Abstract
Clinical manifestations of rheumatoid arthritis (RA), the second most common human autoimmune disease, are primarily focused on the joints, causing disability and requiring life-long treatment to ameliorate signs and symptoms. The etiology of RA is unknown; however, important discoveries in two areas have been made which provide hope that the causal mechanisms can be identified. First, the most severe form of this disease is associated with the presence of humoral and cellular autoimmunity to citrullinated proteins and peptides. Second, in the natural history of RA, autoimmunity to citrullinated antigens appears years prior to the onset of clinically apparent disease. Herein is described a model in which to consider how these two features are linked during very early disease development.
Rheumatoid Arthritis: Clinical and Autoimmune Features
Rheumatoid arthritis (RA) is the second most prevalent autoimmune disorder and presents clinically as a systemic, inflammatory autoimmune disease that most typically affects women (reviewed in [1]). RA exhibits a prevalence of 0.5-0.8 per 100 individuals in the general population, and an ~3-5-fold increase in first-degree relatives (FDRs) (reviewed in [2]). The term RA encompasses two major subsets of disease, seropositive and seronegative (reviewed in [3]). Based on clinical comparisons as well as studies of environmental and genetic associations, it is considered that the two forms of RA likely exhibit overlapping but individually distinctive pathogenic mechanisms [4;5].
RA is considered a prototypic polygenic disorder in which genetic risk factors interact with environmental exposures and, in the context of increasingly understood immune alterations, lead to the development of a severe autoimmune attack against self-tissues [6]. Consistent with this concept are observations that there are many genes which associate with the presence of clinically apparent RA, including high risk HLA-DRB1 alleles containing the shared epitope (SE) [7], and PTPN22 [8], as well as many additional non-HLA genes, a substantial number of whose products are components of immune-related signaling pathways (reviewed in [9]). In addition, there are several well-accepted environmental exposures which increase or decrease the risk of developing RA [10;11]. However, despite these insights into pathogenesis and recent improvements in therapy, only a relatively small number of patients achieve a durable drug free disease remission in standard clinical practice even with the best of current therapies [12].
Because of this situation, in order to achieve more dramatic changes in long term outcomes and prognosis, new approaches to the understanding and treatment of this disorder are needed. With that goal, this overview reviews the emerging evidence that RA does not begin as an articular disease but rather is initiated outside of the joints at sites of local inflammation, likely at mucosal surfaces. The underlying premise of this line of reasoning is that characterizing the relevant causal immune alterations at these sites should lead to the ability to treat this disease very early using therapies designed to beneficially modify these extra-articular processes, and perhaps even prevent the evolution of disease as it progresses from early asymptomatic stages through to the onset of clinically apparent deforming arthritis.
Key Features of Humoral and Cellular Autoimmunity in RA
Several types of autoantibodies are found in patients with clinically apparent RA. One is rheumatoid factor (RF), which is an autoantibody that recognizes the Fc domain of IgG (reviewed in [13]). Of note, in patients IgM, IgG and IgA RF isotypes can all be readily detected, the presence of which indicate that there are ongoing related but immunologically distinct processes [14]. The second type of RA-related autoantibody demonstrates reactivity to citrullinated proteins and peptides, and thus are broadly designated anti-citrullinated protein antibodies (ACPA) (reviewed in [15-17]). Peptidylcitrulline is generated through the conversion of arginine to citrulline by one of a family of enzymes designated peptidyl arginine deiminases (PADs) (reviewed in [18]). This process occurs as a part of normal intracellular homeostasis, but extracellular citrullination of proteins in tissues is a characteristic of many if not all inflammatory conditions. Notably, PADs exhibit different subcellular localizations and target specificities for RA-related antigens [19], and PAD4 is also itself a target of the citrullination process in a manner which changes its functional characteristics [20].
Thus, what is unique to RA is not the presence of citrullinated proteins but rather the development of an autoimmune response to this post-translational modification [6]. In patients with RA, reactivity against many citrullinated proteins has been detected, although much of the research focus has been on modifications of fibrinogen, vimentin, enolase, and Type II collagen. From these complex proteins, specific immunoreactive citrullinated peptides have been identified and applied to multiplex screening, demonstrating substantial heterogeneity across the RA population [21]. In one recent study 17 different subsets within RA patients were identified based on fine specificity profiles, with substantial differences across the subsets in the relationships to genetic and environmental factors [22]. Additionally, one particular reactivity to citrullinated alpha-enolase demonstrated a linkage to both smoking and DR4 SE status [23].
Importantly, a high proportion of B cells in the inflamed joint from patients with RA produce IgG ACPA at high levels in an apparently clonally restricted manner [24]. Finally, it is not only the specific epitope reactivity of ACPA that is important, as there are alterations in avidity [25;26] and the presence of G0 carbohydrates [27;28] as well as differential potential engagement of several pathogenic effector functions, including relative complement-fixing capacity [29], and the ability to engage Fc receptors [30] (reviewed in [31]).
The primary commercially-available tests for ACPAs are in ELISA format with cyclic citrullinated protein/peptide (CCP) or other similarly modified antigens as the substrate. Anti-CCP antibodies were first described as demonstrating a sensitivity of 60-70% with a specificity of ~98% [17;32]; however, the specificity falls modestly when other diseases with arthritis or pulmonary manifestations are evaluated (reviewed in [33]). Recently, a related type of antibody to carbamylated proteins has been described as highly specific for RA but of modestly lower sensitivity, wherein reactivity is found against the post-translational homocitrulline modification of lysine that is similar in structure to citrulline but with one additional carbon [34]. Although there is substantial overlap in reactivity to both targets within individual patients, there are individuals who lack anti-CCP antibodies but exhibit anti-carbamylated protein antibodies, and vice versa [34].
In addition to humoral autoimmunity to citrullinated antigens, patients with RA also demonstrate evidence of cellular immunity to citrullinated autoantigens. In one study, enhanced reactivity to a citrullinated vimentin peptide was detected in HLA-DRB1*0401 SE positive patients through the use of specific peptide:T cell receptor (TCR) tetramers and proliferative responses [35], and in another through enhanced proliferative responses [36]. In another report, T cell proliferative cytokine responses were studied, and secretion of IL-6, IL-17 and tumor necrosis factor (TNF)-alpha by CD4+ cells was found to be present in normal controls and patients with RA, while interferon (IFN) and IL10 were specific for RA, with citrullinated aggrecan generating the most robust responses [37].
