Abstract
Oropharyngeal swallowing is a complex sensorimotor phenomenon that has had decades of research dedicated to understanding it more thoroughly. However, the underlying neural mechanisms responsible for normal and disordered swallowing remain very vague. We consider this gap in knowledge the result of swallowing research that has been broad (identifying phenomena) but not deep (identifying what controls the phenomena). The goals of this review are to address the complexity of motor control of oropharyngeal swallowing and to review the principles of motor learning based on limb movements as a model system. We compare this literature on limb motor learning to what is known about oropharyngeal function as a first step toward suggesting the use of motor learning principles in swallowing research.
Keywords: Neuroscience, Motor learning, Adaptation, Muscle memory, Motor control, Deglutition, Deglutition disorders
Introduction
Swallowing disorders (dysphagia) can be the devastating consequences of many different disorders or diseases, including neurological damage, accidental trauma to head or neck structures, or head and neck cancer treatments. Oropharyngeal swallowing is a complex sensorimotor phenomenon involving numerous meticulously timed events that require the peripheral and central nervous systems and many paired muscles. Given the complexity of the oropharyngeal swallow, in-depth and rigorous scientific inquiry is needed to improve our understanding of the system.
Dysphagia research has examined many phenomena that impact the success or failure of a swallow. This research allows us to understand more about the kinematics of the many structures involved in oropharyngeal swallowing (i.e., jaws, tongue, velum, pharynx, larynx, esophagus), the impact of bolus properties (i.e., consistency, taste, volume, chemesthesis), and the many etiologies of dysfunctional swallowing. Despite this knowledge, our field is open to critiques of being shallow because our understanding of the neural mechanisms responsible for those phenomena is deficient. In other words, scientific inquiry into swallowing and swallowing disorders has been broad, in that it describes many phenomena (i.e., what do we see happening?), but not deep, because it rarely delves into how these phenomena are controlled (i.e., what is controlling what we see?). A complete discipline rests on each of these aspects; effective evidence-based medicine requires both.
A well-defined model of normal and disordered swallowing control has remarkable potential for transforming how we care for our patients with dysphagia. However, when comparing the research in dysphagia with other areas of sensorimotor function, such as movements of the eyes, arms, or legs, those subdisciplines have a deeper comprehension of underlying neural mechanisms. In particular, they have defined the neurological control of accurate movements (motor control) as well as the ways in which inaccurate movements are improved (motor learning). Studies of oropharyngeal function could benefit greatly from understanding how other movements are controlled by the central nervous system.
In this review we first address the complexity of motor control of oropharyngeal swallowing. Then we review the principles of motor learning based on limb movements as a model system. We also compare this literature on motor learning to what is known about oropharyngeal function as a first step toward suggesting using motor learning principles in swallowing research.
Motor Control
Motor control is a very broad discipline that encompasses all of the neurologic aspects of movement. It is the process of creating movement, including the interaction between the central and peripheral nervous systems (CNS and PNS) for creating coordinated and skilled actions. Work in the field of motor control, as applied to swallowing physiology, can be traced back through the last two centuries [1–3]. Wise and Shadmehr [4] present a relatively modern review of the field of motor control, which is accessible to nonspecialists.
Decades of research on motor control in swallowing and swallowing disorders have addressed a broad range of issues related to normal and disordered swallowing, yet we still have a limited understanding of motor control in oropharyngeal swallowing. Although little is known about swallowing behavior relative to important principles of motor control, such as sensory feed-back and levels of control, we discuss some concepts below.
Sensory Feed-Back and Prediction
According to Shadmehr et al. [5], “motor control is the study of how organisms make accurate goal-directed movements.” They expand on this definition by explaining that producing accurate movements over the course of a lifetime is possible because we are born with an adaptable nervous system. This adaptable nervous system recognizes its limitations and continuously compensates for them to prevent systematic movement errors [5].
So how are these limitations perceived and adapted regularly with seemingly little conscious effort? Experts in motor control emphasize the importance of sensory input for accurate motor control. One important concept is that ease of movement is possible due to the steady stream of sensory information that is being processed while planning, executing, and evaluating an action. Thus, constant sensory feed-back is necessary for correcting movement. In addition to using sensory feed-back, the human nervous system also has the capacity to predict sensory consequences of motor commands. According to Shadmehr et al. [5], this means that we make sensory predictions so that the brain will not have to wait for actual sensory feed-back before it can act. Thus, we can estimate the movement and the environment first by predicting what should happen and then by receiving information from our sensory system about what did happen to make comparisons.
These concepts about sensory feed-back and making predictions prior to sensory confirmation are particularly significant for oropharyngeal swallowing. For instance, many of the movements needed to break down a solid bolus in the oral cavity through mastication require a steady stream of constant sensory feed-back about the state and position of the bolus relative to the masticators [6]. Such feed-back ensures that pressure between the teeth is applied to the bolus and not to the tongue or cheeks. Sensory feed-back also ensures that the bolus is propelled into the pharynx with proper timing and force, considering the unique properties (i.e., size, viscosity, texture) of the bolus; this is necessary to trigger a swallow that is appropriate for the bolus. If the swallow is triggered and the swallowing motor plan is not appropriate, it is unlikely that online corrections may be made midswallow, although this has not been directly tested using scientific methods. This suggests that while the bolus is still in the oral cavity, some sensory predictions about bolus readiness for the pharyngeal swallow are being made before pharyngeal or laryngeal sensory feed-back can confirm a successful or unsuccessful swallow [7].
Levels of Control: CNS
The brain is commonly considered to be hierarchical, with cortical regions responsible for volitional, higher-level tasks, and the brain stem is associated with nonvolitional tasks and heavily influenced by cortical input [8]. In other words, a hierarchical model assumes that the direction of control moves from higher to lower centers, with no reciprocity of control, such as brain-stem influence on cortical centers [9]. However, debates over pure hierarchical theories of the human brain are ongoing, with alternative views that the CNS is likely heterarchical and not hierarchical [10]. Heterarchical control, described by Turvey et al. [9, 10], is the principle that control in the CNS is circular so that multiple brain regions at various levels modulate one another, leading to control reciprocity. In other words, although the cortex is responsible for most higher-level tasks (i.e., executive function), it still receives input from lower centers and uses it to derive the best possible commands.
