Abstract
Background
Asthma and atopic dermatitis are both regarded as atopic diseases. Being born too early is associated with increased risk of asthma, but some studies have indicated that the opposite might be true for atopic dermatitis. We explored in more detail the associations between preterm birth, asthma, and atopic dermatitis.
Methods
We analyzed data from Norwegian registries with prospectively collected data. All live births in Norway from 1967 through 2001 were followed through 2005 by linking the Medical Birth Registry of Norway to the National Insurance Scheme and to Statistics Norway. Only severe asthma and atopic dermatitis were registered in the National Insurance Scheme.
Results
Of a total of 1,760,821 children, we identified 9,349 cases (0.5%) with severe asthma and 6,930 cases (0.4%) with severe atopic dermatitis. Compared with children born at term (37–41 weeks’ gestation), preterm birth was associated with increased odds for severe asthma (odds ratio (OR) 1.7 (95% confidence interval (CI): 1.6–1.8) for 32–36 weeks’ gestation and OR 3.6 (95% CI: 3.1–4.2) for 23–31 weeks) and decreased odds for severe atopic dermatitis (OR 0.9 (95% CI: 0.8–1.0) for 32–36 weeks’ gestation and OR 0.7 (95% CI: 0.5–1.0) for 23–31 weeks). Adjustment for perinatal and socio-demographic factors weakened the association between gestational age and severe asthma, while slightly strengthening the association between gestational age and severe atopic dermatitis.
Conclusions
Preterm birth was associated with increased risk for severe asthma and decreased risk for severe atopic dermatitis.
Keywords: Premature birth, Atopic dermatitis, Asthma, Pregnancy complications, Gestational age
Introduction
Asthma and atopic dermatitis are among the most common chronic disorders in childhood. The two diseases share several etiological factors, and children with atopic dermatitis are at increased risk of developing asthma (1). The biological pathways in the development of asthma and atopic dermatitis are poorly understood, but fetal and early life exposures seem to play important roles in complex gene-environment interactions (2).
Being born too early is associated with increased risk of several diseases including asthma (3–5), while reports on the association between preterm birth and atopic dermatitis are inconsistent (6–9). Some of these studies have even reported that preterm birth is associated with decreased risk of atopic dermatitis (6, 8).
We aimed to explore the association of preterm birth with asthma and atopic dermatitis. The Medical Birth Registry of Norway (MBRN) has recorded information on maternal health, pregnancy, and births since 1967 (10). With linkage of the MBRN to other national registries, we were able to assess these associations in a national cohort with wide information on possible confounders.
Methods
Study design
Data were collected from three national registries with use of encrypted personal identification numbers. The Medical Birth Registry in Norway gave information about maternal health, pregnancy, and birth, and Statistics Norway provided data on death, migration, and parental educational attainment (11). Every Norwegian resident is insured in the National Insurance Scheme (12). This insurance program provides a basic benefit to those with disabilities that significantly increase expenses, and an attendance benefit to those who need a substantially increased level of care because of their disabilities (13). Only chronic and severe asthma and atopic dermatitis qualify for basic benefit or attendance benefit, and admittance of benefits is based on the patient’s application and a medical examination. Insurance benefits are independent of the family’s economic situation. We identified the cases of severe asthma and atopic dermatitis among recipients of basic or attendance benefit in the National Insurance Scheme from 1967 through 2005 with International Classification of Diseases codes (493 and 691 from 9th revision, J45 and L20 from 10th revision). To emphasize that cases of the two diseases were serious enough to be compensated through the National Insurance Scheme, we use the terms “severe asthma “ and “severe atopic dermatitis”.
All live births in Norway from 1967 through 2001 were identified. We excluded children with missing data on gestational age (GA) at birth, children without Norwegian residence status, children with birth weight outside 3 standard deviations from the mean of a national, sex-specific birth weight standard for GA (14), children with less than 23 weeks’ or more than 43 weeks’ gestation, children who died before one year of age, and children with missing data on own or mother’s personal identification number. The cohort was followed through 2005.
As possible confounders for the associations between preterm birth and the two diseases, we included pregnancy disorders (placental abruption, chorioamnionitis, prolonged rupture of membranes, multiple birth, unspecified bleeding during pregnancy, urinary tract infection, and cervical conization), caesarean section, maternal asthma and atopic dermatitis, and socio-demographic factors (maternal age, parental education, parity, single mother at birth, sex, and immigrant status).
Approval of the study was given by the Norwegian Data Inspectorate, the Norwegian Labour and Welfare Administration, the National Population Register, and the Norwegian Directorate of Health.
Definitions
GA at birth was estimated from the first day of the last menstrual period. The variable “chorioamnionitis” included recorded chorioamnionitis and fever or sepsis during labor. “Prolonged rupture of membranes” was defined as membranous rupture earlier than 24 hours before birth. “Unspecified bleeding” was defined as bleeding during pregnancy with other causes than placenta previa and placental abruption. “Urinary tract infection” included infectious cystitis and pyelonephritis. “Parity” included previous live births and stillbirths. A person was considered “immigrant” if both parents were born abroad. Attained educational levels for parents were categorized as low (less than 11 years), medium (11–14 years; reference), and high (more than 14 years). Maternal age at birth was defined as low (<18 years), medium (18–39 years; reference), and high (≥ 40 years).