A Detectable ACPA-Positive Preclinical Period Exists Prior to Onset of Arthritis in the Natural History of Rheumatoid Arthritis
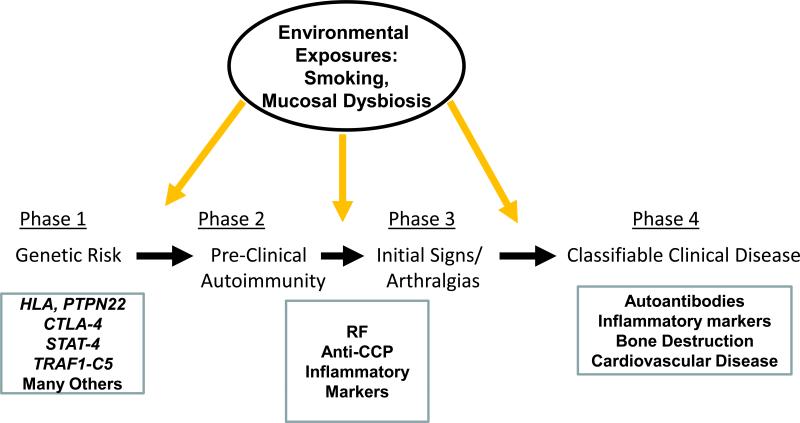
Although one might consider that the onset of RA occurs around the time that clinically apparent arthritis appears, manifest by joint pain and swelling, it has been found instead that there is a prolonged period of highly specific RA-related autoimmunity in patients that occurs for 3-5, and in some instances more than 10, years prior to the onset of clinically apparent disease [reviewed in [38]]. This period is defined as the “preclinical” period of RA in subjects who eventually develop the disease [39], and is now considered to be an intrinsic part of the disease process itself (Figure 1). As described herein the study of this period of time is opening up new opportunities to understand the natural history and pathogenesis of this disease.
Figure 1.
Phases in the development of RA, with known genetic, clinical and biomarker alterations in patients below, and potential environmental exposures that influence the disease course at the top.
Many studies of such early RA pathogenesis have been performed using retrospective serum samples and data bases [reviewed in [38]]. However, because there are not yet sufficient studies of subjects who have transitioned in real time through these periods while under study, much of the current research is being performed on individuals who are at high risk for the future development of disease because they exhibit a pattern of autoantibodies and biomarkers that indicate they are at high-risk for the future development of clinically apparent disease (reviewed in [38]).
With regard to characteristics of subjects as they progress, there are several methods utilized and observations that have been made. First, one can assess individuals who exhibit only a family-based elevated risk of disease because they are FDRs, regardless of the presence or absence of autoantibodies in these at-risk family members. In this instance, there are increases of anti-CCP antibodies as well as multiple cytokines in this population [40]; additionally, ACPA of IgG, IgA and IgM isotypes, as well as IgM and IgA isotype RF are increased in unaffected members of multicase rheumatoid arthritis families [41], although the pattern of reactivity against individual antigens is more restricted than patients with clinically apparent RA [42;43]. When stratifying for the presence or absence of RA-related autoantibodies within FDR populations, a positive autoantibody status shows further preferential associations of cytokine elevations that are typically found in patients with active RA [44], and an increase in the number of individual ACPA peptide reactivities [43].
Finally, one can utilize samples serially collected in the past for other purposes to retrospectively evaluate biomarker evolution during the preclinical period. Using this approach, what is observed prior to the onset of clinically apparent arthritis is an increase in titer and expansion of epitope reactivity to stably encompass a wider variety of specific targets [45;46], an increase in the number of elevated cytokines [47-50], and avidity maturation of ACPA [26].
Notably, the finding that preclinical asymptomatic disease evolves into clinically apparent disease after a prolonged period of highly specific autoantibody positivity is a common feature of many autoimmune diseases. A similar type of progression occurs during the evolution of systemic lupus erythematosus [51], type 1 diabetes mellitus (T1DM) [52], celiac disease [53] and Hashimoto's thyroiditis [54]. What is unusual about RA, though, is that as opposed to other conditions in which disease-associated autoantibodies occur as an apparent manifestation of ongoing subclinical inflammation and organ-specific damage, such as in beta cells of the islets, is that the preclinical period in RA is apparently not characterized by detectable arthritis [55].
Are ACPA Pathogenic or Just Disease-Associated Biomarkers?
Observational studies in humans by their nature can only detect associations, and similarly to other diseases, there is the question of whether ACPA are pathogenic or simply markers of disease. Indeed, the finding that ACPA are present for years in the absence of arthritis suggests they alone are not alone sufficient to cause disease, although there are changes as noted above in their characteristics over time which may exceed a threshold of pathogenic potential. The question of pathogenic potential has been addressed primarily through murine models, wherein several studies have demonstrated that citrullinated autoantigen responses by both autoreactive B and T cells can lead to or amplify arthritis [56-60].
In addition, recently it has been shown that citrullination of mouse type II collagen eliminates the need to utilize adjuvant in the model in order to initiate arthritis in the collagen-induced arthritis model [61]. Finally, using purified human IgG reactive with mutated citrullinated vimentin, induction of osteopenia and bone resorption was demonstrated in vivo, and both binding to osteoclasts and osteoclastogenesis was seen in in vitro [62]. Thus, it appears that ACPA at least have the potential to be pathogenic, especially in the presence of pre-existing inflammation in the joint that leads to the elaboration of citrullinated proteins as “targets” of ACPA.
Where and How Do ACPA Originate at Extra-Articular Sites?
Even though the great majority of subjects with RA-related autoantibodies alone are asymptomatic, one might wonder whether there is subclinical inflammation. This subject has been addressed in several ways, but perhaps most importantly by performing synovial biopsy and magnetic resonance imaging in subjects who exhibit antibodies in the absence of clinically detectable arthritis. In that study, no histologic or MRI-based evidence of synovial inflammation was found in the knee [55].
In contrast to the lack of evidence of synovial inflammation, other studies strongly suggest that the RA-related autoimmunity and biomarker alterations may originate from a mucosal site. As one point in favor of this hypothesis, a substantial number of subjects manifest IgA ACPA and RF in the preclinical state [63-65]. Perhaps more importantly, though, alterations at specific mucosal sites, including the oral cavity, the lungs and intestines, suggest that microbial factors at these sites may affect the mucosal immune response and play an important role in the early pathogenesis of RA.
