Abstract
Interleukin 6 (IL-6) signaling plays a role in inflammation, cancer, and senescence. Here, we identified soluble IL-6 receptor (sIL-6R) as a member of the senescence-associated secretory phenotype (SASP). Senescence-associated sIL-6R upregulation was mediated by mammalian target of rapamycin (mTOR). sIL-6R was mainly generated by a disintegrin and metalloprotease 10 (ADAM10)-dependent ectodomain shedding to enable IL-6 trans-signaling. In vivo, heterozygous PTEN-knockout mice exhibited higher mTOR activity and increased sIL-6R levels. Moreover, aberrant EGF receptor (EGFR) activation triggered IL-6 synthesis. In analogy to senescence, EGFR-induced activation of mTOR also induced IL-6R expression and sIL-6R generation. Hence, mTOR activation reprograms IL-6 non-responder cells into IL-6 responder cells. Our data suggest that mTOR serves as a central molecular switch to facilitate cellular IL-6 classic and trans-signaling via IL-6R upregulation with direct implications for cellular senescence and tumor development.
Keywords: EGFR, Interleukin 6, Interleukin 6 receptor, SASP, mTOR, senescence
Introduction
IL-6 type cytokines are critically involved in physiological and pathophysiological processes, including inflammation, metabolism, aging, and cancer.1-4 IL-6 activates cells via 2 different mechanisms, named classic and trans-signaling. In classic signaling, IL-6 first binds to the non-signaling membrane-bound IL-6 receptor (IL-6R), and the IL-6/IL-6R complex recruits 2 signal-transducing glycoprotein 130 (gp130) receptor proteins which dimerize and subsequently activate the Jak/STAT, PI3K, and MAPK signaling pathways. Whereas gp130 is ubiquitously expressed, IL-6R expression is rather restricted to a limited number of cell types, such as hepatocytes, monocytes, megakaryocytes, leukocytes, and certain T-cell subpopulations.3 Consequently, the expression of IL-6R determines whether a cell is responsive to IL-6 or not. In IL-6 trans-signaling, IL-6 binds to a soluble form of the IL-6R (sIL-6R) and subsequently activates gp130 signal transduction of target cells. Therefore, IL-6 trans-signaling potentially activates all cells of the body. In vivo, IL-6 trans-signaling is of particular importance, because in contrast to classic signaling, it is considered the main driving force of pro-inflammatory responses in chronic inflammatory diseases.5 In humans, the soluble IL-6R is either generated by alternative splicing of the IL-6R mRNA or by limited proteolysis of the membrane-bound IL-6R.3 In pathophysiological situations, the serum level of sIL-6R increases up to 2-fold as a result of increased expression and/or shedding of the membrane-bound IL-6R, contributing to pro-inflammatory IL-6 trans-signaling. A disintegrin and metalloprotease (ADAM) family members ADAM10 and ADAM17 have been identified as the main proteases responsible for sIL-6R generation.6,7
Even though IL-6R expression limits IL-6 classic and trans-signaling, the regulation of IL-6R expression is only incompletely understood. Long-term treatment with the synthetic glucocorticoid dexamethasone upregulated IL-6R mRNA and protein expression,8-10 whereas short-term treatment interfered with the IL-6-induced SOCS3 negative feedback loop.11 In T cells, IL-2 inhibits Th17 cell differentiation by blunting IL-6 responses via downregulation of the IL-6R and gp130.12 Moreover, IL-6R mRNA is destabilized by the tumor suppressor microRNA-125b, leading to downregulation of IL-6R levels and insensitivity to IL-6 signaling. Downregulation of microRNA-125b is often found in hepatocellular carcinoma (HCC) tissues.13 Recently, the transcription factor E2F3, which is commonly overexpressed in tumors, was shown to positively regulate IL-6R expression.14
IL-6 is a major component of the so-called senescence-associated secretory phenotype (SASP), a typical feature of senescent cells characterized by the release of various cytokines, growth factors, and proteases.15 Cellular senescence is linked to age-associated diseases and inflammation and has recently been recognized as an important antitumor barrier.16 The role of the SASP, however, in tumor progression remains unclear. The SASP can signal to the tumor environment and elicit the immune-mediated clearance of tumor cells or, depending on the context, could potentially promote tumor progression. Furthermore, IL-6 has been reported to actively participate in the senescence process by reinforcing the senescence growth arrest in a positive feedback loop.17 In addition to SASP formation, senescence is characterized by a persistent cell cycle arrest, which was first recognized to occur in fibroblasts after having undergone a finite number of cell divisions. Nowadays, senescence is known to be not solely induced in response to telomere shortening, a process referred to as replicative senescence, but also to be triggered by various types of cellular stress. Among others, DNA damage as well as replicative stress caused by the increased expression of oncogenes has been identified to induce stress-induced premature senescence (SIPS). Recently, also downregulation of TACC3 and other mitotic spindle proteins was reported to cause SIPS.18 Another hallmark of SIPS is the activation of mammalian target of rapamycin (mTOR), a serine-threonine kinase involved in the regulation of protein synthesis and cell growth, which might be related to the typically enlarged morphology of senescent cells.19,20 Moreover, blocking the mTOR-complex mTORC1 by rapamycin or rapamycin analogs (rapalogs) has been shown to prevent SIPS.21,22
Here, we show that SIPS induced through TACC3 depletion or HRasG12V expression leads to upregulation of IL-6R expression. SIPS-induced IL-6R expression was dependent on mTOR activation. Furthermore, the increased levels of sIL-6R were mainly generated by ADAM10-mediated ectodomain shedding. Several studies have reported that the IL-6R is overexpressed in human cancer cell lines and in human tumors.23-27 Among other signaling pathways, EGFR signaling leads to sustained mTOR activation. In a subset of tumors, EGFR signaling induces IL-6 secretion, which contributes to tumor development via STAT3 activation.28 In this regard, we demonstrate that EGF-induced mTOR activation also leads to increased IL-6R expression and IL-6 responsiveness, suggesting that mTOR activation is a general mechanism to facilitate cellular IL-6 responsiveness via upregulation of cellular and soluble IL-6R levels both in vitro and in vivo. Consistent with the latter, we show that heterozygous PTEN (phosphatase and tensin homolog)-KO mice, which exhibit increased mTOR activity,29 display significantly increased sIL-6R serum levels. Altogether, our data suggest that mTOR serves as a central mediator of IL-6 effector function by upregulating IL-6R during cellular senescence and EGFR signaling.
Results
SIPS induced by TACC3 depletion and oncogenic HRasG12V causes increased cellular IL-6R level and increased generation of the soluble IL-6R
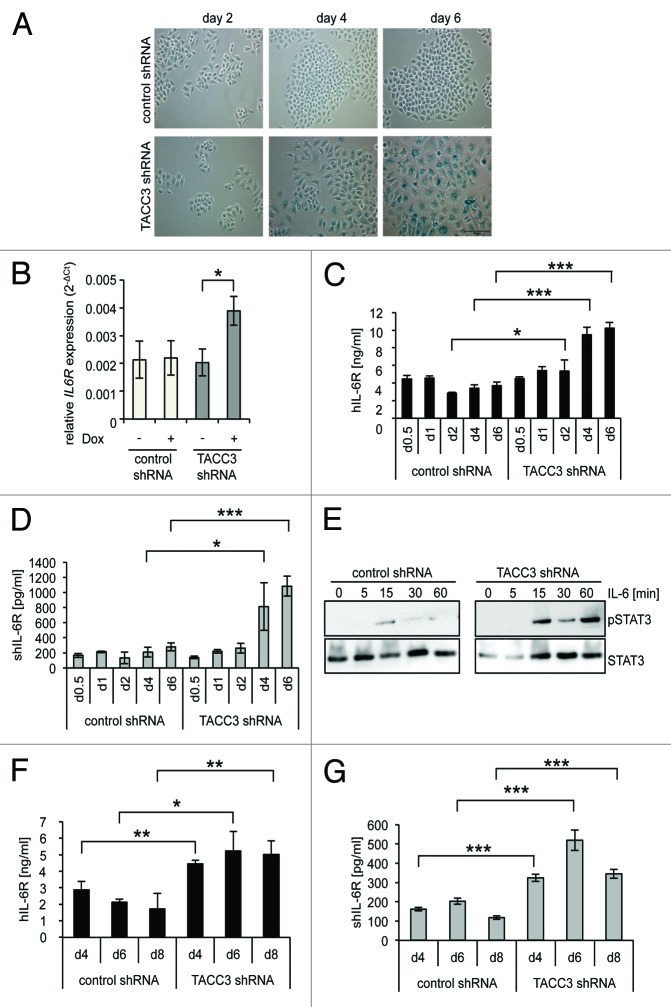
IL-6 is one of the most prominent SASP proteins.15 Therefore, we examined whether the expression of cellular IL-6R is also induced during senescence. This is of particular importance, since IL-6R expression pattern restricts IL-6 signaling to hepatocytes and some subtypes of immune cells. shRNA-mediated depletion of the mitotic spindle protein TACC3 in the epithelial breast cancer cell line MCF7 induced SIPS within 4 to 6 d, as demonstrated by the typical appearance of flat, enlarged cells and induction of senescence-associated β-galactosidase activity, whereas expression of a control shRNA had no effects (Fig. 1A). After 4 d of TACC3 shRNA induction, IL-6R mRNA and protein levels were upregulated by a factor of 2 and 2.8, respectively (Fig. 1B and C). MCF7 cells expressed basal amounts of cellular IL-6R (Fig. 1C), and stimulation of MCF7 cells with physiological concentrations of IL-6 (2 ng/ml)28 led to a slight increase of STAT3 phosphorylation. However, in senescent MCF7 cells STAT3 phosphorylation was strongly induced by IL-6 (Fig. 1E), indicating that the increased expression of the IL-6R sensitizes senescent MCF7 cells to classic IL-6 signaling. Additionally, IL-6 trans-signaling appears to be enabled after TACC3 depletion, since the amount of sIL-6R found in the cell culture supernatant significantly increased up to 3.9 ± 0.3-fold after TACC3 depletion compared with control shRNA-expressing cells (Fig. 1D).