Empirical evidence on swallowing supports the theory of reciprocity of central control or heterarchical control, as multiple levels of the brain, including the cortex, subcortex, cerebellum, and brain stem, may drive swallowing behavior [11–14]. For instance, swallowing behavior can be modified by top-down input (intention-based, i.e., cortical drive to peripheral structures), where the cortex influences lower brain centers, demonstrated by an altered swallowing response by the peripheral structures. An example of top-down modification is evident when reflexive components of oropharyngeal swallowing are altered on command with a Mendelsohn maneuver, a breath hold, or an effortful swallow, which are associated with more cortical involvement [15, 16]. Alternatively, bottom-up input (stimulus-based, i.e. peripheral input to the cortex) is shown by the finding that cortical activity during swallowing is modulated by reflexively initiated swallowing behavior (bolus delivered directly to pharynx) or by targeting peripheral sensory receptors (i.e., sour or flavorful bolus, cutaneous electrical stimulation, air puff) [17–19].
Levels of Control: Reflexive to Volitional Behaviors
One significant issue in motor control is defining movements or systems as reflexive or volitional [20]. Oropharyngeal swallowing is multifaceted, having both volitional and reflexive components [21]. The volitional component is often described as the oral phase, where the bolus is prepared and then transferred to the pharynx. The reflexive component is typically considered the pharyngeal phase where a cascade of events such as pharyngeal squeeze, hyolaryngeal movement, and upper esophageal sphincter opening occur only after a sufficient sensory trigger elicits them [22].
One current view is that rather than two distinct categories of reflex or volition, there is a continuum on which many sensorimotor tasks can be placed. Prochazka et al. [20] provide a thorough overview of this debate in their review. The authors agree that reflexive movements require a trigger to be initiated, are difficult to suppress once in motion, and can be controlled primarily by lower brain areas (i.e., brain stem or spinal cord). While volitional movements differ because they do not require a trigger, they can be interrupted at any time and they are controlled primarily by the cortex [20].
One challenge in understanding oropharyngeal swallowing is that, as mentioned previously, its components fall on more than one place on this reflex-to-volition continuum. For instance, mastication follows the criteria for volitional movement wherein no stimulus is needed—one can simulate chewing without a bolus—and the behavior can be volitionally interrupted at any time. However, it is possible that mastication might move toward the reflexive end of the continuum under certain circumstances. A good example was given by Gibbs and Suit [23], who showed that contact with an unexpected hard bolus during normal mastication caused participants to immediately discontinue mastication. Thus, peripheral sensory input to the central nervous system for mastication (central pattern generator) likely resulted in suppression of this rhythmic behavior. Gibbs and Suit [23] then noted that mastication cycles that immediately followed the unexpected hard bolus were slow and cautious. This careful mastication suggests greater cortical involvement (moving back toward the volitional end of the continuum), given the potential danger, thus temporarily modulating chewing behavior while continued sensory feed-back confirmed that no more hard particles remained. Therefore, during the course of feeding, masticatory behavior moved along the continuum of low and high voluntary control due to the presence of a perturbation. Similar conclusions may be made for components of the pharyngeal swallow that might move toward the volitional end of the continuum during a Mendelsohn maneuver.
Motor Control Summary
There are a number of resources that form a basis of our current understanding of the motor control for swallowing and should be familiar to all researchers and clinicians in the field. Miller's text, The Neuroscientific Principles of Swallowing and Dysphagia [24] (and its subsequent update [25]) is an invaluable resource in its thorough review of the literature. More recently, Steele and Miller [6] reviewed sensory input and motor control of swallowing. Multiple methodologies, including functional magnetic resonance imaging and transcranial magnetic stimulation, have enhanced our understanding of which portions of the cortex are associated with swallowing behavior [12, 26, 27]. Others have provided insight into the relationship between cortex and brain stem [21, 28–31].
Swallowing motor control is understudied compared to the motor control of the limbs, partly because the tongue, larynx, and pharynx are more difficult to access and measure. Although complex, understanding swallowing motor control is critical for better dysphagia treatment and concepts such as sensory feed-back, and levels of control for swallowing movements need to be examined more deeply in scientific experiments.
In the following sections, we discuss motor adaptation, an important and well-studied component in the field of motor learning. These concepts are intriguing for swallowing because they might lead to new discoveries that reveal the mechanism responsible for various types of dysphagia and lead to treatments that directly target those pathophysiologies.
Motor Learning
Motor learning is a field of study that aims to elucidate how motor performance improves and is subsequently retained. Many of its fundamental theories have been investigated through studies of motor adaptation of the limbs. Specific definitions of motor learning, however, are still debated. In fact, one major textbook chooses not to define motor learning explicitly [4]. Generally, though, motor adaptation is the short-term process of reducing errors (or improving accuracy) in a sensory–motor task [32]. It is described further as a gradual or incremental process based on reduction of error with the goal of returning to accurate movements (baseline behavior) using a model that predicts the outcome (feed-forward model, described further below) [33, 34]. In the following subsections we focus on the research supporting motor adaptation and how the principles of motor learning can improve knowledge about swallowing and swallowing disorders.
Motor Adaptation
Motor adaptation is the process by which our neuromuscular system makes accurate movements throughout our lifetime. It is based on the idea that our nervous system constructs a model of a movement and predicts the outcome. After the movement, the nervous system compares the predicted outcome to what actually happened. The difference is called error and adaptation is often called error-based learning [5, 35]. Much of this work is based on pointing or reaching experiments, where research subjects experience an unexpected displacement through devices such as prism glasses or a robot arm changing their trajectory. The subjects see or feel the difference between goals and actions and gradually, over repeated trials, reduce this error (feed-forward adaptation). This type of learning is a robust, well-studied phenomenon seen in different motor behaviors, with the cerebellum as one of the critical neural substrates [36–40].
Motor adaptation can also be demonstrated in the following example of a real-life scenario. You are test-driving a new car. You notice that the car's brakes are much more sensitive than those of your old car and the first several braking instances result in alarming halts in vehicle motion. So, to avoid this error feed-back, you might make trial-by-trial adjustments by tapping the brakes more tentatively throughout the test drive rather than using the same force that you would use with your own car. If you have overcome the braking error by the time you have finished the test drive, then motor adaptation to the new vehicle has occurred. Now, upon returning to your old car, you might discover that you are not using enough force when braking and thus you are braking too slowly. This is evidence of an after-effect that requires deadaptation to brake more appropriately in your own vehicle by recalibrating gradually once again. So, motor adaptation (and deadaptation) can occur over several minutes or hours and involves a continued process of gradual adaptation and deadaptation.