With regard to the oral cavity and periodontal region, there is an elevated occurrence of severe periodontitis in patients with RA, and patients with severe periodontitis exhibit higher disease activity scores [66]. Notably, patients with severe periodontitis manifest higher titers of IgG and IgM antibodies to Porphyromonas gingivalis, which is important because this organism exhibits PAD activity and citrullinates human fibrinogen and alpha-enolase in the presence of bacterial gingipains [67]. In addition, in subjects without RA but who exhibit RA-related autoantibodies in a pattern that is high-risk for future disease, there is a specific elevation of antibodies to Porphyromonas gingivalis but not to related strains [68]. In contrast, other studies have found that Prevotella and Leptotrichia, but not Porphyromonas gingivalis, are specifically expanded in the subgingival microbiota in patients with recent onset untreated RA [69].
Perhaps an even stronger case can be made for changes in the lung that promote the local development of ACPA. For instance, cigarette smoke exposure is highly associated with risk for the development of RA, especially in the presence of HLA expressing the DR4 SE [4;11]. In addition, although standard chest x-rays are typically normal, when assessed by sensitive high-resolution computed tomography, patients with early RA demonstrate a substantial number of pulmonary abnormalities [70]. The latter finding is particularly important because when individuals who do not have RA but exhibit high-risk RA-related autoantibodies, they similarly demonstrate a high rate of inflammatory airway disease that is not associated with smoke or other similar exposures [71]. Supporting the idea that there is local production of RA-related autoantibodies in the lung are studies of the sputa from subjects who exhibit family-based risk for the future development of RA, where RF and ACPA can be readily demonstrated in the sputa but not peripheral blood of a subset of individuals [72]. This is also notable because prior studies of subjects with RA and pulmonary disease had demonstrated local production of ACPA and RF by inducible bronchus associated lymphoid tissue [73].
Finally, the third mucosal site that has garnered attention is the intestine, where gut microbes are well known to be able to influence immune responses, and where experimental arthritis has been modulated by the exposure in the gut to a single organism [74]. Early studies have also suggested that change in the gut microbiota are associated with concurrent RA [75], and studies are being performed to evaluate whether similar changes might exist in subject manifesting high-risk autoantibodies. These studies, plus ongoing single cell analyses of human lymph node biopsies in patients and at-risk subjects [76], should continue to be informative with regard to the architecture of the ACPA response.
How Does Mucosal Autoimmunity Develop and then Transition to Local Inflammatory Arthritis?
With regard to the immune mechanisms that drive the loss of tolerance at mucosal sites, and also allow the evolution of disease from there to the joints, very little is known in patients; therefore, one can only speculate based on other similar situations and the little that is known about human at-risk subjects and very early arthritis. In the mucosal site, possible roles for citrullinated microbial antigens and molecular mimicry [67], Toll-like receptor (TLR) signals [reviewed in [77], and other innate immune activators and danger signals [78] exist. In addition, recent studies suggest that neutrophil extracellular traps (NETs) may play an important role in RA, as this type of neutrophil is found in tissue of patients with RA, circulates at levels that correlate with disease activity in patients, externalize citrullinated autoantigens, and are generated from neutrophils following activation by anti-citrullinated vimentin antibodies [79].
With regard to the transition from an autoimmune and inflammatory reaction at a mucosal site associated with circulating autoantibodies to then attack the joints, similarly little is known in human disease. Nevertheless, several potential mechanisms exist and have been explored either in patients or animal models (reviewed in [6;80]). One possibility, reflecting the presence of circulating immune complexes containing citrullinated fibrinogen in patients with RA [81], which notably can more effectively activate macrophages through the synergistic engagement of TLR4 and Fc receptors [82], is that immune complexes can enter the joint and deposit on cartilage and in synovial tissue. Another possibility is that transient expression of citrullinated antigens allows the ability of circulating autoantibodies [58;60] or T cells [83] to recognize and initiate or amplify inflammation. A third, potentially explaining the link between the early involvement of the lung with later arthritis, may be shared expression of citrullinated vimentin that is recognized by pathogenic autoantibodies at both sites [84].
Can One Use RA-Related Autoantibody Screening to Identify At-Risk Status and Halt Progression to Arthritis?
Because the pathway described out above strongly suggests that one might be able to interrupt the process, and there is a prolonged period of autoantibody positivity, one naturally could consider whether it is possible to screen for risk of developing RA and initiate a prevention therapy, perhaps focused on the mucosal site itself. Several recent publications have addressed this important topic [85;86], and interested readers are referred to these for further information.
Conclusion
RA is a potentially devastating chronic autoimmune disease that is treatable but is only moderately improved in most patients with current therapies. Substantial evidence from many investigators has been accumulated which demonstrates that the disease process begins years prior to the development of clinically apparent arthritis. The mechanisms by which autoantibodies and inflammatory biomarkers develop, in the absence of clinically and radiographically detectable arthritis, are under active investigation. This overview has summarized the evidence supporting the hypothesis that in a substantial number of patients, a dysbiosis at one or more mucosal sites leads to immune alterations and the initial break in self-tolerance to citrullinated autoantigens. This autoimmune process progresses to involve the joints secondarily. Although much work remains to further evaluate this concept, if it is validated there would be many important ramifications with regard to treatment approaches, therapeutic targets, and screening for future risk of disease.
Highlights.
