as DCs) have been widely recognized for their expression of TLRs and their central role in alerting the innate and adaptive immune systems to microbial infection. We recently reported that TLR2 on CD4+ T cells responds to purified PSA to promote TREG function (Round et al., 2011). However, a role for TLR2 expression by innate immune cells during OMV recognition remains unknown. To test the requirement for DC-specific TLR2 in PSA recognition, we treated WT and TLR2-/- DCs with OMVs and subsequently co-cultured DCs with CD4+ T cells. Compared to wild-type DCs, the absence of TLR2 (DCs from TLR2-/- animals) almost completely inhibited IL-10 production in response to vesicles (Figure 4A). TLR2-/- DCs have no defect in OMV uptake (Figure S4A). Both TLR2-sufficient and deficient DCs responded equally to superantigen (SEA) stimulation, demonstrating a specific defect in PSA sensing, not a general lack of T cell activation by TLR2-/- DCs. In addition, when TLR2-/- T cells were cultured with OMV-pulsed WT DCs, production of IL-10 was unaffected (Figure S4B). When OMVs were added continuously to DC-T cell co-cultures (thus both cell types were exposed to OMVs), TLR2 deletion on DCs led to decreased IL-10 induction (Figure S4C). Furthermore, direct treatment of purified CD4+ T cells with PSA-containing OMVs resulted in no IL-10 up-regulation, unlike stimulation with purified PSA, which activated T cells in a TLR2-dependent manner as previously shown (Figure S4D) (Round et al., 2011). Therefore, while purified CD4+ T cells can respond directly to PSA, immune responses to OMV delivery of PSA require DC, and not T cell, expression of TLR2. Using this information, we sought to identify cellular factors involved in PSA-specific, TLR2-specific signaling by DCs.
Figure 4.
TLR2 on DCs is required to sense OMV-associated PSA and induces genes specific for OMVs. (A) ELISA analysis of DC-T cell co-cultures similar to Figure 3(C) from WT and TLR2-/- DCs. SEA: staphylococcal enterotoxin A. Error bars indicate SEM from quadruplicate samples. Results are representative of 3 independent trials. * p<0.05. NS: not significant. (B) Pie chart showing the number of up-regulated genes (red) and down-regulated genes (green) upon stimulation with PSA-containing OMVs during in vitro cultured with CD11c+ BMDCs, using a Whole Mouse Genome Microarray analysis. Unaffected genes (blue). Only genes with a p value <0.01 and fold change >2 were used for subsequent analysis. (C) Gene ontology analysis of changes in gene expression levels in BMDCs for various biological processes or pathways. (D) Heat-map analysis of gene expression in BMDCs from either wild-type or TLR2-/- animals upon OMV stimulation reveals that approximately 30% of the genes are either up-regulated or down-regulated in a PSA dependent manner and that the majority of the genes are TLR2 dependent. (E) Pie charts show that the majority of the genes in BMDCs that respond to PSA are dependent on TLR2. (F) List of select genes of highest fold change of expression level upon OMV stimulation in a PSA- and TLR2-dependent manner. (G) qRT-PCR analysis of the expression level of genes listed in (F) identified and confirmed 6 genes that are significantly up-regulated by OMVs in a PSA- and TLR2-dependent manner from purified CVD11c+ BMDCs. Results are representative of 3 independent experiments. See also Figure S4.
TLR2 Signaling on DCs Induces OMV-specific Gene Expression
OMVs from Gram-negative bacteria have been shown to contain numerous TLR ligands, including lipopolysaccharide (LPS), lipoproteins, peptidoglycan, and bacterial DNA (Beveridge, 1999). These molecular patterns are recognized by DCs to drive pro-inflammatory reactions. PSA is a microbial molecule that induces immune tolerance. To differentiate between the contributions of PSA and other TLR ligands contained within vesicles, we performed global gene expression profiling, comparing mRNA levels induced by WT-OMVs and ΔPSA-OMVs in a TLR2-specific fashion. Using microarray analysis, 4,712 target probes representing mouse transcripts showed expression changes following treatment of DCs with OMVs (Figure 4B). The vast majority of these genes were classified as immune-related by the GO Biological Process enrichment and the KEGG Pathway enrichment algorithms (Figure 4C). Approximately two-thirds of the genes were either up- or down-regulated similarly by both WT-OMVs and ΔPSA-OMVs, representing PSA-independent transcript changes within DCs (Figure 4D and Figure 4E; blue shaded regions). Of the remaining one-third (roughly 1,200), which corresponds to PSA-dependent expression changes, the vast majority (98%) of genes were regulated in a TLR2-dependent fashion (Figure 4E). We currently do not know of other signaling molecules that sense PSA, and further studies are needed to validate the few transcripts that appear to be regulated independently of TLR2. However, a list of the top 12 genes that displayed greatest PSA-specific, TLR2-specific changes again yielded several molecules known to have immune functions (Figure 4F). Of these, transcriptional analysis by qRT-PCR verified the microarray results for 6 genes, which showed a pattern of being up-regulated by WT-OMVs and dependent on TLR2 expression (Figure 4G). Thus, by virtue of delivering PSA to DCs, OMVs signal through TLR2 to induce a highly specific gene expression profile.
