Abstract
Osteoclast-mediated bone resorption precedes osteoblast-mediated bone formation through early adulthood, but formation fails to keep pace with resorption during aging. We previously identified several factors produced by osteoclasts that promote bone formation. In this study, we determined if osteoclast-produced factors contribute to the impaired bone formation with aging. We previously found that mice between the ages of 18 and 22 months develop age-related bone loss. Bone marrow-derived pre-osteoclasts were isolated from 6-week, 12-month, and 18- to 24-month-old mice and differentiated into osteoclasts in vitro. Conditioned media were collected and compared for osteoblast mineralization support. Conditioned medium from osteoclasts from all ages was able to support mineralization of bone marrow stromal cells. Concentrating the conditioned medium from 6-week-old and 12-month-old mouse marrow cells-derived osteoclasts enhanced mineralization support whereas concentrated conditioned medium from 18- to 24-month-old mouse marrow-derived osteoclasts repressed mineralization compared to base medium. This observation suggests that an inhibitor of mineralization was secreted by aged murine osteoclasts. Gene and protein analysis revealed that the Wnt antagonist sclerostin was significantly elevated in the conditioned media from 24-month-old mouse cells compared to 6-week-old mouse cells. Antibodies directed to sclerostin neutralized the influences of the aged mouse cell concentrated conditioned media on mineralization. Sclerostin is primarily produced by osteocytes in young animals. This study demonstrates that osteoclasts from aged mice also produce sclerostin in quantities that may contribute to the age-related impairment in bone formation.
Keywords: OSTEOCLAST, AGING, SCLEROSTIN
When individuals reach adulthood, bone resorption and bone formation are approximately equal, substantiating the hypothesis that bone resorption and bone formation are tightly coupled during optimal periods of bone health [Martin et al., 2009]. It is now well established that osteoblasts control osteoclast differentiation from precursor cells through the production of macrophage colony stimulating factor (M-CSF) and receptor activator of NFκB (RANKL) [Khosla, 2001]. Recent interest has focused on whether the communication is bidirectional and if osteoclasts and/or the process of bone resorption could be involved in regulating bone formation. Cell-cell contact through the Eph/ephrin system and release of matrix bound transforming growth factor beta 1 (TGF-β1) and insulin like growth factor 1 (IGF-I) during bone resorption are implicated in this process [Weisman et al., 1990; Tang et al., 2009; Xian et al., 2012]. Our studies showed that osteoclasts produce bone morphogenic protein 6 (BMP6), sphingosin 1 phosphate (S1P), and Wnt10b, which are candidate secreted factors that may couple bone resorption to subsequent bone formation [Pederson et al., 2008].
Age-related bone loss is associated with a defect in bone formation [Lips et al., 1978], but the underlying mechanisms for this defect remain to be clearly defined. We have documented that 18- to 22-month-old mice exhibit age-related bone loss that is only partially prevented by estrogen stabilization [Syed et al., 2010]. Here we examined the influences of aging on osteoclast support of osteoblast mineralization using this mouse model. Conditioned media produced by osteoclasts from aged mice stimulated osteoblast mineralization; however, concentrating the conditioned media suppressed mineralization below the level of base media mineralization support. This suggested the presence of a mineralization inhibitor(s) in the conditioned media from the aged mouse cells. Additional studies identified that the Wnt signaling inhibitor sclerostin as a potent inhibitor of mineralization in aged mice.
MATERIALS AND METHODS
Unless otherwise indicated, all chemicals were from Sigma Chemical Co. (St. Louis, MO).
OSTEOCLAST CULTURE
All protocols were approved by the Mayo Clinic IACUC prior to the start of the studies. Six-week-old, 12-month-old, and 18- to 24-month-old Balb c or C57Bl/6 mice (National Institutes of Health, National Institute on Aging) were sacrificed and bone marrow harvested as we previously reported [Pederson et al., 2008]. Red blood cells were lysed and the remaining bone marrow cells were cultured in α-minimal essential medium (α-MEM) supplemented with 10% (v/v) fetal bovine serum (FBS) and 25 ng/ml M-CSF and incubated at 37°C, in 5% CO2 [Pederson et al., 2008]. One day after isolation, adherent mesenchymal cells were discarded and the non-adherent cells were cultured in the presence of 100 ng/ml RANKL and 25 ng/ml M-CSF (differentiation medium) for 4 days with a re-feeding on Day 3.
CONDITIONED MEDIA PREPARATION
For mineralization and ELISA analyses, mature osteoclast conditioned medium was harvested and either held for testing or concentrated 10-fold (v/v) using Amicon Centricon Plus-70 centrifugation concentrators. These concentrators retain and concentrate factors that are 10 kDa or greater in size. For western blot analysis of secreted proteins, osteoclasts were rinsed three times with PBS and incubated for 24 h in serum free α-MEM. Serum free conditioned media were harvested and concentrated 50-fold (v/v) as above.
