Abstract
SAD-A kinase is a member of the AMPK-related family of kinases, which are under the control of LKB1 kinase. In the human kinome, SAD-A is most closely related to AMPK, a key energy sensor and master regulator of metabolism. In contrast to AMPK, little is known about the physiological function of the SAD-A kinase in metabolism. Recent studies using knockout mice have revealed a striking role of the SAD-A kinase in regulating dynamic functions of islet β cells, from glucose-stimulated insulin secretion (GSIS), islet β-cell size and mass, to GLP-1 response as the first tissue-specific effector of mTORC1 signaling. These studies suggest that SAD-A and AMPK kinase may function as the positive and negative regulators of mTORC1 signaling in islet β cells. Importantly, these findings have implicated SAD-A kinase as a novel drug target for the treatment of type 2 diabetes.
Keywords: AMPK-related kinase, GLP‑1, LKB1, incretin, insulin secretion, type 2 diabetes
Introduction
The AMPK-related family of kinases, which consists of 13 members, plays an important role in regulating glucose and energy homeostasis.1-8 AMPK is a key regulator of energy homeostasis, being activated in response to an increase in AMP/ATP ratio under low nutrient conditions.1 These functions are partly mediated by its regulatory role in nutrient sensing in hypothalamic neurons.9 AMPK is also required for GSIS and other pancreatic β-cell functions, as suggested by the phenotype of mice with targeted deletion of α1 and α2 subuits of the kinase.10,11 Surprisingly, this phenotype directly contradicts with that of the mice with targeted deletion of LKB1 which activates AMPK and 12 other members in this family.12,13 Accordingly, LKB1 deficiency in adult islet β cells leads to increased pancreatic β-cell mass and insulin secretion,14-16 raising a key question of whether other members in the AMPK-related family of kinases may also regulate islet function. Furthermore, AMPK is a potent inhibitor of the mammalian target of rapamycin (mTOR) signaling, which coordinates nutritional status with protein synthesis in pancreatic β cells.1,17 mTOR is an evolutionarily conserved serine/threonine kinase that functions in 2 complexes: mTORC1 and mTORC2. The mTORC1 complex functions as a sensor of nutritional status and responds by altering metabolic processes, whereas mTORC2 complex is involved in the regulation of cytoskeletal organization.1,32 Thus, targeted deletion of LKB1 in mice also leads to mTORC1 activation.14,16 Recent studies using knockout mice has revealed a surprising role of SAD-A in regulating islet β-cell functions as the first tissue-specific effector kinase of mTORC1 signaling in islet β cells.
SAD-A and Insulin Secretion
SAD-A, also referred to as BRSK2, is a member AMPK-related family of kinases that is most closely related AMPK12 in the human kinome. In contrast to other members of this family, we have recently shown that SAD-A is exclusively expressed in the pancreas and brain,18 implicating a role of the kinase in energy metabolism. Major progress has been made in recent years in elucidating the function of SAD-A kinases in the brain, including neuronal polarity and axon specification.19-21 By contrast, little is known about the physiological function of SAD-A in pancreas, although SAD kinases are activated by stimuli that evoke GSIS, including activation by PKA- and CamKK1-mediated signaling pathways.22,23 Using adenoviral overexpression, our recent studies provide the first evidence for the SAD-A kinase as a key regulator of GSIS.24 We show that SAD-A promotes GSIS in part by activating the p21-activated kinase-1 (PAK1) through phosphorylation of PAK1 at Thr423. PAK1 is an effector of Rho GTPases, and is activated in response to the onset of GSIS through phosphorylation at Thr423. Additionally, SAD-A stimulates cytoskeletal remodeling of F-actin, which is required for the trafficking and fusion of insulin secretory granules to the plasma membrane.