Figure 1. Depletion of TACC3 causes premature senescence and upregulation of the Interleukin 6-Receptor. (A) MCF7 cells stably expressing a doxycycline-inducible shRNA targeting TACC3 or a control shRNA were seeded at day 0 in medium containing 5 µg/ml doxycycline (DOX). Cells were supplemented every 2 d with fresh medium containing doxycycline. Cells were stained for senescence-associated β-galactosidase (SA-βgal) activity at days 2, 4, and 6. Microscopic pictures are representative for 3 independent experiments. (B) IL-6R mRNA levels in MCF7 cells were measured on day 4. Cells expressing either control shRNA or TACC3 shRNA were treated with doxycycline and compared with untreated cells. The results show the mean ± s. d. of 3 independent experiments. (C) Cells were treated as described in (A) and harvested at days 0.5, 1, 2, 4, and 6. The amount of IL-6R in the cell lysate was measured via ELISA. The results show the mean ± s. d. of 3 independent experiments. (D) Cells were treated as described in (A) and harvested at days 0.5, 1, 2, 4, and 6. The amount of IL-6R in the cell supernatant was measured via ELISA. The results show the mean ± s. d. of 3 independent experiments. (E) MCF7 cells stably expressing a DOX-inducible TACC3-specific or a control shRNA were treated as described under panel (A). On day 6, cells were starved for 4 h in serum-free medium and stimulated with IL-6 (2 ng/ml). Cells were lysed at the time points indicated and phosphorylation of STAT3 assessed via western blotting. One of 2 experiments with similar outcome is shown. (F and G) MCF10a cells stably expressing a DOX-inducible shRNA either targeting human TACC3 or an unrelated control were seeded at day 0 in medium containing DOX. Cells were supplemented every 2 d with fresh medium containing DOX. Cells were harvested at days 4, 6, and 8. The amount of IL-6R (F) in the cell lysate or (G) of soluble IL-6R in the supernatant was measured via ELISA. The results show the mean ± s. d. of 3 independent experiments.
Next, we tested whether the induction of IL-6R expression and sIL-6R generation is cell line-specific. Using the same shRNA-based system, TACC3 was depleted in the non-transformed epithelial breast cell line MCF10a.30 The time frame of induction of senescence upon TACC3 depletion in MCF10a cells was somewhat longer compared with MCF7 cells.30 Consistent with the finding in TACC3-depleted senescent MCF7 cells, quantification of IL-6R by ELISA revealed a SIPS-associated upregulation of both IL-6R expression and sIL-6R generation by a factor of 2.9 in MCF10a cells (Fig. 1F and G).
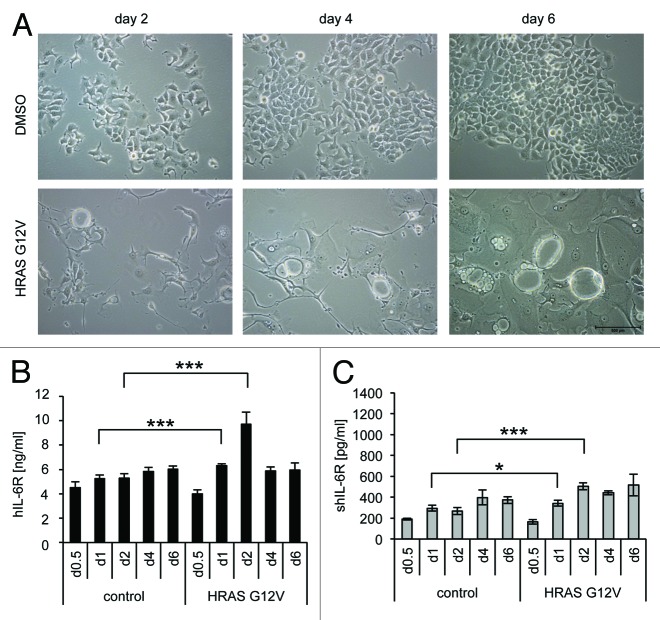
Next, we analyzed upregulation of IL-6R/sIL-6R during oncogene-induced senescence and employed MCF7 cells expressing a doxycycline-inducible HRasG12V protein. Already, 2 d following doxycycline incubation the SIPS phenotype became clearly detectable by the formation of large vacuolated cells (Fig. 2A). Of note, HRasG12V-induced SIPS in MCF7 cells was detectable much faster when compared with TACC3 depletion (2 d compared with 4–6 d, respectively). For HRasG12V-induced SIPS, IL-6R was significantly, but transiently, increased after 1 (1.2 ± 0.07-fold) and 2 d (1.8 ± 0.3-fold) as compared with control cells (Fig. 2B). Furthermore, a significant increase in sIL-6R-level was also observed on day 1 and day 2 (Fig. 2C). Since SIPS, induced by TACC3-depletion, resulted in a stronger upregulation of IL-6R and sIL-6R level, we decided to concentrate on this SIPS model for further experiments.
Figure 2. Ectopic overexpression of oncogenic Ras causes senescence and transient IL-6R upregulation. (A) MCF7 cells stably expressing doxycycline-inducible HRASG12V were seeded on day 0 in medium containing 5 µg/ml DOX. Microscopic pictures were taken at days 2, 4, and 6 and are representative for 3 independent experiments. (B and C) MCF7 cells stably expressing a DOX-inducible HRASG12V were seeded on day 0 in medium containing 5 µg/ml DOX and harvested after the indicated time. The amount of IL-6R (B) in the cell lysate or (C) of soluble IL-6R in the supernatant was measured via ELISA. The results show the mean ± s. d. of 3 independent experiments.
Increased IL-6R and sIL-6R levels in SIPS are dependent on mTOR activity
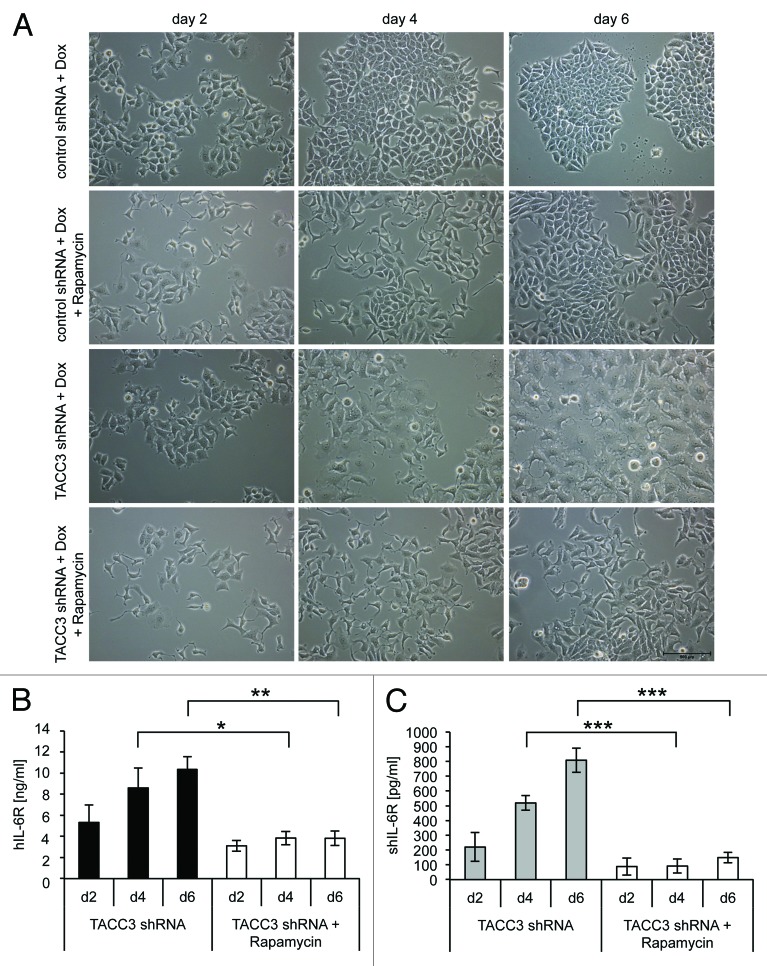
Recently, mammalian target of rapamycin (mTOR) has been connected with the induction of senescence.31 When co-incubated with inducers of senescence, rapamycin, which is a potent inhibitor of the mTOR complex mTORC1, also inhibited the onset of senescence, but failed to revert an established senescence phenotype.31 We therefore analyzed SIPS and IL-6R/sIL-6R levels in TACC3-depleted MCF7 cells after rapamycin treatment. Rapamycin prevented both the development of SIPS as indicated by normal morphology of TACC3-depleted cells (Fig. 3A), as well as the upregulation of cellular and soluble IL-6R levels (Fig. 3B and C). Moreover, rapamycin decreased IL-6R and sIL-6R concentrations below control levels, indicating that mTOR also regulates the expression of endogenous IL-6R expression in MCF7 cells (Fig. 3B and C). These results indicate that the increased IL-6R expression during SIPS is dependent on mTOR signaling.