OSTEOBLAST CULTURE, MINERALIZATION, AND QUANTITATION
Calvarial osteoblastic cells from neonates were obtained and cultured as previously described [Subramaniam et al., 2005]. Cultures were maintained and replated for experiments in α-MEM supplemented with 10% FBS (base medium). For mineralization, 70% confluent cells were treated with base or conditioned media supplemented with ascorbic acid (1 μM) and β-glycerol phosphate (4 μM). Mineralization was assessed by quantifying calcium in the extracellular matrix or staining fixed cells with Alizarin Red [Pederson et al., 2008]. Calcium content of the matrix was assessed by rinsing the cultures with Dulbecco’s Ca2+ free phosphate buffered saline (Gibco Life Technologies), extracting Ca2+ with 0.6 N HCl and using a Teco Diagnostics Calcium Reagent Set that quantifies Ca2+ concentrations against a standard curve. Alizarin red was eluted with 10% cetylpyridinium chloride and levels were determined using a standard curve.
QUANTITATIVE REAL-TIME-POLYMERASE CHAIN REACTION
Cells were rinsed with PBS and RNA harvested from mature osteoclasts using Qiagen’s microRNA purification kit according to the product literature. Following quantitation, cDNA was synthesized and real time PCR analysis carried out as we have reported [Karst et al., 2004]. Primers were:
| Gene | Forward | Reverse |
|---|---|---|
| Sclerostin | 5′-TACTGGGAGAG CTGGCTGTGT-3′ |
5′-GTTTCCTCACC CTCCTCCTCA-3′ |
| BMP6 | 5′-GGTTCTTCAGAC TACAACGG-3′ |
5′-GAAGGAACAC TCTCCATCAC-3′ |
| SPHK1 | 5′-GACTTTGTCCTG GTGCTGGT-3′ |
5′-CCGCACGTACG TAGAACAGA-3′ |
| Wnt10b | 5′-CCACTGGTGCT GTTATGTGC-3′ |
5′-CAGTGCTTCTC CTCCTCGTC-3′ |
| Tubulin A1A | 5′-GAGTGCATCTC CATCCACGTT-3′ |
5′-TAGAGCTCCCA GCAGGCATT-3′ |
Fluorescence was quantified as the Ct value. The differences between the mean Ct values of the genes were denoted (ΔCt) and the difference between the ΔCt value of the Tubulin A1A was calculated as ΔΔCt. The log2(ΔΔCt) resulted in the relative quantification value of gene expression. Expression is calculated for each gene relative to expression of that gene in cells from 6-week-old mice.
SCLEROSTIN QUANTITATION
Osteoclast conditioned media were concentrated 10-fold as above and assayed using the mouse Sclerostin ELISA from Alpco Immunoassays. The protocol for conditioned media analysis was modified from the provider’s instructions in that the samples were assayed without the directed 10-fold dilution and the incubation of the samples was at 4°C for 18 h with rocking instead of 4.5 h at room temperature.
WESTERN BLOT ANALYSIS
Proteins were separated using 10% SDS–PAGE followed by electroblotting to Immobilon-P membranes (Millipore, Bedford, MA). To verify comparable lane loading, the blots were stained for total protein with 0.1% (w/v) Ponceau S in 5% (v/v) acetic acid for 5 min, rinsed with deionized water, and scanned with a CanoScan LiDE 100 scanner. Membranes were probed as described with antibodies to mouse Sclerostin (1:1,000 dilution; R&D) with secondary antibodies to goat (1:5,000 dilution; Abcam) [Pederson et al., 2008]. Signals were visualized using the ECL Plus detection system (Amersham Biosciences, Buckinghamshire, England) according to the manufacturer’s instructions.
NEUTRALIZING ANTIBODY TREATMENT
Osteoclast conditioned media from 6-week-old to 24-month-old mice were concentrated 10-fold as above and was pre-treated with 10 μl/ml isotope matched control or anti-sclerostin antibodies (Acris Antibodies) prior to addition to calvarial cells. Other controls were base medium and untreated concentrated aged mouse osteoclast conditioned media. The mineralization assay was carried out as outlined above with analysis for Ca2+ incorporation into the matrix analyzed 7-10 days after initiation.
STATISTICS
Each experiment had at least three replicates and was repeated at least three times. These data are representative of the results. Data were analyzed using a one way analysis of variance (ANOVA) as compared to controls as indicated in each figure legend and are presented as mean ± SEM. Significance was determined at P < 0.05 using KaleidaGraph software (Synergy Software, Reading PA).
