Abstract
This study examined, among children, the associations among chaos in the home, diurnal cortisol patterns, eating behaviors and being overweight. Participants included 331 low-income children aged 3–4 years. Mean salivary cortisol-intercept (representing morning peak, 60 minutes since waking) and cortisol-slope (representing diurnal decline after peak) were calculated using mixed models from samples obtained across 3 days. Parents reported chaos in the home by questionnaire and responded to the Children’s Eating Behavior Questionnaire, generating subscales Food Responsiveness (FR), Emotional Overeating (EO), Enjoyment of Food (EF), and Satiety Responsiveness (SR). Body mass index was categorized as overweight versus not. Path analysis evaluated associations among chaos, cortisol patterns, eating behaviors, and weight status. Children living in more chaotic homes had lower morning cortisol levels, consistent with “hypocortisolism” reported among individuals who have experienced significant allostatic load as a result of substantial early life chronic stress. Among girls, the hypocortisolism pattern predicted a higher likelihood of being overweight both directly and mediated through reduced Satiety Responsiveness; in boys, the association of the hypocortisolism pattern with being overweight was mediated entirely through Emotional Overeating. In summary, our results provide support for the conceptual model that psychosocial stress contributes to hypocortisolism, which contributes directly to a higher likelihood of being overweight in girls, and indirectly through reduced Satiety Responsiveness in girls and through increased Emotional Overeating in boys.
Keywords: child, obesity, overweight, stress, eating behavior, cortisol
Introduction
More than one in four US preschool-aged children is overweight (Ogden, Carroll, Kit, & Flegal, 2012), and children living in poverty are at even greater risk (Shrewsbury & Wardle, 2008). The chronic stress resulting from living in poverty has been one hypothesized contributor to these disparities (Repetti, Taylor, & Seeman, 2002). Changes in the hypothalamic-pituitary-adrenal (HPA) axis under conditions of chronic stress have been proposed as one explanation for how stress contributes to poor health.
The HPA axis has a strong autonomous circadian diurnal pattern such that cortisol levels typically peak in the early waking hours and decrease through the progression of the day, reaching a nadir within a few hours of sleep onset. Under conditions of chronic stress, regulation of the HPA axis can be altered. Hypocortisolism, characterized by low morning cortisol levels flattening the diurnal rhythm, is one possible adaptation to chronic stress (Gunnar & Vazquez, 2001). Adults who have had stressful experiences in childhood and children experiencing chronic stress often display this hypocortisolism diurnal pattern, which has led to the hypothesis that hypocortisolism results from allostasis or adaptation of the HPA axis following prolonged and repeated activation as a result of early childhood chronic stress (Gunnar & Vazquez, 2001).
Cortisol increases appetite and shifts food preferences to so-called “comfort foods” foods high in fat and added sugars) (Pasquali, Vicennati, Agostini, & Pagotto, 2010) that may reduce feelings of stress via dampening of HPA axis activity (Dallman et al., 2003). How cortisol may shape the eating behavior of preschool-age children has not been previously described. However, preschool-aged children who are irritable and difficult to soothe are more likely to tantrum over food and to become overweight (Agras, Hammer, McNicholas, & Kraemer, 2004).
Psychological stress has been linked to a higher risk of obesity in children (Koch, Sepa, & Ludvigsson, 2008; Moens, Braet, Bosmans, & Rosseel, 2009). However, no prior study has examined associations among psychosocial stress, diurnal cortisol, eating behaviors, and weight status among children. Prior studies have examined subsets of these potential associations, with often conflicting results (Barat et al., 2007; Chalew, Lozano, Armour, Zadik, & Kowarski, 1991; Kiess et al., 1995; Knutsson et al., 1997; Li, Power, Kelly, Kirschbaum, & Hertzman, 2007; Mårin et al., 1992; Netherton, Goodyer, Tamplin, & Herbert, 2004; Oskis, Loveday, Hucklebridge, Thorn, & Clow, 2009; Rosmalen et al., 2005; Rosmond & Björntorp, 2000; Rosmond, Dallman, & Björntorp, 1998; Törnhage & Alfven, 2006). Interpretation of this prior work is limited by the relatively high socioeconomic status (Barat et al., 2007; Knutsson et al., 1997; Li et al., 2007; Mårin et al., 1992; Netherton et al., 2004; Rosmalen et al., 2005; Rosmond & Björntorp, 2000; Rosmond et al., 1998; Törnhage & Alfven, 2006) and low (Netherton et al., 2004; Rosmond & Björntorp, 2000; Rosmond et al., 1998; Törnhage & Alfven, 2006) (or unreported (Knutsson et al., 1997; Li et al., 2007)) obesity prevalence within study cohorts. Many studies have been in a laboratory setting (Barat et al., 2007; Chalew et al., 1991; Knutsson et al., 1997), which may spuriously elevate stress and cortisol. Just six studies have evaluated the association between cortisol and weight status in children, finding positive (Barat et al., 2007), negative (Chalew et al., 1991; Törnhage & Alfven, 2006), and no association (Knutsson et al., 1997; Netherton et al., 2004; Rosmalen et al., 2005). To our knowledge, no study has evaluated cortisol and specific eating behaviors in early childhood.
The present study sought to test the conceptual model that exposure to psychosocial stress is associated with hypocortisolism, which is associated with specific eating behaviors that contribute to higher weight status among young low-income children. Support for the hypotheses within this conceptual model would suggest that the high prevalence of overweight in low-income children is at least partially explained by a progressive adaptation of the intrinsic daily cortisol rhythm to chronic stress, which also drives specific eating behaviors.
Methods and Procedures
Study Design and Participants
Children attending Head Start, a free, federally-funded preschool program for low-income children, and their primary caregiver and legal guardian were invited to participate in a study about children’s eating behaviors. Exclusion criteria were: parent with ≥ 4 year college degree; parent or child not English-speaking; child in foster care, with food allergies, significant medical problems or perinatal complications, gestational age < 35 weeks, or use of medication known or hypothesized to affect cortisol. The study was approved by the University of Michigan Institutional Review Board. Written informed consent was provided by parents and age appropriate assent was obtained from children; families were compensated for their time. The study was explained to parents as seeking to understand whether children with different levels of the stress hormone cortisol eat differently; families were not aware of the study hypotheses. All questionnaires were interviewer-administered and anthropometry and saliva collection were performed by trained study staff.