Figure 3. Inhibition of mTOR by rapamycin prevents IL-6R upregulation and premature senescence. (A) MCF7 cells stably expressing DOX-inducible TACC3-specific or control shRNAs were each seeded at day 0 in medium containing 5 µg/ml DOX and either 500 ng/ml rapamycin or the solvent DMSO. Medium was exchanged every 2 d and fresh DOX and 500 ng/ml rapamycin or DMSO added. Microscopic pictures were taken at days 2, 4, and 6 and are representative for 3 independent experiments. (B and C) Cells were treated as described under panel (A). Cells were harvested at the indicated time points and the amount of IL-6R (B) in the cell lysate or (C) of soluble IL-6R in the supernatant was measured via ELISA. The results show the mean ± s. d. of 3 independent experiments.
Senescence-induced generation of soluble IL-6R is mainly mediated by ADAM10 shedding
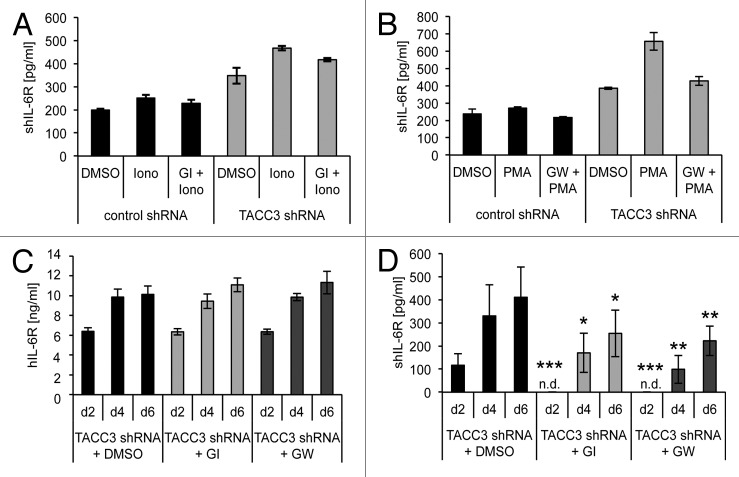
Shedding of human IL-6R is mediated by ADAM10 and ADAM17. ADAM10 is responsible for constitutive and induced shedding of the IL-6R, whereas ADAM17 is considered as inducible sheddase of the IL-6R.7 We therefore asked to which extent IL-6R can be released from senescent cells by ectodomain shedding. Treatment with ionomycin, an inducer of ADAM10,7 led to an increase of sIL-6R levels from TACC3-depleted MCF7 cells as compared with control cells (Fig. 4A). Ionomycin-induced sIL-6R generation was slightly inhibited by the ADAM10-specific inhibitor GI254023X (GI). Also PMA, an inducer of ADAM17, led to elevated sIL-6R levels, and this increase was inhibited by the ADAM10/ADAM17-specific inhibitor GW280264X (GW) (Fig. 4B). Overall, these data demonstrate that senescent cells can shed IL-6R via activation of ADAM10 and ADAM17.
Figure 4. The soluble IL-6R is partly generated through constitutive shedding by the metalloprotease ADAM10. (A) MCF7 cells stably expressing a DOX-inducible TACC3-specific or a control shRNA were seeded at day 0 in medium containing 5 µg/ml DOX. Medium was exchanged every 2 d and fresh DOX added. On day 6, medium was replaced with 1 ml serum-free medium and cells were stimulated with 1 µM ionomycin or DMSO as control for 60 min. Where indicated, cells were pre-incubated with the ADAM10-specific inhibitor GI254023X (GI) for 30 min. The amount of shIL-6R in the supernatant was quantified via ELISA. (B) Cells were treated as described under panel (A), but stimulated with PMA for 2 h and pre-incubated with the combined ADAM10/ADAM17-specific inhibitor GW280264X (GW) where indicated. All results show the mean ± s. d. of 3 independent experiments. (C and D) MCF7 cells stably expressing a DOX-inducible shRNA targeting human TACC3 were seeded at day 0 in medium containing 5µg/ml doxycycline and either 3 µM of the ADAM10-specific inhibitor GI, 3 µM of the combined ADAM10/ADAM17-specific inhibitor GW or the solvent DMSO. Medium was exchanged every 2 d and fresh DOX and 3 µM GI, 3 µM GW, or DMSO added. Cells were harvested at the indicated time points and the amount of IL-6R (C) in the cell lysate or (D) of soluble IL-6R in the supernatant was measured via ELISA. The results show the mean ± s. d. of 3 independent experiments. n.d., not detectable.
To identify the protease responsible for the observed sIL-6R generation during cellular senescence (Fig. 1D), TACC3-depleted MCF7 cells were treated every 24 h for 6 d with GI or GW (3 µM) and IL-6R/sIL-6R levels were determined by ELISA. As shown in Figure 4C, GI and GW had no effect on the overall cellular IL-6R level, but significantly reduced sIL-6R levels at all time points examined (Fig. 4D). These results indicate that ADAM10-mediated ectodomain shedding of the IL-6R contributes to SIPS-induced sIL-6R generation.
Dexamethasone-induced IL-6R expression is mTOR-independent
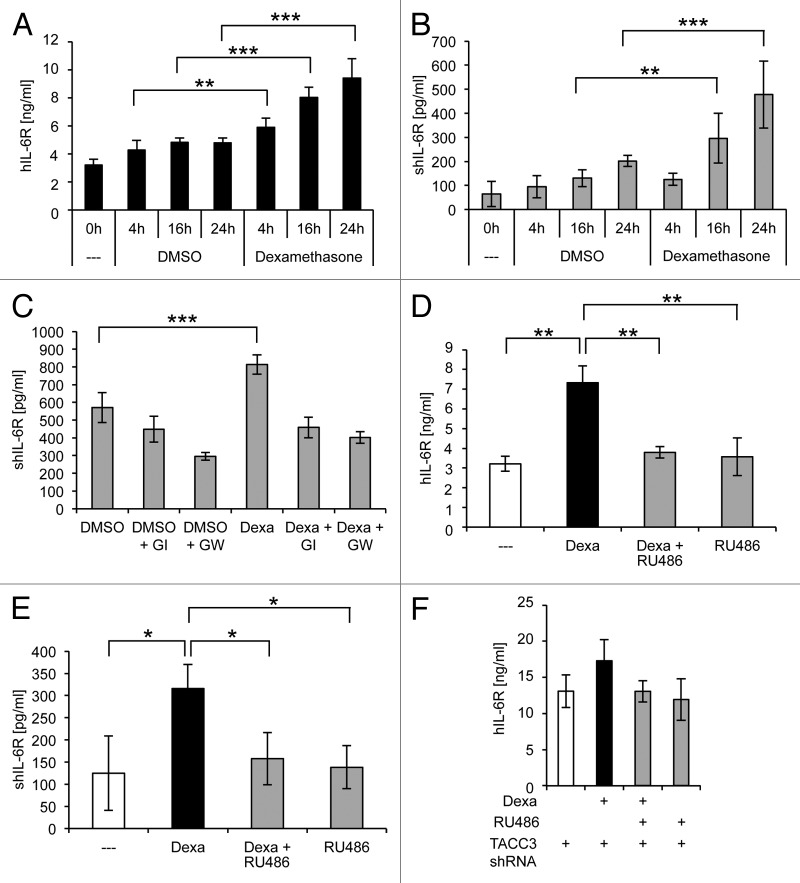
The glucocorticoid analog dexamethasone was shown to induce IL-6R mRNA and protein expression in hepatocytes.8-10 In MCF7 cells, the levels of IL-6R mRNA (Fig. S1) and protein (Fig. 5A) were increased up to 2.0 ± 0.07-fold and 1.9 ± 0.2-fold, respectively, after treatment with dexamethasone for 24 h, indicating that dexamethasone treatment promotes IL-6R expression not only in hepatocytes. In analogy to TACC3 depletion, the level of soluble IL-6R was also increased up to 2.4 ± 0.6-fold after dexamethasone treatment (Fig. 5B). Next, cells were incubated with GI and GW in combination with dexamethasone for 20 h. Inhibition of the proteases did not influence the dexamethasone-induced increase in IL-6R mRNA expression (Fig. S1B). However, both GI and GW significantly reduced the amount of sIL-6R, indicating a major role of ADAM10 in generation of the sIL-6R increase after dexamethasone treatment (Fig. 5C).