RESULTS
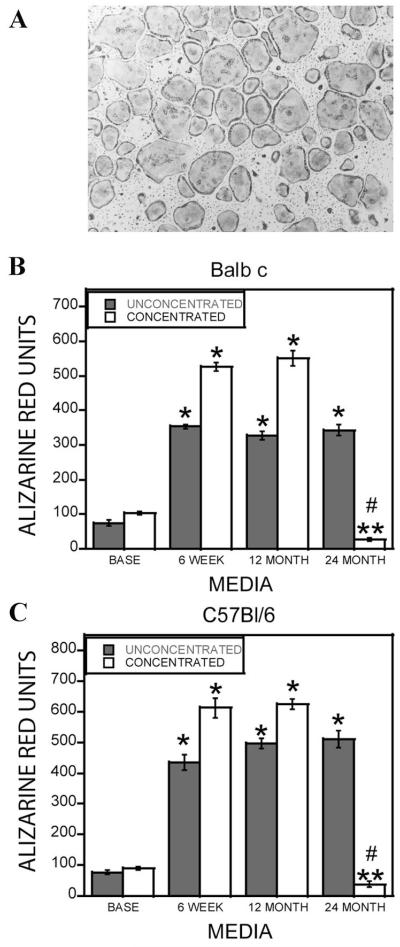
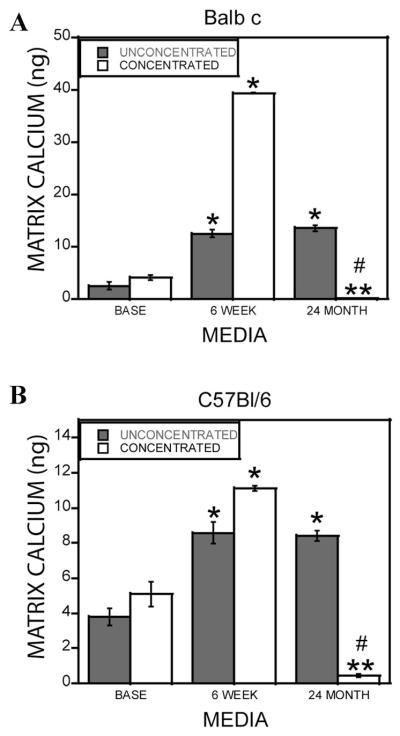
Aging is associated with a defect in bone formation [Lips et al., 1978]. We evaluated whether differences existed in the ability of osteoclasts from young and aged Balb c and C57Bl/6 mouse marrow to promote osteoblastic cell mineralization in vitro. Marrow harvested from the mice efficiently differentiated into osteoclasts (Fig. 1A). In previous studies, 10-fold concentrated conditioned media from osteoclasts from 6- to 12-week-old mice stimulated osteogenesis of mesenchymal cells [Pederson et al., 2008]. In these experiments, unconcentrated conditioned media was compared to 10-fold concentrated media to evaluate the contributions of candidate factors larger than 10,000 Da. Mineralization was assessed with Alizarin red staining (Fig. 1B,C) and by quantitating Ca2+ incorporation into the extracellular matrix (Fig. 2). There was no detectable difference in mineralization between any age of mouse cell sources when unconcentrated conditioned media was examined. However, 10-fold concentrated conditioned media from 18- to 24-month, but not 6-week or 12-month-old, mouse marrow inhibited mineralization in both assays. Mineralization levels were significantly below that supported by concentrated base medium. A similar pattern was observed with cells from either the Balb c or the C57Bl/6 mouse strains.
Fig. 1.
A: Marrow from 18-month-old Balb c mice was cultured to generate osteoclasts as detailed. Cultures were fixed and stained for tartrate resistant acid phosphatase. B,C: Alizarin red quantitation of osteoclast support of mineralization. Base medium (BASE), conditioned media from 6-week, 12-month, and 24-month-old mouse marrow-derived osteoclasts from Balb c (B) and C57Bl/6 (C) mice were collected. The media were left unconcentrated or concentrated 10-fold. Calvarial osteoblasts were treated for 1–2 weeks with the indicated media in the presence of ascorbic acid and β-glycerol phosphate. Cultures were fixed and stained with alizarin red, and extracted as detailed in the Materials and Methods Section. **P < 0.05 comparing conditioned medium to corresponding base medium; ****P < 0.05 comparing 24-month-old cell source to corresponding 6-week or 12-month-old medium; #P < 0.05 decrease in conditioned medium response compared to corresponding base medium. Results with 18- to 22-month-old C57Bl/6 and Balb c mice were similar to the 24-month-old mouse cell conditioned medium.
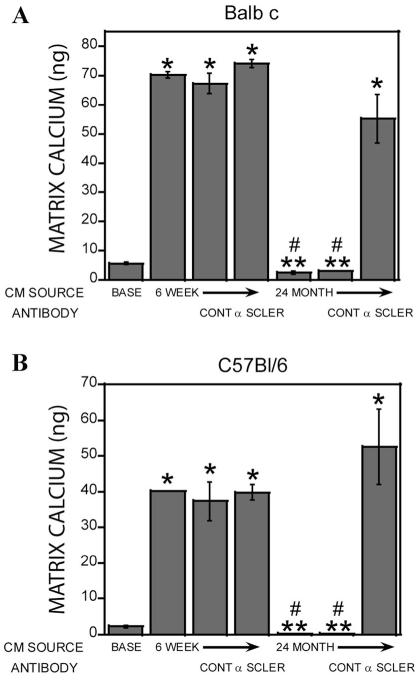
Fig. 2.
Extracellular matrix calcium content stimulated by osteoclast conditioned media. Base medium or conditioned medium from 6-week and 24-month-old mouse marrow-derived osteoclasts from Balb c (A) or C57Bl/6 (B) mice were collected. The media were left untreated or concentrated 10-fold as described. Calvarial osteoblasts were treated for 1-week with the indicated media in the presence of ascorbic acid and β-glycerol phosphate as described. Cultures were extracted and calcium bound to the extracellular matrix was quantitated as detailed in the Materials and Methods Section. **P < 0.05 comparing conditioned medium to corresponding base medium; ****P < 0.05 comparing 24-month-old cell source to corresponding 6-week or 12-month-old medium; #P < 0.05 decrease in conditioned medium response compared to corresponding base medium. Results with 18- to 22-month-old mice were similar to the 24-month-old C57Bl/6 and Balb c mouse cell conditioned medium.