Measures
Primary caregivers completed the Chaos, Hubbub, and Order Scale (CHAOS) (Matheny Jr, Wachs, Ludwig, & Phillips, 1995), a 15-item true/false measure. To capture psychosocial stress in the home, we created an Emotional CHAOS subscale score that included the 8 items on the CHAOS scale reflective of emotional stress (Cronbach’s α = 0.80): “There is very little commotion in our home” (reverse scored); “It’s a real “zoo” in our home”; “At home we can talk to each other without being interrupted” (reverse scored); “There is often a fuss going on at our home”; “You can’t hear yourself think in our home”; “I often get drawn into other people’s arguments at home”; “Our home is a good place to relax” (reverse scored): and “The atmosphere in our home is calm” (reverse scored).
Salivary cortisol is highly correlated with free serum cortisol (Kirschbaum & Hellhammer, 1994). Children provided saliva samples 3 times per day (on arrival to preschool, before breakfast, about 8:30am; before lunch, about 11:30am; and at 4:30pm) on 3 consecutive days by drooling in a tube or chewing on a piece of cotton. Daily logs included primary caregiver report of any medication use, illness, unusually good or bad events, exact time of morning awakening and if it was the usual time, napping or eating prior to the saliva sample; and location at the time of the afternoon sample. Saliva was stored at −20 degree Celsius until extracted and assayed in duplicate using an Expanded Range High Sensitivity Salivary Cortisol Enzyme Immunoassay Kit (Salimetrics LLC, PA, USA) with a detection limit of 0.007µg/dL and intra and inter-assay coefficients of variation of 7%, respectively.
The child’s primary caregiver completed the Children’s Eating Behavior Questionnaire (CEBQ) (Wardle, Guthrie, Sanderson, & Rapoport, 2001), a validated and reliable 35-item questionnaire. For this analysis, subscales examined included Food Responsiveness, Emotional Overeating, Enjoyment of Food, and Satiety Responsiveness. Children and their primary caregiver (usually the mother) were weighed and measured without shoes or heavy clothing by trained staff according to standard protocols on a +/− 0.1kg calibrated scale (Detecto Physician’s Scale Model DR550) and a +/− 0.1 cm calibrated stadiometer (Seca 217/213).
Primary caregivers reported children’s sex, race/ethnicity (categorized for this report as non-Hispanic white vs. not), primary caregiver education (< high school, high school diploma or generalized equivalency diploma (GED), or some college), family structure (single parent vs. not) and children’s birth weight. Primary caregivers completed the US Department of Agriculture 18-item Household Food Security Survey that categorizes households as food secure vs. not (Bickel, Nord, Price, Hamilton, & Cook, 2000). Primary caregivers reported annual household income; this value was divided by the federal poverty line for a family of a specific size to generate the income-to-needs ratio. An income-to-needs ratio <1.00 indicates that the family was living below the federal poverty line. An income-to-needs ratio < 1.85 is often used to define “low-income”. Primary caregivers reported whether or not the family received income from “food stamps” or “Temporary Assistance for Needy Families” (i.e. “welfare”)”. “Food stamps” is the term popularly used to refer to the United States Supplemental Nutrition Assistance Program that provides financial assistance for purchasing food to low-income people. The Temporary Assistance to Needy Families program is one of the US federal assistance programs providing cash assistance to families with dependent children; it is often referred to simply as “welfare”.
Statistical Analysis
Data analysis was performed using SAS 9.2 (SAS Institute Inc., Cary, NC). Univariate and bivariate statistics were used to describe the sample. Outliers as described below were excluded from the analysis and log transformation was implemented to ensure normality for the residuals.
Cortisol values were excluded if: (1) the child took a medication known to affect cortisol on the day of saliva collection; (2) the value was > 3 SD’s from the mean and the data did not reflect a consistent diurnal pattern for the child; (3) the value was > 2 SD’s from the mean and either did not fit the child’s diurnal pattern and/or there was an unusual circumstance (i.e., child was reported “getting sick”). Of the 3010 samples assayed, 66 (2.2%) were excluded for these reasons. To be included in the analysis, a child needed to have at least 5 valid cortisol data points over ≥ 2 days; the number of valid cortisol data points per child was mean 8.4 (SD 1.2).
The diurnal cortisol curve follows a well-established pattern, where cortisol increases initially after morning awakening, reaches a peak usually in the first 30 minutes and after that is followed by an exponential decay over the course of the day. Thus, using the log transformed cortisol as the outcome and the time (since awakening) at which the cortisol sampling occurred as the independent variable, the diurnal cortisol would be linear on time in a log-scale (for time > 60 minutes). Such a linear trajectory can then be captured by two parameters, intercept and slope. We used hierarchical linear models (HLM) using random parameters to capture individual diurnal cortisol curves for each participant. The HLM approach is a powerful modeling technique for estimating individual trajectories, provided that trajectories have a known parametric form (e.g. linear, log-linear, quadratic) (Hruschka, Kohrt, & Worthman, 2005). This approach is also powerful because it accounts for the time differential in the measurement of the cortisol in a direct way using the parametric function of the diurnal cortisol. The random intercept is an estimate of the expected cortisol level at 60 minutes after awakening for a given individual, and the random slope is the expected rate of decay on cortisol after 60 minutes post-awakening. Thus, both the random intercept and the random slope capture the diurnal cortisol patterns of an individual.
Data recorded in the daily logs obtained at the time of cortisol collection (primary caregiver report of any medication use, illness, unusually good or bad events, exact time of morning awakening and if it was the usual time, napping or eating prior to the saliva sample; and location at the time of the afternoon sample) were not associated with cortisol diurnal patterns and these data were therefore not included as controls in analyses.