Another example of motor learning is the process that happens with speech following the placement of an orthodontic or oral dental plate [41]. Such a device effectively lowers the palate and initially tongue movements for correct speech are not accurate. However, an individual quickly adjusts to the device and their tongue movements, and speech, return to normal. Following removal of the device, however, the tongue movements are not quite enough, as the individual has learned to accommodate to the oral device. However, in only a short time, the individual adjusts and speech is once again back to normal.
An example of experimental testing of motor adaptation is described in Fig. 1. In this example the task requires a subject to rapidly lift a barbell to shoulder height over several trials where new demands from the weight of the barbell are unexpectedly introduced throughout three consecutive phases, including (1) preperturbation (5 lb.), (2) perturbation (15 lb.) with an unexpected catch trial (5 lb.), and (3) post-perturbation (5 lb.).
Fig. 1.
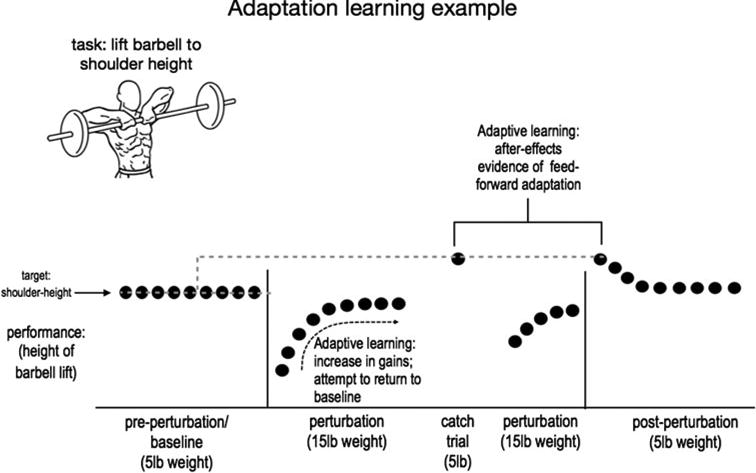
Adaptive learning example showing a typical study design used to explore evidence of adaptation in a resistance task of lifting a barbell to a shoulder-height target level in the presence of ongoing perturbations. Participants are not expecting the onset of the perturbation, the catch trial, or the onset of the post-perturbation changes [7] (image: http://fitnessanddefense.com/upright-rows/)
Feed-Back and Feed-Forward Control Loops (Fig. 2)
Fig. 2.
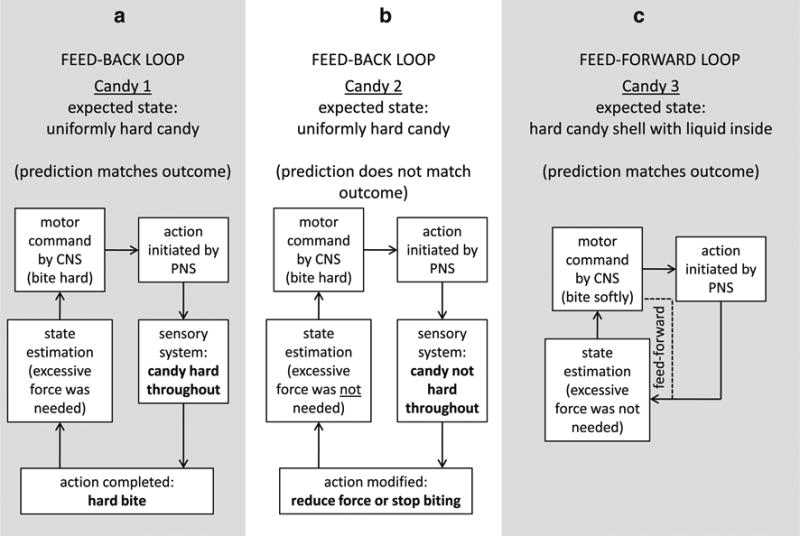
Feed-forward and feed-back loops are shown. a Hard-wired feedback loop where what is expected occurs. b Another feed-back loop where the unexpected texture was used to modify the motor command. c Predictive feed-forward loop with no consideration of the outcome provided by sensory input
Feed-back and feed-forward control loops are often used to explain how the brain sends and receives information in expected and unexpected states. A feed-back loop shows how the outcome is integrated into the control command signals [42]. Expected input and output circuits have a hard-wired loop. An expected state could be described as an outcome that matches the predictions. Consider the example of the first bite into a hard candy that has the same texture throughout, such as a peppermint, which requires some force to crack. An expected state could occur when biting into a hard candy and experiencing what was predicted (Bite 1; Fig. 2a). If the candy is uniformly hard, as expected, then excessive force to crack the candy was necessary.
An unexpected state could be described as an outcome that does not match the predictions. For instance, if a uniformly hard texture is expected again for candy number 2, but instead, the candy has a hard shell with liquid inside, an error occurs in this unexpected state. In this circumstance, accuracy is compromised because the neural signal for motor output and the actual movement are not perfectly matched because of an unexpected physical property of the candy [5]. As a result, the bite is immediately slowed or stopped when the candy shell easily breaks with excessive force to avoid potential damage to the teeth. Slowing or stopping in the midst of a bite is also evidence of a feedback loop because the outcome (easily breaking candy due to excessive force) was used to modify the control command signals (Fig. 2b).
To make accurate movements, the brain must compensate for sensory input that can be relatively slow and noisy. In candy number 2, the liquid inside of the candy was detected and compensation occurred after the bite had been initiated. To avoid this problem in candy number 3, an internal predictive model is constructed by the neural system called a feed-forward loop (dotted line, Fig. 2c). A feed-forward loop does not integrate the actual outcome into the control command signals. In other words, when biting candy number 3, an unexpected state will be assumed and incorporated into the motor command without regard for the outcome of biting candy number 3. Feed-forward control estimates the state of the mismatch between the motor command and the predicted outcome, with the goal of reducing errors by matching them. In other words, the outcome of biting candy number 3 will not be used to influence the motor command for this bite. The bite into candy number 3 will be slow and careful because of the predictions that the candy is not uniformly hard and that it will require less force to crack. The system learns, or adapts, by comparing the errors in prediction with the sensory feed-back information [43]. The map of this schema onto specific parts of the neural system and its application to limb control has proved useful for understanding normal function and perceiving and predicting the outcomes of pathological neural deficits [5, 35].