DCs Require Gadd45α for Induction of IL-10
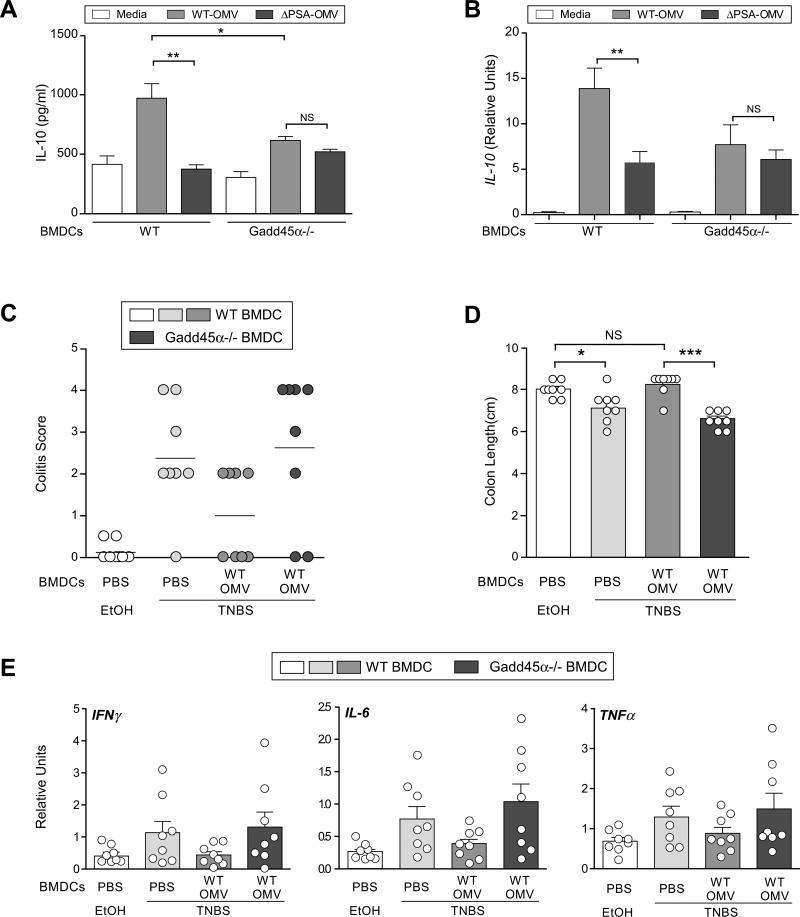
Downstream of TLR2, factors involved in the intracellular signaling pathway(s) that respond to PSA remain completely unknown. Based on its reported role in the immune system, we assessed the function of Gadd45α (Growth Arrest and DNA-Damage-Inducible protein), whose expression by DCs has been shown to promote T cell responses (Jirmanova et al., 2007). Gadd45α was induced by OMVs in a PSA-specific, TLR2- specific manner (see Figure 4G). Gadd45α-/- DCs have no defect in OMV internalization (Figure S5A). Co-culture of Gadd45α-/- DCs with WT CD4+ T cells resulted in reduced IL-10 production compared to WT DCs treated with WT-OMVs (Figure 5A). The level of IL-10 expression upon co-culture of Gadd45α-/- DCs and CD4+ T cells was identical following treatment with either WT-OMVs or ΔPSA-OMVs. IL-10 production from BMDCs alone treated with OMV was not detectable by ELISA (see Figure 3C); thus, using qRT-PCR analysis, we found that IL-10 expression by BMDCs was increased by WT-OMVs, whereas Gadd45α-/- DCs displayed reduced IL-10 expression when treated with WT-OMVs or ΔPSA-OMVs (Figure 5B). Gene expression profiling had identified several other genes that were co-regulated with Gadd45α (see Figure 4F and 4G). Of these, BpiI2 and Chac1 are downstream of Gadd45α signaling as their expression is lost in Gadd45α-/- DCs following treatment with OMVs (Figure S5B). Gadd45α thus appears to be a central signaling component of the TLR2 response to OMVs by DCs.
Figure 5.
Gadd45α on DCs is required for in vitro IL-10 production and protection from colitis by OMVs. (A) ELISA analysis of DC-T co-cultures similar to Figure 3(C), but also including Gadd45α-/- DCs. Error bars indicate SEM from quadruplicate samples. Results are representative of 3 independent trials. *p<0.05. ** p<0.01. NS: not significant. (B) qRT-PCR analysis of IL-10 transcript levels from either WT or Gadd45α-/- BMDCs after 16-18hrs incubation with OMVs in vitro. Error bars indicate SEM from triplicate samples. Results are representative of 3 independent trials. ** p<0.01. NS: not significant. (C) Colitis scores from DC recipient animals induced for TNBS colitis. Each symbol represents an individual animal. Results are shown from 2 combined experiments, each performed independently. (D) Colon length from mice, at day 2 following TNBS treatment. Each symbol represents an individual animal. Error bars indicate SEM from 8 animals. Results are shown from 2 combined experiments each performed independently. * p<0.05. *** p<0.001. NS: not significant. (E) Cytokine analysis by qRT-PCR of RNA recovered from whole colon. Each symbol represents an individual animal. Error bars indicate SEM from 8 animals. Results are shown from 2 combined experiments, each performed independently. See also Figure S5.