Body mass index (BMI) was calculated and weight status categorized as overweight (BMI ≥ 85th and < 99.5th percentile) or normal weight (BMI < 85th percentile and > 5th percentile) based on US Centers for Disease Control reference growth curves for age and sex. We excluded from this analysis children with a BMI <5th percentile or ≥ 99.5th percentile because diurnal cortisol patterns have been reported to differ among individuals who are either underweight or extremely obese (Kumari, Chandola, Brunner, & Kivimaki, 2010) and extreme adiposity has been reported to affect peripheral cortisol metabolism (Anagnostis, Athyros, Tziomalos, Karagiannis, & Mikhailidis, 2009; Rask et al., 2001; Wake & Walker, 2004).
Correlations and t-tests were used to examine the associations of Emotional CHAOS score with overweight, cortisol-intercept, and cortisol-slope, as well as CEBQ subscales Food Responsiveness, Emotional Overeating, Enjoyment of Food, and Satiety Responsiveness. Associations were tested in the total sample, as well as stratified by child sex using t-tests and Pearson correlation coefficients.
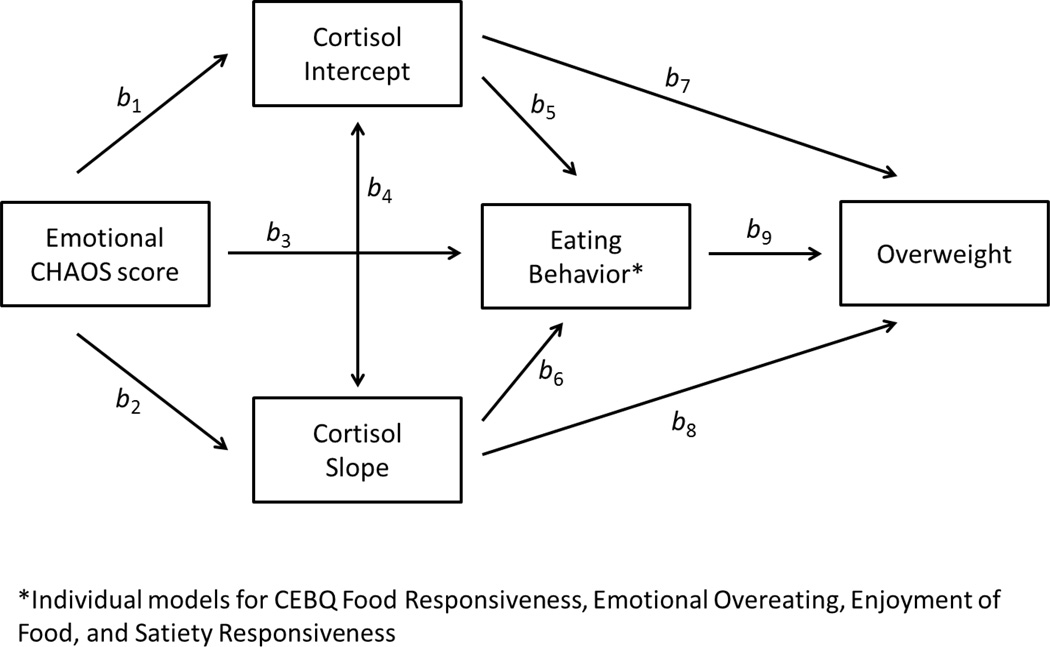
Path models were conducted (using MPLUS version 4.1 (Muthen & Muthen, Los Angeles, CA)) to test both the direct and indirect associations between Emotional CHAOS, diurnal cortisol pattern (both intercept and slope), eating behaviors, and child overweight (see Figure 1). The path models were repeated testing each of the 4 eating behaviors (CEBQ subscales Food Responsiveness, Emotional Overeating, Enjoyment of Food, and Satiety Responsiveness) individually in the model, separately in boys and girls. Bayesian estimation technique in MPLUS was used to fit path models which contained both continuous and binary variables. The path between cortisol intercept and cortisol slope in all models for boys was highly non-significant and was therefore removed from the models for boys to obtain a more parsimonious model with a better fit. Bayesian posterior predictive checks (PPC) using Chi-square statistics and the corresponding posterior predictive p-values were used to assess the goodness of fit in each model (Gelman, 2004).
Figure 1.
Conceptual model for hypothesized associations between diurnal cortisol, Emotional CHAOS score, eating behavior, and child overweight
Results
Characteristics of the sample are shown in Table 1. Most (66.2%) families had a poverty-to-income ratio <1.00 and were therefore living below the federal poverty line; 92.0% had a poverty-to-income ratio < 1.85 and were therefore “low-income”. The morning saliva sample was collected at a mean of 1.5 hours (SD 0.6, range 0.2 – 4.4 hours, interquartile range 1.2 – 1.8 hours) since awakening.
Table 1.