RA exhibits a long preclinical period with autoantibodies and other biomarkers
Highly specific preclinical autoimmunity to citrullinated antigens develops early
RA appears to be initiated by processes outside of the joints
Inflammation at mucosal sites may drive the early phases of RA development
Acknowledgements
Work presented herein from the laboratory of the author has been supported by NIH U01 AI101981, NIH R21 AI61479, NIH U19 AI50864, NIH R01 AR051394, and a Disease Targeted Research Initiative Grant from the Rheumatology Research Foundation. The author acknowledges the important contributions of many colleagues to the concepts presented herein, including Drs. Jill Norris, Kevin Deane, Bill Robinson, Katie Haskins, Van Willis, Kristen Cordova, Kristen Demoruelle, Hani El-Gabalawy, Beth Karlson, Bill Arend, our many colleagues in the research groups led by Drs. Tom Huizinga and Lars Klareskog, and the leadership of the Studies of the Etiology of Rheumatoid Arthritis (SERA) consortium (Drs. Jim O'Dell, Ted Mikuls, Michael Weisman, Jane Buckner, Richard Keating and Peter Gregersen).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature Cited
○ Of special or outstanding interest
- 1.Lee DM, Weinblatt ME. Rheumatoid arthritis. Lancet. 2001;359:903–911. doi: 10.1016/S0140-6736(01)06075-5. [DOI] [PubMed] [Google Scholar]
- 2.Silman AJ. Epidemiology of rheumatoid arthritis. APMIS. 1994;102:721–728. doi: 10.1111/j.1699-0463.1994.tb05226.x. [DOI] [PubMed] [Google Scholar]
- 3.Fuchs HA, Sergent JS. Rheumatoid Arthritis: The Clinical Picture. In: Koopman WJ, editor. Arthritis and Allied Conditions: A Textbook of Rheumatology. edn 13 Williams and Wilkins; Baltimore, MD: 1997. pp. 1041–1070. [Google Scholar]
- 4.Klareskog L, Malmstrom V, Lundberg K, Padyukov L, Alfredsson L. Smoking, citrullination and genetic variability in the immunopathogenesis of rheumatoid arthritis. Seminars in Immunology. 2011;23:92–98. doi: 10.1016/j.smim.2011.01.014. [DOI] [PubMed] [Google Scholar]
- 5.Padyukov L, Silva C, Stolt P, Alfredsson L, Klareskog L. A gene-environment interaction between smoking and shared epitope genes in HLA-DR provides a high risk of seropositive rheumatoid arthritis. Arth.Rheum. 2004;50:3085–3092. doi: 10.1002/art.20553. [DOI] [PubMed] [Google Scholar]
- 6.Klareskog L, Ronnelid J, Lundberg K, Padyukov L, Alfredsson L. Immunity to citrullinated proteins in rheumatoid arthritis. Ann.Rev.Immunol. 2008;26:651–675. doi: 10.1146/annurev.immunol.26.021607.090244. [DOI] [PubMed] [Google Scholar]
- 7.Nepom GT, Nepom BS. Prediction of susceptibility to rheumatoid arthritis by human leukocyte antigen phenotyping. Rheum.Dis.Clin.North Am. 1992;18:785–792. [PubMed] [Google Scholar]
- 8.Begovich AB, Carlton VE, Honigberg LA, Schrodi SJ, Chokkalingam AP, Alexander HC, Ardlie KG, Huang Q, Smith AM, Spoerke JM, Conn MT, Chang M, Saiki RK, Catanese JJ, Leong DU, Garcia VE, McAllister LB, Jeffery DA, Lee AT, Batliwalla FM, Remmers E, Criswell LA, Seldin MF, Kastner DL, Amos CI, Sninsky JJ, Gregersen PK. A missense single-nucleotide polymorphism in a gene encoding a protein tyrosine phosphatase (PTPN22) is associated with rheumatoid arthritis. American J.Human Genetics. 2004;75:330–337. doi: 10.1086/422827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bax M, van Heemst J, Huizinga TWJ, Toes REM. Genetics of rheumatoid arthritis: what have we learned? Immunogenetics. 2011;63:459–466. doi: 10.1007/s00251-011-0528-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Karlson EW, Deane KD. Environmental and gene-environment interactions and risk of rheumatoid arthritis. Rheum.Dis.Clin.North Am. 2012;38:405–426. doi: 10.1016/j.rdc.2012.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11○.Klareskog L, Stolt P, Lundberg K, Kallberg H, Bengtsson C, Grunewald J, Ronnelid J, Harris HE, Ulfgren AK, Rantapaa Dahlqvist S, Eklund A, Padyukov L, Alfredsson L. A new model for an etiology of rheumatoid arthritis: smoking may trigger HLA-DR (shared epitope)-restricted immune reactions to autoantigens modified by citrullination. Arth.Rheum. 2006;54:38–46. doi: 10.1002/art.21575. [Seminal contribution to the understanding of the genetic epidemiology of RA] [DOI] [PubMed] [Google Scholar]
- 12.Prince FH, Bykerk VP, Shadick NA, Iannaccone CK, Weinblatt ME, Solomon DH. Sustained rheumatoid arthritis remission is uncommon in clinical practice. Arth.Care Res. 2012 Mar 19;14(2):R68. doi: 10.1186/ar3785. doi:10.1186/ar3785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schrohenloher RE, Bridget SL, Koopman WJ. Rheumatoid Factor. In: Koopman WJ, editor. Arthritis and Allied Conditions: A Textbook of Rheumatology. edn 13 Williams and Wilkins; Baltimore, MD: 1997. pp. 1109–1130. [Google Scholar]
- 14.Newkirk MM. Rheumatoid factors: what do they tell us? J.Rheum. 2000;29:2034–2040. [PubMed] [Google Scholar]
- 15.Girbal-Neuhauser E, Durieux JJ, Arnaud M, Dalbon P, Sebbag M, Vincent C, Simon M, Senshu T, Masson-Bessiere C, Jolivet-Reynaud C, Jolivet M, Serre G. The epitopes targeted by the rheumatoid arthritis-associated antifilaggrin autoantibodies are posttranslationally generated on various sites of (pro)filaggrin by deimination of arginine residues. J.Immunol. 1999;162:585–594. [PubMed] [Google Scholar]