DCs Mediate Protection from Colitis Through a Requirement for Gadd45α
Colonization of animals with B. fragilis or treatment with PSA ameliorates experimental colitis (Mazmanian et al., 2008; Round and Mazmanian, 2010). However, the role of DCs during PSA-mediated protection from disease remains unknown. We first tested OMVs in Gadd45α-/- animals induced for TNBS colitis. While OMV restored the colon length and suppressed pro-inflammatory cytokine production in Gadd45aα+/- (wild-type) littermate controls, Gadd45α-/- mice were not protected (Figure S5C and S5D). To demonstrate whether DCs require Gadd45α for in vivo PSA activity, we incubated BMDCs with vesicles and transferred cells into mice that were induced for TNBS colitis. WT-OMV-treated DCs protected animals from disease compared to control (PBS) treated cells (Figure 5C). However, treatment of Gadd45α-/- DCs with OMVs prior to transfer into wild-type mice resulted in disease scores similar to untreated cells (last column of Figure 5C), demonstrating that Gadd45α is required by DCs for PSA-mediated protection. Representative histopathology is shown in Figure S5E. Accordingly, reduction in colon length is rescued by OMV treatment of WT, but not Gadd45α-deficient, DCs (Figure 5D). Finally, we analyzed pro-inflammatory molecules within intestinal tissues of TNBS colitis-induced animals following DC transfer. The cytokines IFNγ, IL-6 and TNFα were reduced in the colon following transfer of WT DCs incubated with OMVs (Figure 5E). Transfer of OMV-treated Gadd45α-/- DCs into animals resulted in cytokine and chemokine levels as high as untreated DCs (last column of Figure 5E), although these trends did not reach statistical significance. Collectively, these findings show that OMVs promote tolerogenic DC function during protection from colitis and further identify Gadd45α is a factor required for PSA activity, revealing a previously unknown signaling pathway by which the human mutualist B. fragilis mediates immunologic health.
Discussion
Molecular Communication Between the Microbiota and the Immune System
Bacteria shape diverse environments on land, in the oceans, and within plants and animals; often, these processes are mediated by secretion of bacterial molecules. Regarding interactions with mammals, decades of research has uncovered molecules produced by pathogens that promote bacterial invasion into tissues and cells, nutrient acquisition and subversion of the immune system. We know of numerous mechanisms by which virulence factors are secreted or delivered to mammalian targets. Only recently has it become appreciated that symbiotic bacteria also establish molecular communication with mammals. The archetypal symbiosis factor of the human microbiota is Polysaccharide A (PSA) from Bacteroides fragilis, which ameliorates inflammatory disease in animals by inducing immunologic tolerance (Lee and Mazmanian, 2010). However, it has remained unknown how PSA is delivered to the immune system. The studies described herein illustrate that outer membrane vesicles (OMVs) released from the bacterial surface are sufficient to mediate inter-kingdom interactions between B. fragilis and the immune system by delivering PSA to DCs. Oral treatment of mice with OMVs prevents experimental colitis, whereas vesicles from B. fragilisΔPSA are unable to protect animals from wasting disease, histopathology and pro-inflammatory cytokine production. As attempts to delete OMVs from bacteria have thus far been unsuccessful, as they may be necessary for viability (Deatherage et al., 2009; McBroom and Kuehn, 2007), the in vivo relevance of B. fragilis OMVs during intestinal colonization requires further investigation. However, our work reveals a paradigm by which a beneficial bacterium of the human microbiota selectively delivers a microbial molecule to its host. Future studies will aim to understand how other components of B. fragilis OMVs interact with the mammalian immune system.
OMVs Mediate Immune Tolerance through Activation of Dendritic Cells
In response to sampling both self and foreign antigens, DCs interact with a variety of immune cells to coordinate diverse biological responses. The presentation of microbial molecules from pathogens to CD4+ T cells is a critical component of a productive immune response during infection. Conversely, antigens of the microbiota must be either ignored or actively tolerated during long-term colonization to prevent deleterious reactions that may adversely affect both microbes and mammals. Indeed, it is believed that a critical feature of IBD is uncontrolled immune reactivity to the microbiota. Our laboratory has previously shown that PSA induces immunologic tolerance through IL-10-producing Foxp3+ regulatory T cells (TREGS), a process that protects animals from colitis and promotes colonization by B. fragilis (Round et al., 2011; Round and Mazmanian, 2010). Here we show that OMVs internalized by DCs induce tolerogenic DCs that produce IL-10, which in turn drive the development of IL-10-producing TREGS. In contrast, OMVs from B. fragilisΔPSA do not promote TREGS. Thus, unlike immunity that results from recognition of microbial molecules from pathogens, PSA programs DCs to adopt an anti-inflammatory profile leading to T cell-mediated tolerance and protection from experimental colitis.