Characteristics of the sample
| Demographic Characteristics | Total N = 331 |
Girls N =167 |
Boys N =164 |
|---|---|---|---|
| Child birth weight, mean (SD), kilograms | 3.33 (0.49) | 3.32 (0.53) | 3.33 (0.45) |
| Male sex, No. (%) | 164 (49.6) | - | - |
| Primary caregiver education, No. (%) | |||
| < high school diploma | 55 (16.6) | 27 (16.2) | 28 (17.1) |
| High school or general equivalency diploma | 107 (32.3) | 60 (35.9) | 47 (28.7) |
| > high school diploma, < 4-year college degree | 169 (51.1) | 80 (47.9) | 89 (54.3) |
| Household food security status, No. (%) | |||
| Food Secure | 245 (74.0) | 122 (73.0) | 123 (75.0) |
| Food Insecure | 86 (26.0) | 45 (27.0) | 41 (25.0) |
| Family structure, No. (%) | |||
| Single parent household | 114 (34.6) | 54 (32.3) | 60 (36.8) |
| Not single parent household | 216 (65.4) | 113 (67.7) | 103 (63.2) |
| Income-to-needs ratio, mean (SD) | 0.88 (0.79) | 0.94 (0.91) | 0.82 (0.66) |
| Family participates in US Supplemental Nutrition Assistance Program, No. (%) | |||
| Yes | 267 (81.4) | 131 (79.4) | 136 (83.4) |
| No | 61 (18.6) | 34 (20.6) | 27 (16.6) |
| Family participates in US Temporary Assistance for Needy | |||
| Families (“welfare”) program, No. (%) | |||
| Yes | 78 (23.7) | 34 (20.5) | 44 (27.0) |
| No | 251 (76.3) | 132 (79.5) | 119 (73.0) |
| Emotional CHAOS score | 2.78 (2.37) | 2.76 (2.43) | 2.80 (2.32) |
| Child overweight, No. (%) | 120 (36.2) | 71 (42.5) | 49 (29.9) |
| Mother overweight, No. (%) | 241 (75.1) | 126 (77.8) | 115 (72.3) |
| Children’s Eating Behavior Questionnaire Subscales, mean (SD) | |||
| Emotional Overeating | 1.97 (0.71) | 1.94 (0.68) | 2.01 (0.74) |
| Food Responsiveness | 2.47 (0.90) | 2.45 (0.88) | 2.49 (0.93) |
| Satiety Responsiveness | 3.00 (0.69) | 3.02 (0.67) | 2.97 (0.71) |
| Enjoyment of Food | 3.77 (0.79) | 3.76 (0.75) | 3.78 (0.82) |
| Cortisol-intercept (µg/dL), mean (SD) | 0.19 (0.07) | 0.19 (0.07) | 0.20 (0.08) |
| Cortisol-slope ((µg/dL/hour), mean (SD) | −0.07 (0.03) | −0.07 (0.04) | −0.07 (0.03) |
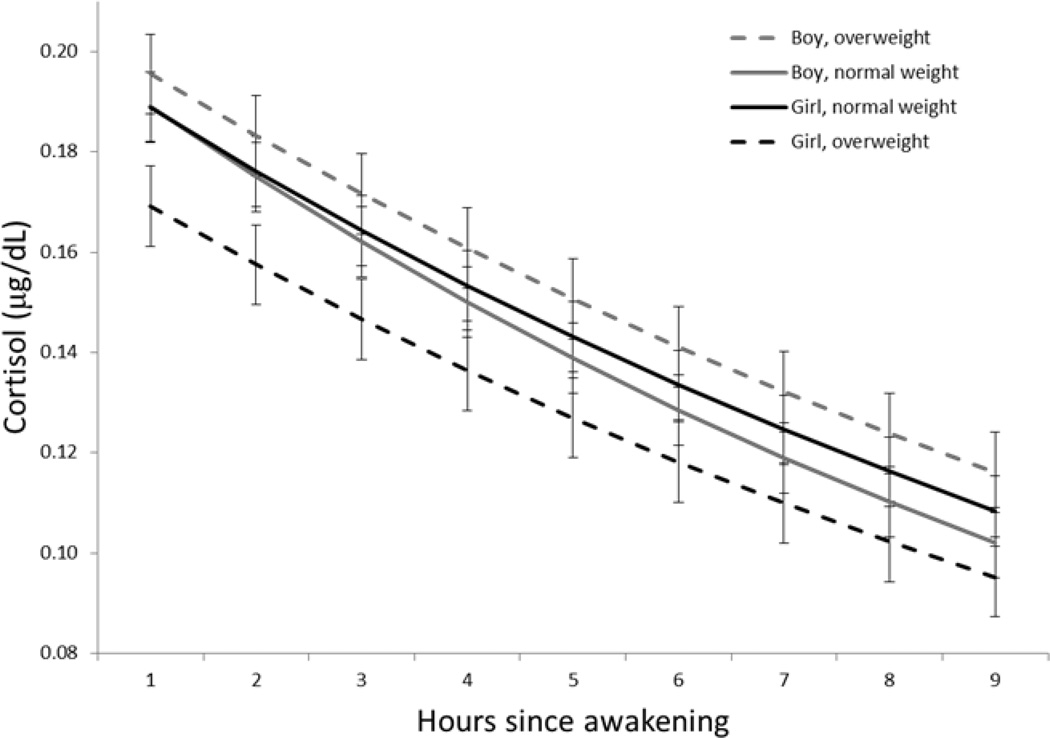
The bivariate associations of the Emotional CHAOS score with overweight, cortisol-intercept, and cortisol-slope, as well as each of the eating behaviors, are shown in Table 2 for the total sample as well as stratified by child sex. Based on t-tests, the Emotional CHAOS score was not associated with child weight status in the total sample, or among either boys or girls. However, based on Pearson correlation coefficients, the Emotional CHAOS score was associated with diurnal cortisol pattern, as well as with many of the eating behaviors in the total sample, as well as in boys and girls separately. Cortisol diurnal pattern in boys and girls by weight status is shown in Figure 2.
Table 2.
Associations of Emotional CHAOS with other variables in total sample and stratified by child sex
| Total | Girls | Boys | |
|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean (SD) | |
| Child weight status‡ | |||
| Overweight | 2.84 (2.30) | 2.82 (2.34) | 2.88 (2.27) |
| Not overweight | 2.74 (2.42) | 2.72 (2.50) | 2.77 (2.36) |
| r | r | r | |
| Cortisol intercept | −0.20† | −0.16* | −0.24** |
| Cortisol slope | 0.06 | 0.18* | −0.07 |
| CEBQ Food Responsiveness Subscale score | 0.21† | 0.20** | 0.24** |
| CEBQ Emotional Overeating Subscale score | 0.20† | 0.25** | 0.15 |
| CEBQ Enjoyment of Food Subscale score | 0.004 | 0.03 | −0.01 |
| CEBQ Satiety Responsiveness Subscale score | 0.05 | 0.03 | 0.07 |
p < .05;
< .01;
< .001;
There were no significant differences in Emotional CHAOS by weight status in any group. Statistical significance is based on the t-test and Pearson correlation.
