Abstract
BACKGROUND
The goal of the Carolinas Cancer Education and Screening (CARES) Project was to improve colorectal cancer (CRC) screening among low-income women in subsidized housing communities in 11 cities in North and South Carolina who were traditionally underserved by cancer control efforts.
METHODS
Cross-sectional samples were randomly selected from housing authority lists at 5 timepoints in this nonrandomized community-based intervention study. Face-to-face interviews focused on CRC knowledge, beliefs, barriers to screening, and screening behaviors. The intervention components were based on a previous evidence-based program.
RESULTS
A total of 2098 surveys were completed. Seventy-eight percent of the respondents were African American, 62% were 65+ years, and 4% were married. At baseline, the rate of CRC screening within guidelines was 49.3% and physician recommendation was the strongest predictor (odds ratio [OR] = 21.9) of being within guidelines. There was an increase in positive beliefs about CRC screening (P =.010) and in the intention to complete CRC screening in the next 12 months (P =.053) after the intervention. The odds of being within CRC screening guidelines for women living in a city that had received the intervention were not significantly different from women living in a city that had not received the intervention (P =.496).
CONCLUSIONS
Although CRC screening rates were not significantly better after the intervention, there was a positive change in beliefs about screening and intention to be screened. The results suggest that the dissemination of an evidence-based behavioral intervention may require a longer duration to engage hard-to-reach populations and change behaviors.
Keywords: colorectal cancer, cancer screening, vulnerable populations, disparities
Colorectal cancer (CRC) is the third leading type of cancer and the third leading cause of cancer death among females in the US.1 African American females have a CRC incidence rate of 56.1 per 100,000 compared with 46.8 per 100,000 White females, and African American females have a CRC mortality rate of 24.5 per 100,000 compared with 17.1 per 100,000 White females.2 More African Americans present with increased late-stage CRC tumors compared with Whites and have decreased survival rates.2–4
One factor contributing to these rates among African Americans is that they are overrepresented in the socioeconomically disadvantaged or underserved segment of the population.5,6
National policy-making expert organizations recognize the importance of CRC screening and support a variety of CRC screening test strategies among average-risk adults 50+ years.7– 11 These recommendations are based on strong evidence that screening decreases CRC mortality and may also reduce CRC incidence.7–9,12,13 However, even with these recommended guidelines for CRC screening, barriers exist to widespread utilization, especially among minority and underserved populations.
According to the 2004 Behavioral Risk Factor Surveillance System only 19.0% of adults, 51+ years, had a fecal occult blood test (FOBT) within the last year, and 52.1% of adults had a sigmoidoscopy/colonoscopy within the past 5 years.14 Decreased CRC screening rates are also documented among African Americans compared with Whites15,16 and in patients without health insurance.17,18 In general, low CRC screening rates are due to patient, provider, and system level factors.19–28
The goal of the Carolinas Cancer Education and Screening (CARES) Project was to improve CRC screening among low-income women living in North and South Carolina who were traditionally underserved by cancer control efforts by: 1) identifying knowledge, beliefs, and barriers to CRC screening; and 2) addressing identified barriers using an evidence-based program. The current study was designed to assess the effects of an intervention that consisted of community-based out-reach and clinic-based in-reach strategies delivered by American Cancer Society (ACS) volunteers. This article reports the results in terms of CRC screening intention and rates.
MATERIALS AND METHODS
The intervention components of the CARES Project were based on the Forsyth County Cancer Screening (FoCaS) Project, which included community outreach and community health clinic-based in-reach components to improve breast and cervical cancer screening.29The present study was approved by the Institutional Review Boards of Wake Forest University School of Medicine and The Ohio State University.