Recently, it was shown that intestinal colonization of animals with B. fragilis prevents and treats experimental autoimmune encephalomyelitis (EAE), an animal model for human multiple sclerosis (MS) (Ochoa-Reparaz et al., 2010a; Ochoa-Reparaz et al., 2010b). Both EAE and MS involve inflammation in the central nervous system (CNS). How does PSA, a symbiosis factor produced in the gut, mediate protection from immunity in the brain and spinal cord of animals? Mice protected from disease display an increase in the proportions of CD11c+CD103+ DCs, a subset that is known to promote TREG differentiation (Ochoa-Reparaz et al., 2010a; Ochoa-Reparaz et al., 2010b). Interestingly, increased CD103+ DCs and Foxp3+ TREG proportions are only observed in the cervical lymph nodes that drain the CNS, but not in other systemic compartments. It is currently unknown if the gut is the site of tolerogenic DC induction followed by cell migration to the CNS, or if PSA somehow activates DCs outside the intestine. Further, it will be interesting to know the subset of DCs that respond to PSA/OMVs. As tolerogenic DCs promote TREG development, and TREGS have been shown to suppress inflammation in many tissues of the body, it is conceivable that PSA may represent a therapy for immunologic diseases beyond IBD and MS.
TLR2 Signaling by DCs is Required for OMV Sensing
Immune cells recognize microbial ligands through pattern recognition receptors to initiate immunologic responses. Previous studies have identified toll-like receptor 2 (TLR2) as being required for PSA-mediated induction of interferon (IFNγ) (Wang et al., 2006), as well as IL-10 production from TREG cells (Round and Mazmanian, 2010). Furthermore, TLR2-deficient animals are not protected from TNBS colitis by PSA treatment (Round and Mazmanian, 2010). Our studies now reveal that TLR2 expression by DCs is necessary for the induction of anti-inflammatory responses by OMVs. This phenotype is largely dependent on PSA, although OMVs contain other molecules (perhaps lipoproteins) that also increase IL-10 expression through TLR2 (see Figure 4A). In contrast, we have previously shown that purified PSA is able to directly induce IL-10 from CD4+ T cells in the absence of DCs. The DC-dependent pathway described here is specific for OMVs, as treatment of T cells alone with OMVs (containing PSA) is unable to promote IL-10 production from CD4+ T cells, unlike purified PSA. Furthermore, TLR2 deletion on DCs abrogates IL-10 production during in vitro culture with OMVs. The biological context by which DCs and T cells contact PSA during colonization by B. fragilis remains unresolved, and further studies involving in vivo metabolic labeling of PSA are required to distinguish when, where and how each cell type ‘sees’ PSA. However, we speculate the possibility that DCs internalize PSA associated with OMVs, which traffic through the endocytic pathway to contact T cells at the immunologic synapse. This notion is supported by evidence that PSA is presented to CD4+ T cells by major histocompatibility complex (MHC II) following internalization and processing in the endosome. Perhaps deletion of TLR2 on T cells abrogates PSA recognition at the immunologic synapse following initial association of DCs with OMVs. Thus, both cell types may require TLR2, and addition of purified PSA directly to T cells bypasses the requirement for DC expression of TLR2 (Round et al., 2011). Other models are conceivable as well, and the details of the mechanism by which the immune system responds to anti-inflammatory microbial ligands are currently being addressed. What is clear from our results is that PSA, in the context of OMVs, directly activates DCs and not T cells, in a process that requires TLR2. The consequence of this interaction leads to the induction of tolerogenic DCs that enhance Treg function and promote protection from inflammatory disease.
Identification of Gadd45α in the PSA Signaling Pathway
Following internalization into DCs and engagement of TLR2, OMVs initiate a gene expression program that results in IL-10 production by DCs. Although it is known that the outcome of PSA sensing by the immune system is TREG induction, nothing is known about the intracellular signaling pathway(s) activated by PSA within DCs. We analyzed the gene expression profile of DCs treated with OMVs to uncover factors that are expressed in a PSA-dependent, TLR2-dependent manner. Of the handful of candidates, we focused on Gadd45α (growth arrest and DNA-damage-inducible protein) due to its known role in immune responses. In addition to a function in stress responses and cell cycle control, Gadd45α has been shown to affect T cell activation by inhibiting the alternative p38 pathway in CD4+ T cells (Salvador et al., 2005). More recently, Gadd45α expression by DCs was implicated in Th1 polarization in response to Toxoplasma gondii antigen stimulation (Jirmanova et al., 2007). Levels of the Th1 skewing cytokine IL-12 are reduced in Gadd45α-/- DCs, resulting in a non-T cell intrinsic reduction in IFNγ+ T cells. We show that Gadd45α is up-regulated upon OMV stimulation in a TLR2-specific fashion, and that absence of Gadd45α in OMV-treated DCs leads to a defect in IL-10 production. Furthermore, this results in a lack of IL-10 expression from CD4+ T cells co-cultured with Gadd45α-/- DCs. Transfer of DCs treated with OMVs into wild-type animals ameliorates TNBS colitis, demonstrating the protective effects of PSA directly on DCs. Transfer of OMV-treated DCs deficient in Gadd45α results in no protection from the histopathologic damage and cytokine production associated with colitis, revealing that PSA signaling in DCs requires Gadd45α. Intriguingly, Gadd45α deficient animals die at late ages from lupus-like autoimmunity (Salvador et al., 2002), a pathology that has been linked to defective Treg function (Buckner, 2010). Collectively, these results suggest a model whereby PSA from OMVs activates TLR2 to induce expression of Gadd45α, which in turn modulates intracellular signaling cascades to promote tolerogenic DC function and Treg development during protection from autoimmune and/or inflammatory disease.