Figure 2.
Association between cortisol diurnal pattern and overweight among boys and girls
Path analysis results for the conceptual model depicted in Figure 1 are presented in Tables 3 and 4. Results for each of the 4 eating behaviors are presented separately for girls (Table 3) and boys (Table 4) All of the models showed good fit, with posterior predictive p-values ranging from .583 to .667, well within the 0.05 – 0.95 range.
Table 3.
Standardized path coefficients for each of 4 path analysis models shown in Figure 1, individually testing eating behaviors Food Responsiveness, Emotional Overeating, Enjoyment of Food, and Satiety Responsiveness, in girls (n = 167)
| Path | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Eating Behavior Variable |
Emotional CHAOS → Cortisol Intercept |
Emotional CHAOS → Cortisol Slope |
Emotional CHAOS → Eating Behavior |
Cortisol Intercept → Cortisol Slope |
Cortisol Intercept → Eating Behavior |
Cortisol Slope → Eating Behavior |
Cortisol Intercept → Overweight |
Cortisol Slope → Overweight |
Eating Behavior → Overweight |
| b1 | b2 | b3 | b4 | b5 | b6 | b7 | b8 | b9 | |
| Food Responsiveness | −.16** | .18** | .21** | .28** | .01 | −.07 | −.21** | .02 | .32** |
| Emotional Overeating | −.16** | .18** | .25** | .28** | .01 | −.06 | −.22** | .02 | −.04 |
| Enjoyment of Food | −.16* | .18** | −.01 | .28** | −.11† | .09 | −.18* | −.02 | .29** |
| Satiety Responsiveness | −.16** | .18** | .03 | .28** | .17* | .09 | −.18* | .05 | −.30** |
p < .01,
p < .05,
p < .10
Table 4.
Standardized path coefficients for each of 4 path analysis models shown in Figure 1, individually testing eating behaviors Food Responsiveness, Emotional Overeating, Enjoyment of Food, and Satiety Responsiveness, in boys (n = 164)
| Path | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Eating Behavior Variable |
Emotional CHAOS → Cortisol Intercept |
Emotional CHAOS → Cortisol Slope |
Emotional CHAOS → Eating Behavior |
Cortisol Intercept → Cortisol Slope |
Cortisol Intercept → Eating Behavior |
Cortisol Slope → Eating Behavior |
Cortisol Intercept → Overweight |
Cortisol Slope → Overweight |
Eating Behavior → Overweight |
| b1 | b2 | b3 | b4 | b5 | b6 | b7 | b8 | b9 | |
| Food Responsiveness | −.11** | −.03 | .09* | - | .08 | .03 | .10 | .21† | .34** |
| Emotional Overeating | −.25** | −.08 | .12 | - | −.15* | .02 | .05 | .18† | .26** |
| Enjoyment of Food | −.22** | −.08 | .01 | - | .03 | .14* | .05 | .16 | .25** |
| Satiety Responsiveness | −.25** | −.08 | .05 | - | −.07 | −.10 | .04 | .17† | −.19† |
p < .01,
p < .05,
p < .10
As shown in Table 3, among girls, the Emotional CHAOS score predicted lower cortisol intercept and flatter slope across all models, regardless of which of the 4 eating behaviors was being tested in the model. A higher Emotional CHAOS score predicted greater Food Responsiveness and Emotional Overeating. A lower cortisol intercept predicted greater Enjoyment of Food (approaching statistical significance), and less Satiety Responsiveness. A lower cortisol intercept predicted a higher likelihood of being overweight regardless of which of the 4 eating behaviors was being tested in the model. Greater Food Responsiveness, greater Enjoyment of Food, and less Satiety Responsiveness each predicted a greater likelihood of being overweight. In summary, among girls, the Emotional CHAOS score predicted a flatter pattern of diurnal cortisol, which predicted a higher likelihood of being overweight both directly and mediated through reduced Satiety Responsiveness; there was a trend towards mediation through greater Enjoyment of Food as well.
As shown in Table 4, among boys, the Emotional CHAOS score predicted lower cortisol intercept but there was no association with cortisol slope across any of the models, regardless of which of the 4 eating behaviors was being tested in the model. A higher Emotional CHAOS score predicted greater Food Responsiveness. A lower cortisol intercept predicted greater Emotional Overeating. A flatter slope predicted more Enjoyment of Food. A lower cortisol intercept was not associated with a higher likelihood of being overweight in any of the models, but a flatter slope was associated with a trend towards a higher likelihood of being overweight in 3 of the 4 models. Greater Food Responsiveness, greater Emotional Overeating, greater Enjoyment of Food, and less Satiety Responsiveness (approaching statistical significance) each predicted a greater likelihood of being overweight. In summary, among boys, the Emotional CHAOS score predicted a lower cortisol intercept, which predicted a higher likelihood of being overweight mediated entirely through increased Emotional Overeating. There was no association of the Emotional CHAOS score with cortisol slope, but a flatter pattern of diurnal cortisol (indicated by a higher slope) increased the likelihood of being overweight mediated through Enjoyment of Food as well as directly for the models with greater Food Responsiveness, Emotional Overeating, and Satiety Responsiveness.
We reran the path analysis models described in Tables 3 and 4, but replacing categorical overweight status with child body mass index z-score. The associations shown in Tables 3 and 4 were essentially unchanged, with the exception of the associations between cortisol intercept and child adiposity in girls. Specifically, the significant association between cortisol intercept and child overweight became statistically non-significant with adiposity was modeled as body mass index z-score instead, suggesting that the association in girls is non-linear.