SAD-A Controls GLP-1 Response in Islet β Cells
GLP-1 is an incretin hormone that improves glucose responsiveness or sensing of islet β cells. GLP-1 is secreted in response to oral ingestion of nutrients and strongly enhances GSIS through activation of the adenylate cyclase coupled with incretin receptors, leading to increased production of cAMP.25,26 The increase in [cAMP]i exerts its powerful potentiating effect on GSIS through both PKA-independent and PKA-dependent mechanisms, the former involving activation of Epac2 (cAMP-GEFII) pathways.27,28 Long-acting GLP-1 analogs, such as Byetta and Liraglutide, are used in the clinic worldwide for the treatment of type 2 diabetes by improving multiple β-cell functions.25 Increased GLP-1 action is also attributed to the complete remission of type 2 diabetes in obese patients who undergo a gastric bypass surgery.29-31 However, the distal signaling pathways that mediate GLP-1 effects in islet β cells remain poorly defined. We have recently identified SAD-A as a pancreas-specific regulator of GLP-1 effect in islet β cells.18 We show that SAD-A is activated in islet β cells in response to treatment with GLP-1 and forskolin, and that overexpression of SAD-A greatly enhances GLP-1 effect on GSIS from isolated mouse islets. Consequently, targeted deletion of SAD-A from the pancreas causes glucose intolerance from impaired incretin response in SAD-A knockout mice.18 Furthermore, we identified Thr443 of SAD-A as a novel auto-inhibitory phosphorylation site, which negatively regulates GLP-1 effect. Thus, ablation of Thr443 significantly enhances SAD-A activity and incretin effect on GSIS .
SAD-A as a Tissue-Specific Mediator of mTORC1 Signaling
Cumulative evidence suggests a critical role of mTORC1 in regulating pancreatic β cells’ mass and function, which is highlighted by the phenotypes of multiple mouse models of constitutive activation or inhibition of mTORC1 signaling. Accordingly, targeted deletion of TSC1 and TSC2, repressors of mTORC1, leads to increased islet β-cell mass and enhanced GSIS.33-35 Conversely, targeted deletion of S6K1, an effector of mTORC1 signaling, or ablation of S6K1 phosphorylation site in ribosomal protein S6, leads to hypoinsulinemia, defective GSIS, and reduction in islet β-cell size.36,37 Furthermore, inhibition of mTORC1 with rapamycin also causes reduction in islet mass and insulin content, leading to exacerbation of type 2 diabetes.38,39 However, the molecular mechanisms linking mTOR effect to dynamic islet β-cell functions remains elusive.
Our most recent study identifies a key role SAD-A kinase in regulating islet β-cell function and size as the first tissue-specific mediator of mTORC1 signaling in islet β cells.40 We demonstrate that global SAD-A deletion causes multiple defects in islet β-cell function, which are highly reminiscent of defects observed in mice with global deletion of S6K1, including growth retardation, hypoinsulinemia, insulin deficiency, petite islets, and diminished β-cell mass.36 These findings are further supported by the phenotype of mice with selective deletion of SAD-A in pancreas.40 These mice exhibit significantly decreased islet β-cell size, islet mass, and a defective incretin response, leading to glucose intolerance. Conversely, SAD-A overexpression significantly increases the size of MIN6 β cells. In direct support of SAD-A as a novel mediator of mTORC1 signaling in islet β cells, glucose dramatically stimulates SAD-A protein translation in isolated mouse islets, which are potently inhibited by rapamycin. The results suggest that mTORC1 regulates SAD-A protein expression primarily at translational level. Indeed, we further demonstrate that the 5′-untranslated region (5′-UTR) of SAD-A mRNA is highly structured and requires mTORC1 signaling for its translation initiation. Accordingly, we show that the onset of GSIS greatly stimulates the SAD-A 5′-UTR luciferase reporter activity in pancreatic β