Perspective
Eons of co-evolution have formed an inextricable connection between mammals and our microbiota. With over 100-fold more unique genes in the microbiome than the human genome, symbiotic bacteria posses the vast potential to produce molecules, destined for their host, which mediate various biological processes. We reveal that B. fragilis actively delivers PSA via outer membrane vesicles, uncovering a previously unrecognized mechanism by which the immense molecular coding potential of the microbiome can be utilized during mutualism. Sustained, life-long molecular communication between the microbiota and mammals bridges the boundaries between kingdoms of life, and supports the notion that animals may be viewed as holobionts: a single unit of evolutionary selection comprised of a host and its associated microbes (Rosenberg et al., 2009).
Experimental Procedures
Bacterial Strains and Culture Conditions
Bacteroides fragilis strain NCTC9343 was obtained from the American Type Culture Collection, and B. fragilisΔPSA has been described (Coyne et al., 2001). Bacteria were grown either in brain heart infusion (BHI) broth (BD Biosciences) or a minimum medium containing 8g Glucose, 1% FBS, 0.5μg Hemin, and 0.5μg/ml Vitamin K in 1L of RPMI (Invitrogen).
Mice
C57BL/6 and BALB/c mice were purchased from Taconic Farms. TLR2 knockout and IL-10 knockout mice were purchased from Jackson laboratories. Gadd45α knockout mice were maintained in an NCI pathogen free animal facility. IL-10-eGFP mice (Vert-X mice) have been described previously (Sun et al., 2009). Foxp3-eGFP mice were obtained from Talal A. Chatila (University of California Los Angeles) (Lin et al., 2007). All procedures were performed in accordance with the guidelines and the approved protocol from the Institutional Animal Care and Use Committee at California Institute of Technology.
OMV Purification and Labeling
B. fragilis OMV isolation was adapted from a previously described protocol for the preparation of OMVs from Escherichia coli (Horstman and Kuehn, 2002). Briefly, EDL-enriched B. fragilis (Patrick and Reid, 1983) was grown in customized minimal media. OMVs were recovered from the bacteria-free culture supernatant by centrifugation. FITC-labeled OMVs were prepared as previously described (Kesty and Kuehn, 2004).
Electron Microscopy of Bacterial Ultrathin Sections
Ultrathin sections of EDL-enriched B. fragilis were prepared as previously described (Patrick et al., 1996). For immunogold labeling, purified OMVs were applied to formvar/carbon coated gold grids (EMS). Samples were blocked in 10% FBS for 10 minutes after 5 minute incubation in 0.12% glycine. After blocking, samples were incubated with anti-PSA. Secondary anti-IgG conjugated with 5nm gold was applied to the samples for 20 minutes, and washed. After labeling, samples were fixed in 1% glutaraldehyde for 5 minutes and washed extensively by transferring grids to drops of PBS and H2O. Contrast staining was performed by placing the grids on drops of 3-5% uranyl acetate in 2% methylcellulose for 10 minutes on ice. Samples covered by a thin film of methylcellulose were removed from loop and used for visualization by TEM.
Chemical (TNBS)-induced Experimental Colitis
BALB/c mice were orally treated with PBS, WT-OMV (5μg) or ΔPSA-OMV (5μg) every other day for one week before TNBS administration. Mice were anesthetized with isofluorane and rectal administration of 2% TNBS (in 50% EtOH, Sigma) was applied through a 3.5F catheter (Instech Solomon; SIL-C35) as previously described (Wirtz et al., 2007). For DC transfer-TNBS experiments, wild-type or Gadd45α-/- BMDCs were treated with WT-OMVs or ΔPSA-OMVs for 16-18 hours. 2×106 cells were intra-peritoneally injected into each recipient (C57Bl/6) mouse 2 hours before TNBS (125mg/kg) administration.
Tissue Pathology Analysis
Mouse colons were fixed in neutral buffered 10% formalin (ScyTek Laboratories), embedded in paraffin, sectioned and stained by Pacific Pathology (San Diego, CA). Colitis scores for each colon section were evaluated in a blinded fashion by a veterinary pathologist (G.W.S.) using a standard scoring system (Wirtz et al., 2007): 0: No signs of inflammation; 1: Low level of inflammation; 2: Low level of leukocytic infiltration; 3: High level of leukocytic infiltration, high vascular density thickening of colon wall; 4: loss of goblet cells, high vascular density, thickening of colon wall. Histology images were taken using light microscopy at 20× magnification.
Colon Lamina Propria Lymphocytes (LPLs) isolation and Intracellular Cytokine Staining (ICCS)
Performed as previously described (Round et al., 2011; Round and Mazmanian, 2010). Full-length colon was dissected and luminal contents were carefully flushed. Colon epithelium was dissociated, digested with collagenase D (0.5mg/ml), dispase (3 units/ml) and DNase I (0.5mg/ml). Finally, a 40%-80% Percoll gradient was used to isolate LPLs. ICCS was performed on LPLs that were re-stimulated in vitro with PMA/Ionomycin in the presence of golgi-plug for 4.5 hours, according to manufacturer's protocol (BD Biosciences).