Discussion
Results provided support for our hypothesized conceptual model, but highlighted that the paths of association differ substantially in girls versus boys and involve only certain eating behaviors. First, within this low-income cohort, children living in more emotionally stressful homes had lower morning cortisol levels and girls had a diminished diurnal decline, consistent with “hypocortisolism” reported among individuals who have experienced significant allostatic load as a result of substantial early life chronic stress (Gunnar & Vazquez, 2001). Second, psychosocial stress was associated most robustly in boys and girls with Food Responsiveness, which in turn was robustly associated with being overweight. Third, the hypocortisolism pattern was associated with being overweight in girls and there was a trend towards this association in boys. Fourth, in girls, the hypocortisolism pattern predicted a higher likelihood of being overweight both directly and mediated through reduced Satiety Responsiveness; in boys, the association of the hypocortisolism pattern with being overweight was mediated entirely through Emotional Overeating. In summary, our results provide support for the conceptual model that psychosocial stress contributes to hypocortisolism, which contributes directly to a higher likelihood of being overweight in girls, and indirectly through reduced Satiety Responsiveness in girls and through increased Emotional Overeating in boys.
Increased food intake is the final common outcome of a number of different eating behaviors with separate neuroanatomical correlates. The finding that different types of eating behaviors served as mediators is notable given that prior work on the physiologic underpinnings of stress-related eating has generally reported only increased food intake, but not the specific eating behaviors that ultimately lead to the increased intake.
In this study, among girls, parent-reported satiety responsiveness mediated links between stress-associated diurnal cortisol patterns and overweight. Notably, the items contributing to satiety responsiveness in the measure used in this study largely related to the child not feeling “full” during a meal. This suggests that stress-associated cortisol patterning may be leading to overweight in girls through disruption of one of the many satiety signals to the hindbrain (e.g. ghrelin, cholecystokinin, etc). This finding suggests that limiting portion sizes and interventions focused on attending to satiety cues during a meal may be effective in disrupting the pathways linking stress to overweight among preschool-aged girls.
In contrast, among boys, parent-reported emotional overeating mediated links between stress-associated diurnal cortisol patterns and overweight. The items contributing to the emotional overeating measure in this study were questions relating to the child eating when anxious, annoyed, worried, or bored. This observation suggests that interventions focused on providing boys with alternative strategies for coping with unpleasant emotional states may hold the greatest promise for disrupting these pathways of association.
In this study, the Food Responsiveness subscale may be serving as an indicator of “incentive salience”, which is the motivational “wanting” for a stimulus. This subscale includes items such as, “My child is always asking for food,” and “Given the chance, my child would eat most of the time.” “Wanting” food has distinct neuroanatomical mechanisms from “liking” food (Mela, 2006). In the current study, among both boys and girls, emotional stress in the home led to increased “wanting” of food, which led to an increased likelihood of overweight. However, this pathway did not occur through diurnal cortisol patterning. In prior work, stress has been linked with increased motivation to attain food, though in animal studies glucocorticoids have increased “wanting” (Adam & Epel, 2007; Dallman, 2010). These discrepant findings indicate that further work is need to understand the biological mechanism linking emotional stress and increased “wanting” of food in preschool-aged children. Reducing food cue exposure (i.e., removing the stimulus that is triggering “wanting”) may be helpful in disrupting the link between emotional stress and overweight in young children.
In contrast to “wanting”, “liking” is the pleasure gained from consuming a stimulus, and has been found to have a different neuroanatomical signature from “wanting” (Mela, 2006). In the current study, the Enjoyment of Food subscale may serve as an indicator of “liking”. This subscale includes items such as, “My child loves food”, and “My child enjoys eating”. In the current study there was a marginal association between diurnal cortisol patterning and Enjoyment of Food among girls, suggesting that emotional stress in the home predicted increased “liking” via diurnal cortisol patterning, which led to an increased likelihood of being overweight. Among boys, a flatter diurnal cortisol pattern predicted greater “liking”, which led to an increased likelihood of being overweight. Prior work with adults has shown that obese individuals demonstrate increased “wanting”, but not necessarily “liking” of food (Mela, 2006), which is counter to our findings. This may be because the Enjoyment of Food subscale is not capturing “liking” specifically, or because there are developmental differences between children and adults. Nonetheless, our results suggest that if emotional stress increases the hedonic value of food for young girls, efforts to provide young girls with alternative coping mechanisms that do not involve food will be important to obesity prevention.
Finally, it is noteworthy that among girls, the link between hypocortisolism and overweight also occurred directly, and not entirely through eating behavior. Cortisol is known to have direct effects on metabolism affecting energy balance (Dallman et al., 2003), suggesting that intervening upon eating behaviors alone may not be enough to prevent the effects of stress on overweight risk. These observations suggest that reducing emotional stress directly, and not just the resulting eating behavior, may also be an important obesity prevention strategy.
Our study has several limitations. The sample was limited to low-income preschool-aged children enrolled in Head Start, thus limiting generalizability to children of other ages and socioeconomic strata. Due to limitations of our study setting, our measurement of morning cortisol did not occur at a fixed time after awakening, and therefore our estimates of each child’s cortisol intercept and slope include some degree of error, but are unbiased. Notably, however, we reran our models altering the fixed time after awakening (i.e., 30 minutes, 45 minutes) at which cortisol intercept was estimated and our results did not differ. The study was also cross-sectional and therefore cannot inform the understanding of the temporality of the identified associations; it is possible that being overweight causes changes to HPA axis functioning. Longitudinal studies are needed to disentangle the sequence of these changes. Study strengths include the relatively large, diverse, high-risk sample with a large proportion of overweight children and a biological measure of chronic stress exposure.
Conclusions
There are several implications of our findings. First, results suggest that chronic stress in early childhood may promote changes in eating behaviors or cortisol patterns that are associated with the child being overweight. One novel focus for obesity intervention and prevention programming may be to help parents better understand children’s eating behaviors, how they may be related to psychosocial stress, and to give them strategies to respond effectively to children’s food requests. A second focus for obesity intervention and prevention may also be to reduce chaotic home environments. A third implication is that while stress in the lives of children living in poverty (Evans & English, 2002) may not be reduced, their ability to cope with daily stressors could be improved; indeed, behavioral interventions have effectively restored healthy HPA axis functioning in very young children who live in high-stress conditions (Dozier, Peloso, Lewis, Laurenceau, & Levine, 2008; Fisher, Stoolmiller, Gunnar, & Burraston, 2007). Finally, HPA axis functioning is associated with a myriad of physiologic functions. The findings here raise questions regarding the implications of the identified differences in HPA axis function in overweight children for their future health and well-being.