Setting
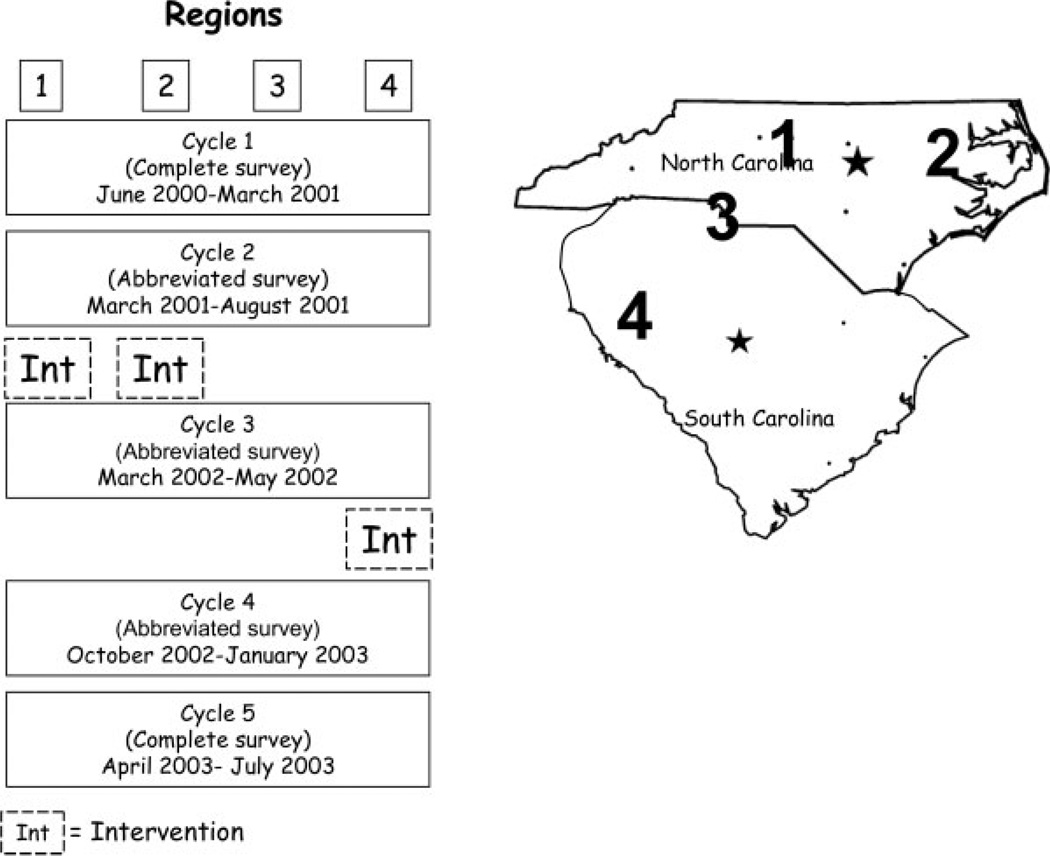
Eleven cities in North Carolina (NC) and South Carolina (SC) with subsidized housing communities were identified and grouped together in 4 regions that share media markets and are represented by the Southeast Division of the American Cancer Society (Fig. 1). Region 1 included Greensboro, High Point, and Winston-Salem, NC. Rocky Mount, Wilson, and Greenville, North Carolina comprised Region 2. Region 3 included Charlotte, North Carolina, and Rock Hill, South Carolina, and Region 4 included Anderson, Spartanburg, and Greenville, South Carolina.
FIGURE 1.
Carolinas Cancer Education and Screening (CARES) Project: regions in North and South Carolina, survey cycles, and timing of intervention.
★ Capitals of North Carolina and South Carolina.
Research Design
The study was designed as a community-based intervention trial delivered and evaluated across time within regions. Whereas communities were not randomly assigned to intervention or control, each city acted as its own control comparing outcomes before (or without) intervention to outcomes after the intervention. The intervention was delivered at different timepoints in each region (Fig. 1) and outcomes were evaluated through repeated cross-sectional surveys at 5 timepoints or ‘cycles’ of the study. Regions 1 and 2 received the intervention after Cycle 2 (beginning September and October 2001, respectively). Region 4 received the intervention after Cycle 3 (beginning April 2002), and Region 3 did not receive the intervention, which provided a control to adjust for any secular trends in the area.
Participant Selection and Surveys
At each cycle a cross-sectional sample of women was randomly selected from housing authority resident lists in each region. Independent samples were taken at each cycle and women were interviewed only once. Approximately 800 women were sampled in each cycle to obtain at least 400 interviews, 100 from each of the 4 regions. A letter introducing the project was sent to each woman selected and an interviewer contacted each woman to determine eligibility (≥50 years of age, resident of housing community), willingness to participate, and arrange a time for the interview. A total of 5 attempts were made to contact each selected woman.
All face-to-face interviews were conducted in the participant’s home and, on average, were completed in 50–60 minutes for the comprehensive survey and 20–30 minutes for the abbreviated survey. The surveys at Cycles 1 and 5 collected demographics, medical history, social support, sources of health information, and CRC screening intention and behaviors and an assessment of knowledge, beliefs, and barriers associated with CRC and CRC screening. The questions were modified from surveys used in a previous study to increase mammography screening.30 An abbreviated survey that focused on demographic information and CRC screening behaviors was used for Cycles 2, 3, and 4. Participants were mailed a $10 grocery store gift certificate in appreciation of their time.
Interviewer training
Women (n = 38) from the communities with various career backgrounds (ie, social worker, sales clerk, homemaker, marketing research interviewer) were hired as interviewers. The interviewers received a 1 to 2-day training session by the investigators at the beginning of each cycle. The training included general project information and procedures, interviewing techniques, research ethics, the importance of informed consent, a review of the survey, and the importance of the documentation of administrative procedures. The interviewers were responsible for completing all cycles of the survey using contact lists provided by the research team.
Intervention
Theoretical framework
The theoretical framework for the community-based interventions included the PRECEDE-PROCEED Model for planning and evaluating the program including a social, epidemiologic, behavioral, environmental, educational, and ecological assessment,31 Social Learning Theory in terms of using lay health advisors to deliver education messages and to increase self-efficacy,32 Health Belief Model for identifying and addressing barriers,33 and the Transtheoretical Model for addressing the stage of readiness to change.34 For the clinic-based intervention, messages focused on provider-patient communication about CRC screening.