In vitro BMDC-T Cell Co-culture
Performed as previously described (Round et al., 2011; Round and Mazmanian, 2010). Briefly, DCs were differentiated from bone marrow. OMV-pulsed BMDCs (10μg/ml OMVs, 1×105 cells/ml, 12-24 hours) were incubated with splenic CD4+ T cells. BMDCs, for the OMV uptake assay or activation assay, were collected and blocked in 5% mouse serum for 30 minutes on ice. Cells were stained with specific antibodies for 30 minutes on ice and washed twice with FACS buffer at 4°C. Cells from in vitro BMDC-T cell co-culture were blocked, and stained similarly except that the cells were re-stimulated using PMA/Ionomycin for 4 hours before collecting. All FC was performed with BD FACSCalibur and results were analyzed using the FlowJo software (BD Biosciences). In some experiments, BMDCs were pre-treated with 5μM Cytochalasin D for 1hr before adding FITC-labeled OMVs.
In vitro Suppression Assay
CD4+CD25+Treg cells purified from BMDC-T cell co-cultures using magnetic microbeads (Miltenyi Biotec). CD4-depleted mouse splenocytes treated with Mitomycin C (Sigma) were used as APCs (1×105 cells/ml). CD4+CD25-responder T cells directly purified from mouse spleen were pulsed with CFSE for 10minutes at 37°C, followed by first wash with PBS and a second wash with culture media, and used immediately (5×105 cells/ml), referred to as effector T cells (Teff). This assay was conducted in round bottom 96 well plates with an addition of 5μg/ml of anti-CD3 (eBiosciences) in 200μl. Teff:Treg ratio was titrated and cells were collected after 2-3 days of culture for FACS analysis. Anti-IL-10R (20μg/ml, R&D Systems) was added as indicated in the results.
Microarray Hybridization and Data Analysis
RNA samples (1ug total RNA) from BMDCs were labeled with fluorescent dyes using the Quick Amp Labeling Kit (Agilent). Microarray (AgilentWhole Mouse Genome chip) hybridizations (65°C for 16 hours) and washes were performed with Agilent reagents following standard protocols. Microarrays were analyzed using an Agilent DNA Microarray Scanner G2565CA, and data were acquired using Agilent's Feature Extraction Software version 10.1.1.1. Significant genes were selected based on p< 0.01 and fold change >2.0. For enrichment analysis of biological process ontology, probe lists were analyzed in DAVID (The Database for Annotation, Visualization and Integrated Discovery annotation tools (http://david.abcc.ncifcrf.gov/)) and selected based on p< 0.01. Microarray data have been deposited in the GEO database with the accession number GSE39563
Statistical Analyses
Student's t test and one-way ANOVA were applied for pair-wise comparisons and comparisons among >2 groups, respectively. Significant differences among groups detected by ANOVA were analyzed using Newman-Keuls test as the post-hoc test to identify groups exhibiting statistically significant differences.
Supplementary Material
Highlights.
Bacteroides fragilis packages PSA into outer membrane vesicles (OMVs)
OMV-mediated delivery of PSA to dendritic cells (DCs) promotes regulatory T cells
Toll-like receptor 2 (TLR2) on DCs is required to sense OMV-associated PSA
Gadd45α expression in DCs is required for PSA containing OMV activity
Acknowledgments
We are indebted to M. Kuehn (Duke University) for helpful discussions regarding OMVs. We thank P. Webster (House Ear Institute) for help with electron microscopy. Proteomic mass spectrometry of OMVs was performed and analyzed at Proteome Exploration Laboratory (Caltech). Mouse whole genome microarray was performed at Millard and Muriel Jacobs Genetics and Genomics Laboratory (Caltech). We are grateful to members of the Mazmanian laboratory for their critical review of the manuscript. Y.S. acknowledges support from a National Institutes of Health (NIH) pre-doctoral training grant (GM007616). This work was funded by grants from the NIH (DK078938) and the Crohn's and Colitis Foundation of America to S.K.M.