cells, which is also inhibited by rapamycin. Likewise, activation of mTORC1 through overexpression of constitutively active Rheb leads to a great enhancement in the SAD-A 5′-UTR luciferase reporter activity, which is again abolished by rapamycin treatment. Our findings are consistent with previous reports that mTORC1 complex plays an important role in regulating the translation of mRNAs with highly structured 5′-UTR, including genes encoding MYC, HIF1, ODC1, cyclin D1, and VEGF.41 mTORC1 does so through S6K1-mediated activation of eIF4A helicase activity, which is essential in unwinding a structured 5′-UTR for initiation of translation.41
Concluding Remarks
Both SAD-A and AMPK are under the control of LKB1 kinase, yet it is not clear how the LKB1 kinase balances its action on these 2 kinases in islet β cells. In contrast to SAD-A, which mediates mTORC1 effect, AMPK is an inhibitor of mTORC1 signaling. Consistent of LKB1 as an activator of AMPK, LKB1 deletion leads to activation of mTORC1 signaling in islet β cells.14-16 Although other members of AMPK-related kinases may play a role in the process, it can be envisaged that AMPK and SAD-A function as the “yin and yang” of mTORC1 signaling in islet β cells (Fig. 1). However, conflicting results have been reported on the role of AMPK in regulating GSIS and islet function.10,11,15-17,42,43 Key questions remain as to why targeted deletion of AMPK inhibits GSIS from islet β cells in the knockout mice. Additionally, a short isoform of SAD-B kinase was recently shown to localize to centrosomes, where it controls centrosome duplication, suggesting a potential role of SAD-A in regulating cell cycle of islet β cells.44 Therefore, future studies will reveal whether the SAD-A kinase also plays a role in regulating β-cell proliferation and survival, since GLP-1 stimulates islet β-cell regeneration in rodents and humans.25,45 In support of this speculation, our recent studies show that SAD-A depletion causes β-cell deficiency in global SAD-A KO mice.45 In addition to pancreas, SAD-A also expressed in brain, where it controls neuronal polarity and axon specification. It can be speculated that SAD-A may also play a role in controlling satiety, since SAD-A is exclusively expressed in the primary targeting tissue of GLP-1, which causes weight loss in part by suppressing appetite.25,26,45-48
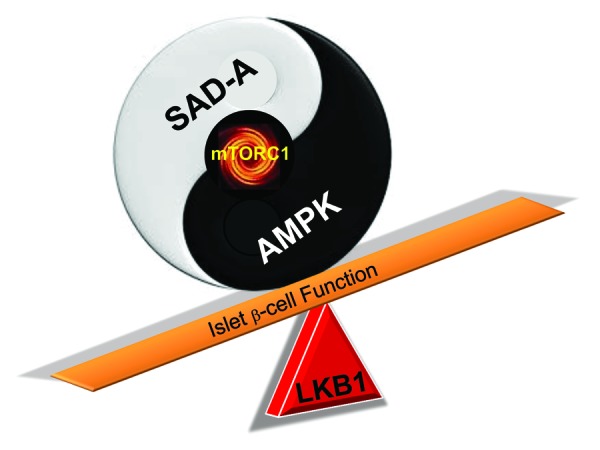
Figure 1. A hypothetic model depicting how LKB1 may balance its act on SAD-A and AMPK to regulate mTORC1 signaling and β-cell functions. Accordingly, AMPK and SAD-A function as the “yin and yang” regulators of mTORC1 activity in islet β cells, which is further fine-tuned by LKB1 kinase, a bona fide activator of both kinases.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/cc/article/26496
References
- 1.Inoki K, Kim J, Guan KL. AMPK and mTOR in cellular energy homeostasis and drug targets. Annu Rev Pharmacol Toxicol. 2012;52:381–400. doi: 10.1146/annurev-pharmtox-010611-134537. [DOI] [PubMed] [Google Scholar]
- 2.Long YC, Zierath JR. AMP-activated protein kinase signaling in metabolic regulation. J Clin Invest. 2006;116:1776–83. doi: 10.1172/JCI29044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rutter GA, Leclerc I. The AMP-regulated kinase family: enigmatic targets for diabetes therapy. Mol Cell Endocrinol. 2009;297:41–9. doi: 10.1016/j.mce.2008.05.020. [DOI] [PubMed] [Google Scholar]
- 4.Hurov JB, Huang M, White LS, Lennerz J, Choi CS, Cho YR, Kim HJ, Prior JL, Piwnica-Worms D, Cantley LC, et al. Loss of the Par-1b/MARK2 polarity kinase leads to increased metabolic rate, decreased adiposity, and insulin hypersensitivity in vivo. Proc Natl Acad Sci U S A. 2007;104:5680–5. doi: 10.1073/pnas.0701179104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hurov J, Piwnica-Worms H. The Par-1/MARK family of protein kinases: from polarity to metabolism. Cell Cycle. 2007;6:1966–9. doi: 10.4161/cc.6.16.4576. [DOI] [PubMed] [Google Scholar]