Feed-forward loops, or predictive internal models, are thought to be the basis of motor adaptation. In motor adaptation studies, the goal is to measure trial-by-trial error reduction that occurs after a perturbation to learn more about the predictive internal model that is being used to adapt. Much of the research of motor adaptation has focused on the limbs, particularly the arms and hands. This work requires research participants to move a lever or have a device on their forearm that limits or modifies movement. A classic paper in this field [44] first shows that over several repeated reaching trials, people learn that the lever was impeding their movements and then gradually correct to reach a specified target by moving away from the force of the perturbation and toward their baseline. Second, when the impediment was unexpectedly removed, people over-corrected, i.e., they overshot the target in the direction away from the perturbation and beyond the baseline target. This is called an after-effect. The conclusions from these experiments were that a subject would build an “internal model” to predict changes in the environment, and that the model, which could be used for motor activities, is rooted in neurologically based feed-forward loops (Fig. 1).
Advances and Sophistication of Adaptation Models
The advances and sophistication of adaptation models for understanding reaching has grown tremendously since Shadmehr and Mussa-Ivaldi [44]. A wide range of review articles use reaching as a paradigm to understand how motor control works and how the CNS functions to produce accurate movements [5, 33, 34, 45, 46]. Shadmehr et al. [5] use an engineering problem-solving approach to define two significant dilemmas that individuals face in making accurate movements. One is a sensory problem: that feed-back to the CNS is slow and noisy. The other is a motor problem: that the signals from the CNS to the muscles are subject to a changing environment and body. After decades of experimental work, they clearly lay out the current consensus that a feed-forward computational model provides a mechanism for determining accurate motor behavior.
Adaptation in Head and Neck Functions
Adaptation is known to occur in two areas of oropharyngeal function: mastication, speech and swallowing. Speech and chewing do rely on the same anatomical substrates as swallowing, including hard tissue structures, muscles, and peripheral nerve supply [47–49]. Differences in central neurological processing are considerable and likely to produce differences in motor control [49]. Yet understanding what is possible with the peripheral anatomy can give us some insights into swallowing motor control.
Feed-forward models exist for the control of jaw movement during mastication [50–57]. The force generated by the muscles of mastication does rely on feed-back mechanisms through the periodontal ligaments, muscle spindles, and joint receptors [52]. However, the delays in sensory feed-back are significant relative to masticatory cycles and can produce errors because the conditions at the periphery are changing [52]. As Ross et al. [52, 53] emphasize, feed-forward loops relying on periodontal afferents to predict and avoid chewing errors likely minimize tooth wear and risk of tooth breakage during the trituration of food items [54, 58, 59]. Avoiding chewing errors might also prevent damage to softer tissues, including the cheeks and tongue.
Adaptive models for learning speech are better developed than those for mastication [60–63]. Houde et al.'s original work [61] drew an explicit analogy between reaching movements with a forelimb and articulatory movements associated with speech. They perturbed what subjects heard when they produced a particular formant and determined that not only did adaptation occur for the particular perturbed vowel sound, but also for that sound in other words. Subsequent work [63] has refined these original results, indicating that it is not just motor learning and output but auditory representations of the speech sounds that are modified during the learning process. In contrast to these results, work with motor learning in profoundly deaf individuals [64] suggests that adaptation occurs even without auditory sensory feed-back.
Little is known about adaptation in swallowing or oropharyngeal function. Some of the extensive literature on neuroplasticity in the oral cavity touches on related subjects by examining the amount of neuroplasticity in cortical motor representations with changes in sensory input [65–68]. Overall, results showed changes in the cortical representations of digastric and genioglossus muscles after modifications due to tooth extraction or trimming. These muscles are significant in swallowing and there are links between sensation in the oral cavity and pharyngeal function [69].
The only explicit work that tested motor adaptation in swallowing is Humbert et al. [7]. We continuously reduced peak hyolaryngeal elevation using electrical stimulation over the course of multiple volitional swallows in healthy adults. Evidence of adaptive motor learning of hyolaryngeal movements was evident when participants showed systematic, gradual increases in peak elevation against the force of electrical stimulation. Feed-forward adaptation was evident when the electrical stimulation was unexpectedly removed and subsequently peak hyolaryngeal elevation overshot the baseline (preperturbation) peak elevation means, showing behavioral after-effects. We concluded that hyolaryngeal kinematics demonstrate adaptive, error-reducing movements in the presence of changing and unexpected demands. Thus, the promise and potential of motor learning is evident for swallowing based on these findings, because patients may be taught to predict the presence of perturbations and reduce errors in swallowing before they occur (feed-forward adaptation).
Adaptation was also tested in swallowing kinematics in our published work that investigated the effects of repeated swallowing of the same liquid bolus on hyoid bone and laryngeal kinematics. In that investigation, the bolus was delivered either to the oral cavity or directly to the pharyngeal cavity [7]. Our findings showed that only repeated pharyngeal bolus delivery resulted in gradual adaptation of hyolaryngeal kinematics, suggesting that without oral sensory information, the events of the pharyngeal phase vary over time. Also, our results show that the oral cavity plays a crucial role in providing a motor plan for hyolaryngeal movements of the pharyngeal phase so that its response during swallowing can be consistent and perform optimally. On the other hand, hyolaryngeal kinematics responded to bolus volume differences only with oral delivery and not pharyngeal delivery. This again shows the importance of oral sensory-motor integration for kinematics of the components of the pharyngeal swallow [7].
Motor adaptation is important for rehabilitation processes. We want patients to show adaptation for improved accuracy for a motor task, even though there is a process of error reduction associated with adaptation. By the end of rehabilitation, the best outcome would be for patients to exhibit motor learning where they no longer exhibit errors but can access what they have learned during motor adaptation processes for full rehabilitation. For a thorough review of motor adaptation for rehabilitation, read Bastian [32].