Figure 5. Activation of the glucocorticoid receptor causes IL-6R upregulation and increased sIL-6R generated by ADAM10. (A and B) MCF7 cells were seeded on day 0.1 µM dexamethasone or the solvent DMSO as control were added 24 h later, and the cells were harvested at the indicated time points. The amount of IL-6R (A) in the cell lysate or (B) of soluble IL-6R in the supernatant was measured via ELISA. The results show the mean ± s. d. of 3 independent experiments. (C) MCF7 cells were treated as described under panel (A). Besides 1 µM dexamethasone or DMSO as control, cells were incubated with the ADAM10-specific inhibitor GI254023X (GI) or the combined ADAM10/ADAM17-specific inhibitor GW280264X (GW) where indicated. Cells were harvested after 20 h, and the amount of sIL-6R in the supernatant was measured via ELISA. The results show the mean ± s. d. of 3 independent experiments. (D and E) MCF7 cells were seeded on day 0 and stimulated with either 1 µM dexamethasone, 1 µM dexamethasone together with 5 µM of the glucocorticoid receptor antagonist RU486, or 5 µM RU486 alone. Cells were harvested at the indicated time points and the amount of IL-6R (D) in the cell lysate or (E) of soluble IL-6R in the supernatant was measured via ELISA. The results show the mean ± s. d. of 3 independent experiments. (F) MCF7 cells stably expressing a doxycycline-inducible shRNA targeting TACC3 were seeded at day 0 in medium containing 5 µg/ml DOX. Medium was exchanged every 2 d, and fresh DOX was added. On day 6, medium was replaced by fresh medium containing doxycycline with either DMSO, 1 µM dexamethasone, 1 µM dexamethasone plus 5 µM RU486, or 5 µM RU486 alone. Cells were harvested 24 h after treatment on day 7. The results show the mean ± s. d. of 3 independent experiments.
Treatment of MCF7 cells with RU486, a glucocorticoid receptor antagonist, completely prevented the dexamethasone-induced increase of IL-6R and sIL-6R levels (Fig. 5D and E). In contrast, RU486 was not able to significantly reduce SIPS-induced IL-6R expression and sIL-6R generation (Fig. S1C and D). Vice versa, rapamycin did not significantly reduce the dexamethasone-induced increase in IL-6R and sIL-6R levels (Fig. S1E and F). Moreover, TACC3-depletion in combination with dexamethasone further increased IL-6R levels (Fig. 5F). These results indicate that dexamethasone and mTOR-induced IL-6R expression are independent regulatory processes.
IL-6R expression via mTOR is also induced by epidermal growth factor (EGF) stimulation in MCF7, HepG2 cells, and primary murine hepatocytes
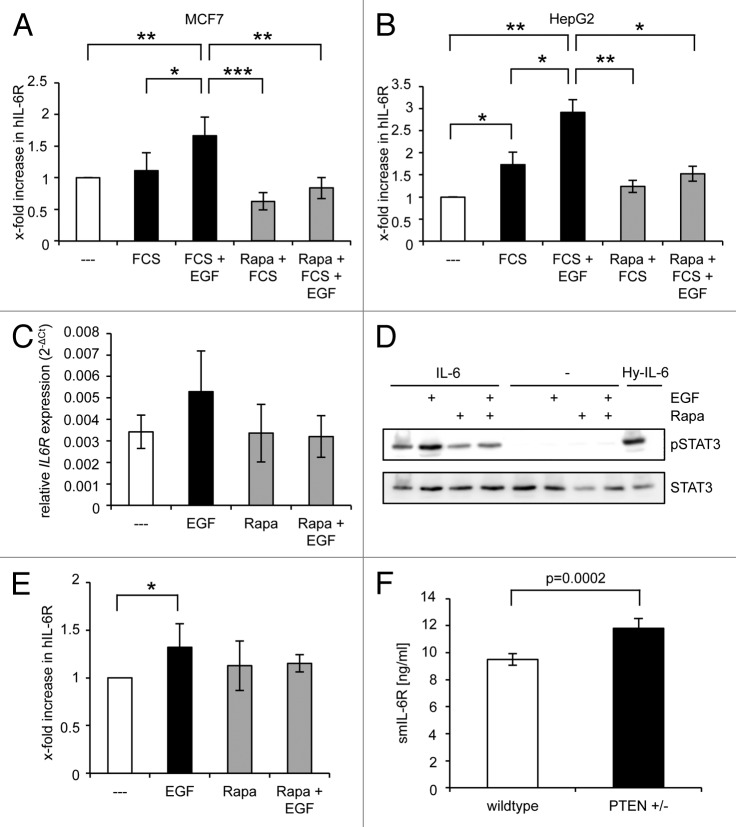
Next, we analyzed whether the increase of the IL-6R level by the mTOR pathway is restricted to senescence or represents a general regulatory mechanism. Therefore, we determined IL-6R levels after stimulation of MCF7 and HepG2 cells as well as primary murine hepatocytes with epidermal growth factor (EGF), which, among other signaling pathways, also leads to activation of mTOR.32 As depicted in Figure 6A, treatment of MCF7 cells with EGF led to a 1.6 ± 0.5-fold increase in IL-6R levels. Moreover, EGF-induced IL-6R expression was reduced below basal levels in the presence of rapamycin, indicating that basal IL-6R expression is partly maintained by mTOR activity, whereas EGF-induced IL-6R expression completely depends on mTOR signaling. Basal expressed IL-6R levels in HepG2 hepatoma cells are sufficient to induce IL-6 classic signaling.33 EGF treatment led also in HepG2 cells to a 2.9 ± 0.3-fold increase of IL-6R levels, which was again completely prevented by rapamycin treatment (Fig. 6B). Interestingly, rapamycin did not reduce IL-6R level below basal levels, indicating that in HepG2 cells additional regulatory mechanisms must exist that maintain basal IL-6R expression. A comparable pattern emerged when IL-6R mRNA was quantified via real-time PCR (Fig. 6C). To test whether the observed IL-6R upregulation after EGF-treatment renders the cells responsive toward IL-6 classic signaling, we stimulated MCF7 cells for 24 h with EGF and/or rapamycin. After serum starvation, classic signaling was induced by IL-6 stimulation for 15 min. As shown in Figure 6D, IL-6 induced stronger STAT3 phosphorylation when cells were pre-treated with EGF. STAT3 phosphorylation was completely prevented when mTOR was blocked, thus confirming our ELISA data. No detectable STAT3 phosphorylation was observed without IL-6 stimulation. Furthermore, stimulation of primary murine hepatocytes with EGF led also to a significant increase in IL-6R expression that was rapamycin-sensitive, i.e., mTOR-dependent (Fig. 6E).
Figure 6. Activation of mTOR by EGF drives IL-6R expression. (A and B) MCF7 (A) or HepG2 (B) cells were starved 24 h post-seeding for one day in serum-free medium. Medium was then replaced either with serum-free medium or medium supplemented with 10% serum. Recombinant EGF (20 ng/ml) and/or rapamycin (500 nM) were added when indicated. Cells were harvested 24 h later. (C) HepG2 cells were treated as described above and harvested after 24 h. The amounts of IL-6R mRNA were determined as described under “Materials and Methods”. The results show the mean ± s. d. of 3 independent experiments, each performed in triplicates. (D) MCF7 cells were treated as described under panel (B), serum starved for 3 h, and stimulated with 2 ng/ml recombinant IL-6 for 15 min. Stimulation with hyper-IL-6 served as positive control. Phosphorylation of STAT3 was assessed by western blotting. One of 2 performed experiments with similar outcome is shown. (E) Primary murine hepatocytes were stimulated with recombinant EGF (20 ng/ml) and/or rapamycin (500 nM) where indicated or left untreated. Cells were harvested 24 h later and IL-6R levels determined via ELISA. The amount of IL-6R in unstimulated cells was considered 100%, and the other samples were calculated accordingly. The results show the mean ± s.d. of 3 independent experiments. (F) The amount of smIL-6R in the serum of wild-type and PTEN+/− mice (n = 3 each) was determined via ELISA (mean ± s. d.).