- 6.Lennerz JK, Hurov JB, White LS, Lewandowski KT, Prior JL, Planer GJ, Gereau RW, 4th, Piwnica-Worms D, Schmidt RE, Piwnica-Worms H. Loss of Par-1a/MARK3/C-TAK1 kinase leads to reduced adiposity, resistance to hepatic steatosis, and defective gluconeogenesis. Mol Cell Biol. 2010;30:5043–56. doi: 10.1128/MCB.01472-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bricambert J, Miranda J, Benhamed F, Girard J, Postic C, Dentin R. Salt-inducible kinase 2 links transcriptional coactivator p300 phosphorylation to the prevention of ChREBP-dependent hepatic steatosis in mice. J Clin Invest. 2010;120:4316–31. doi: 10.1172/JCI41624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sun C, Tian L, Nie J, Zhang H, Han X, Shi Y. Inactivation of MARK4, an AMP-activated protein kinase (AMPK)-related kinase, leads to insulin hypersensitivity and resistance to diet-induced obesity. J Biol Chem. 2012;287:38305–15. doi: 10.1074/jbc.M112.388934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kahn BB, Alquier T, Carling D, Hardie DG. AMP-activated protein kinase: ancient energy gauge provides clues to modern understanding of metabolism. Cell Metab. 2005;1:15–25. doi: 10.1016/j.cmet.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 10.Beall C, Piipari K, Al-Qassab H, Smith MA, Parker N, Carling D, Viollet B, Withers DJ, Ashford ML. Loss of AMP-activated protein kinase alpha2 subunit in mouse beta cells impairs glucose-stimulated insulin secretion and inhibits their sensitivity to hypoglycaemia. Biochem J. 2010;429:323–33. doi: 10.1042/BJ20100231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sun G, Tarasov AI, McGinty J, McDonald A, da Silva Xavier G, Gorman T, Marley A, French PM, Parker H, Gribble F, et al. Ablation of AMP-activated protein kinase alpha1 and alpha2 from mouse pancreatic beta cells and RIP2.Cre neurons suppresses insulin release in vivo. Diabetologia. 2010;53:924–36. doi: 10.1007/s00125-010-1692-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lizcano JM, Göransson O, Toth R, Deak M, Morrice NA, Boudeau J, Hawley SA, Udd L, Mäkelä TP, Hardie DG, et al. LKB1 is a master kinase that activates 13 kinases of the AMPK subfamily, including MARK/PAR-1. EMBO J. 2004;23:833–43. doi: 10.1038/sj.emboj.7600110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sakamoto K, McCarthy A, Smith D, Green KA, Grahame Hardie D, Ashworth A, Alessi DR. Deficiency of LKB1 in skeletal muscle prevents AMPK activation and glucose uptake during contraction. EMBO J. 2005;24:1810–20. doi: 10.1038/sj.emboj.7600667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fu A, Ng AC, Depatie C, Wijesekara N, He Y, Wang GS, Bardeesy N, Scott FW, Touyz RM, Wheeler MB, et al. Loss of Lkb1 in adult beta cells increases beta cell mass and enhances glucose tolerance in mice. Cell Metab. 2009;10:285–95. doi: 10.1016/j.cmet.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 15.Granot Z, Swisa A, Magenheim J, Stolovich-Rain M, Fujimoto W, Manduchi E, Miki T, Lennerz JK, Stoeckert CJ, Jr., Meyuhas O, et al. LKB1 regulates pancreatic beta cell size, polarity, and function. Cell Metab. 2009;10:296–308. doi: 10.1016/j.cmet.2009.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sun G, Tarasov AI, McGinty JA, French PM, McDonald A, Leclerc I, Rutter GA. LKB1 deletion with the RIP2.Cre transgene modifies pancreatic beta-cell morphology and enhances insulin secretion in vivo. Am J Physiol Endocrinol Metab. 2010;298:E1261–73. doi: 10.1152/ajpendo.00100.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gleason CE, Lu D, Witters LA, Newgard CB, Birnbaum MJ. The role of AMPK and mTOR in nutrient sensing in pancreatic beta cells. J Biol Chem. 2007;282:10341–51. doi: 10.1074/jbc.M610631200. [DOI] [PubMed] [Google Scholar]