Discussion
Dysphagia research will benefit from the application of the motor learning paradigm in several ways. Unlike the paradigm of hand movement, where the understanding of the relevant aspects of the CNS are well understood, swallowing is complex in that we do not understand the various levels of CNS control. Furthermore, the role of sensory feed-back in swallowing motor control is only somewhat understood. The neurophysiologic basis of oropharyngeal function lacks an overall model that can be used to predict the outcomes of specific insults or disruptions. This emphasizes the need for swallowing research that examines both the phenomena (what do we see happening?) as well as the underlying mechanism that controls the phenomena (what is responsible for what we see?)
The effects of taste on swallowing behavior highlight the need for studies of both the phenomena as well as the underlying mechanisms responsible for them. For example, many previous studies have reported differences in submental electromyography (EMG) recordings when swallowing a sour bolus compared to water [70–74]. Then the underlying neural mechanism for these taste effects was investigated with functional neuroimaging studies that showed differences in cortical responses with sour taste or flavor compared to water during swallowing [18, 19]. This evidence has led to the conclusion that significant taste effects are found in swallowing kinematics. However, when hyoid bone kinematics, which are subject to submental muscle activity, were examined for taste effects using videofluoroscopic imaging, no significant differences were found [7]. Therefore, it is possible that both the EMG signal and the cortical signal are more sensitive to taste effects; hyoid bone kinematics does not show the same effects. This dissimilarity is peculiar considering the expected role of the cortex and submental muscles on hyoid bone movement. We suggest that these differences do not reflect problems in design or execution but rather are like the blind men describing different parts of the elephant.1 The differences in findings for taste effects may be in the measurement specificity of the aspects of oropharyngeal swallowing.
An example of measurement specificity is arm movement, where the EMG of the biceps and triceps are much more precisely linked to arm movements than is the EMG of hyoid musculature to hyoid movements [75]. Thus, while bicep contraction often corresponds with lifting the arm in a particular trajectory, submental activity could reflect lingual movement, hyoid movement, or stabilization against infrahyoid musculature. Both hyoid and lingual kinematics might have clear taste effects that correspond to submental EMG, while hyoid bone kinematics alone might not. These concerns strongly support the need for study designs and measures that respect the complexity of oropharyngeal swallowing in a deeper way.
The field of motor learning for arm movements is deep because the understanding and prediction of movements are based on solid research about the neurophysiology and motor control that governs those movements. This depth of understanding provides an underlying physiologic mechanism for those movements, changes in those movements, and the potential for external modification in the form of post-insult or injury rehabilitation. Such depth is possible because of the simplicity of the system (fewer moving parts) and its ease of study (it is easier to visualize movements of the arm without instrumentation). In fact, arm movements have become the mouse model of the motor learning world because of it is straightforward function (fewer movement trajectories than the hyoid bone). This “effector chauvinism” is based on the idea of using a model system from which generalizations can easily be extracted [33]. However, just as not all diseases in mice translate directly to humans, so the model of arm movement does not necessarily generalize to motor learning for all neurogenic issues and physiologic systems.
Currently, there are tantalizing data that suggest various facets of oropharyngeal motor control and the interactions at various levels of the CNS during the normal swallow [76]. However, the debate of whether the pharyngeal portion of the swallow is a reflex continues because our study designs do not delve into these issues to the depth that investigators on motor learning in hand or eye movement have. Given our argument above that there are multiple levels of control for oropharyngeal swallowing that can be anywhere on the continuum from reflex to control, we first need to define each of the components of oropharyngeal swallowing along these axes. Motor learning experimental paradigms deeply probe a particular movement of a particular system in multiple ways to understand it more completely, both in isolation and within a larger system.
In other fields, the integration of basic experiments and knowledge about motor learning has been explicitly connected to clinical studies and the ultimate design of successful rehabilitation [32, 77, 78]. We maintain that bringing these concepts to dysphagia research will not only broaden our knowledge but provide the basis for a synthesis of existing data. Most importantly, this depth of understanding will be the basis for the development of successful rehabilitation strategies for the complex pathophysiologies associated with dysphagia.
Acknowledgments
This study was funded by The National Institutes of Health grants NIDCD 1K23DC010776 (IH) and DC009980 (RZG).
Footnotes
An old tale, common to Jain, Buddhist, Sufi Muslim, and Hindu traditions, tells of three blind men who describe an elephant. The first, feeling the trunk, said that an elephant is like a snake, a muscular rope. The second, feeling an ear, said that an elephant is like a soft piece of cloth and very flexible. The third, feeling a leg, said that an elephant is like a strong pillar, covered in tough material.
Conflict of interest: There are no financial or other conflicts of interest related to this study by any author.
Contributor Information
Ianessa A. Humbert, Department of Physical Medicine and Rehabilitation, Johns Hopkins University, School of Medicine, 98 North Broadway, Suite 409, Baltimore, MD 21231, USA, Department of Neuroscience, Johns Hopkins University, School of Medicine, 98 North Broadway, Suite 409, Baltimore, MD 21231, USA.
Rebecca Z. German, Email: rzgerman@jhu.edu, Department of Physical Medicine and Rehabilitation, Johns Hopkins University, School of Medicine, 98 North Broadway, Suite 409, Baltimore, MD 21231, USA, Department of Neuroscience, Johns Hopkins University, School of Medicine, 98 North Broadway, Suite 409, Baltimore, MD 21231, USA.