Footnotes
The authors have no competing financial interests related to this publication.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Beveridge TJ. Structures of gram-negative cell walls and their derived membrane vesicles. J Bacteriol. 1999;181:4725–4733. doi: 10.1128/jb.181.16.4725-4733.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner JH. Mechanisms of impaired regulation by CD4(+)CD25(+)FOXP3(+) regulatory T cells in human autoimmune diseases. Nature reviews Immunology. 2010;10:849–859. doi: 10.1038/nri2889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerdeno-Tarraga AM, Patrick S, Crossman LC, Blakely G, Abratt V, Lennard N, Poxton I, Duerden B, Harris B, Quail MA, et al. Extensive DNA inversions in the B. fragilis genome control variable gene expression. Science. 2005;307:1463–1465. doi: 10.1126/science.1107008. [DOI] [PubMed] [Google Scholar]
- Chung H, Pamp SJ, Hill JA, Surana NK, Edelman SM, Troy EB, Reading NC, Villablanca EJ, Wang S, Mora JR, et al. Gut immune maturation depends on colonization with a host-specific microbiota. Cell. 2012;149:1578–1593. doi: 10.1016/j.cell.2012.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyne MJ, Tzianabos AO, Mallory BC, Carey VJ, Kasper DL, Comstock LE. Polysaccharide biosynthesis locus required for virulence of Bacteroides fragilis. Infect Immun. 2001;69:4342–4350. doi: 10.1128/IAI.69.7.4342-4350.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deatherage BL, Lara JC, Bergsbaken T, Rassoulian Barrett SL, Lara S, Cookson BT. Biogenesis of bacterial membrane vesicles. Mol Microbiol. 2009;72:1395–1407. doi: 10.1111/j.1365-2958.2009.06731.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galan JE. Common themes in the design and function of bacterial effectors. Cell Host Microbe. 2009;5:571–579. doi: 10.1016/j.chom.2009.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horstman AL, Kuehn MJ. Bacterial surface association of heat-labile enterotoxin through lipopolysaccharide after secretion via the general secretory pathway. J Biol Chem. 2002;277:32538–32545. doi: 10.1074/jbc.M203740200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishiguro K, Ando T, Maeda O, Hasegawa M, Kadomatsu K, Ohmiya N, Niwa Y, Xavier R, Goto H. Paeonol attenuates TNBS-induced colitis by inhibiting NF-kappaB and STAT1 transactivation. Toxicol Appl Pharmacol. 2006;217:35–42. doi: 10.1016/j.taap.2006.07.002. [DOI] [PubMed] [Google Scholar]
- Izcue A, Coombes JL, Powrie F. Regulatory lymphocytes and intestinal inflammation. Annu Rev Immunol. 2009;27:313–338. doi: 10.1146/annurev.immunol.021908.132657. [DOI] [PubMed] [Google Scholar]
- Jirmanova L, Jankovic D, Fornace AJ, Jr, Ashwell JD. Gadd45alpha regulates p38-dependent dendritic cell cytokine production and Th1 differentiation. J Immunol. 2007;178:4153–4158. doi: 10.4049/jimmunol.178.7.4153. [DOI] [PubMed] [Google Scholar]
- Kesty NC, Kuehn MJ. Incorporation of heterologous outer membrane and periplasmic proteins into Escherichia coli outer membrane vesicles. J Biol Chem. 2004;279:2069–2076. doi: 10.1074/jbc.M307628200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krinos CM, Coyne MJ, Weinacht KG, Tzianabos AO, Kasper DL, Comstock LE. Extensive surface diversity of a commensal microorganism by multiple DNA inversions. Nature. 2001;414:555–558. doi: 10.1038/35107092. [DOI] [PubMed] [Google Scholar]
- Lee YK, Mazmanian SK. Has the microbiota played a critical role in the evolution of the adaptive immune system? Science. 2010;330:1768–1773. doi: 10.1126/science.1195568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ley RE, Hamady M, Lozupone C, Turnbaugh PJ, Ramey RR, Bircher JS, Schlegel ML, Tucker TA, Schrenzel MD, Knight R, et al. Evolution of mammals and their gut microbes. Science. 2008;320:1647–1651. doi: 10.1126/science.1155725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin W, Haribhai D, Relland LM, Truong N, Carlson MR, Williams CB, Chatila TA. Regulatory T cell development in the absence of functional Foxp3. Nat Immunol. 2007;8:359–368. doi: 10.1038/ni1445. [DOI] [PubMed] [Google Scholar]
- Lindsay J, Van Montfrans C, Brennan F, Van Deventer S, Drillenburg P, Hodgson H, Te Velde A, Sol Rodriguez Pena M. IL-10 gene therapy prevents TNBS-induced colitis. Gene Ther. 2002;9:1715–1721. doi: 10.1038/sj.gt.3301841. [DOI] [PubMed] [Google Scholar]
- Liu CH, Lee SM, Vanlare JM, Kasper DL, Mazmanian SK. Regulation of surface architecture by symbiotic bacteria mediates host colonization. Proc Natl Acad Sci U S A. 2008;105:3951–3956. doi: 10.1073/pnas.0709266105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mashburn-Warren LM, Whiteley M. Special delivery: vesicle trafficking in prokaryotes. Mol Microbiol. 2006;61:839–846. doi: 10.1111/j.1365-2958.2006.05272.x. [DOI] [PubMed] [Google Scholar]
- Mazmanian SK, Liu CH, Tzianabos AO, Kasper DL. An immunomodulatory molecule of symbiotic bacteria directs maturation of the host immune system. Cell. 2005;122:107–118. doi: 10.1016/j.cell.2005.05.007. [DOI] [PubMed] [Google Scholar]