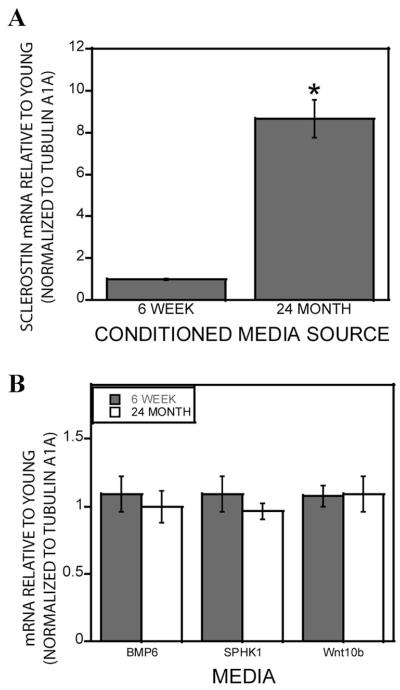
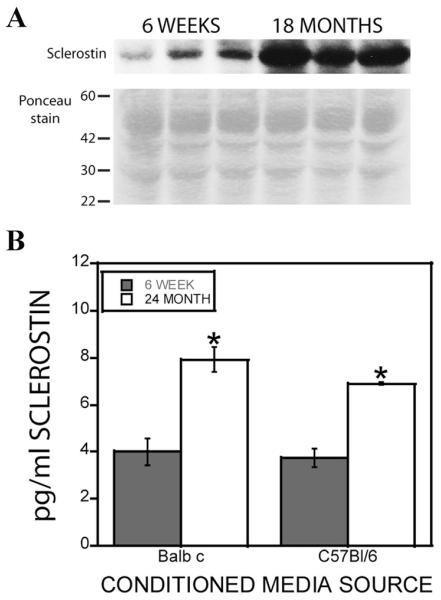
The observation that concentrated conditioned media was required to observe reduced support of mineralization suggested that the concentration process was increasing the levels of a mineralization inhibitor larger than 10 kDa. We documented that early osteoclast precursors expressed and secreted the Wnt inhibitor sclerostin, which rapidly decreases as the cells differentiate [Pederson et al., 2008]. We therefore examined osteoclasts from 6-week and 24-month-old mice for sclerostin mRNA expression and observed significantly higher expression in cells from aged mice (Fig. 3A). In contrast, the expression of previously identified coupling factors, BMP6, Wnt10b, or S1P, did not change during aging (Fig. 3B). Sclerostin protein was significantly increased in the conditioned media derived from 24-month-old mouse marrow compared to osteoclasts obtained from 6-week-old mouse marrow as measured by both Western blotting (Fig. 4A) and a quantitative ELISA (Fig. 4B). Ponceau S staining indicated no overall apparent differences in protein secretion between the young and aged mouse cells (Fig. 4A lower panel).
Fig. 3.
Effect of aging on coupling factor gene expression. A: Marrow-derived osteoclast RNA was assessed for sclerostin mRNA expression in cells from 6-week-old to 24-month-old Balb c mice were analyzed by real-time-PCR. **P < 0.05 compared to mRNA levels in 6-week-old mouse cells. B: Expression of BMP6, SPHK1, and Wnt10b in osteoclasts from 6-week to 24-month-old Balb c mouse marrow. Results similar to the 24-month-old mouse data were obtained with 18- to 22-month-old C57Bl/6 and Balb c mouse cells.
Fig. 4.
Sclerostin secretion by young and aged mouse osteoclasts. A: Marrow-derived osteoclasts from three 6-week-old to three 18-month-old Balb c mice were rinsed with PBS and cultured for an additional 24 h with serum free media as detailed in the Materials and Methods Section. Conditioned media was harvested and concentrated 50-fold for Western blot analysis. The top panel is probing blot for sclerostin levels. The bottom panel is the Ponceau red stained region of the blot between 30 and 60 kDa (size marker migration is indicated on the left of the blot image; the predicted sclerostin molecular weight is 40 kDa). Similar results were obtained with 18- to 24-month-old C57Bl/6 mouse cells. B: Conditioned media from marrow derived osteoclasts from Balb c and C57Bl/6 mice of the indicated ages were collected and concentrated 10-fold prior to analysis of sclerostin protein levels as described. **P < 0.05 compared to 6-week-old levels.
To evaluate whether the elevated sclerostin in the conditioned media from 24-month-old mouse osteoclasts contributed to suppression of mineralization, concentrated conditioned medium from 6-week and 24-month-old mouse osteoclasts was treated with control or an anti-sclerostin antibody prior to addition to calvarial cells (Fig. 5). Sclerostin neutralizing antibodies restored support of calvarial cell mineralization, but the control antibodies did not.
Fig. 5.