References
- 1.Marshall-Hall E. On the reflex function of the medulla oblongata and medulla spinalis. Philos Trans. 1833;26:635–65. [Google Scholar]
- 2.Miller RF, Sherrington CS. Some observations on the buccopharyngeal stage of reflex deglutition in the cat. Q J Exp Physiol. 1916;9:147–86. [Google Scholar]
- 3.Negus VE. The mechanism of swallowing. Proc R Soc Med. 1942;36:85–92. [PMC free article] [PubMed] [Google Scholar]
- 4.Wise S, Shadmehr R. Motor control. In: Ramachandran VS, editor. Encyclopedia of the Human Brain. San Diego: Academic Press; 2002. pp. 137–57. [Google Scholar]
- 5.Shadmehr R, Smith MA, Krakauer JW. Error correction, sensory prediction, and adaptation in motor control. Annu Rev Neurosci. 2010;33:89–108. doi: 10.1146/annurev-neuro-060909-153135. [DOI] [PubMed] [Google Scholar]
- 6.Steele CM, Miller AJ. Sensory input pathways and mechanisms in swallowing: a review. Dysphagia. 2010;25(4):323–33. doi: 10.1007/s00455-010-9301-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Humbert IA, Lokhande A, Christopherson H, German R, Stone A. Adaptation of swallowing hyo-laryngeal kinematics is distinct in oral versus pharyngeal sensory processing. J Appl Physiol. 2012;112(10):1698–705. doi: 10.1152/japplphysiol.01534.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kandel R, Schwartz J, Jessell T. Principles of neuroscience. 4th. New York: McGraw-Hill; 2002. [Google Scholar]
- 9.Turvey MT, Fonseca S. Nature of motor control: perspectives and issues. Adv Exp Med Biol. 2009;629:93–123. doi: 10.1007/978-0-387-77064-2_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Turvey M, Shaw R, Mace W. Issues in the theory of action: degrees of freedom, coordinative structures and coalitions. Hillsdale: Lawrence Erlbaum Associates; 1978. [Google Scholar]
- 11.Han DS, et al. Comparison of disordered swallowing patterns in patients with recurrent cortical/subcortical stroke and first-time brainstem stroke. J Rehabil Med. 2005;37(3):189–91. doi: 10.1080/16501970410024163. [DOI] [PubMed] [Google Scholar]
- 12.Michou E, Hamdy S. Cortical input in control of swallowing. Curr Opin Otolaryngol Head Neck Surg. 2009;17(3):166–71. doi: 10.1097/MOO.0b013e32832b255e. [DOI] [PubMed] [Google Scholar]
- 13.Mosier K, Bereznaya I. Parallel cortical networks for volitional control of swallowing in humans. Exp Brain Res. 2001;140(3):280–9. doi: 10.1007/s002210100813. [DOI] [PubMed] [Google Scholar]
- 14.Suzuki M, Asada Y, Ito J, Hayashi K, Inoue H, Kitano H. Activation of cerebellum and basal ganglia on volitional swallowing detected by functional magnetic resonance imaging. Dysphagia. 2003;18(2):71–7. doi: 10.1007/s00455-002-0088-x. [DOI] [PubMed] [Google Scholar]
- 15.Martin BJ, Logemann JA, Shaker R, Dodds WJ. Normal laryngeal valving patterns during three breath-hold maneuvers: a pilot investigation. Dysphagia. 1993;8(1):11–20. doi: 10.1007/BF01351472. [DOI] [PubMed] [Google Scholar]
- 16.Boden K, Hallgren A, Witt Hedstrom H. Effects of three different swallow maneuvers analyzed by videomanometry. Acta Radiol. 2006;47(7):628–33. doi: 10.1080/02841850600774043. [DOI] [PubMed] [Google Scholar]
- 17.Kern MK, Jaradeh S, Arndorfer RC, Shaker R. Cerebral cortical representation of reflexive and volitional swallowing in humans. Am J Physiol Gastrointest Liver Physiol. 2001;280(3):G354–60. doi: 10.1152/ajpgi.2001.280.3.G354. [DOI] [PubMed] [Google Scholar]
- 18.Babaei A, Kern M, Antonik S, Mepani R, Ward BD, Li SJ, Hyde J, Shaker R. Enhancing effects of flavored nutritive stimuli on cortical swallowing network activity. Am J Physiol Gastrointest Liver Physiol. 2010;299(2):G422–9. doi: 10.1152/ajpgi.00161.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Humbert IA, Joel S. Tactile, gustatory, and visual biofeed-back stimuli modulate neural substrates of deglutition. Neuroimage. 2012;59(2):1485–90. doi: 10.1016/j.neuroimage.2011.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Prochazka A, Clarac F, Loeb GE, Rothwell JC, Wolpaw JR. What do reflex and voluntary mean? Modern views on an ancient debate. Exp Brain Res. 2000;130(4):417–32. doi: 10.1007/s002219900250. [DOI] [PubMed] [Google Scholar]
- 21.Ertekin C. Voluntary versus spontaneous swallowing in man. Dysphagia. 2011;26(2):183–92. doi: 10.1007/s00455-010-9319-8. [DOI] [PubMed] [Google Scholar]
- 22.Jean A. Brainstem organization of the swallowing network. Brain Behav Evol. 1984;25(2–3):109–16. doi: 10.1159/000118856. [DOI] [PubMed] [Google Scholar]
- 23.Gibbs CH, Suit SR. Movements of the jaw after unexpected contact with a hard object. J Dent Res. 1973;52(4):810–4. doi: 10.1177/00220345730520042701. [DOI] [PubMed] [Google Scholar]
- 24.Miller AJ. The Neuroscientific principles of swallowing and dysphagia (dysphagia series) San Diego: Singular Publishing Group; 1999. p. 284. [Google Scholar]
- 25.Miller AJ. The neurobiology of swallowing and dysphagia. Dev Disabil Res Rev. 2008;14(2):77–86. doi: 10.1002/ddrr.12. [DOI] [PubMed] [Google Scholar]
- 26.Martin RE. Neuroplasticity and swallowing. Dysphagia. 2009;24(2):218–29. doi: 10.1007/s00455-008-9193-9. [DOI] [PubMed] [Google Scholar]
- 27.Humbert IA, Robbins J. Normal swallowing and functional magnetic resonance imaging: a systematic review. Dysphagia. 2007;22(3):266–75. doi: 10.1007/s00455-007-9080-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ertekin C, Aydogdu I. Neurophysiology of swallowing. Clin Neurophysiol. 2003;114(12):2226–44. doi: 10.1016/s1388-2457(03)00237-2. [DOI] [PubMed] [Google Scholar]