Finally, we asked whether the mTOR/IL-6R axis might play a role in the generation of the sIL-6R in vivo. Therefore, sIL-6R was quantified in serum samples obtained from mice with a heterozygous deletion of PTEN and wild-type control animals. PTEN is a negative regulator of the mTOR pathway and heterozygous PTEN-knockout mice (PTEN+/−) exhibit hyperactivity of the mTOR pathway.34 In consistency with our results above, PTEN heterozygosity resulted in a significant 25% upregulation of the sIL-6R serum level. This suggests that the increased mTOR activity in PTEN+/− mice contributes to IL-6R expression and sIL-6R generation in vivo (Fig. 6F).
Discussion
IL-6 is critically involved in inflammation and cancer development.3 More recently, IL-6 has been identified as a key component of the senescence secretome, which enables senescent cells to communicate with their microenvironment. The role of the SASP in tumor progression remains unclear and can be beneficial or deleterious, depending on the biological context. IL-6 and other SASP factors could support tumorigenesis by promoting cell proliferation, angiogenesis, epithelial-to-mesenchymal transition, and invasiveness. In addition, the SASP may exert tumor-suppressive functions and trigger an immune response, thereby favoring tumor cell clearance and cancer regression.35 In this context, IL-6 was recently shown to actively contribute to the senescence process by re-enforcing cell cycle arrest in an autocrine feedback loop.17 Certainly, the secretory profile and function of the SASP are highly dependent on the cell type and context, such as the type of cell stress, its level and duration.
It was suggested that the composition of the IL-6 target cell decides whether IL-6 acts as tumor suppressor or promoter.36,37 Since gp130 is ubiquitously expressed, IL-6R expression determines whether a cell is responsive to classic signaling or trans-signaling. Here, for the first time, we provide evidence that cellular and soluble IL-6R levels are upregulated via the mTOR pathway. We identified that: (1) stress-induced premature senescence and (2) EGF triggered IL-6R expression occurs in a mTOR-dependent manner, whereas glucocorticoid-induced IL-6R expression was not regulated by mTOR.9,11
We found that IL-6R levels were substantially increased in the breast cancer cell line MCF7 and the untransformed breast epithelial cell line MCF10a in the course of senescence induced by TACC3 depletion and oncogene expression. Progression of SIPS upon HRasG12V-expression was faster compared with TACC3 depletion (1–2 d compared with 4–6 d, respectively). Induction of p53 restricts mTOR activity to the early phase of senescence,38 and the half-life of cellular IL-6R is only about 2–3 h.39 We assume that during oncogene-induced senescence, the transiently increased IL-6R levels were caused within a limited time window of mTOR activation. TACC3 depletion leads to much slower SIPS development and consequently to a more sustained IL-6R upregulation, which is presumably caused by the longer time window of mTOR activation. EGFR stimulation also leads to mTOR activation.40 Long-term stimulation of MCF7 cells, HepG2 cells, and primary hepatocytes with EGF also led to a sustained increase of cellular IL-6R levels. Both senescence and EGF-induced IL-6R expression were efficiently inhibited by pre-incubation with the mTOR inhibitor rapamycin.
Unstimulated MCF7 cells did not exhibit detectable STAT3 phosphorylation and were almost unresponsive to exogenous IL-6. SIPS-triggered, mTOR-dependent expression of IL-6R rendered MCF7 cells responsive to IL-6. Interestingly, even the endogenous low basal IL-6R levels in unstimulated MCF7 cells were reduced by rapamycin, indicating that endogenous IL-6R expression is under the control of mTOR signaling. IL-6R expression in non-stimulated HepG2 cells or primary murine hepatocytes was, however, not reduced below control levels by rapamycin, indicating that endogenous IL-6R levels in hepatic cells are regulated at least partially by an mTOR-independent mechanism.
Senescent MCF7 cells were principally able to induce shedding of the IL-6R by ADAM10 and ADAM17, as shown by PMA and ionomycin treatment. However, mTOR-induced IL-6R expression in senescent and EGFR-stimulated cells resulted in increased soluble IL-6R levels mainly caused by the constitutive IL-6R sheddase ADAM10.
Tumorigenic IL-6-induced STAT3 phosphorylation regulates proliferation, survival, and angiogenesis of growing tumors.41 Consequently, IL-6-deficient mice develop less and smaller tumors in colitis-associated cancer.42 Recently, mTOR activation was shown to have a driving role in cytokine-dependent tumor promotion via the gp130 pathway.43 Inhibition of mTORC1 with the rapalog RAD001 suppressed colitis-associated cancer, suggesting that both STAT3 and mTOR are mechanistically involved in tumor development.43 EGF was shown to be an important inducer of tumor-promoting IL-6 secretion and IL-6-dependent STAT3 phosphorylation in epithelial cancer cells.28 Moreover, increased TGF-β-dependent IL-6 secretion unleashed previously addicted lung tumor cells from their EGFR dependency, resulting in tumor resistance against anti-EGFR therapeutics.44 Consequently, IL-6 emerges as a promising therapeutic target in tumorigenesis. Recently, the IL-23R was also shown to be upregulated by IL-1-mediated mTOR activation, thereby highlighting the link between IL-1 and Th17 development.45 IL-6R expression is, however, restricted to hepatocytes and some immune cells; other cells such as epithelial and endothelial cells do normally not express detectable amounts of IL-6R and are therefore almost unresponsive to IL-6. The epithelial cell lines MCF7 and MCF10a became IL-6 responsive due to increased cellular IL-6R level after mTOR activation. PTEN is an efficient suppressor of the mTOR pathway. PTEN+/− mice display hyperactivation of the mTOR pathway34 and have about 25% higher sIL-6R level as compared with wild-type mice. PTEN activity is frequently abrogated in tumors, supporting mTOR activity and tumor progression. Our data suggest that during tumor development, senescence-mTOR and/or EGFR-mTOR pathways might not only induce IL-6 expression, but also convert previously non-transformed IL-6 unresponsive cells into transformed IL-6-responsive cells to enable sustained IL-6/IL-6R signaling.
In summary, our data indicate that mTOR activation represents a general mechanism to upregulate cellular IL-6R and sIL-6R levels and highlight the importance of ADAM10 in the generation mTOR-dependent sIL-6R. mTOR activation might lead to IL-6 classic signaling on senescent and tumor cells and to IL-6 trans-signaling within the senescence and tumor microenvironment. Thus, mTOR might be a promising therapeutic target to suppress IL-6R expression and IL-6 sensitivity of tumor cells but at the same time leaving endogenous expression of IL-6R on professional IL-6R expressing cells such as hepatocytes unaffected.
Materials and Methods
Cells, mice, and reagents
HepG2 and MCF7 cells were obtained from DSMZ. Immortalized MCF10a breast epithelial cells were kindly provided by Miguel Pujana (ICO). MCF7 cells expressing doxycycline-inducible HRasG12V protein were described previously.46 Dexamethasone, doxycycline, doxorubicin, RU486, and rapamycin were purchased from Sigma-Aldrich. Recombinant EGF was purchased from ImmunoTools GmbH. Anti-phospho STAT3 mAb (Tyr705) and anti-STAT3 mAb (124H6) were purchased from Cell Signaling Technology. The ADAM10-specific inhibitor GI254023X and the combined ADAM10/ADAM17-specific inhibitor GW280264X were a kind gift from Glaxo Smith Kline.47,48 Human IL-6 was expressed and purified as described previously.49 PTEN+/− mice have been described previously.50,51 Blood samples were taken from 12–16-wk-old female mice on a C57/BL6 genetic background. The experiments were approved by the responsible local authorities.
Cell culture
MCF7 breast carcinoma cells and HepG2 cells were cultured in Dulbecco modified Eagle Medium supplemented with 10% FCS (Invitrogen), 2 mM L-glutamine, 100 U/ml penicillin, and 100 µg/ml of streptomycin. MCF10a cells were cultured in Dulbecco modified Eagle Medium/F12 (1:1) high glucose, supplemented with 10% FCS (Invitrogen), 20 ng/ml EGF, 0.5 µg/ml hydrocortisone, 10 µg/ml insulin, 100 U/ml penicillin, and 100 µg/ml of streptomycin. All cells were kept at 37 °C in an incubator with 5% CO2 in a water-saturated atmosphere.