Focus groups and baseline surveys
A focus group of community providers (n = 10) was conducted to identify CRC screening practices and procedures and current CRC screening educational materials used in clinics. In addition, women (n = 40) from the community participated in 4 focus groups that focused on CRC and CRC screening knowledge, screening barriers, and patient-provider communication issues associated with CRC screening. The women evaluated educational materials (brochures, etc) for cultural acceptability and ease of understanding. Intervention materials were revised based on the suggestions (minor changes in wording, preferred format, etc) from the women participating in the focus groups.
The educational intervention was designed to address barriers experienced by women while being culturally acceptable to all racial groups. The goal of the intervention was to increase awareness of the benefits of early detection of CRC and to encourage women to reduce their own risk of CRC mortality by identifying and reducing barriers to obtaining screening. The American Cancer Society’s (ACS) screening guidelines were used in designing educational materials. Results from the surveys from Cycles 1 and 2 were used to provide baseline information on CRC knowledge, beliefs, attitudes, and screening practices.
ACS volunteer training
One ACS coordinator was hired and trained to serve as a liaison to the research team. The ACS coordinator trained ACS area coordinators who in turn trained the local ACS project volunteers (n = 179) throughout North and South Carolina. The volunteers lived in the communities and came from various career backgrounds (ie, nurses, teachers, sorority member). ACS volunteer training included general project information, the role of a community education volunteer, cancer rates among minority and underserved populations, and an introduction to CRC that included risk factors, screening, diagnosis, and treatment. During the training the project protocol, procedures (ie, checking with clinics about educational materials for waiting rooms and examination rooms, chart reminders), documentation of materials and events (ie, type and quantity of clinic educational materials distributed, activity attendance sheets), and the importance of completing administrative documents was stressed.
Intervention delivery
All intervention components were delivered by trained ACS volunteers. Out-reach strategies (educational classes, direct mailings, brochures, media campaigns by community newspapers and local radio stations) focused on providing messages to the public and in-reach strategies (waiting-room posters, monthly examination-room messages) were directed to healthcare providers and clinics.
Process evaluation
During the duration of the CARES Project, evaluation methods aimed at documenting the training process, monitoring the progress of the project, and refining the intervention components were used. The total number of ACS volunteers and the number of hours spent in program activities were tracked. Tracking of events that could directly affect screening practices in each city participating in this project were also maintained by the ACS volunteers. In addition, process evaluation occurred at the various educational workshops and in the medical clinics.
Data Analysis
Women within guidelines versus not within guidelines
Cycles 1 and 2 of the surveys were considered ‘base-line’ because these were collected before the intervention in any region and were used to analyze the differences between women who were within or not within CRC screening guidelines. CRC screening within guidelines was determined by self-report using ACS-recommended guidelines: a Fecal Occult Blood Test (FOBT) annually, a flexible sigmoidoscopy (FS) every 5 years, double contrast barium enema every 5 years, or a colonoscopy every 10 years.1 Composite CRC knowledge, beliefs, and barrier scores were created by summing the scores for individual items in each category and raw summary scores were then transformed to a 0 to 10 scale for analyses. High scores indicated better knowledge, positive beliefs, and more barriers. Participants within and out of screening guidelines were compared on demographic factors, health behaviors, and knowledge, beliefs about, and barriers to CRC screening.
The GLIMMIX macro35 in the SAS System for Windows36 v. 9.1 was used to fit generalized linear mixed models with a logit link and binomial error function on screening within guidelines (Yes/No), testing the effect of each factor (demographic factors, health behaviors, and knowledge, beliefs, and barriers of CRC screening) after adjusting for Cycle (1 and 2) as a linear fixed effect and city as a random effect (with random intercept and slope and an unstructured covariance structure). Denominator degrees of freedom for group-level effects (cycle) were based on the number of cities instead of the number of individuals.37,38 Odds ratios (ORs), 95% confidence intervals (CIs), and P-values were calculated from the models to compare the odds of screening between the levels within each factor.
The demographic characteristics tested were: race, age, education, work status, marital status, any health insurance, smoking status, medical condition requiring regular physician visits, personal history of polyps or CRC, perceived risk of CRC, and physician recommendation for CRC screening. Based on the results of univariate analyses, purposeful forward-selection modeling39 was performed to identify the set of factors most associated with CRC screening at baseline. This was done by adding each variable to the ‘GLIMMIX’ model 1-at-a-time and deciding whether or not to keep it in the model based on both statistical evidence and substantive grounds. Two-way interactions were considered between factors included in the main effects model in the same way.
This modeling procedure was then performed in the same manner to identify the best set of factors associated with intention to screen. Intention was defined by participants who were not currently within guidelines and indicated being very likely or slightly likely to have a flexible sigmoidoscopy in the next 12 months.
Analysis of intervention effects
To evaluate the effect of the intervention data from all 5 cycles were used and generalized linear mixed models (described above) were used with screening, intention to receive screening, physician’s recommendation for screening, and knowledge, belief, and barrier items as separate binary outcomes, with intervention as the main predictor. The variable testing the intervention effect was an indicator variable equal to 1 for surveys done after the intervention had begun in the participant’s city and equal to 0 if it was before the intervention began or in a city that never received the intervention (cities in Region 3). Again, cycle was included as a fixed effect (linear 1 to 5), random intercepts and slopes were included for each city (unstructured covariance), and the denominator degrees of freedom for cycle and intervention were based on the number of observations at the city level. Linear mixed models were similarly used to test the intervention effect on continuous knowledge, belief, and barrier scores. ORs, 95% CIs, and P-values were calculated to describe the odds of each response for participants postintervention compared with those without intervention.