- 29.Lang IM. Brain stem control of the phases of swallowing. Dysphagia. 2009;24(3):333–48. doi: 10.1007/s00455-009-9211-6. [DOI] [PubMed] [Google Scholar]
- 30.Thexton AJ, Crompton AW, German RZ. Electromyographic activity during the reflex pharyngeal swallow in the pig: Doty and Bosma (1956) revisited. J Appl Physiol. 2007;102(2):587–600. doi: 10.1152/japplphysiol.00456.2006. [DOI] [PubMed] [Google Scholar]
- 31.German RZ, Crompton AW, Thexton AJ. Integration of the reflex pharyngeal swallow into rhythmic oral activity in a neurologically intact pig model. J Neurophysiol. 2009;102(2):1017–25. doi: 10.1152/jn.00100.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bastian AJ. Understanding sensorimotor adaptation and learning for rehabilitation. Curr Opin Neurol. 2008;21(6):628–33. doi: 10.1097/WCO.0b013e328315a293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Krakauer JW, Mazzoni P. Human sensorimotor learning: adaptation, skill, and beyond. Curr Opin Neurobiol. 2011;21(4):636–44. doi: 10.1016/j.conb.2011.06.012. [DOI] [PubMed] [Google Scholar]
- 34.Wolpert DM, Diedrichsen J, Flanagan JR. Principles of sensorimotor learning. Nat Rev Neurosci. 2011;12(12):739–51. doi: 10.1038/nrn3112. [DOI] [PubMed] [Google Scholar]
- 35.Shadmehr R, Krakauer JW. A computational neuroanatomy for motor control. Exp Brain Res. 2008;185(3):359–81. doi: 10.1007/s00221-008-1280-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aasland WA, Baum SR, McFarland DH. Electropalatographic, acoustic, and perceptual data on adaptation to a palatal perturbation. J Acoust Soc Am. 2006;119(4):2372–81. doi: 10.1121/1.2173520. [DOI] [PubMed] [Google Scholar]
- 37.Gritsenko V, Kalaska JF. Rapid online correction is selectively suppressed during movement with a visuomotor transformation. J Neurophysiol. 2010;104(6):3084–104. doi: 10.1152/jn.00909.2009. [DOI] [PubMed] [Google Scholar]
- 38.Noguchi K, Fujii H, Yamabe Y, Tanaka M, Shimada A, Torisu T, Suenaga H. Anticipation and motor control on repetitive tooth tapping produced by open–close jaw movements. J Oral Rehabil. 2008;35(1):20–6. doi: 10.1111/j.1365-2842.2007.01780.x. [DOI] [PubMed] [Google Scholar]
- 39.Vasudevan EV, Bastian AJ. Split-belt treadmill adaptation shows different functional networks for fast and slow human walking. J Neurophysiol. 2010;103(1):183–91. doi: 10.1152/jn.00501.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jayaram G, Galea JM, Bastian AJ, Celnik P. Human locomotor adaptive learning is proportional to depression of cerebellar excitability. Cereb Cortex. 2011;21(8):1901–9. doi: 10.1093/cercor/bhq263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Searl J, Evitts P, Davis WJ. Perceptual and acoustic evidence of speaker adaptation to a thin pseudopalate. Logoped Phoniatr Vocol. 2006;31(3):107–16. doi: 10.1080/14015430500390961. [DOI] [PubMed] [Google Scholar]
- 42.Latash M. Neurophysiological basis of movement. 2nd. Champaign: Human Kinetics; 2008. [Google Scholar]
- 43.Krakauer JW, Shadmehr R. Towards a computational neuropsychology of action. Prog Brain Res. 2007;165:383–94. doi: 10.1016/S0079-6123(06)65024-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shadmehr R, Mussa-Ivaldi FA. Adaptive representation of dynamics during learning of a motor task. J Neurosci. 1994;14(5 Pt 2):3208–24. doi: 10.1523/JNEUROSCI.14-05-03208.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Franklin DW, Wolpert DM. Computational mechanisms of sensorimotor control. Neuron. 2011;72(3):425–42. doi: 10.1016/j.neuron.2011.10.006. [DOI] [PubMed] [Google Scholar]
- 46.Shmuelof L, Krakauer JW. Are we ready for a natural history of motor learning? Neuron. 2011;72(3):469–76. doi: 10.1016/j.neuron.2011.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Donner MW, Bosma JF, Robertson DL. Anatomy and physiology of the pharynx. Gastrointest Radiol. 1985;10(3):196–212. [PubMed] [Google Scholar]
- 48.Smith KK. The evolution of the mammalian pharynx. Zool J Linn Soc. 1992;104:313–49. [Google Scholar]
- 49.Ludlow CL. Recent advances in laryngeal sensorimotor control for voice, speech and swallowing. Curr Opin Otolaryngol Head Neck Surg. 2004;12(3):160–5. doi: 10.1097/01.moo.0000120302.58882.13. [DOI] [PubMed] [Google Scholar]
- 50.Komuro A, Masuda Y, Iwata K, Kobayashi M, Kato T, Hidaka O, Morimoto T. Influence of food thickness and hardness on possible feed-forward control of the masseteric muscle activity in the anesthetized rabbit. Neurosci Res. 2001;39(1):21–9. doi: 10.1016/s0168-0102(00)00192-9. [DOI] [PubMed] [Google Scholar]
- 51.Komuro A, Morimoto T, Iwata K, Inoue T, Masuda Y, Kato T, Hidaka O. Putative feed-forward control of jaw-closing muscle activity during rhythmic jaw movements in the anesthetized rabbit. J Neurophysiol. 2001;86(6):2834–44. doi: 10.1152/jn.2001.86.6.2834. [DOI] [PubMed] [Google Scholar]
- 52.Ross CF, Baden AL, Georgi J, Herrel A, Metzger KA, Reed DA, Schaerlaeken V, Wolff MS. Chewing variation in lepidosaurs and primates. J Exp Biol. 2010;213(4):572–84. doi: 10.1242/jeb.036822. [DOI] [PubMed] [Google Scholar]
- 53.Ross CF, Dharia R, Herring SW, Hylander WL, Liu ZJ, Rafferty KL, Ravosa MJ, Williams SH. Modulation of mandibular loading and bite force in mammals during mastication. J Exp Biol. 2007;210:1046–63. doi: 10.1242/jeb.02733. [DOI] [PubMed] [Google Scholar]
- 54.Hidaka O, Morimoto T, Kato T, Masuda Y, Inoue T, Takada K. Behavior of jaw muscle spindle afferents during cortically induced rhythmic jaw movements in the anesthetized rabbit. J Neurophysiol. 1999;82(5):2633–40. doi: 10.1152/jn.1999.82.5.2633. [DOI] [PubMed] [Google Scholar]