- 16○.Schellekens GA, de Jong BAW, van den Hoogen FHJ, van de Putte LBA, van Venrooij WJ. Citrulline is an essential constituent of antigenic determinants recognized by rheumatoid arthritis-specific autoantibodies. J.Clin.Invest. 1998;101:273–281. doi: 10.1172/JCI1316. [Classic identification of the target of disease-specific autoantibodies as citrullinated epitopes] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schellekens GA, Visser H, de Jong BAW, van den Hoogen FHJ, Hazes JMW, Breedveld FC, van Venrooij WJ. The diagnostic properties of rheumatoid arthritis antibodies recognizing a cyclic citrullinated peptide. Arth.Rheum. 2000;43:155–163. doi: 10.1002/1529-0131(200001)43:1<155::AID-ANR20>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 18.Bicker KL, Thompson PR. The protein arginine deiminases: Structure, function, inhibition, and disease. Biopolymers. 2013;99:155–163. doi: 10.1002/bip.22127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Darrah E, Rosen A, Giles JT, Andrade F. Peptidylarginine deiminase 2, 3 and 4 have distinct specificities against cellular substrates: novel insights into autoantigen selection in rheumatoid arthritis. Ann.Rheum.Dis. 2012;71:92–98. doi: 10.1136/ard.2011.151712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Andrade F, Darrah E, Gucek M, Cole RN, Rosen A, Zhu X. Autocitrullination of human peptidyl arginine deiminase type 4 regulates protein citrullination during cell activation. Arth.Rheum. 2010;62:1630–1640. doi: 10.1002/art.27439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hueber W, Kidd BA, Tomooka BH, Lee BJ, Bruce B, Fries JF, Sonderstrup G, Monach P, Drijfhout JW, vanVenrooij WJ, Utz PJ, Genovese MC, Robinson WH. Antigen microarray profiling of autoantibodies in rheumatoid arthritis. Arth.Rheum. 2005;52:2645–2655. doi: 10.1002/art.21269. [DOI] [PubMed] [Google Scholar]
- 22○.Lundberg K, Bengtsson C, Kharlamova N, Reed E, Jiang X, Kallberg H, Pollak-Dorocic I, Israelsson L, Kessel C, Padyukov L, Holmdahl R, Alfredsson L, Klareskog L. Genetic and environmental determinants for disease risk in subsets of rheumatoid arthritis defined by the anticitrullinated protein/peptide antibody fine specificity profile. Ann.Rheum.Dis. 2013;72:652–658. doi: 10.1136/annrheumdis-2012-201484. [Demonstrates complexity of genetic and environmental relationships as they relate to the fine specificity of the ACPA response] [DOI] [PubMed] [Google Scholar]
- 23○.Mahdi H, Fisher BA, Kallberg H, Plant D, Malmstrom V, Ronnelid J, Charles P, Ding B, Alfredsson L, Padyukov L, Symmons DP, Venables PJ, Klareskog L, Lundberg K. Specific interaction between genotype, smoking and autoimmunity to citrullinated alpha-enolase in the etiology of rheumatoid arthritis. Nat.Genetics. 2009;41:1319–1324. doi: 10.1038/ng.480. [Additional evidence that epitope-specific ACPA responses are strongly influenced by genotype and environmental exposures] [DOI] [PubMed] [Google Scholar]
- 24.Amara K, Steen J, Murray F, Morbach H, Fernandez-Rodriguez BM, Joshua V, Engstrom M, Snir O, Israelsson L, Catrina AI, Wardemann H, Corti D, Meffre E, Klareskog L, Malmstrom V. Monoclonal IgG antibodies generated from joint-derived B cells of RA patients have a strong bias toward citrullinated autoantigen recognition. J.Exp.Med. 2013;210:445–455. doi: 10.1084/jem.20121486. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 25.Suwannalai P, Scherer HU, ver der Woude D, Ioan-Facsinay A, Jol-van der Zijde CM, van Tol MJ, Drijfhout AW, Huizinga TW, Toes REM, Trouw LA. Anti-citrullinated protein antibodies have a low avidiy compared with antibodies against recall antigens. Ann.Rheum.Dis. 2011;70:373–379. doi: 10.1136/ard.2010.135509. [DOI] [PubMed] [Google Scholar]
- 26.Suwannalai P, van de Stadt LA, Radner H, Steiner G, El-Gabalawy HS, Jol-van der Zijde CM, Toes REM, Trouw LA. Avidity maturation of anti-citrullinated protein antibodies in rheumatoid arthritis. Arth.Rheum. 2012;64:1323–1328. doi: 10.1002/art.33489. [DOI] [PubMed] [Google Scholar]
- 27.Ercan A, Cui J, Chatterton DEW, Deane KD, Hazen MM, Brintnell W, O'Donnell CI, Derber LA, Weinblatt ME, Shadick NA, Bell DA, Cairns E, Solomon DH, Holers VM, Rudd PM, Lee DM. IgG galactosylation aberrancy precedes disease onset, correlates with disease activity and is prevalent in autoantibodies in rheumatoid arthritis. Arth.Rheum. 2010;62:2238–2248. doi: 10.1002/art.27533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scherer HU, van der Woude D, Ioan-Facsinay A, el Bannoudi H, Trouw LA, Wang J, Haupl T, Burmester G-R, Deelder AM, Huizinga TW, Wuhrer M, Toes REM. Glycan profiling of anti-citrullinaed protein antibodies isolated from human serum and synovial fluid. Arth.Rheum. 2010;62:1620–1629. doi: 10.1002/art.27414. [DOI] [PubMed] [Google Scholar]
- 29.Trouw LA, Haisma EM, Levarht EWN, van der Woude D, Ioan-Facsinay A, Daha MR, Huizinga TW, Toes REM. Anti-cyclic peptide antibodies from rheumatoid arthritis patients activate complement via both the classical and alternative pathways. Arth.Rheum. 2009;60:1923–1931. doi: 10.1002/art.24622. [DOI] [PubMed] [Google Scholar]
- 30.Laurent L, Clavel C, Lemaire O, Anquetil F, Cornillet M, Zabraniecki L, Nogueira L, Fournie B, Serre G, Sebbag M. Fc-gamma receptor profile of monocytes and macrophages from rheumatoid arthritis patients and their response to immune complexes formed with autoantibodies to citrullinated proteins. Ann.Rheum.Dis. 2011;70:1052–1059. doi: 10.1136/ard.2010.142091. [DOI] [PubMed] [Google Scholar]
- 31.Willemze A, Trouw LA, Toes REM, Huizinga TWJ. The influence of ACPA status and characteristics on the course of RA. Nat.Rev.Rheum. 2012;8:144–152. doi: 10.1038/nrrheum.2011.204. [DOI] [PubMed] [Google Scholar]