Isolation and culture of primary murine hepatocytes
Hepatocytes from male C57/BL6 wild-type mice (8–10 wk), which were obtained directly from Janvier Labs (France) and fed ad libitum with a standard diet, were isolated by a collagenase perfusion technique described previously.52 Cells were plated on collagen I-coated 6-well culture plates at a density of 2 × 106 and maintained in bicarbonate-buffered Krebs-Henseleit medium (115 mmol/L NaCl; 25 mmol/L NaHCO3; 5.9 mmol/L KCl; 1.18 mmol/L MgCl2; 1.23 mmol/L NaH2PO4; 1.2 mmol/L Na2SO4; 1.25 mmol/L CaCl2), supplemented with 6 mmol/L glucose in a humidified atmosphere of 5% CO2 and 95% air at 37 °C. After 2 h, the medium was removed, and the cells were washed twice. Subsequently the culture was continued for 24 h in William E medium (Biochrom), supplemented with 5 mmol/L glutamine, 100 U/mL penicillin, 0.1 mg/mL streptomycin, 100 nmol/L dexamethasone and HEPES. The viability of the hepatocytes was more than 95% as assessed by trypan blue exclusion. The experiments were approved by the responsible local authorities.
Cellular TACC3 depletion in MCF7 and MCF10a cells
The centrosomal protein TACC3 was depleted as described.30,53 MCF7 cells were plated at a density of 6.7 × 103/cm2, 3.3 × 103/cm2, 0.8 × 103/cm2 (control shRNA) and 6.7 × 103/cm2, 6.7 × 103/cm2, 5 × 103/cm2 (TACC3 shRNA) and harvested for analysis on days 2, 4, and 6 after doxycycline treatment. Expression of control or TACC3 shRNA was induced by adding doxycycline (5 μg/ml) to the culture medium for the time of the experiment. MCF10a cells were plated at a density of 3.3 × 103/cm2, 1.7 × 103/cm2, 0.8 × 103/cm2 (control shRNA) and 6.7 × 103/cm2, 5 × 103/cm2, 3.3 × 103/cm2 (TACC3 shRNA), and harvested for analysis on days 4, 6, and 8 after doxycycline treatment.
Ionizing γ-irradiation (γIR) of MCF7 and MCF10a cells
Trypsinized cells were exposed to γIR (20 Gy) using a Gammacell 1000 Elite irradiator (Nordion International, Inc). After irradiation, MCF7 cells were plated at a density of 6.7 × 103/cm2, 3.3 × 103/cm2, 0.8 × 103/cm2 (control) and 8.3 × 103/cm2, 8.3 × 103/cm2, 8.3 × 103/cm2 (20 Gy) and harvested for analysis on days 2, 4, and 6 after treatment. MCF10a cells were plated at a density of 3.3 × 103/cm2, 1.7 × 103/cm2, 0.8 × 103/cm2 (control) and 10 × 103/cm2, 10 × 103/cm2, 10 × 103/cm2 (20 Gy) and harvested for analysis on days 4, 6, and 8 after treatment.
Treatment of MCF7 cells with Doxorubicin
Cells were plated at a density of 6.7 × 103/cm2, 3.3 × 103/cm2, 0.8 × 103/cm2 (DMSO), and 8.3 × 103/cm2, 8.3 × 103/cm2, 8.3 × 103/cm2 (50 nM doxorubicin) and harvested for analysis on days 2, 4, and 6 after treatment. Culture medium containing DMSO or doxorubicin (50 nM) was changed every second day.
Induction of senescence by ectopic expression of oncogenic ras in MCF7 cells
MCF7 cells expressing an inducible HrasG12V were described previously.46 In brief, cells were plated at a density of 6.7 × 103/cm2, 3.3 × 103/cm2, 0.8 × 103/cm2 (control), and 6.7 × 103/cm2, 6.7 × 103/cm2, 5 × 103/cm2 (HRASG12V), and harvested for analysis on days 2, 4, and 6 after treatment. Expression of HRASG12V was induced by adding doxycycline (5 μg/ml) to the culture medium for the time of the experiment. Culture medium was changed every second day.
Cell lysis
After removal of the culture medium cells were washed once with PBS and collected in ice-cold PBS by scraping. Cells were pelleted by centrifugation and afterwards lysed in mild lysis buffer (50 mM Tris, pH 7.5, 150 mM NaCl, 1% Triton X-100, supplemented with complete protease inhibitor cocktail [Roche Applied Science]) for 60 min at 4 °C under constant agitation. After centrifugation, the resulting supernatant was transferred into a fresh tube, and protein concentrations were determined using Pierce BCA Protein Assay (Thermo Scientific). Protein lysates were stored at −20 °C for subsequent analysis.
Enzyme-linked immunosorbent assay (ELISA)
To quantify the amount of IL-6R in cell lysates or as soluble protein in cell culture supernatants, an ELISA specific for the human IL-6R was used for supernatants and 1:10 diluted cell lysates as described previously.7,54 For the enzymatic reaction, the peroxidase substrate BM blue POD (Roche Diagnostics) was used. The reaction was stopped by adding 1.8 M sulfuric acid. The absorbance was read at 450 nm on an infinite M200 pro (Tecan GmbH). An ELISA specific for murine IL-6R was from R&D Systems was used according to the manufacturer’s instructions.
Senescence-associated β-galactosidase (SA-β-gal) staining
On days 2, 4, and 6 after treatment, cells were fixed for 4 min with 2% formaldehyde/0.2% glutaraldehyde in PBS and stained with β-gal staining solution (2 mM MgCl2, 150 mM NaCl, 5 mM K4Fe[CN]6, 5 mM K3Fe[CN]6, 40 mM citric acid pH 6.0, 1 mg/ml 5-bromo-4-chloro-3-indolyl α-D-galactopyranoside) at 37 °C for 24 h. A senescence cell histochemical staining kit (Sigma-Aldrich) was used as indicated by the supplier.
SDS-PAGE and western blotting
SDS-PAGE and western blotting were performed as described previously.55 In brief, 50 µg cell lysate were separated on an 8% SDS-PAGE and western blotting was performed with the indicated antibodies. Signal detection was performed using ECL Prime Western Blotting Detection Reagent (GE Healthcare) on a ChemoCam Imager (Intas GmbH).
RNA extraction from cultured and irradiated cells and quantitative real-time PCR analysis
Cell lysates were homogenized using Qiashredder spin columns (Qiagen). Total RNA was extracted using the RNeasy Plus Mini Kit (Qiagen) following the standard manufacturer’s protocol. Nucleic acid concentrations were measured at 260 nm by spectrophotometry using a NanoDrop 1000 Spectrophotometer (Thermo Scientific). Extracted RNA samples were stored at −80 °C. For relative quantification of mRNAs, total RNA was reversely transcribed to cDNA using the ImProm-II™ Reverse Transcription System (Promega) according to the manufacturer’s instructions. In brief, up to 1 μg RNA was reverse transcribed to cDNA in a final volume of 20 μl using Oligo (dT)15 primers (0.5 μg/reaction) and 3.75 mM MgCl2. Each quantitative real-time PCR reaction mixture (20 μl) contained 1 μl of RT product (cDNA transcribed from 50 ng of total RNA), 10 μl of TaqMan Universal PCR Master Mix (Applied Biosystems), and 1 μl of IL-6R TaqMan Gene Expression Assay (Hs01075666_m1*, Applied Biosystems) containing primers and probe for the mRNA of interest. The mixture was initially incubated at 95 °C for 10 min, followed by 40 cycles of 95 °C for 15 s and 60 °C for 60 s. PCR reactions were performed on a 7500 Real-Time PCR System (Applied Biosystems) in triplicate. Samples were normalized relative to human large ribosomal protein PO (RPLPO, Hs99999902_m1), which served as endogenous control. Relative gene expression levels were calculated using the 2−ΔCt method.
Statistical analysis
Statistical significance was calculated using Student t tests. Results are given as means ± SD. Significant P values are shown in the figures as follows: below 0.05 (*), below 0.01 (**), or below 0.001 (***).