Neutralizing sclerostin reduced aged osteoclast conditioned media suppression of mineralization. Six-week-old and 24-month-old C57Bl/6 mouse marrow-derived osteoclast conditioned media was collected and concentrated 10-fold. Calvarial osteoblasts were cultured as described with conditioned media with and without the indicated antibody as detailed in the Materials and Methods Section. Mineralization was assessed by determine calcium incorporation in to the matrix. Similar results were obtained with 18- to 22-month-old Balb c and C57Bl/6 mouse cells. **P < 0.05 comparing conditioned medium to base medium; ****P < 0.05 comparing 24-month-old cell source to corresponding 6-week-old medium; #P < 0.05 decrease in conditioned medium response compared to base medium.
DISCUSSION
Bone metabolism in young adults is tightly coupled in that the bone that is removed by osteoclasts is precisely replaced in both location and amount by new bone. With aging, bone formation is impaired [Lips et al., 1978], leading to a net deficit in bone formation as compared to bone resorption and subsequent bone loss. In this study, we investigated how aging influences the ability of osteoclasts to promote osteoblast mineralization. Our results demonstrate that, while production of coupling factors remains constant with aging, osteoclasts from aged animals secrete substantially more sclerostin, which is a potent suppressor of bone formation. Sclerostin, the product of the gene Sost, is primarily produced by osteocytes [Winkler et al., 2003; van Bezooijen et al., 2004]. Mice lacking sclerostin exhibit increased Wnt signaling, bone density, and bone strength whereas mice overexpressing sclerostin are osteopenic [Winkler et al., 2003; Loots et al., 2005; Li et al., 2008; Krause et al., 2010]. Mutations in Sost cause two rare bone disorders: sclerosteosis and van Buchem disease, which involve increased osteoblast activity and bone formation [Wergedal et al., 2003]. Sclerostin binds to the Wnt receptors Lrp5 and Lrp6, and inhibits Lrp association with the required co-receptors, Frizzleds, and Wnts [Li et al., 2005; Semenov et al., 2005]. Consequently, sclerostin inhibits osteoblast proliferation and stimulates osteoblast apoptosis [Winkler et al., 2003; Sutherland et al., 2004; van Bezooijen et al., 2004]. To our knowledge no studies to date have investigated whether osteocyte sclerostin expression changes with aging, although serum sclerostin levels do increase markedly with age in women and in men [Modder et al., 2011]. The observation that osteoclasts from aged mice express sclerostin at higher levels than cells from young mice suggest that further exploration of sclerostin expression changes with aging are warranted.
Recently, Qing et al. [2012] found that lactating, but not virgin, mouse osteocytes express cathepsin K, which has been regarded at highly restricted within the bone environment to osteoclasts [Teitelbaum, 2007]. Our data support an interesting parallel in that osteoclasts from aged mice express a protein whose expression is mostly restricted to osteocytes [Winkler et al., 2003].
Impairment of osteoblast functions in the aging skeleton is well documented. Rubin et al. [1992] observed that load-induced osteogenesis was reduced in aged animals compared to young animals. In vitro studies of osteoblasts taken from aged animals have sought to resolve the cause of this defect. Parathyroid hormone stimulation of cAMP accumulation and stimulation of gap junction communication in osteoblasts decrease with aging [Donahue et al., 1997; Genetos et al., 2012]. Osteoblasts from aged animals also respond poorly to fluid flow and flow-induced intracellular calcium oscillations [Donahue et al., 2001]. In a mouse model of accelerated aging, osteoblasts from aged animals exhibited impaired telomere maintenance and reduced differentiation capacity that was independent of proliferation [Wang et al., 2012]. Expression of the nuclear receptor Rorβ in mouse bone marrow-derived osteoblast precursors is elevated in cells from aged mice compared to young mice and expression of Rorβ suppresses osteoblast differentiation in vitro, implicating Rorβ in age related reduced bone formation [Roforth et al., 2012]. It is possible that some of these osteoblast-autonomous aging phenotypes may, in part, be due to paracrine sclerostin exposure in vivo prior to cell harvesting. Resolution of this will require additional studies.
While concentrated base and osteoclast conditioned media derived from 6-week and 12-month-old mice resulted in stimulation of osteoblast mineralization when compared to unconcentrated media, there were clear differences with concentration of conditioned media from 24-month-old mice. The concentrated 24-month-old mouse osteoclast conditioned media suppressed mineralization below that observed with concentrated base medium. This reduction below base medium support indicated the presence of one or more mineralization inhibitors. Given the proximity of osteoblasts and osteoclasts in the closed bone remodeling compartment (BRC), which may serve to locally concentrate secreted factors facilitating communication between osteoblasts and osteoclasts [Khosla et al., 2008], it is plausible that the concentrated conditioned media more accurately reflects the in vivo cross-talk occurring between osteoclasts and osteoblasts. We recognize, however, that further validation of our findings requires extension of these data to in vivo models involving, for example, selective deletion of the SOST gene in osteoclasts from aged mice and the consequences of that on the impaired bone formation with aging. This study is currently on going.