- 32.Goldbach-Mansky R, Lee J, McCoy A, Hoxworth J, arboro C, Smolen J, Steiner G, Rosen A, Zhang C, Menard HA, Zhou ZJ, Palosuo T, van Venrooij WJ, Wilder RL, Klippel HH, Schumacher HR, El-Gabalawy HS. Rheumatoid arthritis associated autoantibodies in patients wtih synovitis of recent onset. Arth.Res.Ther. 2000;2:236–243. doi: 10.1186/ar93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Demoruelle MK, Deane KD. Antibodies to citrullinated protein antigens (ACPAs): clinical and pathophysiologic significance. Curr.Rheumatol.Rep. 2011;13:421–430. doi: 10.1007/s11926-011-0193-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34○.Shi J, Knevel R, Suwannalai P, van der Linden MPM, Janssen GM, van Veelen PA, Levarht NE, van der Helm-van Mil AHM, Cerami A, Huizinga TW, Toes RE, Trouw LA. Autoantibodies recognizing carbamylated proteins are present in sera of patients with rheumatoid arthritis and predict joint damage. PNAS. 2011;108:17372–17377. doi: 10.1073/pnas.1114465108. [New RA-related autoantibody system that is distinct from ACPA but likely also generated by neutrophil-dependent processes] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35○.Snir O, Rieck M, Gebe JA, Yue BB, Rawlings CA, Nepom GT, Malmstrom V, Buckner JH. Identification and functional characterization of T cells reactive to citrullinated-vimentin in HLA-DRB1*0401 humanized mice and RA patients. Arth.Rheum. 2011;63:2873–2883. doi: 10.1002/art.30445. [Demonstration of peptide-specific T cell responses in patients with RA using state-of-the art techniques] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Feitsma AL, van der Voort EI, Franken KL, el Bannoudi H, Elferink BG, Drijfhout JW, Huizinga TW, de Vries RR, Toes RE, Ioan-Facsinay A. Identification of citrullinated vimentin peptides as T cell epitopes in HLA-DR4-positive in patients with rheumatoid arthritis. Arth.Rheum. 2010;62:117–125. doi: 10.1002/art.25059. [DOI] [PubMed] [Google Scholar]
- 37.Law SC, Street S, Yu CH, Capini C, Ramnoruth S, Nel HJ, van Gorp E, Hyde C, Lau K, Pahau H, Purcell AW, Thomas R. T-cell autoreactivity to citrullinated autoantigenic peptides in rheumatoid arthritis patients carrying HLA-DRB1 shared epitope alleles. Arth.Res.Ther. 2012;14:R118. doi: 10.1186/ar3848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Deane KD, Norris JM, Holers VM. Pre-clinical rheumatoid arthritis: identification, evaluation and future directions for investigation. Rheum.Dis.Clin.North Am. 2010:236–241. doi: 10.1016/j.rdc.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gerlag DM, Raza K, van Baarsen LGM, Brouwer E, Buckley CD, Burmester GR, Gabay C, Pope AP, Cornelis F, Dahlqvist SR, Emery P, Eyre S, Finckh A, Gay S, Hazes JMW, van der Helm-van Mil AHM, Huizinga TW, Klareskog L, Kvien TK, Lewis C, Machold KP, Ronnelid J, van Schaardenburg D, Schett G, Smolen JS, Thomas S, Worthington J, Tak PP. EULAR recommendations for terminology and research in individuals at risk of rheumatoid arthritis: report from the Study Group for Risk Factors for Rheumatoid Arthritis. Ann.Rheum.Dis. 2012;71:638–641. doi: 10.1136/annrheumdis-2011-200990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.El-Gabalawy HS, Robinson DB, Smolik I, Hart D, Elias B, Wong K, Peschken CA, Hitchon CA, Li X, Bernstein CN, Newkirk MM, Fritzler MJ. Familial clustering of the serum cytokine profile in the relatives of rheumatoid arthritis patients. Arth.Rheum. 2012;64:1720–1729. doi: 10.1002/art.34449. [DOI] [PubMed] [Google Scholar]
- 41.Arlestig L, Mullazehi M, Kokkonen H, Rocklov J, Ronnelid J, Dahlqvist SR. Antibodies against cyclic citrullinated peptides of IgG, IgA and IgM isotype and rheumatoid factor of IgM and IgA isotype are increased in unaffected members of multicase rheumatoid arthritis families from northern Sweden. Ann.Rheum.Dis. 2012;71:825–829. doi: 10.1136/annrheumdis-2011-200668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Barra L, Scinocca M, Saunders S, Bhayana R, Rohekar S, Racape M, Coles R, Cairns E, Bell DA. Anti-citrullinated protein antibodies in unaffected first-degree relatives of rheumatoid arthritis patients. Arth.Rheum. 2013;65:1439–1447. doi: 10.1002/art.37911. [DOI] [PubMed] [Google Scholar]
- 43.Young KA, Deane KD, Derber LA, Hughes-Austin J, Wagner CA, Sokolove J, Weisman MH, Buckner JH, Mikuls TR, O'Dell JR, Keating RM, Gregersen PK, Robinson WH, Holers VM, Norris JM. Relatives without rheumatoid arthritis show reactivity to anti-citrullinated protein/peptide antibodies that are associated with arthritis-related traits: studies of the etiology of rheumatoid arthritis. Arth.Rheum. 2013;65:1995–2004. doi: 10.1002/art.38022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hughes-Austin J, Deane KD, Derber LA, Kolfenbach JR, Zerbe GO, Sokolov J, Lahey LJ, Weisman MH, Buckner JH, Mikuls TR, O'Dell JR, Keating RM, Gregersen PK, Robinson WH, Holers VM, Norris JM. Multiple cytokines and chemokines are associated with rheumatoid arthritis-related autoimmunity in first-degree relatives without rheumatoid arthritis: Studies of the Aetiology of Rheumatoid Arthritis (SERA). Ann.Rheum.Dis. 2012;72:901–907. doi: 10.1136/annrheumdis-2012-201505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brink M, Hansson M, Mathsson L, Jakobsson PJ, Holmdahl R, Hallmans G, Stenlund H, Ronnelid J, Klareskog L, Rantapaa Dahlqvist S. Multiplex analyses of antibodies against citrullinated peptides in individuals prior to the development of rheumatoid arthritis. Arth.Rheum. 2013;65:899–910. doi: 10.1002/art.37835. [DOI] [PubMed] [Google Scholar]
- 46○.Sokolove J, Bromberg R, Deane KD, Lahey LJ, Derber LA, Chandra PE, Edison JD, Gilliland WR, Tibshirani RJ, Norris JM, Holers VM, Robinson WH. Autoantibody epitope spreading in the pre-clinical phase predicts progression to rheumatoid arthritis. PLoS one. 2012;7:e35296. doi: 10.1371/journal.pone.0035296. [Elegant study of epitope spreading in the ACPA response during the early development of RA in the preclinical stage] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47○.Deane KD, O'Donnell CI, Hueber W, Majka DS, Lazar AA, Derber LA, Gilliland WR, Edison JD, Norris JM, Robinson WH, Holers VM. The number of elevated cytokines and chemokines in preclinical seropositive rheumatoid arthritis predicts time to diagnosis in an age-dependent manner. Arth.and Rheum. 2010;62:3161–3172. doi: 10.1002/art.27638. [First quantitative model that proposes a method whereby the likelihood and timing of onset of clinically apparent RA can be defined] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jorgensen KT, Wiik a, Pedersen M, Hedegaard CJ, Gislefoss RE, Kvien TK, Wohlfahrt J, Bendtzen KFM. Cytokines, autoantibodies and viral antibodies in premorbid and postdiagnostic sera from patients with rheumatoid arthritis: case-control study nested in a cohort of Norwegian blood donors. Ann.Rheum.Dis. 2008;67:860–866. doi: 10.1136/ard.2007.073825. [DOI] [PubMed] [Google Scholar]