In addressing the main hypothesis of the intervention effect on screening, risk-factor modeling38 was performed to determine the intervention effect after accounting for important confounders and effect modifiers. The same model was used as described above and variables for age and race were added first due to their known importance. To determine possible confounders, variables were considered in the model 1-at-a-time while evaluating the change in the parameter estimate for the intervention effect (instead of the significance of the factor added). To identify any effect modifiers, interactions between the intervention effect and each main effect were tested in the model and included if both clinically and statistically significant. Similarly, risk-factor modeling was also used to determine the intervention effect on participants’ intention to have a flexible sigmoidoscopy in the next 12 months if they were not currently within CRC screening guidelines, after adjusting for any important confounders. Predictive forward-selection models and risk-factor models were performed both using cycle as the linear effect for time and using the month of the survey as the linear effect for time.
The results were consistent, so only the results based on cycle are reported. Effects for knowledge, belief, and barriers were tested in the risk-factor models and caused a slight decrease in the ORs for the treatment effects. Because they are on the causal pathway of the treatment effect, they were determined to be mediators and not included in the final models.
RESULTS
Characteristics of the Sample
A total of 2283 surveys were completed from a random sample of 3127 eligible women from all regions during the 5 cycles, resulting in a participation rate of 73.0%. Only participants ages 51 years and older were included (N = 2259). Where CRC screening could not be determined because of missing (n = 80) or “don’t know” (n = 81) responses, these women were removed from the analysis for a total of 2098 participants (530 surveys from Region 1; 537 from Region 2; 537 from Region 3; and 494 surveys from Region 4).
Demographic characteristics (n = 888) at baseline are listed in Table 1. About 3-quarters (78%) of the participants were African American, 62% were 65 years and older, and very few (4%) were married or living with a partner. Almost 3-quarters (71%) had not graduated from high school, only 9% worked full or part-time, 64% had medical conditions that required regular physician visits, and 15% reported having no health insurance. A physician recommendation for a CRC screening test was reported by 64% of the women.
TABLE 1.
Demographic Characteristics of Participants at Baseline and Differences in CRC Screening Within Guidelines
| Characteristic | CRC Screening within guidelines | Total no. (%) | OR* | 95% Confidence interval |
||
|---|---|---|---|---|---|---|
| No no. (%) | Yes no. (%) | |||||
| Race | White | 76 (46) | 90 (54) | 166 (19) | 1.00 | |
| African American | 360 (52) | 337 (48) | 697 (78) | 0.80 | 0.57,1.14 | |
| Other | 14 (56) | 11(44) | 25 (3) | 0.69 | 0.29,1.63 | |
| Age, y | 50–64 | 175 (51) | 165 (49) | 340 (38) | 1.00 | |
| 65–74 | 127 (48) | 140 (52) | 267 (30) | 1.15 | 0.83,1.60 | |
| 75–84 | 97 (49) | 101 (51) | 198 (22) | 1.10 | 0.77,1.57 | |
| 85+ | 51 (61) | 32 (39) | 83 (9) | 0.63 | 0.38,1.03 | |
| Education | < 8th grade | 179 (54) | 154 (46) | 333 (38) | 1.00 | |
| 9th–10th grade | 151 (52) | 139 (48) | 290 (33) | 1.07 | 0.78,1.48 | |
| High school, GED | 92 (46) | 109 (54) | 201 (23) | 1.34 | 0.93,1.92 | |
| Some college | 27 (44) | 35 (56) | 62 (7) | 1.41 | 0.80,2.46 | |
| Work status | Full/part time | 47 (58) | 34 (42) | 81 (9) | 1.00 | |
| Volunteer/other | 12 (52) | 11 (48) | 23 (3) | 1.11 | 0.43,2.84 | |
| Unemployed | 26 (70) | 11 (30) | 37 (4) | 0.43 | 0.18,1.02 | |
| Retired | 181 (50) | 180 (50) | 361 (41) | 1.30 | 0.79,2.14 | |
| Unable to work | 183 (48) | 202 (52) | 385 (43) | 1.51 | 0.92,2.49 | |
| Marital status | Married/living together | 14 (36) | 25 (64) | 39 (4) | 1.00 | |
| Separated/divorced | 129 (49) | 134 (51) | 263 (30) | 0.58 | 0.28,1.17 | |
| Widowed | 231 (51) | 225 (49) | 456 (52) | 0.54 | 0.27,1.08 | |
| Never married | 75 (60) | 51 (40) | 126 (14) | 0.39† | 0.18,0.83 | |
| Insurance | No | 77 (58) | 55 (42) | 132 (15) | 1.00 | |
| Yes | 373 (49) | 382 (51) | 755 (85) | 1.51† | 1.02,2.25 | |
| Medical condition requiring regular visits | No | 197 (63) | 118 (37) | 315 (36) | 1.00 | |