Sclerostin inhibitors are under development as an anabolic therapy to promote bone health. Both pre-clinical and clinical studies of sclerostin neutralizing antibodies have shown promising results in post-menopausal females [Li et al., 2009; Padhi et al., 2011; Rachner et al., 2011]. It will be of interest to evaluate whether a component of the positive influences of neutralizing sclerostin is due to influencing osteoclast-derived sclerostin in the aging skeleton. Deregulation of Wnt signaling is involved in the development and progression of several malignancies including breast cancer [Olson and Papkoff, 1994; Wong et al., 1994, 2002; Bankfalvi et al., 1999; Jonsson et al., 2000; Li et al., 2000; Karayiannakis et al., 2001; Hatsell et al., 2003; Ayyanan et al., 2006; Benhaj et al., 2006; Zardawi et al., 2009]. Thus, sclerostin-mediated suppression of Wnt signaling would be beneficial in targeting tumor cells, given the importance of Wnt in promoting tumor progression. To balance the positive and negative impacts of altering Wnt signaling, it is imperative to fully understand the myriad effects of Wnts in health and disease. Our findings implicate suppressed Wnt signaling in age-related bone loss, which lessens the desirability of global suppression of Wnt to suppress tumor development over extended periods of time.
Given the complexity of Wnt influences, it is important to resolve their roles in normal and pathological conditions in order to design more effective therapies to target cancer and aging. The study reported here supports that the age-related impairment in bone formation may be due, at least in part, to increased osteoclast sclerostin production. Targeting this sclerostin source may prove to be beneficial in the aging skeleton whereas mimicking this deregulation could be an effective therapy to target osteolytic tumor progression.
ACKNOWLEDGMENTS
We wish to acknowledge Christine Hachfeld for her assistance. P.Q. was supported by NIH training grant DK07352.
Grant sponsor: National Institutes of Health; Grant number: P01 AG004875Grant number: DK07352; Grant sponsor: The Mayo Foundation.
Footnotes
The authors have no conflicts of interest.
REFERENCES
- Ayyanan A, Civenni G, Ciarloni L, Morel C, Mueller N, Lefort K, Mandinova A, Raffoul W, Fiche M, Dotto GP, Brisken C. Increased Wnt signaling triggers oncogenic conversion of human breast epithelial cells by a Notch-dependent mechanism. Proc Natl Acad Sci USA. 2006;103:3799–3804. doi: 10.1073/pnas.0600065103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bankfalvi A, Terpe HJ, Breukelmann D, Bier B, Rempe D, Pschadka G, Krech R, Lelle RJ, Boecker W. Immunophenotypic and prognostic analysis of E-cadherin and beta-catenin expression during breast carcinogenesis and tumour progression: A comparative study with CD44. Histopathology. 1999;34:25–34. doi: 10.1046/j.1365-2559.1999.00540.x. [DOI] [PubMed] [Google Scholar]
- Benhaj K, Akcali KC, Ozturk M. Redundant expression of canonical Wnt ligands in human breast cancer cell lines. Oncol Rep. 2006;15:701–707. [PubMed] [Google Scholar]
- Donahue HJ, Zhou Z, Li Z, McCauley LK. Age-related decreases in stimulatory G protein-coupled adenylate cyclase activity in osteoblastic cells. Am J Physiol. 1997;273:E776–E781. doi: 10.1152/ajpendo.1997.273.4.E776. [DOI] [PubMed] [Google Scholar]
- Donahue SW, Jacobs CR, Donahue HJ. Flow-induced calcium oscillations in rat osteoblasts are age, loading frequency, and shear stress dependent. Am J Physiol. Cell Physiol. 2001;281:C1635–C1641. doi: 10.1152/ajpcell.2001.281.5.C1635. [DOI] [PubMed] [Google Scholar]
- Genetos DC, Zhou Z, Li Z, Donahue HJ. Age-related changes in gap junctional intercellular communication in osteoblastic cells. J Orthop Res. 2012;30:1979–1984. doi: 10.1002/jor.22172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatsell S, Rowlands T, Hiremath M, Cowin P. Beta-catenin and Tcfs in mammary development and cancer. J Mammary Gland Biol Neoplasia. 2003;8:145–158. doi: 10.1023/a:1025944723047. [DOI] [PubMed] [Google Scholar]
- Jonsson M, Borg A, Nilbert M, Andersson T. Involvement of adenomatous polyposis coli (APC)/beta-catenin signalling in human breast cancer. Eur J Cancer. 2000;36:242–248. doi: 10.1016/s0959-8049(99)00276-2. [DOI] [PubMed] [Google Scholar]
- Karayiannakis AJ, Nakopoulou L, Gakiopoulou H, Keramopoulos A, Davaris PS, Pignatelli M. Expression patterns of beta-catenin in in situ and invasive breast cancer. Eur J Surg Oncol. 2001;27:31–36. doi: 10.1053/ejso.1999.1017. [DOI] [PubMed] [Google Scholar]