- 49.Karlson EW, Chisnik LB, Tworoger SS, Li I-M, Buring JE, Shadick NA, Manson JE, Costenbader KH. Biomarkers of inflammation and development of rheumatoid arthritis in women from two prospective cohort studies. Arth.Rheum. 2009;60:641–652. doi: 10.1002/art.24350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kokkonen H, Soderstrom I, Rocklov J, Hallmans G, Lejon K, Rantapaa Dahlqvist S. Up-regulation of cytokines and chemokines predates the onset of rheumatoid arthritis. Arth.Rheum. 2010;62:383–391. doi: 10.1002/art.27186. [DOI] [PubMed] [Google Scholar]
- 51.Arbuckle MR, McClain MT, Rubertone MV, Scofield RH, Dennis GJ, James JA, Harley JB. Development of autoantibodies before the clinical onset of Systemic Lupus Erythematosus. NEJM. 2003;349:1526–1533. doi: 10.1056/NEJMoa021933. [DOI] [PubMed] [Google Scholar]
- 52.Gottlieb PA, Eisenbarth GS. Diagnosis and treatment of pre-insulin dependent diabetes. Ann.Rev.Med. 1998;49:391–405. doi: 10.1146/annurev.med.49.1.391. [DOI] [PubMed] [Google Scholar]
- 53.Barton SH, Murray JA. Celiac disease and autoimmunity in the gut and elsewhere. Gastro.Clin.North Am. 2008;37:411–428. doi: 10.1016/j.gtc.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Burek CL, Rose NR. Autoimmune thyroiditis and ROS. Autoimmunity Reviews. 2008;7:530–537. doi: 10.1016/j.autrev.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 55.van de Sande MGH, de Hair MJH, van der Leij C, Klarenbeek CL, Bos WH, Smith MD, Maas M, de Vries N, van Schaardenburg D, Dijkmans BAC, Gerlag DM, Tak P-P. Different stages of rheumatoid arthritis: features of the synovium in the preclinical phase. Ann.Rheum.Dis. 2011;70:772–777. doi: 10.1136/ard.2010.139527. [DOI] [PubMed] [Google Scholar]
- 56.Hill JA, Bell DA, Brintnell W, Yue D, Wehrli B, Jevnikar AM, Lee DM, Hueber W, Robinson WH, Cairns E. Arthritis induced by posttranslationally modified (citrullinated) fibrinogen in DR4-IE transgenic mice. J.Exp.Med. 2008;205:967–979. doi: 10.1084/jem.20072051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ho PP, Lee LY, Zhao X, Tomooka B, Paniagua RT, Sharpe O, Bencivenga R, Chandrasekaran A, Hueber W, Steinman L, Robinson WH. Autoimmunity against fibrinogen mediates inflammatory arthritis in mice. J.Immunol. 2010;184:379–390. doi: 10.4049/jimmunol.0901639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kuhn KA, Kulik L, Tomooka B, Braschler KJ, Arend WP, Robinson WH, Holers VM. Antibodies to citrullinated proteins enhance tissue injury in experimental arthritis. J.Clin.Invest. 2006;116:961–973. doi: 10.1172/JCI25422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lundberg K, Nijenhuis S, Vossenaar ER, Palmblad K, van Venrooij WJ, Klareskog L, Zendman AJ, Harris HE. Citrullinated proteins have increased immunogenicity and arthritogenicity and their presence in arthritic joints correlates with disease severity. Arth.Res.Ther. 2005;7:R458–R467. doi: 10.1186/ar1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Uysal H, Bockermann R, Nandakumar KS, Sehnert B, Bajtner E, Engstrom A, Serre G, Burkhardt H, Thunnissen MMGM, Holmdahl R. Structure and pathogenicity of antibodies specific for citrullinated collagen type II in experimental arthritis. J.Exp.Med. 2009;206:449–462. doi: 10.1084/jem.20081862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Thiele GM, Duryee MJ, Dusad A, Hunter CD, Lacy JP, Anderson DR, Wang D, O'Dell JR, Mikuls TR, Klassen LW. Citrullinated mouse collagen administered to DBA/1J mice in the absence of adjuvant initiates arthritis. Intl.Immunopharmacology. 2012;13:424–431. doi: 10.1016/j.intimp.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 62○.Harre U, Georgess D, Bang H, Bozec A, Axmann R, Ossipova E, Jakobsson PJ, Baum W, Nimmerjahn F, Szarka E, Sarmay G, Krumbholz G, Neumann E, Toes RE, Scherer HU, Catrina A, Klareskog L, Jurdic P, Schett G. Induction of osteoclastogenesis and bone loss by human autoantibodies against citrullinated vimentin. J.Clin.Invest. 2012;122:1791–1802. doi: 10.1172/JCI60975. [Demonstration that ACPA are able to lead to direclty bone loss in vivo, expanding the understanding of potential pathogenic mechansims operative even in the absence of arthritis] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kokkonen H, Mullazehi M, Berglin E, Hallmans G, Wadell G, Ronnelid J, Rantapaa Dahlqvist S. Antibodies of IgG, IgA and IgM isotypes against cyclic citrullinated peptide precede the development of rheumatoid arthritis. Arth.Res.Ther. 2011;13:R13. doi: 10.1186/ar3237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nielen MMJ, van Schaardenburg D, Reesink HW, van de Stadt RJ, van der Horst-Bruinsma IE, de Koning MHMT, Habibuw MR, Vandenbroucke JP, Dijkmans BAC. Specific autoantibodies precede the symptoms of rheumatoid arthritis. Arth.Rheum. 2004;50:380–386. doi: 10.1002/art.20018. [DOI] [PubMed] [Google Scholar]
- 65.Rantapaa Dahlqvist S, de Jong BAW, Hallmans G, Wadell G, Sundin U, vanVenrooij WJ. Antibodies against citrullinated peptides (CCP) predict the development of rheumatoid arthritis. Arth.Rheum. 2003;48:2701–2705. doi: 10.1002/art.11223. [DOI] [PubMed] [Google Scholar]