| Yes | 253 (44) | 319 (56) | 572 (64) | 1.89† | 1.41,2.53 | |
| Doctor recommended CRC screening | No | 284 (90) | 33 (10) | 317 (36) | 1.00 | |
| Yes | 164 (29) | 403 (71) | 567 (64) | 21.9† | 14.7,32.9 | |
| Perceived risk of CRC | Much lower | 73 (56) | 58 (44) | 131 (15) | 1.00 | |
| Somewhat lower | 58 (45) | 70 (55) | 128 (14) | 1.44 | 0.87,2.38 | |
| About the same | 164 (48) | 178 (52) | 342 (39) | 1.21 | 0.80,1.83 | |
| Somewhat higher | 26 (46) | 30(54) | 56 (6) | 1.32 | 0.69,2.49 | |
| Much higher | 3 (15) | 17 (85) | 20 (2) | 7.04† | 1.95,25.5 | |
| Don’t know | 126 (60) | 85 (40) | 211 (24) | 0.89 | 0.56,1.42 | |
| Personal history of polyps | No | 445 (56) | 356 (44) | 801 (91) | 1.00 | |
| Yes | 5 (6) | 79 (94) | 84 (9) | 19.3† | 7.59,48.9 | |
| Personal history of CRC | No | 446 (51) | 425 (49) | 871 (99) | 1.00 | |
| Yes | 2 (15) | 11 (85) | 13 (1) | 6.33† | 1.38,29.1 | |
CRC indicates colorectal cancer; OR, odds ratio.
OR is modeling odds of being within screening guidelines from a generalized linear mixed model with a logit link and binomial error function, adjusting for cycle (1/2) as a linear fixed effect and city as a random effect (with random intercept and slope and an unstructured covariance structure). Denominator degrees of freedom for group-level effects (cycle) were based on the number of observations at the city level.
P <.05.
Screening Rates
At baseline, the CRC screening rate within guidelines was 49.3% (95% CI, 46.0%, 52.6%) among the participants. The CRC screening rates differed by city ranging from 29.5% in Greenville, NC to 66.7% in Anderson, SC (P < .001). The odds of being within CRC screening guidelines were significantly higher for participants who reported a physician recommendation for CRC screening (OR = 21.9; P < .001), for individuals with a history of polyps (OR = 19.3; P < .001) or colon cancer (OR = 6.33; P =.018), for individuals who perceived their CRC risk as “much higher” (OR = 7.04; P =.003) compared with “much lower,” for participants who had a medical condition requiring regular physician visits (OR = 1.89; P < .001), and for individuals having health insurance (OR = 1.51; P = .042). The odds of being within CRC screening guidelines were significantly lower for participants who had never been married (OR = 0.39; P = .014) compared with those who were currently married or living with a partner.
After the intervention the odds of being within CRC screening guidelines for women living in a city that had received the intervention were 1.27 times (95% CI, 0.90, 1.78; P = .172) the odds of women living in a city that had not received the intervention, after adjusting for the cycle and city in a generalized linear mixed model. The estimated CRC screening rates within guidelines were 55.6% (95% CI, 47.9%, 63.0%) for women receiving the intervention and 49.7% (95% CI, 42.9%, 56.6%) for women not receiving the intervention. In risk-factor modeling, age group, insurance status, and cancer history confounded the relation between intervention and CRC screening. After adjusting for these variables, as well as the participant’s race, the OR for completing CRC screening for women living in the cities receiving the intervention was 1.13 times (95% CI, 0.80, 1.59; P = .496) the odds of women living in a city that had not received the intervention.
Among participants not currently within recommended screening guidelines, women exposed to the intervention had 1.96 times (95% CI, 1.21, 3.18; P = .007) the odds of reporting an intention to complete screening in the next 12 months compared with women not exposed to the intervention. After adjusting for confounders of age, race, and insurance status the OR of reporting the intention for completing CRC screening decreased to 1.56 (95% CI, 0.99, 2.44; P = .053).
Colorectal Cancer Knowledge, Beliefs, and Barriers
At baseline the association between knowledge, beliefs, and barriers and CRC screening within guidelines revealed being within screening guidelines was higher for women who had better CRC knowledge (OR = 1.20; 95% CI, 1.11, 1.29; P < .001), more positive beliefs about CRC screening (OR = 1.26; 95% CI, 1.17, 1.36; P < .001), and had fewer barriers to completing CRC screening (OR = 0.70; 95% CI, 0.65, 0.76; P < .001). The intervention had a significant impact only on the belief score (β = .36, 95% CI, 0.09, 0.64; P = .010), with participants in the cities exposed to the intervention having more positive belief about CRC screening after receiving the intervention.