- Karst M, Gorny G, Galvin RJ, Oursler MJ. Roles of stromal cell RANKL, OPG, and M-CSF expression in biphasic TGF-beta regulation of osteoclast differentiation. J Cell Physiol. 2004;200:99–106. doi: 10.1002/jcp.20036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khosla S. Minireview: The OPG/RANKL/RANK system. Endocrinology. 2001;142:5050–5055. doi: 10.1210/endo.142.12.8536. [DOI] [PubMed] [Google Scholar]
- Khosla S, Westendorf JJ, Oursler MJ. Building bone to reverse osteoporosis and repair fractures. J Clin Invest. 2008;118:421–428. doi: 10.1172/JCI33612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause C, Korchynskyi O, de Rooij K, Weidauer SE, de Gorter DJ, van Bezooijen RL, Hatsell S, Economides AN, Mueller TD, Lowik CW, ten Dijke P. Distinct modes of inhibition by sclerostin on bone morphogenetic protein and Wnt signaling pathways. J Biol Chem. 2010;285:41614–41626. doi: 10.1074/jbc.M110.153890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Ominsky MS, Niu QT, Sun N, Daugherty B, D’Agostin D, Kurahara C, Gao Y, Cao J, Gong J, Asuncion F, Barrero M, Warmington K, Dwyer D, Stolina M, Morony S, Sarosi I, Kostenuik PJ, Lacey DL, Simonet WS, Ke HZ, Paszty C. Targeted deletion of the sclerostin gene in mice results in increased bone formation and bone strength. J Bone Miner Res. 2008;23:860–869. doi: 10.1359/jbmr.080216. [DOI] [PubMed] [Google Scholar]
- Li X, Ominsky MS, Warmington KS, Morony S, Gong J, Cao J, Gao Y, Shalhoub V, Tipton B, Haldankar R, Chen Q, Winters A, Boone T, Geng Z, Niu QT, Ke HZ, Kostenuik PJ, Simonet WS, Lacey DL, Paszty C. Sclerostin antibody treatment increases bone formation, bone mass, and bone strength in a rat model of postmenopausal osteoporosis. J Bone Miner Res. 2009;24:578–588. doi: 10.1359/jbmr.081206. [DOI] [PubMed] [Google Scholar]
- Li X, Zhang Y, Kang H, Liu W, Liu P, Zhang J, Harris SE, Wu D. Sclerostin binds to LRP5/6 and antagonizes canonical Wnt signaling. J Biol Chem. 2005;280:19883–19887. doi: 10.1074/jbc.M413274200. [DOI] [PubMed] [Google Scholar]
- Li Y, Hively WP, Varmus HE. Use of MMTV-Wnt-1 transgenic mice for studying the genetic basis of breast cancer. Oncogene. 2000;19:1002–1009. doi: 10.1038/sj.onc.1203273. [DOI] [PubMed] [Google Scholar]
- Lips P, Courpron P, Meunier PJ. Mean wall thickness of trabecular bone packets in the human iliac crest: Changes with age. Calcified Tissue Res. 1978;26:13–17. doi: 10.1007/BF02013227. [DOI] [PubMed] [Google Scholar]
- Loots GG, Kneissel M, Keller H, Baptist M, Chang J, Collette NM, Ovcharenko D, Plajzer-Frick I, Rubin EM. Genomic deletion of a long-range bone enhancer misregulates sclerostin in Van Buchem disease. Genome Res. 2005;15:928–935. doi: 10.1101/gr.3437105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin T, Gooi JH, Sims NA. Molecular mechanisms in coupling of bone formation to resorption. Critic Rev Eukaryot Gene Expr. 2009;19:73–88. doi: 10.1615/critreveukargeneexpr.v19.i1.40. [DOI] [PubMed] [Google Scholar]
- Modder UI, Hoey KA, Amin S, McCready LK, Achenbach SJ, Riggs BL, Melton LJ, III, Khosla S. Relation of age, gender, and bone mass to circulating sclerostin levels in women and men. J Bone Miner Res. 2011;26:373–379. doi: 10.1002/jbmr.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson DJ, Papkoff J. Regulated expression of Wnt family members during proliferation of C57mg mammary cells. Cell Growth Differ. 1994;5:197–206. [PubMed] [Google Scholar]
- Padhi D, Jang G, Stouch B, Fang L, Posvar E. Single-dose, placebo-controlled, randomized study of AMG 785, a sclerostin monoclonal antibody. J Bone Miner Res. 2011;26:19–26. doi: 10.1002/jbmr.173. [DOI] [PubMed] [Google Scholar]
- Pederson L, Ruan M, Westendorf JJ, Khosla S, Oursler MJ. Regulation of bone formation by osteoclasts involves Wnt/BMP signaling and the chemokine sphingosine-1-phosphate. Proc Natl Acad Sci USAm. 2008;105:20764–20769. doi: 10.1073/pnas.0805133106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qing H, Ardeshirpour L, Pajevic PD, Dusevich V, Jahn K, Kato S, Wysolmerski J, Bonewald LF. Demonstration of osteocytic perilacunar/canalicular remodeling in mice during lactation. J Bone Miner Res. 2012;27:1018–1029. doi: 10.1002/jbmr.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rachner TD, Khosla S, Hofbauer LC. Osteoporosis: Now and the future. Lancet. 2011;377:1276–1287. doi: 10.1016/S0140-6736(10)62349-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roforth MM, Liu G, Khosla S, Monroe DG. Examination of nuclear receptor expression in osteoblasts reveals Rorbeta as an important regulator of osteogenesis. J Bone Miner Res. 2012;27:891–901. doi: 10.1002/jbmr.1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin CT, Bain SD, McLeod KJ. Suppression of the osteogenic response in the aging skeleton. Calcified Tissue Int. 1992;50:306–313. doi: 10.1007/BF00301627. [DOI] [PubMed] [Google Scholar]