- Mazmanian SK, Round JL, Kasper DL. A microbial symbiosis factor prevents intestinal inflammatory disease. Nature. 2008;453:620–625. doi: 10.1038/nature07008. [DOI] [PubMed] [Google Scholar]
- McBroom AJ, Kuehn MJ. Release of outer membrane vesicles by Gram-negative bacteria is a novel envelope stress response. Mol Microbiol. 2007;63:545–558. doi: 10.1111/j.1365-2958.2006.05522.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medzhitov R. Recognition of microorganisms and activation of the immune response. Nature. 2007;449:819–826. doi: 10.1038/nature06246. [DOI] [PubMed] [Google Scholar]
- O'Garra A, Vieira PL, Vieira P, Goldfeld AE. IL-10-producing and naturally occurring CD4+ Tregs: limiting collateral damage. J Clin Invest. 2004;114:1372–1378. doi: 10.1172/JCI23215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochoa-Reparaz J, Mielcarz DW, Ditrio LE, Burroughs AR, Begum-Haque S, Dasgupta S, Kasper DL, Kasper LH. Central nervous system demyelinating disease protection by the human commensal Bacteroides fragilis depends on polysaccharide A expression. J Immunol. 2010a;185:4101–4108. doi: 10.4049/jimmunol.1001443. [DOI] [PubMed] [Google Scholar]
- Ochoa-Reparaz J, Mielcarz DW, Wang Y, Begum-Haque S, Dasgupta S, Kasper DL, Kasper LH. A polysaccharide from the human commensal Bacteroides fragilis protects against CNS demyelinating disease. Mucosal Immunol. 2010b;3:487–495. doi: 10.1038/mi.2010.29. [DOI] [PubMed] [Google Scholar]
- Patrick S, McKenna JP, O'Hagan S, Dermott E. A comparison of the haemagglutinating and enzymic activities of Bacteroides fragilis whole cells and outer membrane vesicles. Microb Pathog. 1996;20:191–202. doi: 10.1006/mpat.1996.0018. [DOI] [PubMed] [Google Scholar]
- Patrick S, Reid JH. Separation of capsulate and non-capsulate Bacteroides fragilis on a discontinuous density gradient. J Med Microbiol. 1983;16:239–241. doi: 10.1099/00222615-16-2-239. [DOI] [PubMed] [Google Scholar]
- Rescigno M, Urbano M, Valzasina B, Francolini M, Rotta G, Bonasio R, Granucci F, Kraehenbuhl JP, Ricciardi-Castagnoli P. Dendritic cells express tight junction proteins and penetrate gut epithelial monolayers to sample bacteria. Nat Immunol. 2001;2:361–367. doi: 10.1038/86373. [DOI] [PubMed] [Google Scholar]
- Rosenberg E, Sharon G, Zilber-Rosenberg I. The hologenome theory of evolution contains Lamarckian aspects within a Darwinian framework. Environ Microbiol. 2009;11:2959–2962. doi: 10.1111/j.1462-2920.2009.01995.x. [DOI] [PubMed] [Google Scholar]
- Round JL, Lee SM, Li J, Tran G, Jabri B, Chatila TA, Mazmanian SK. The Toll-like receptor 2 pathway establishes colonization by a commensal of the human microbiota. Science. 2011;332:974–977. doi: 10.1126/science.1206095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Round JL, Mazmanian SK. The gut microbiota shapes intestinal immune responses during health and disease. Nat Rev Immunol. 2009 doi: 10.1038/nri2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Round JL, Mazmanian SK. Inducible Foxp3+ regulatory T-cell development by a commensal bacterium of the intestinal microbiota. Proc Natl Acad Sci U S A. 2010;107:12204–12209. doi: 10.1073/pnas.0909122107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubtsov YP, Rasmussen JP, Chi EY, Fontenot J, Castelli L, Ye X, Treuting P, Siewe L, Roers A, Henderson WR, Jr, et al. Regulatory T cell-derived interleukin-10 limits inflammation at environmental interfaces. Immunity. 2008;28:546–558. doi: 10.1016/j.immuni.2008.02.017. [DOI] [PubMed] [Google Scholar]
- Salvador JM, Hollander MC, Nguyen AT, Kopp JB, Barisoni L, Moore JK, Ashwell JD, Fornace AJ., Jr Mice lacking the p53-effector gene Gadd45a develop a lupus-like syndrome. Immunity. 2002;16:499–508. doi: 10.1016/s1074-7613(02)00302-3. [DOI] [PubMed] [Google Scholar]
- Salvador JM, Mittelstadt PR, Belova GI, Fornace AJ, Jr, Ashwell JD. The autoimmune suppressor Gadd45alpha inhibits the T cell alternative p38 activation pathway. Nat Immunol. 2005;6:396–402. doi: 10.1038/ni1176. [DOI] [PubMed] [Google Scholar]
- Sun J, Madan R, Karp CL, Braciale TJ. Effector T cells control lung inflammation during acute influenza virus infection by producing IL-10. Nat Med. 2009;15:277–284. doi: 10.1038/nm.1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, McLoughlin RM, Cobb BA, Charrel-Dennis M, Zaleski KJ, Golenbock D, Tzianabos AO, Kasper DL. A bacterial carbohydrate links innate and adaptive responses through Toll-like receptor 2. J Exp Med. 2006;203:2853–2863. doi: 10.1084/jem.20062008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirtz S, Neufert C, Weigmann B, Neurath MF. Chemically induced mouse models of intestinal inflammation. Nat Protoc. 2007;2:541–546. doi: 10.1038/nprot.2007.41. [DOI] [PubMed] [Google Scholar]
- Xavier RJ, Podolsky DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007;448:427–434. doi: 10.1038/nature06005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.