Process Evaluation
Extensive process evaluation documented the CARES project activities, the educational materials provided, staff involvement, participants’ experiences, and the strengths and weakness of the intervention. The following activities occurred: 211 community classes with 2519 participants, 6 community presentations with 77 participants, 24 churches held classes or distributed bulletins, a booth was set up at 4 community health fairs, 109 information centers were set up in local businesses (beauty and barber shops, drug-store, grocery stores, public housing resource centers) and distributed 15,400 brochures throughout the 4 geographic regions, and 5 community clinics participated in the project where 4169 brochures and posters were distributed. In addition, 7 local radio stations were recruited and played public service announcements during the duration of the project.
DISCUSSION
Colorectal cancer is a significant public health problem, especially among minority populations. This study used ACS partnerships to deliver the intervention that was designed to improve CRC screening rates among low-income, minority women in North and South Carolina. The current study was modeled after a previous successful community-based project in North Carolina that significantly increased cervical and breast cancer screening rates.29
The outcome, CRC screening within guidelines, in this study had similar predictors as identified in previous studies conducted among different racial, socioeconomic status, and cultural groups.19–26 In this study the strongest predictor of completing CRC screening within guidelines was a recommendation by a physician (OR = 21.9), which is similar to a report of a 2001 telephone survey of a random sample of Medicare consumers residing in North and South Carolina.26 There was not, however, a significant increase in the completion of CRC screening in women who lived in a community that received the intervention compared with women living in a community that had not received the intervention. There was, however, a significant increase in positive beliefs about CRC screening and in the intention to complete CRC screening in the future among women living in a community that had received the intervention. Intention to be screened has been reported to be a strong factor associated with future CRC screening.40 Conceivably, if the participants in this study were followed for a longer period of time a significant difference in CRC screening rates may have emerged.
Previous community-based cancer prevention research has identified that an effective method of reaching underserved populations is with lay health advisors (LHA), informal educators, or natural helpers.41,42 In this study only ACS volunteers were used, and they were not paid for work associated with the CARES Project. Similar results were reported by Campbell et al.,43 who found that LHA assigned to African-American churches in rural North Carolina did not significantly increase FOBT screening because of suboptimal intervention exposure and limited information diffusion. In contrast, Paskett et al.29 used paid LHA in 2 previous studies among low-income minority populations, urban and rural, to significantly increase breast (P < .001) and cervical cancer screening (P = .003), and mammography screening (P < .01).44 The last study assigned LHA to visit specific women in the intervention condition to deliver educational messages and barriers counseling. Thus, LHA may be effective in changing health behaviors if they are paid and provide individual attention to assigned study participants or community members.
This study has several strengths. It included a medically underserved population of low-income women and was modeled after an evidence-based intervention that improved breast and cervical cancer screening rates. Women from the community assisted with the design of the educational materials and helped plan and implement the intervention. The study also included a region that did not receive the intervention so that adjustment for any secular trends in CRC screening could be controlled. The varying CRC screening rates by city may be due to several reasons (medical practices, community culture, population access to screening services, etc) that were not necessarily captured in the study. This is 1 reason each community served as its own control in the study design. Finally, this study represents diffusion and dissemination of an evidence-based intervention by a cancer-focused volunteer organization.
Limitations of this study include assessment of CRC screening based on women’s self-report, which has been shown to vary in accuracy compared with medical records.45 The women included in this study did not represent all women living in low-income housing in the US. Volunteers have limited ability to penetrate communities unless directed to specific community residents to educate, and there was also a lack of control of the intervention delivery as well as limited ability to track the fidelity of the intervention. Because the duration of the intervention was short (3 years), it may not have saturated the communities or captured completion of all CRC screening that was initiated by the intervention. Finally, this was a community-based intervention study and was not a randomized trial.
In conclusion, this study describes a large community-based intervention to increase CRC screening among low-income women. Although there was no effect on CRC screening rates, there was a significant increase in positive beliefs about CRC screening and in intention to complete CRC screening in the future among women living in a community that had received the intervention. Future community-based intervention studies to increase CRC screening should be more intensive and longer in duration to engage hard-to-reach populations.
Acknowledgments
Supported by the American Cancer Society, Grant #TIOG-99-361-02 and NCI 1 K07 CA107079.
Footnotes
All women provided written informed consent, and the study was approved by the institutional review boards at Wake Forest University Medical Center and Ohio State University.