- Semenov M, Tamai K, He X. SOST is a ligand for LRP5/LRP6 and a Wnt signaling inhibitor. J Biol Chem. 2005;280:26770–26775. doi: 10.1074/jbc.M504308200. [DOI] [PubMed] [Google Scholar]
- Subramaniam M, Gorny G, Johnsen SA, Monroe DG, Evans GL, Fraser DG, Rickard DJ, Rasmussen K, van Deursen JM, Turner RT, Oursler MJ, Spelsberg TC. TIEG1 null mouse-derived osteoblasts are defective in mineralization and in support of osteoclast differentiation in vitro. Mol Cell Biol. 2005;25:1191–1199. doi: 10.1128/MCB.25.3.1191-1199.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland MK, Geoghegan JC, Yu C, Turcott E, Skonier JE, Winkler DG, Latham JA. Sclerostin promotes the apoptosis of human osteoblastic cells: A novel regulation of bone formation. Bone. 2004;35:828–835. doi: 10.1016/j.bone.2004.05.023. [DOI] [PubMed] [Google Scholar]
- Syed FA, Modder UI, Roforth M, Hensen I, Fraser DG, Peterson JM, Oursler MJ, Khosla S. Effects of chronic estrogen treatment on modulating age-related bone loss in female mice. J Bone Miner Res. 2010;25:2438–2446. doi: 10.1002/jbmr.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y, Wu X, Lei W, Pang L, Wan C, Shi Z, Zhao L, Nagy TR, Peng X, Hu J, Feng X, Van Hul W, Wan M, Cao X. TGF-beta1-induced migration of bone mesenchymal stem cells couples bone resorption with formation. Nat Med. 2009;15:757–765. doi: 10.1038/nm.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teitelbaum SL. Osteoclasts: What do they do and how do they do it? Am J Pathol. 2007;170:427–435. doi: 10.2353/ajpath.2007.060834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Bezooijen RL, Roelen BA, Visser A, van der Wee-Pals L, de Wilt E, Karperien M, Hamersma H, Papapoulos SE, ten Dijke P, Lowik CW. Sclerostin is an osteocyte-expressed negative regulator of bone formation, but not a classical BMP antagonist. J Exp Med. 2004;199:805–814. doi: 10.1084/jem.20031454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Chen Q, Lee SH, Choi Y, Brad Johnson F, Pignolo RJ. Impairment of osteoblast differentiation due to proliferation-independent telomere dysfunction in mouse models of accelerated aging. Aging Cell. 2012;11:704–713. doi: 10.1111/j.1474-9726.2012.00838.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisman LS, Emr SD, Wickner WT. Mutants of Saccharomyces cerevisiae that block intervacuole vesicular traffic and vacuole division and segregation. Proc Natl Acad Sci USA. 1990;87:1076–1080. doi: 10.1073/pnas.87.3.1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wergedal JE, Veskovic K, Hellan M, Nyght C, Balemans W, Libanati C, Vanhoenacker FM, Tan J, Baylink DJ, Van Hul W. Patients with Van Buchem disease, an osteosclerotic genetic disease, have elevated bone formation markers, higher bone density, and greater derived polar moment of inertia than normal. J Clin Endocrinol Metabol. 2003;88:5778–5783. doi: 10.1210/jc.2003-030201. [DOI] [PubMed] [Google Scholar]
- Winkler DG, Sutherland MK, Geoghegan JC, Yu C, Hayes T, Skonier JE, Shpektor D, Jonas M, Kovacevich BR, Staehling-Hampton K, Appleby M, Brunkow ME, Latham JA. Osteocyte control of bone formation via sclerostin, a novel BMP antagonist. EMBO J. 2003;22:6267–6276. doi: 10.1093/emboj/cdg599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong GT, Gavin BJ, McMahon AP. Differential transformation of mammary epithelial cells by Wnt genes. Mol Cell Biol. 1994;14:6278–6286. doi: 10.1128/mcb.14.9.6278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong SC, Lo SF, Lee KC, Yam JW, Chan JK, Wendy Hsiao WL. Expression of frizzled-related protein and Wnt-signalling molecules in invasive human breast tumours. J Pathol. 2002;196:145–153. doi: 10.1002/path.1035. [DOI] [PubMed] [Google Scholar]
- Xian L, Wu X, Pang L, Lou M, Rosen CJ, Qiu T, Crane J, Frassica F, Zhang L, Rodriguez JP, Xiaofeng J, Shoshana Y, Shouhong X, Argiris E, Mei W, Xu C. Matrix IGF-1 maintains bone mass by activation of mTOR in mesenchymal stem cells. Nat Med. 2012;18:1095–1101. doi: 10.1038/nm.2793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zardawi SJ, O’Toole SA, Sutherland RL, Musgrove EA. Dysregulation of Hedgehog, Wnt and Notch signalling pathways in breast cancer. Histol Histopathol. 2009;24:385–398. doi: 10.14670/HH-24.385. [DOI] [PubMed] [Google Scholar]