- 18.Nie J, Lilley BN, Pan YA, Faruque O, Liu X, Zhang W, Sanes JR, Han X, Shi Y. SAD-A potentiates glucose-stimulated insulin secretion as a mediator of glucagon-like peptide 1 response in pancreatic β cells. Mol Cell Biol. 2013;33:2527–34. doi: 10.1128/MCB.00285-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kishi M, Pan YA, Crump JG, Sanes JR. Mammalian SAD kinases are required for neuronal polarization. Science. 2005;307:929–32. doi: 10.1126/science.1107403. [DOI] [PubMed] [Google Scholar]
- 20.Barnes AP, Lilley BN, Pan YA, Plummer LJ, Powell AW, Raines AN, Sanes JR, Polleux F. LKB1 and SAD kinases define a pathway required for the polarization of cortical neurons. Cell. 2007;129:549–63. doi: 10.1016/j.cell.2007.03.025. [DOI] [PubMed] [Google Scholar]
- 21.Lilley BN, Pan YA, Sanes JR. SAD kinases sculpt axonal arbors of sensory neurons through long- and short-term responses to neurotrophin signals. Neuron. 2013;79:39–53. doi: 10.1016/j.neuron.2013.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fujimoto T, Yurimoto S, Hatano N, Nozaki N, Sueyoshi N, Kameshita I, Mizutani A, Mikoshiba K, Kobayashi R, Tokumitsu H. Activation of SAD kinase by Ca2+/calmodulin-dependent protein kinase kinase. Biochemistry. 2008;47:4151–9. doi: 10.1021/bi702528r. [DOI] [PubMed] [Google Scholar]
- 23.Guo Z, Tang W, Yuan J, Chen X, Wan B, Gu X, Luo K, Wang Y, Yu L. BRSK2 is activated by cyclic AMP-dependent protein kinase A through phosphorylation at Thr260. Biochem Biophys Res Commun. 2006;347:867–71. doi: 10.1016/j.bbrc.2006.06.178. [DOI] [PubMed] [Google Scholar]
- 24.Nie J, Sun C, Faruque O, Ye G, Li J, Liang Q, Chang Z, Yang W, Han X, Shi Y. Synapses of amphids defective (SAD-A) kinase promotes glucose-stimulated insulin secretion through activation of p21-activated kinase (PAK1) in pancreatic β cells. J Biol Chem. 2012;287:26435–44. doi: 10.1074/jbc.M112.378372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Drucker DJ. The biology of incretin hormones. Cell Metab. 2006;3:153–65. doi: 10.1016/j.cmet.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 26.MacDonald PE, El-Kholy W, Riedel MJ, Salapatek AM, Light PE, Wheeler MB. The multiple actions of GLP-1 on the process of glucose-stimulated insulin secretion. Diabetes. 2002;51(Suppl 3):S434–42. doi: 10.2337/diabetes.51.2007.S434. [DOI] [PubMed] [Google Scholar]
- 27.Ozaki N, Shibasaki T, Kashima Y, Miki T, Takahashi K, Ueno H, Sunaga Y, Yano H, Matsuura Y, Iwanaga T, et al. cAMP-GEFII is a direct target of cAMP in regulated exocytosis. Nat Cell Biol. 2000;2:805–11. doi: 10.1038/35041046. [DOI] [PubMed] [Google Scholar]
- 28.Seino S, Shibasaki T, Minami K. Dynamics of insulin secretion and the clinical implications for obesity and diabetes. J Clin Invest. 2011;121:2118–25. doi: 10.1172/JCI45680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Laferrère B. Diabetes remission after bariatric surgery: is it just the incretins? Int J Obes (Lond) 2011;35(Suppl 3):S22–5. doi: 10.1038/ijo.2011.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thaler JP, Cummings DE. Minireview: Hormonal and metabolic mechanisms of diabetes remission after gastrointestinal surgery. Endocrinology. 2009;150:2518–25. doi: 10.1210/en.2009-0367. [DOI] [PubMed] [Google Scholar]
- 31.Torekov SS, Madsbad S, Holst JJ. Obesity - an indication for GLP-1 treatment? Obesity pathophysiology and GLP-1 treatment potential. Obes Rev. 2011;12:593–601. doi: 10.1111/j.1467-789X.2011.00860.x. [DOI] [PubMed] [Google Scholar]
- 32.Howell JJ, Manning BD. mTOR couples cellular nutrient sensing to organismal metabolic homeostasis. Trends Endocrinol Metab. 2011;22:94–102. doi: 10.1016/j.tem.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mori H, Inoki K, Opland D, Münzberg H, Villanueva EC, Faouzi M, Ikenoue T, Kwiatkowski DJ, Macdougald OA, Myers MG, Jr., et al. Critical roles for the TSC-mTOR pathway in β-cell function. Am J Physiol Endocrinol Metab. 2009;297:E1013–22. doi: 10.1152/ajpendo.00262.