REFERENCES
- 1.American Cancer Society: Cancer Facts and Figures, 2006. Atlanta, GA: American Cancer Society; 2006. [Google Scholar]
- 2.American Cancer Society Cancer: Facts and Figures for African Americans, 2005–2006. Atlanta, GA: American Cancer Society; 2005. [Google Scholar]
- 3.Bradley CJ, Givens CW, Roberts C. Disparities in cancer diagnosis and survival. Cancer. 2001;91:178–188. doi: 10.1002/1097-0142(20010101)91:1<178::aid-cncr23>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 4.Irby K, Anderson WF, Henson DE, Devesa SS. Emerging and widening colorectal carcinoma disparities between Blacks and Whites in the United States (1975–2002) Cancer Epidemiol Biomarkers Prev. 2006;15:792–797. doi: 10.1158/1055-9965.EPI-05-0879. [DOI] [PubMed] [Google Scholar]
- 5.Brawley OW. Some perspective on Black-White cancer statistics. CA Cancer J Clin. 2002;52:322–325. doi: 10.3322/canjclin.52.6.322. [DOI] [PubMed] [Google Scholar]
- 6.Freeman HP. Cancer in the socioeconomically disadvantaged. CA CancerJ Clin. 1989;39:266–288. doi: 10.3322/canjclin.39.5.266. [DOI] [PubMed] [Google Scholar]
- 7.Winawer S, Fletcher R, Rex D, et al. Colorectal cancer screening and surveillance: clinical guidelines and rationale—update based on new evidence. Gastroenterology. 2003;124:544–560. doi: 10.1053/gast.2003.50044. [DOI] [PubMed] [Google Scholar]
- 8.Pignone M, Rich M, Teutsch SM, Berg AO, Lohr KN. Screening for colorectal cancer in adults at average risk: a summary of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med. 2002;137:132–141. doi: 10.7326/0003-4819-137-2-200207160-00015. [DOI] [PubMed] [Google Scholar]
- 9.Smith RA, Cokkinides V, Eyre HJ. American Cancer Society Guidelines for the early detection of cancer, 2006. CA Cancer J Clin. 2006;56:11–25. doi: 10.3322/canjclin.56.1.11. [DOI] [PubMed] [Google Scholar]
- 10.Coffield AB, Maciosek MV, McGinnis JM, et al. Priorities among recommended clinical preventive services. Am J Prev Med. 2001;21:1–9. doi: 10.1016/s0749-3797(01)00308-7. [DOI] [PubMed] [Google Scholar]
- 11.Maciosek MV, Solberg LI, Coffield AB, Edwards NM, Goodman MJ. Colorectal cancer screening. Health impact and cost effectiveness. Am J Prev Med. 2006;31:80–89. doi: 10.1016/j.amepre.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 12.Mandel JS, Church TR, Bond JH, et al. The effect of fecal occult-blood screening on the incidence of colorectal cancer. N Engl J Med. 2000;343:1603–1607. doi: 10.1056/NEJM200011303432203. [DOI] [PubMed] [Google Scholar]
- 13.Chu KC, Tarone RE, Chow WH, Hankey BF, Ries LA. Temporal patterns in colorectal cancer incidence, survival, and mortality from 1950 through 1990. J Natl Cancer Inst. 1994;86:997–1006. doi: 10.1093/jnci/86.13.997. [DOI] [PubMed] [Google Scholar]
- 14.Centers for Disease Control and Prevention. National Center for Chronic Disease Prevention and Health Promotion. Behavioral Risk Factor Surveillance System (2004) 2006 Available from URL: http://apps.nccd.cdc.gov/brfss.
- 15.James TM, Griener KA, Ellerbeck EF, Feng C, Ahluwalia JS. Disparities in colorectal cancer screening: a guideline-based analysis of adherence. Ethn Dis. 2006;16:228–233. [PubMed] [Google Scholar]
- 16.Schenck AP, Klabunde CN, Davis WW. Racial differences in colorectal cancer test use by Medicare consumers. Am J Prev Med. 2006;30:320–326. doi: 10.1016/j.amepre.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 17.Matthews BA, Anderson RC, Nattinger AB. Colorectal cancer screening behavior and health insurance status (United States) Cancer Causes Control. 2005;16:735–742. doi: 10.1007/s10552-005-1228-z. [DOI] [PubMed] [Google Scholar]
- 18.Meissner HI, Breen N, Klabunde CN, Vernon SW. Patterns of colorectal cancer screening uptake among men and women in the United States. Cancer Epidemiol Biomarkers Prev. 2006;15:389–394. doi: 10.1158/1055-9965.EPI-05-0678. [DOI] [PubMed] [Google Scholar]
- 19.Paskett ED, Rushing J, D’Agostino R, Jr, Tatum C, Velez R. Cancer screening behaviors of low-income women: the impact of race. Women’s Health. 1997;3:203–226. [PubMed] [Google Scholar]
- 20.Vernon SW. Participation in colorectal cancer screening: a review. J Natl Cancer Inst. 1997;89:1406–1422. doi: 10.1093/jnci/89.19.1406. [DOI] [PubMed] [Google Scholar]
- 21.Holmes-Rovner M, Williams GA, Hoppough S, Quillan L, Butler R, Given CW. Colorectal cancer screening barriers in persons with low income. Cancer Practice. 2002;10:240–247. doi: 10.1046/j.1523-5394.2002.105003.x. [DOI] [PubMed] [Google Scholar]