Highlights.
Among low-income preschoolers psychosocial stress predicted hypocortisolism.
Hypocortisolism predicted a higher risk of overweight.
Reduced satiety responsiveness mediated the association in girls.
Emotional overeating mediated the association in boys.
ACKNOWLEDGEMENTS
All phases of this study were supported by a grant from NIH, grant #5RC1DK086376 to Dr. Lumeng.
Abbreviations
- BMI
body mass index
- HPA
hypothalamic-pituitary-adrenal axis
- ITN
income-to-needs
- SD
standard deviation
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adam TC, Epel ES. Stress, eating and the reward system. Physiology & Behavior. 2007;91(4):449–458. doi: 10.1016/j.physbeh.2007.04.011. http://dx.doi.org/10.1016/j.physbeh.2007.04.011. [DOI] [PubMed] [Google Scholar]
- Agras WS, Hammer LD, McNicholas F, Kraemer HC. Risk factors for childhood overweight: A prospective study from birth to 9.5 years. The Journal of Pediatrics. 2004;145(1):20–25. doi: 10.1016/j.jpeds.2004.03.023. [DOI] [PubMed] [Google Scholar]
- Anagnostis P, Athyros VG, Tziomalos K, Karagiannis A, Mikhailidis DP. Clinical review: The pathogenetic role of cortisol in the metabolic syndrome: a hypothesis. Journal of Clinical Endocrinology & Metabolism. 2009;94(8):2692–2701. doi: 10.1210/jc.2009-0370. [DOI] [PubMed] [Google Scholar]
- Barat P, Gayard-Cros M, Andrew R, Corcuff J-B, Jouret B, Barthe N, Duclos M. Truncal distribution of fat mass, metabolic profile and hypothalamic-pituitary adrenal axis activity in prepubertal obese children. The Journal of Pediatrics. 2007;150(5):535–539. doi: 10.1016/j.jpeds.2007.01.029. e531. [DOI] [PubMed] [Google Scholar]
- Bickel G, Nord M, Price C, Hamilton W, Cook J. United States Department of Agriculture, Food and Nutrition Service. Alexandria, VA: Office of Analysis, Nutrition, and Evaluation; 2000. Measuring food security in the United States: Guide to Measuring Household Food Security. March 2000. Retrieved from http://www.ers.usda.gov/topics/food-nutrition-assistance/food-security-in-the-us/survey-tools.aspx#guide. [Google Scholar]
- Chalew SA, Lozano RA, Armour KM, Zadik Z, Kowarski AA. Reduction of plasma cortisol levels in childhood obesity. The Journal of Pediatrics. 1991;119(5):778–780. doi: 10.1016/s0022-3476(05)80302-6. [DOI] [PubMed] [Google Scholar]
- Dallman MF. Stress-induced obesity and the emotional nervous system. Trends in Endocrinology & Metabolism. 2010;21(3):159–165. doi: 10.1016/j.tem.2009.10.004. doi: http://dx.doi.org/10.1016/j.tem.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallman MF, Pecoraro N, Akana SF, la Fleur SE, Gomez F, Houshyar H, Manalo S. Chronic stress and obesity: A new view of "comfort food". Proceedings of the National Academy of Sciences. 2003;100(20):11696–11701. doi: 10.1073/pnas.1934666100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dozier M, Peloso E, Lewis E, Laurenceau J-P, Levine S. Effects of an attachment-based intervention on the cortisol production of infants and toddlers in foster care. Development and Psychopathology. 2008;20(03):845–859. doi: 10.1017/S0954579408000400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans GW, English K. The environment of poverty: Multiple stressor exposure, psychophysiological stress, and socioemotional adjustment. Child Development. 2002;73(4):1238–1248. doi: 10.1111/1467-8624.00469. [DOI] [PubMed] [Google Scholar]
- Fisher PA, Stoolmiller M, Gunnar MR, Burraston BO. Effects of a therapeutic intervention for foster preschoolers on diurnal cortisol activity. Psychoneuroendocrinology. 2007;32(8–10):892–905. doi: 10.1016/j.psyneuen.2007.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelman A. Bayesian data analysis. Boca Raton, Fla.: Chapman & Hall/CRC; 2004. [Google Scholar]
- Gunnar MR, Vazquez DM. Low cortisol and a flattening of expected daytime rhythm: Potential indices of risk in human development. Development and Psychopathology. 2001;13(03):515–538. doi: 10.1017/s0954579401003066. [DOI] [PubMed] [Google Scholar]
- Hruschka DJ, Kohrt BA, Worthman CM. Estimating between- and within-individual variation in cortisol levels using multilevel models. Psychoneuroendocrinology. 2005;30(7):698–714. doi: 10.1016/j.psyneuen.2005.03.002. [DOI] [PubMed] [Google Scholar]
- Kiess W, Meidert A, Dressendörfer RA, Schriever K, Kessler U, Köunig A, Strasburger CJ. Salivary cortisol levels throughout childhood and adolescence: Relation with age pubertal stage, and weight. Pediatric Research. 1995;37(4):502–506. doi: 10.1203/00006450-199504000-00020. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Hellhammer DH. Salivary cortisol in psychoneuroendocrine research: Recent developments and applications. Psychoneuroendocrinology. 1994;19(4):313–333. doi: 10.1016/0306-4530(94)90013-2. [DOI] [PubMed] [Google Scholar]
- Knutsson U, Dahlgren J, Marcus C, Rosberg S, Brönnegård M, Stierna P, Albertsson-Wikland K. Circadian cortisol rhythms in healthy boys and girls: Relationship with age growth, body composition, and pubertal development. Journal of Clinical Endocrinology & Metabolism. 1997;82(2):536–540. doi: 10.1210/jcem.82.2.3769. [DOI] [PubMed] [Google Scholar]