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rachdi L, Balcazar N, Osorio-Duque F, Elghazi L, Weiss A, Gould A, Chang-Chen KJ, Gambello MJ, Bernal-Mizrachi E. Disruption of Tsc2 in pancreatic beta cells induces beta cell mass expansion and improved glucose tolerance in a TORC1-dependent manner. Proc Natl Acad Sci U S A. 2008;105:9250–5. doi: 10.1073/pnas.0803047105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shigeyama Y, Kobayashi T, Kido Y, Hashimoto N, Asahara S, Matsuda T, Takeda A, Inoue T, Shibutani Y, Koyanagi M, et al. Biphasic response of pancreatic beta-cell mass to ablation of tuberous sclerosis complex 2 in mice. Mol Cell Biol. 2008;28:2971–9. doi: 10.1128/MCB.01695-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pende M, Kozma SC, Jaquet M, Oorschot V, Burcelin R, Le Marchand-Brustel Y, Klumperman J, Thorens B, Thomas G. Hypoinsulinaemia, glucose intolerance and diminished beta-cell size in S6K1-deficient mice. Nature. 2000;408:994–7. doi: 10.1038/35050135. [DOI] [PubMed] [Google Scholar]
- 37.Ruvinsky I, Sharon N, Lerer T, Cohen H, Stolovich-Rain M, Nir T, Dor Y, Zisman P, Meyuhas O. Ribosomal protein S6 phosphorylation is a determinant of cell size and glucose homeostasis. Genes Dev. 2005;19:2199–211. doi: 10.1101/gad.351605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fraenkel M, Ketzinel-Gilad M, Ariav Y, Pappo O, Karaca M, Castel J, Berthault MF, Magnan C, Cerasi E, Kaiser N, et al. mTOR inhibition by rapamycin prevents beta-cell adaptation to hyperglycemia and exacerbates the metabolic state in type 2 diabetes. Diabetes. 2008;57:945–57. doi: 10.2337/db07-0922. [DOI] [PubMed] [Google Scholar]
- 39.Yang SB, Lee HY, Young DM, Tien AC, Rowson-Baldwin A, Shu YY, Jan YN, Jan LY. Rapamycin induces glucose intolerance in mice by reducing islet mass, insulin content, and insulin sensitivity. J Mol Med. 2011 doi: 10.1007/s00109-011-0834-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nie J, Liu X, Lilley BN, Zhang H, Pan YA, Kimball SR, Zhang J, Zhang W, Wang L, Jefferson LS, et al. SAD-A kinase controls islet β-cell size and function as a mediator of mTORC1 signaling. Proc Natl Acad Sci U S A. 2013;110:13857–62. doi: 10.1073/pnas.1307698110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ma XM, Blenis J. Molecular mechanisms of mTOR-mediated translational control. Nat Rev Mol Cell Biol. 2009;10:307–18. doi: 10.1038/nrm2672. [DOI] [PubMed] [Google Scholar]
- 42.da Silva Xavier G, Leclerc I, Varadi A, Tsuboi T, Moule SK, Rutter GA. Role for AMP-activated protein kinase in glucose-stimulated insulin secretion and preproinsulin gene expression. Biochem J. 2003;371:761–74. doi: 10.1042/BJ20021812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Richards SK, Parton LE, Leclerc I, Rutter GA, Smith RM. Over-expression of AMP-activated protein kinase impairs pancreatic beta-cell function in vivo. J Endocrinol. 2005;187:225–35. doi: 10.1677/joe.1.06413. [DOI] [PubMed] [Google Scholar]
- 44.Alvarado-Kristensson M, Rodríguez MJ, Silió V, Valpuesta JM, Carrera AC. SADB phosphorylation of gamma-tubulin regulates centrosome duplication. Nat Cell Biol. 2009;11:1081–92. doi: 10.1038/ncb1921. [DOI] [PubMed] [Google Scholar]
- 45.Campbell JE, Drucker DJ. Pharmacology, physiology, and mechanisms of incretin hormone action. Cell Metab. 2013;17:819–37. doi: 10.1016/j.cmet.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 46.Baggio LL, Drucker DJ. Biology of incretins: GLP-1 and GIP. Gastroenterology. 2007;132:2131–57. doi: 10.1053/j.gastro.2007.03.054. [DOI] [PubMed] [Google Scholar]
- 47.Sandoval D, Barrera JG, Stefater MA, Sisley S, Woods SC, D’Alessio DD, Seeley RJ. The anorectic effect of GLP-1 in rats is nutrient dependent. PLoS One. 2012;7:e51870. doi: 10.1371/journal.pone.0051870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Burmeister MA, Ayala J, Drucker DJ, Ayala JE. Central glucagon-like peptide 1 receptor-induced anorexia requires glucose metabolism-mediated suppression of AMPK and is impaired by central fructose. Am J Physiol Endocrinol Metab. 2013;304:E677–85. doi: 10.1152/ajpendo.00446.2012. [DOI] [PubMed] [Google Scholar]