Supplementary Material
Acknowledgments
The authors would like to thank Hakima Ezzahoini for excellent technical assistance. Grant support: DFG grant SCHE 907/2-1 (to JS); the Collaborative Research Center SFB 728 (TP A5 to RPP) funded by the DFG; and the Research Commission of the Medical Faculty of the Heinrich-Heine-University (to CG, JS, and RPP). SR-J was supported by the DFG (SFB877, project A1) and the Cluster of Excellence “Inflammation at Interfaces.” PAL was supported by the Alexander von Humboldt Foundation (SKA 2010). DB was supported by a postdoctoral fellowship of the German Research Foundation (DFG).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Supplemental Materials
Supplemental materials may be found here: www.landesbioscience.com/journals/cc/article/26431
Footnotes
Previously published online: www.landesbioscience.com/journals/cc/article/26431
References
- 1.Scheller J, Chalaris A, Schmidt-Arras D, Rose-John S. The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim Biophys Acta. 2011;1813:878–88. doi: 10.1016/j.bbamcr.2011.01.034. [DOI] [PubMed] [Google Scholar]
- 2.Garbers C, Hermanns HM, Schaper F, Müller-Newen G, Grötzinger J, Rose-John S, Scheller J. Plasticity and cross-talk of interleukin 6-type cytokines. Cytokine Growth Factor Rev. 2012;23:85–97. doi: 10.1016/j.cytogfr.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 3.Chalaris A, Garbers C, Rabe B, Rose-John S, Scheller J. The soluble Interleukin 6 receptor: generation and role in inflammation and cancer. Eur J Cell Biol. 2011;90:484–94. doi: 10.1016/j.ejcb.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 4.Garbers C, Scheller J. Interleukin-6 and interleukin-11: same same but different. Biol Chem. 2013;394:1145–61. doi: 10.1515/hsz-2013-0166. [DOI] [PubMed] [Google Scholar]
- 5.Jones SA, Scheller J, Rose-John S. Therapeutic strategies for the clinical blockade of IL-6/gp130 signaling. J Clin Invest. 2011;121:3375–83. doi: 10.1172/JCI57158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Matthews V, Schuster B, Schütze S, Bussmeyer I, Ludwig A, Hundhausen C, Sadowski T, Saftig P, Hartmann D, Kallen KJ, et al. Cellular cholesterol depletion triggers shedding of the human interleukin-6 receptor by ADAM10 and ADAM17 (TACE) J Biol Chem. 2003;278:38829–39. doi: 10.1074/jbc.M210584200. [DOI] [PubMed] [Google Scholar]
- 7.Garbers C, Jänner N, Chalaris A, Moss ML, Floss DM, Meyer D, Koch-Nolte F, Rose-John S, Scheller J. Species specificity of ADAM10 and ADAM17 proteins in interleukin-6 (IL-6) trans-signaling and novel role of ADAM10 in inducible IL-6 receptor shedding. J Biol Chem. 2011;286:14804–11. doi: 10.1074/jbc.M111.229393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rose-John S, Schooltink H, Lenz D, Hipp E, Dufhues G, Schmitz H, Schiel X, Hirano T, Kishimoto T, Heinrich PC. Studies on the structure and regulation of the human hepatic interleukin-6 receptor. Eur J Biochem. 1990;190:79–83. doi: 10.1111/j.1432-1033.1990.tb15548.x. [DOI] [PubMed] [Google Scholar]
- 9.Snyers L, De Wit L, Content J. Glucocorticoid up-regulation of high-affinity interleukin 6 receptors on human epithelial cells. Proc Natl Acad Sci U S A. 1990;87:2838–42. doi: 10.1073/pnas.87.7.2838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ladenburger A, Seehase M, Kramer BW, Thomas W, Wirbelauer J, Speer CP, Kunzmann S. Glucocorticoids potentiate IL-6-induced SP-B expression in H441 cells by enhancing the JAK-STAT signaling pathway. Am J Physiol Lung Cell Mol Physiol. 2010;299:L578–84. doi: 10.1152/ajplung.00055.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dittrich A, Khouri C, Sackett SD, Ehlting C, Böhmer O, Albrecht U, Bode JG, Trautwein C, Schaper F. Glucocorticoids increase interleukin-6-dependent gene induction by interfering with the expression of the suppressor of cytokine signaling 3 feedback inhibitor. Hepatology. 2012;55:256–66. doi: 10.1002/hep.24655. [DOI] [PubMed] [Google Scholar]
- 12.Liao W, Lin J-X, Wang L, Li P, Leonard WJ. Modulation of cytokine receptors by IL-2 broadly regulates differentiation into helper T cell lineages. Nat Immunol. 2011;12:551–9. doi: 10.1038/ni.2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gong J, Zhang JP, Li B, Zeng C, You K, Chen MX, Yuan Y, Zhuang SM. MicroRNA-125b promotes apoptosis by regulating the expression of Mcl-1, Bcl-w and IL-6R. Oncogene. 2013;32:3071–9. doi: 10.1038/onc.2012.318. [DOI] [PubMed] [Google Scholar]
- 14.Libertini SJ, Chen H, al-Bataina B, Koilvaram T, George M, Gao AC, Mudryj M. The interleukin 6 receptor is a direct transcriptional target of E2F3 in prostate tumor derived cells. Prostate. 2012;72:649–60. doi: 10.1002/pros.21468. [DOI] [PubMed] [Google Scholar]
- 15.Coppé J-P, Desprez P-Y, Krtolica A, Campisi J. The senescence-associated secretory phenotype: the dark side of tumor suppression. Annu Rev Pathol. 2010;5:99–118. doi: 10.1146/annurev-pathol-121808-102144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ohtani N, Takahashi A, Mann DJ, Hara E. Cellular senescence: a double-edged sword in the fight against cancer. Exp Dermatol. 2012;21(Suppl 1):1–4. doi: 10.1111/j.1600-0625.2012.01493.x. [DOI] [PubMed] [Google Scholar]
- 17.Kuilman T, Michaloglou C, Vredeveld LC, Douma S, van Doorn R, Desmet CJ, Aarden LA, Mooi WJ, Peeper DS. Oncogene-induced senescence relayed by an interleukin-dependent inflammatory network. Cell. 2008;133:1019–31. doi: 10.1016/j.cell.2008.03.039. [DOI] [PubMed] [Google Scholar]
- 18.Schmidt S, Essmann F, Cirstea IC, Kuck F, Thakur HC, Singh M, Kletke A, Jänicke RU, Wiek C, Hanenberg H, et al. The centrosome and mitotic spindle apparatus in cancer and senescence. Cell Cycle. 2010;9:4469–73. doi: 10.4161/cc.9.22.13684. [DOI] [PubMed] [Google Scholar]
- 19.Blagosklonny MV. Cell cycle arrest is not yet senescence, which is not just cell cycle arrest: terminology for TOR-driven aging. Aging (Albany NY) 2012;4:159–65. doi: 10.18632/aging.100443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leontieva OV, Lenzo F, Demidenko ZN, Blagosklonny MV. Hyper-mitogenic drive coexists with mitotic incompetence in senescent cells. Cell Cycle. 2012;11:4642–9. doi: 10.4161/cc.22937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kolesnichenko M, Hong L, Liao R, Vogt PK, Sun P. Attenuation of TORC1 signaling delays replicative and oncogenic RAS-induced senescence. Cell Cycle. 2012;11:2391–401. doi: 10.4161/cc.20683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cornu M, Albert V, Hall MN. mTOR in aging, metabolism, and cancer. Curr Opin Genet Dev. 2013;23:53–62. doi: 10.1016/j.gde.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 23.Hobisch A, Rogatsch H, Hittmair A, Fuchs D, Bartsch GJ, Jr., Klocker H, Bartsch G, Culig Z. Immunohistochemical localization of interleukin-6 and its receptor in benign, premalignant and malignant prostate tissue. J Pathol. 2000;191:239–44. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH633>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 24.Leu CM, Wong FH, Chang C, Huang SF, Hu CP. Interleukin-6 acts as an antiapoptotic factor in human esophageal carcinoma cells through the activation of both STAT3 and mitogen-activated protein kinase pathways. Oncogene. 2003;22:7809–18. doi: 10.1038/sj.onc.1207084. [DOI] [PubMed] [Google Scholar]
- 25.Noda M, Yamakawa Y, Matsunaga N, Naoe S, Jodoi T, Yamafuji M, Akimoto N, Teramoto N, Fujita K, Ohdo S, et al. IL-6 Receptor Is a Possible Target against Growth of Metastasized Lung Tumor Cells in the Brain. Int J Mol Sci. 2012;14:515–26. doi: 10.3390/ijms14010515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Siegall CB, Schwab G, Nordan RP, FitzGerald DJ, Pastan I. Expression of the interleukin 6 receptor and interleukin 6 in prostate carcinoma cells. Cancer Res. 1990;50:7786–8. [PubMed] [Google Scholar]
- 27.Garcia-Tuñón I, Ricote M, Ruiz A, Fraile B, Paniagua R, Royuela M. IL-6, its receptors and its relationship with bcl-2 and bax proteins in infiltrating and in situ human breast carcinoma. Histopathology. 2005;47:82–9. doi: 10.1111/j.1365-2559.2005.02178.x. [DOI] [PubMed] [Google Scholar]