- 22.Cornelius LJ, Smith PL, Simpson GM. What factors hinder women of color from obtaining preventive health care? Am J Public Health. 2002;92:535–539. doi: 10.2105/ajph.92.4.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.O’Malley AS, Beaton E, Yabroff KR, Abramson R, Mandelblatt J. Patient and provider barriers to colorectal cancer screening in the primary care safety-net. Prev Med. 2004;39:56–63. doi: 10.1016/j.ypmed.2004.02.022. [DOI] [PubMed] [Google Scholar]
- 24.Klabunde CN, Vernon SW, Nadel MR, Breen N, Seef LC, Brown ML. Barriers to colorectal cancer screening: a comparison of reports from primary care physicians and average-risk adults. Med Care. 2005;43:939–944. doi: 10.1097/01.mlr.0000173599.67470.ba. [DOI] [PubMed] [Google Scholar]
- 25.Greisinger A, Hawley ST, Bettencourt JL, Perz CA, Vernon SW. Primary care patients’ understanding of colorectal cancer screening. Cancer Detect Prev. 2006;30:67–74. doi: 10.1016/j.cdp.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 26.Klabunde CN, Schenck AP, Davis WW. Barriers to colorectal cancer screening among medicare consumers. Am J Prev Med. 2006;30:313–319. doi: 10.1016/j.amepre.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 27.Dulai GS, Farmer MM, Ganz PA, et al. Primary care provider perceptions of barriers to and facilitators of colorectal cancer screening in managed care setting. Cancer. 2004;100:1843–1852. doi: 10.1002/cncr.20209. [DOI] [PubMed] [Google Scholar]
- 28.Stone EG, Morton SC, Hulscher ME. Interventions that increase use of adult immunization and cancer screening services: a meta-analysis. Ann Intern Med. 2002;136:641–651. doi: 10.7326/0003-4819-136-9-200205070-00006. [DOI] [PubMed] [Google Scholar]
- 29.Paskett ED, Tatum CM, D’Agostino R, et al. Community-based interventions to improve breast and cervical cancer screening: results of the Forsyth County Cancer Screening (FoCaS) Project. Cancer Epidemiol Biomarkers Prev. 1999;8:453–459. [PubMed] [Google Scholar]
- 30.Paskett ED, Tatum C, Rushing, et al. Randomized trial of an intervention to improve mammography utilization among a triracial rural population of women. J Natl Cancer Inst. 2006;98:1226–1237. doi: 10.1093/jnci/djj333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Green LW, Kreuter MW. Health Promotion Planning. An Educational and Ecological Approach. 3rd ed. New York: McGraw-Hill; 1999. [Google Scholar]
- 32.Baranowski T, Perry CL, Parcel GS. Social Cognitive Theory. In: Glanz K, Rimer BK, Lewis FM, editors. Health Behavior and Health Education. Theory, Research, and Practice. 3rd ed. San Francisco: Jossey-Bass; 2002. pp. 165–184. [Google Scholar]
- 33.Rosenstock IM, Strecher VJ, Becker MH. Social learning theory and the health belief model. Health Educ Q. 1988;15:175–183. doi: 10.1177/109019818801500203. [DOI] [PubMed] [Google Scholar]
- 34.Prochaska JO, Velicer WF, Rossi JS, et al. Stages of change and decisional balance for 12 problem behaviors. Health Psychol. 1994;13:39–46. doi: 10.1037//0278-6133.13.1.39. [DOI] [PubMed] [Google Scholar]
- 35.Wolfinger R, O’Connell M. Generalized linear mixed models: a pseudo-likelihood approach. J Stat Comput Simul. 1993;48 [Google Scholar]
- 36.SAS/STAT Software, v 9.1. SAS System for Windows. Cary, NC: SAS Institute; 2002. [Google Scholar]
- 37.Raudenbush SW, Bryk AS. Hierarchical Linear Models: Applications and Data Analysis Methods. 2nd ed. Newbury Park, CA: Sage; 2002. [Google Scholar]
- 38.Murray DM. Design and analysis of group-randomized trials. New York: Oxford University Press; 1998. [Google Scholar]
- 39.Hosmer DW, Lemeshow S. Applied Logistic Regression. 2nd ed. New York: Wiley-Interscience; 2000. [Google Scholar]
- 40.Myers RE, Balshem AM, Wolf TA, Ross EA, Millner L. Adherence to continuous screening for colorectal neoplasia. Med Care. 1993;31:508–519. doi: 10.1097/00005650-199306000-00004. [DOI] [PubMed] [Google Scholar]
- 41.Earp JL, Flax VL. What lay health advisors do. An evaluation of advisor’s activities. Cancer Pract. 1999;7:16–21. doi: 10.1046/j.1523-5394.1999.07104.x. [DOI] [PubMed] [Google Scholar]
- 42.Altpeter M, Earp JL, Bishop C, Eng E. Lay health advisor activity levels: definitions from the field. Health Educ Behav. 1999;26:495–512. doi: 10.1177/109019819902600408. [DOI] [PubMed] [Google Scholar]
- 43.Campbell MK, James A, Hudson MA, et al. Improving multiple behaviors for colorectal cancer prevention among African American church members. Health Psychol. 2004;23:492–502. doi: 10.1037/0278-6133.23.5.492. [DOI] [PubMed] [Google Scholar]
- 44.Paskett ED, Tatum C, Rushing J, et al. Racial differences in knowledge, attitudes and cancer screening practices among a triracial rural population. Cancer. 2004;101:2650–2659. doi: 10.1002/cncr.20671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Baier M, Calonge N, Cutter G, et al. Validity of self-reported colorectal cancer screening behavior. Cancer Epidemiol Biomarkers Prev. 2000;9:229–232. [PubMed] [Google Scholar]