- Koch FS, Sepa A, Ludvigsson J. Psychological stress and obesity. Journal of Pediatrics. 2008;153(6):839–844. doi: 10.1016/j.jpeds.2008.06.016. [DOI] [PubMed] [Google Scholar]
- Kumari M, Chandola T, Brunner E, Kivimaki M. A nonlinear relationship of generalized and central obesity with diurnal cortisol secretion in the Whitehall II study. Journal of Clinical Endocrinology & Metabolism. 2010;95(9):4415–4423. doi: 10.1210/jc.2009-2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Power C, Kelly S, Kirschbaum C, Hertzman C. Life-time socio-economic position and cortisol patterns in mid-life. Psychoneuroendocrinology. 2007;32(7):824–833. doi: 10.1016/j.psyneuen.2007.05.014. [DOI] [PubMed] [Google Scholar]
- Mårin P, Darin N, Amemiya T, Andersson B, Jern S, Björntorp P. Cortisol secretion in relation to body fat distribution in obese premenopausal women. Metabolism. 1992;41(8):882–886. doi: 10.1016/0026-0495(92)90171-6. [DOI] [PubMed] [Google Scholar]
- Matheny AP, Jr, Wachs TD, Ludwig JL, Phillips K. Bringing order out of chaos: Psychometric characteristics of the confusion, hubbub, and order scale. Journal of Applied Developmental Psychology. 1995;16(3):429–444. [Google Scholar]
- Mela DJ. Eating for pleasure or just wanting to eat? Reconsidering sensory hedonic responses as a driver of obesity. Appetite. 2006;47(1):10–17. doi: 10.1016/j.appet.2006.02.006. [DOI] [PubMed] [Google Scholar]
- Moens E, Braet C, Bosmans G, Rosseel Y. Unfavourable family characteristics and their associations with childhood obesity: a cross-sectional study. European Eating Disorders Review. 2009;17(4):315–323. doi: 10.1002/erv.940. [DOI] [PubMed] [Google Scholar]
- Netherton C, Goodyer I, Tamplin A, Herbert J. Salivary cortisol and dehydroepiandrosterone in relation to puberty and gender. Psychoneuroendocrinology. 2004;29(2):125–140. doi: 10.1016/s0306-4530(02)00150-6. [DOI] [PubMed] [Google Scholar]
- Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity and trends in body mass index among us children and adolescents: 1999–2010. JAMA. 2012;307(5):483–490. doi: 10.1001/jama.2012.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oskis A, Loveday C, Hucklebridge F, Thorn L, Clow A. Diurnal patterns of salivary cortisol across the adolescent period in healthy females. Psychoneuroendocrinology. 2009;34(3):307–316. doi: 10.1016/j.psyneuen.2008.09.009. [DOI] [PubMed] [Google Scholar]
- Pasquali R, Vicennati V, Agostini A, Pagotto U. Glucocorticoids, stress and obesity. Expert Review of Endocrinology & Metabolism. 2010;5(3):425–434. doi: 10.1586/eem.10.1. [DOI] [PubMed] [Google Scholar]
- Rask E, Olsson T, Soderberg S, Andrew R, Livingstone DEW, Johnson O, Walker BR. Tissue-specific dysregulation of cortisol metabolism in human obesity. Journal of Clinical Endocrinology & Metabolism. 2001;86(3):1418–1421. doi: 10.1210/jcem.86.3.7453. [DOI] [PubMed] [Google Scholar]
- Repetti RL, Taylor SE, Seeman TE. Risky families: Family social environments and the mental and physical health of offspring. Psychological Bulletin. 2002;128(2):330–366. [PubMed] [Google Scholar]
- Rosmalen JGM, Oldehinkel AJ, Ormel J, de Winter AF, Buitelaar JK, Verhulst FC. Determinants of salivary cortisol levels in 10–12 year old children; a population-based study of individual differences. Psychoneuroendocrinology. 2005;30(5):483–495. doi: 10.1016/j.psyneuen.2004.12.007. [DOI] [PubMed] [Google Scholar]
- Rosmond R, Björntorp P. The hypothalamic–pituitary–adrenal axis activity as a predictor of cardiovascular disease, type 2 diabetes and stroke. Journal of Internal Medicine. 2000;247(2):188–197. doi: 10.1046/j.1365-2796.2000.00603.x. [DOI] [PubMed] [Google Scholar]
- Rosmond R, Dallman MF, Björntorp P. Stress-related cortisol secretion in men: Relationships with abdominal obesity and endocrine, metabolic and hemodynamic abnormalities. Journal of Clinical Endocrinology & Metabolism. 1998;83(6):1853–1859. doi: 10.1210/jcem.83.6.4843. [DOI] [PubMed] [Google Scholar]
- Shrewsbury V, Wardle J. Socioeconomic status and adiposity in childhood: A systematic review of cross-sectional Studies 1990–2005. Obesity. 2008;16(2):275–284. doi: 10.1038/oby.2007.35. [DOI] [PubMed] [Google Scholar]
- Törnhage C-J, Alfven G. Diurnal salivary cortisol concentration in school-aged children: Increased morning cortisol concentration and total cortisol concentration negatively correlated to body mass index in children with recurrent abdominal pain of psychosomatic origin. Journal of Pediatric Endocrinology and Metabolism. 2006;19(6):843–854. doi: 10.1515/jpem.2006.19.6.843. [DOI] [PubMed] [Google Scholar]
- Wake DJ, Walker BR. 11β-Hydroxysteroid dehydrogenase type 1 in obesity and the metabolic syndrome. Molecular and Cellular Endocrinology. 2004;215(1–2):45–54. doi: 10.1016/j.mce.2003.11.015. [DOI] [PubMed] [Google Scholar]
- Wardle J, Guthrie CA, Sanderson S, Rapoport L. Development of the Children's Eating Behaviour Questionnaire. Journal of Child Psychology and Psychiatry. 2001;42(7):963–970. doi: 10.1111/1469-7610.00792. [DOI] [PubMed] [Google Scholar]