- 55.Ottenhoff FA, van der Bilt A, van der Glas HW, Bosman F. Control of human jaw elevator muscle activity during simulated chewing with varying bolus size. Exp Brain Res. 1993;96(3):501–12. doi: 10.1007/BF00234118. [DOI] [PubMed] [Google Scholar]
- 56.van der Bilt A, Ottenhoff FA, van der Glas HW, Bosman F, Abbink JH. Modulation of the mandibular stretch reflex sensitivity during various phases of rhythmic open–close movements in humans. J Dent Res. 1997;76(4):839–47. doi: 10.1177/00220345970760040401. [DOI] [PubMed] [Google Scholar]
- 57.Lund JP. Chew before you swallow (chap 15) Prog Brain Res. 2011;188:219–28. doi: 10.1016/B978-0-444-53825-3.00020-6. [DOI] [PubMed] [Google Scholar]
- 58.Hidaka O, Iwasaki M, Saito M, Morimoto T. Influence of clenching intensity on bite force balance, occlusal contact area, and average bite pressure. J Dent Res. 1999;78(7):1336–44. doi: 10.1177/00220345990780070801. [DOI] [PubMed] [Google Scholar]
- 59.Hidaka O, Morimoto T, Masuda Y, Kato T, Matsuo R, Inoue T, Kobayashi M, Takada K. Regulation of masticatory force during cortically induced rhythmic jaw movements in the anesthetized rabbit. J Neurophysiol. 1997;77(6):3168–79. doi: 10.1152/jn.1997.77.6.3168. [DOI] [PubMed] [Google Scholar]
- 60.Nasir SM, Ostry DJ. Auditory plasticity and speech motor learning. Proc Natl Acad Sci USA. 2009;106(48):20470–5. doi: 10.1073/pnas.0907032106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Houde JF, Jordan MI. Sensorimotor adaptation in speech production. Science. 1998;279(5354):1213–6. doi: 10.1126/science.279.5354.1213. [DOI] [PubMed] [Google Scholar]
- 62.Houde JF, Jordan MI. Sensorimotor adaptation of speech I: Compensation and adaptation. J Speech Lang Hear Res. 2002;45(2):295–310. doi: 10.1044/1092-4388(2002/023). [DOI] [PubMed] [Google Scholar]
- 63.Shiller DM, Sato M, Gracco VL, Baum SR. Perceptual recalibration of speech sounds following speech motor learning. J Acoust Soc Am. 2009;125(2):1103–13. doi: 10.1121/1.3058638. [DOI] [PubMed] [Google Scholar]
- 64.Nasir SM, Ostry DJ. Speech motor learning in profoundly deaf adults. Nat Neurosci. 2008;11(10):1217–22. doi: 10.1038/nn.2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Avivi-Arber L, Lee JC, Sessle BJ. Face sensorimotor cortex neuroplasticity associated with intraoral alterations (chap 9) Prog Brain Res. 2011;188:135–50. doi: 10.1016/B978-0-444-53825-3.00014-0. [DOI] [PubMed] [Google Scholar]
- 66.Adachi K, Lee JC, Hu JW, Yao D, Sessle BJ. Motor cortex neuroplasticity associated with lingual nerve injury in rats. Somatosens Mot Res. 2007;24(3):97–109. doi: 10.1080/08990220701470451. [DOI] [PubMed] [Google Scholar]
- 67.Avivi-Arber L, Lee JC, Sessle BJ. Cortical orofacial motor representation: effect of diet consistency. J Dent Res. 2010;89(10):1142–7. doi: 10.1177/0022034510373767. [DOI] [PubMed] [Google Scholar]
- 68.Sessle BJ, Hannam AG. Mastication and swallowing: biological and chemical correlates. Toronto: University of Toronto Press; 1976. p. 194. [Google Scholar]
- 69.German RZ, Crompton AW, Owerkowicz T, Thexton AJ. Volume and rate of milk delivery as determinants of swallowing in an infant model (Sus scrofia) Dysphagia. 2004;19:147–54. doi: 10.1007/s00455-004-0001-x. [DOI] [PubMed] [Google Scholar]
- 70.Chee C, Arshad S, Singh S, Mistry S, Hamdy S. The influence of chemical gustatory stimuli and oral anaesthesia on healthy human pharyngeal swallowing. Chem Senses. 2005;30(5):393–400. doi: 10.1093/chemse/bji034. [DOI] [PubMed] [Google Scholar]
- 71.Ding R, Logemann JA, Larson CR, Rademaker AW. The effects of taste and consistency on swallow physiology in younger and older healthy individuals: a surface electromyographic study. J Speech Lang Hear Res. 2003;46(4):977–89. doi: 10.1044/1092-4388(2003/076). [DOI] [PubMed] [Google Scholar]
- 72.Leow LP, Huckabee ML, Sharma S, Tooley TP. The influence of taste on swallowing apnea, oral preparation time, and duration and amplitude of submental muscle contraction. Chem Senses. 2007;32(2):119–28. doi: 10.1093/chemse/bjl037. [DOI] [PubMed] [Google Scholar]
- 73.Palmer PM, McCulloch TM, Jaffe D, Neel AT. Effects of a sour bolus on the intramuscular electromyographic (EMG) activity of muscles in the submental region. Dysphagia. 2005;20(3):210–7. doi: 10.1007/s00455-005-0017-x. [DOI] [PubMed] [Google Scholar]
- 74.Pelletier CA, Dhanaraj GE. The effect of taste and palatability on lingual swallowing pressure. Dysphagia. 2006;21(2):121–8. doi: 10.1007/s00455-006-9020-0. [DOI] [PubMed] [Google Scholar]
- 75.German RZ, Campbell-Malone R, Crompton AW, Ding P, Holman S, Konow N, Thexton AJ. The concept of hyoid posture. Dysphagia. 2011;26(2):97–8. doi: 10.1007/s00455-011-9339-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Luo P, Zhang J, Yang R, Pendlebury W. Neuronal circuitry and synaptic organization of trigeminal proprioceptive afferents mediating tongue movement and jaw–tongue coordination via hypoglossal premotor neurons. Eur J Neurosci. 2006;23(12):3269–83. doi: 10.1111/j.1460-9568.2006.04858.x. [DOI] [PubMed] [Google Scholar]
- 77.Krakauer JW, Carmichael ST, Corbett D, Wittenberg GF. Getting neurorehabilitation right: what can be learned from animal models? Neurorehabil Neural Repair. 2012;26(8):923–31. doi: 10.1177/1545968312440745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Krakauer JW. Motor learning: its relevance to stroke recovery and neurorehabilitation. Curr Opin Neurol. 2006;19(1):84–90. doi: 10.1097/01.wco.0000200544.29915.cc. [DOI] [PubMed] [Google Scholar]