- 28.Gao SP, Mark KG, Leslie K, Pao W, Motoi N, Gerald WL, Travis WD, Bornmann W, Veach D, Clarkson B, et al. Mutations in the EGFR kinase domain mediate STAT3 activation via IL-6 production in human lung adenocarcinomas. J Clin Invest. 2007;117:3846–56. doi: 10.1172/JCI31871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Song MS, Salmena L, Pandolfi PP. The functions and regulation of the PTEN tumour suppressor. Nat Rev Mol Cell Biol. 2012;13:283–96. doi: 10.1038/nrm3330. [DOI] [PubMed] [Google Scholar]
- 30.Schmidt S, Schneider L, Essmann F, Cirstea IC, Kuck F, Kletke A, Jänicke RU, Wiek C, Hanenberg H, Ahmadian MR, et al. The centrosomal protein TACC3 controls paclitaxel sensitivity by modulating a premature senescence program. Oncogene. 2010;29:6184–92. doi: 10.1038/onc.2010.354. [DOI] [PubMed] [Google Scholar]
- 31.Demidenko ZN, Zubova SG, Bukreeva EI, Pospelov VA, Pospelova TV, Blagosklonny MV. Rapamycin decelerates cellular senescence. Cell Cycle. 2009;8:1888–95. doi: 10.4161/cc.8.12.8606. [DOI] [PubMed] [Google Scholar]
- 32.Lurje G, Lenz HJ. EGFR signaling and drug discovery. Oncology. 2009;77:400–10. doi: 10.1159/000279388. [DOI] [PubMed] [Google Scholar]
- 33.Schooltink H, Stoyan T, Lenz D, Schmitz H, Hirano T, Kishimoto T, Heinrich PC, Rose-John S. Structural and functional studies on the human hepatic interleukin-6 receptor. Molecular cloning and overexpression in HepG2 cells. Biochem J. 1991;277:659–64. doi: 10.1042/bj2770659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Song MS, Salmena L, Pandolfi PP. The functions and regulation of the PTEN tumour suppressor. Nat Rev Mol Cell Biol. 2012;13:283–96. doi: 10.1038/nrm3330. [DOI] [PubMed] [Google Scholar]
- 35.Cichowski K, Hahn WC. Unexpected pieces to the senescence puzzle. Cell. 2008;133:958–61. doi: 10.1016/j.cell.2008.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kuilman T, Michaloglou C, Vredeveld LC, Douma S, van Doorn R, Desmet CJ, Aarden LA, Mooi WJ, Peeper DS. Oncogene-induced senescence relayed by an interleukin-dependent inflammatory network. Cell. 2008;133:1019–31. doi: 10.1016/j.cell.2008.03.039. [DOI] [PubMed] [Google Scholar]
- 37.Yun UJ, Park SE, Jo YS, Kim J, Shin DY. DNA damage induces the IL-6/STAT3 signaling pathway, which has anti-senescence and growth-promoting functions in human tumors. Cancer Lett. 2012;323:155–60. doi: 10.1016/j.canlet.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 38.Dulic V. Senescence regulation by mTOR. Methods Mol Biol. 2013;965:15–35. doi: 10.1007/978-1-62703-239-1_2. [DOI] [PubMed] [Google Scholar]
- 39.Gerhartz C, Dittrich E, Stoyan T, Rose-John S, Yasukawa K, Heinrich PC, Graeve L. Biosynthesis and half-life of the interleukin-6 receptor and its signal transducer gp130. Eur J Biochem. 1994;223:265–74. doi: 10.1111/j.1432-1033.1994.tb18991.x. [DOI] [PubMed] [Google Scholar]
- 40.Freudlsperger C, Burnett JR, Friedman JA, Kannabiran VR, Chen Z, Van Waes C. EGFR-PI3K-AKT-mTOR signaling in head and neck squamous cell carcinomas: attractive targets for molecular-oriented therapy. Expert Opin Ther Targets. 2011;15:63–74. doi: 10.1517/14728222.2011.541440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rose-John S, Mitsuyama K, Matsumoto S, Thaiss WM, Scheller J. Interleukin-6 trans-signaling and colonic cancer associated with inflammatory bowel disease. Curr Pharm Des. 2009;15:2095–103. doi: 10.2174/138161209788489140. [DOI] [PubMed] [Google Scholar]
- 42.Grivennikov S, Karin E, Terzic J, Mucida D, Yu GY, Vallabhapurapu S, Scheller J, Rose-John S, Cheroutre H, Eckmann L, et al. IL-6 and Stat3 are required for survival of intestinal epithelial cells and development of colitis-associated cancer. Cancer Cell. 2009;15:103–13. doi: 10.1016/j.ccr.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thiem S, Pierce TP, Palmieri M, Putoczki TL, Buchert M, Preaudet A, Farid RO, Love C, Catimel B, Lei Z, et al. mTORC1 inhibition restricts inflammation-associated gastrointestinal tumorigenesis in mice. J Clin Invest. 2013;123:767–81. doi: 10.1172/JCI65086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yao Z, Fenoglio S, Gao DC, Camiolo M, Stiles B, Lindsted T, Schlederer M, Johns C, Altorki N, Mittal V, et al. TGF-beta IL-6 axis mediates selective and adaptive mechanisms of resistance to molecular targeted therapy in lung cancer. Proc Natl Acad Sci U S A. 2010;107:15535–40. doi: 10.1073/pnas.1009472107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chang J, Burkett PR, Borges CM, Kuchroo VK, Turka LA, Chang CH. MyD88 is essential to sustain mTOR activation necessary to promote T helper 17 cell proliferation by linking IL-1 and IL-23 signaling. Proc Natl Acad Sci U S A. 2013;110:2270–5. doi: 10.1073/pnas.1206048110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Alexander E, Hildebrand DG, Kriebs A, Obermayer K, Manz M, Rothfuss O, Schulze-Osthoff K, Essmann F. IκBζ is a regulator of the senescence-associated secretory phenotype in DNA damage- and oncogene-induced senescence. J Cell Sci. 2013;126:3738–45. doi: 10.1242/jcs.128835. [DOI] [PubMed] [Google Scholar]
- 47.Hundhausen C, Misztela D, Berkhout TA, Broadway N, Saftig P, Reiss K, Hartmann D, Fahrenholz F, Postina R, Matthews V, et al. The disintegrin-like metalloproteinase ADAM10 is involved in constitutive cleavage of CX3CL1 (fractalkine) and regulates CX3CL1-mediated cell-cell adhesion. Blood. 2003;102:1186–95. doi: 10.1182/blood-2002-12-3775. [DOI] [PubMed] [Google Scholar]
- 48.Ludwig A, Hundhausen C, Lambert MH, Broadway N, Andrews RC, Bickett DM, Leesnitzer MA, Becherer JD. Metalloproteinase inhibitors for the disintegrin-like metalloproteinases ADAM10 and ADAM17 that differentially block constitutive and phorbol ester-inducible shedding of cell surface molecules. Comb Chem High Throughput Screen. 2005;8:161–71. doi: 10.2174/1386207053258488. [DOI] [PubMed] [Google Scholar]
- 49.Mackiewicz A, Schooltink H, Heinrich PC, Rose-John S. Complex of soluble human IL-6-receptor/IL-6 up-regulates expression of acute-phase proteins. J Immunol. 1992;149:2021–7. [PubMed] [Google Scholar]
- 50.Suzuki A, de la Pompa JL, Stambolic V, Elia AJ, Sasaki T, del Barco Barrantes I, Ho A, Wakeham A, Itie A, Khoo W, et al. High cancer susceptibility and embryonic lethality associated with mutation of the PTEN tumor suppressor gene in mice. Curr Biol. 1998;8:1169–78. doi: 10.1016/S0960-9822(07)00488-5. [DOI] [PubMed] [Google Scholar]
- 51.Stambolic V, Suzuki A, de la Pompa JL, Brothers GM, Mirtsos C, Sasaki T, Ruland J, Penninger JM, Siderovski DP, Mak TW. Negative regulation of PKB/Akt-dependent cell survival by the tumor suppressor PTEN. Cell. 1998;95:29–39. doi: 10.1016/S0092-8674(00)81780-8. [DOI] [PubMed] [Google Scholar]
- 52.Reinehr R, Graf D, Fischer R, Schliess F, Häussinger D. Hyperosmolarity triggers CD95 membrane trafficking and sensitizes rat hepatocytes toward CD95L-induced apoptosis. Hepatology. 2002;36:602–14. doi: 10.1053/jhep.2002.35447. [DOI] [PubMed] [Google Scholar]
- 53.Schneider L, Essmann F, Kletke A, Rio P, Hanenberg H, Wetzel W, Schulze-Osthoff K, Nürnberg B, Piekorz RP. The transforming acidic coiled coil 3 protein is essential for spindle-dependent chromosome alignment and mitotic survival. J Biol Chem. 2007;282:29273–83. doi: 10.1074/jbc.M704151200. [DOI] [PubMed] [Google Scholar]
- 54.Chalaris A, Rabe B, Paliga K, Lange H, Laskay T, Fielding CA, Jones SA, Rose-John S, Scheller J. Apoptosis is a natural stimulus of IL6R shedding and contributes to the proinflammatory trans-signaling function of neutrophils. Blood. 2007;110:1748–55. doi: 10.1182/blood-2007-01-067918. [DOI] [PubMed] [Google Scholar]
- 55.Garbers C, Thaiss W, Jones GW, Waetzig GH, Lorenzen I, Guilhot F, Lissilaa R, Ferlin WG, Grötzinger J, Jones SA, et al. Inhibition of classic signaling is a novel function of soluble glycoprotein 130 (sgp130), which is controlled by the ratio of interleukin 6 and soluble interleukin 6 receptor. J Biol Chem. 2011;286:42959–70. doi: 10.1074/jbc.M111.295758. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.