- 66.Smit MD, Westra J, Vissink A, Doombos-van der Meer B, Brouwer E, van Winkelhoff AJ. Periodontitis in established rheumatoid arthritis patients: a cross-sectional clinical, microbiological and serological study. Arth.Res.Ther. 2012;14:R222. doi: 10.1186/ar4061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wegner N, Wait R, Sroka A, Eick S, Nguyen KA, Lundberg K, Kinloch A, Culshaw S, Potempa J, Venables PJ. Peptidylarginine deiminase from Porphyromonas gingivalis citrullinates human fibrinogen and a-enolase: implications for autoimmunity in rheumatoid arthritis. Arth.Rheum. 2010;62:2662–2672. doi: 10.1002/art.27552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mikuls TR, Thiele GM, Deane KD, Payne JB, O'Dell JR, Yu F, Sayles H, Weisman MH, Gregersen PK, Buckner JH, Keating RM, Derber LA, Robinson WH, Holers VM, Norris JM. Porphyromonas gingivalis and disease-related autoantibodies in individuals at increased risk of rheumatoid arthritis. Arth.Rheum. 2012;64:3522–3530. doi: 10.1002/art.34595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Scher JU, Ubeda C, Equinda M, Khanin R, Buischi Y, Viale A, Lipuma L, Attur M, Pillinger MH, Weissman G, Littman DR, Pamer EG, Bretz WA, Abramson SB. Periodontal disease and the oral microbiota in new-onset rheumatoid arthritis. Arth.Rheum. 2012;64:3083–3094. doi: 10.1002/art.34539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Metafratzi ZM, Georgiadis AN, Ioannidou CV, Alamanos Y, Vassiliou MP, Zikou AK, Raptis G, Drosos AA, Efremidis SC. Pulmonary involvement in patients with early rheumatoid arthritis. Scand.J.Rheum. 2007;38:338–344. doi: 10.1080/03009740701393957. [DOI] [PubMed] [Google Scholar]
- 71.Demoruelle MK, Weisman MH, Simonian PL, Lynch DA, Sachs PB, Pedraza IF, Harrington AR, Kolfenbach JR, Striebich CC, Pham QN, Strickland CD, Petersen BD, Parish MC, Derber LA, Holers VM, Norris JM, Deane KD. Airways abnormalities and rheumatoid arthritis-related autoantibodies in subjects without arthritis: Early injury or initiating site of autoimmunity? Arth.Rheum. 2012;64:1756–1761. doi: 10.1002/art.34344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Willis VC, Demoruelle MK, Derber LA, Chartier-Logan CJ, Parish MC, Pedraza IF, Weisman MH, Norris JM, Holers VM, Deane KD. Sputa autoantibodies in patients with established rheumatoid arthritis and subjects at-risk for future clinically apparent disease. Arth.Rheum. 2013 Jul 1; doi: 10.1002/art.38066. doi: 10.1002/art.38066. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rangel-Moreno J, Hartson L, Navarro C, Gaxiola M, Selman M, Randall TD. Inducible bronchus-associated lymphoid tissue (iBALT) in patients with pulmonary complications of rheumatoid arthritis. J.Clin.Invest. 2006;116:3183–3194. doi: 10.1172/JCI28756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wu HJ, Ivanov Il, Darce J, Hattori KST, Umesaki Y, Littman DR, Benoist C, Mathis D. Gut-residing segmented filamentous bacteria drive autoimmune arthritis via T helper 17 cells. Immunity. 2010;32:815–827. doi: 10.1016/j.immuni.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Vaahtovuo J, Munukka E, Korkeamaki M, Toivanen P. Fecal microbiota in early rheumatoid arthritis. J.Rheum. 2008;35:1500–1505. [PubMed] [Google Scholar]
- 76.de Hair MJH, Zijlstra IAJ, Boumans MJH, van de Sande MGH, Mass M, Gerlag DM, Tak PP. Hunting for the pathogenesis of rheumatoid arthritis: core-needle biopsy of inguinal lymph nodes as a new research tool. Ann.Rheum.Dis. 2012;71:1191–1192. doi: 10.1136/annrheumdis-2012-201540. [DOI] [PubMed] [Google Scholar]
- 77.Bekeredjian-Ding I, Jego G. Toll-like receptors - sentries in the B-cell response. Immunology. 2009;128:311–323. doi: 10.1111/j.1365-2567.2009.03173.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Seong SY, Matzinger P. Hydrophobocity: an ancient damage-associated molecular pattern that initiates innae immune responses. Nature Rev.Immunol. 2004;4:469–478. doi: 10.1038/nri1372. [DOI] [PubMed] [Google Scholar]
- 79○.Khandpur R, Carmona-Rivera C, Vivekanandan-Giri A, Gizinski AM, Yalavarthi S, Knight JS, Friday S, Li S, Patel RM, Subramanian V, Thompson P, Chen P, Fox DA, Pennathur S, Kaplan MJ. NETs are a source of citrullinated autoantigens and stimulate inflammatory responses in rheumatoid arthritis. Sci.Trans.Med. 2013 Mar 27;5(178):178ra40. doi: 10.1126/scitranslmed.3005580. doi: 10.1126/scitranslmed.3005580. [Potential source of citrullinated antigens derived from neutrophil NETs] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Arend WP, Firestein GS. Pre-rheumatoid arthritis: predisposition and transition to clinical synovitis. Nature Rev.Rheum. 2012;8:573–576. doi: 10.1038/nrrheum.2012.134. [DOI] [PubMed] [Google Scholar]
- 81.Zhao X, Okeke NL, Sharpe O, Batliwalla FM, Lee AT, Ho PP, Tomooka BH, Gregersen PK, Robinson WH. Circulating immune complexes contain citrullinated fibrinogen in rheumatoid arthritis. Arth.Res.Ther. 2008;10:R94. doi: 10.1186/ar2478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sokolove J, Zhao X, Chandra PE, Robinson WH. Immune complexes containing citrullinated fibrinogen costimulate macrophages via Toll-like receptor 4 and Fc-gamme receptor. Arth.Rheum. 2011;63:53–62. doi: 10.1002/art.30081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cordova KN, Willis VC, Haskins K, Holers VM. A citrullinated fibrinogen-specific T cell line enhances autoimmune arthritis in a mouse model of rheumatoid arthritis. J.Immunol. 2013;190:1457–1465. doi: 10.4049/jimmunol.1201517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ytterberg AJ, Ossipova E, Rutishauser D, Hensvold A, Eklund A, Skold M, Grunewald J, Lundberg K, Malmstrom V, Jakobsson PJ, Zubarev R, Klareskog L, Catrina A. Identification of shared citrullinated immunological targets in the lungs and joints of patients with rheumatoid arthritis. Ann.Rheum.Dis. 2012:A19. doi: 10.1136/annrheumdis-2013-204912. [European Workshop for Rheumatology Research] [DOI] [PubMed] [Google Scholar]
- 85.Demoruelle MK, Deane KD. Treatment strategies in early rheumatoid arthritis and prevention of rheumatoid arthritis. Curr.Rheumatol.Rep. 2012;14:472–480. doi: 10.1007/s11926-012-0275-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Klareskog L, Gregersen PK, Huizinga TW. Prevention of autoimmune rheumatic disease: state of the art and future perspectives. Ann.Rheum.Dis. 2010;69:2062–2069. doi: 10.1136/ard.2010.142109. [DOI] [PubMed] [Google Scholar]