Statistical methods
We estimated absolute risks of severe asthma and atopic dermatitis for every week of gestation. Next, we analyzed how the possible confounders were related to preterm birth and the two diseases. Since cervical conization was not recorded before 1987, the variable “cervical conization” was analyzed in a sub-cohort with children born from 1987 through 2001. “Year of birth” was used as a continuous variable. Finally, we evaluated odds ratios for severe asthma and atopic dermatitis within four GA categories; 23–31 weeks, 32–36 weeks, 37–41 weeks (referent group), and 42–43 weeks. Because of the potential temporal influences of the 35 years eligibility period, we also assessed these associations in three different periods of time (1967–1977, 1978–1989, and 1990–2001). All adjusted analyses were performed with a logistic regression model (SPSS, version 21.0).
Results
From 1967 through 2001, there were a total of 2,024,215 live births in Norway. Among these, 1,760,821 (87%) met the inclusion criteria (Fig. 1). We identified 9,349 cases (0.5%) of severe asthma and 6,930 cases (0.4%) of severe atopic dermatitis. The prevalence of severe asthma increased from 0.1% in the period 1967–1978 to 0.7% in the period 1979–1990 and to 0.8% in the period 1991–2001. The corresponding prevalences for severe atopic dermatitis were 0.2%, 0.5%, and 0.5%.
Figure 1.
Establishment of the cohort
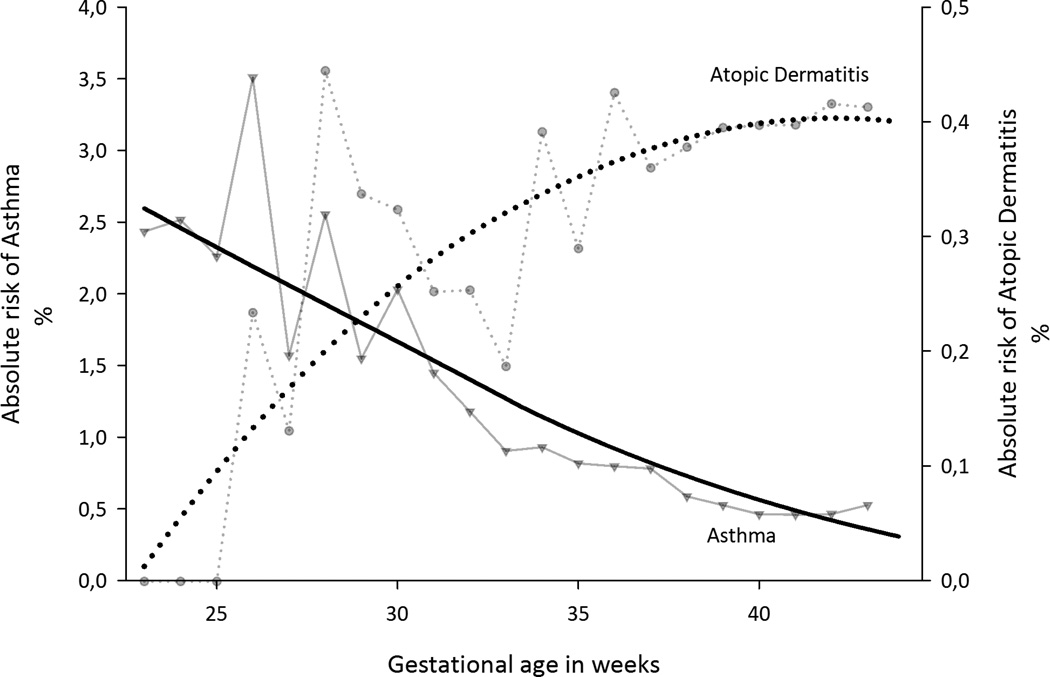
Absolute risk of asthma decreased and absolute risk of atopic dermatitis increased with increasing GA (Fig. 2). We explored the possible role of perinatal and socio-demographic factors as confounders of these associations (Table 1). Bleeding, placental abruption, multiple births, caesarean section, low parental education, single mother at birth, and male gender were associated with increased odds of preterm birth and severe asthma. Atopic dermatitis was generally less strongly associated with pregnancy disorders and socio-demographic factors, and only parity and high maternal education were associated with decreased odds of both preterm birth and atopic dermatitis.
Figure 2.
Absolute risks of asthma and atopic dermatitis according to gestational age among 1,760,821 live births. The smoothed curves were fitted using a negative exponential smoothing.
Table 1.
Logistic regression model with odds ratios for preterm birth, asthma and atopic dermatitis among 1,760,821 live births.
| Preterm birth | Asthma | Atopic dermatitis | ||||||
|---|---|---|---|---|---|---|---|---|
| n = 91,889 | n = 9,349 | n = 6,930 | ||||||
| n | Crude OR (95% CI) |
Adjusted OR* (95% CI) |
Crude OR (95% CI) |
Adjusted OR* (95% CI) |
Crude OR (95% CI) |
Adjusted OR* (95% CI) |
||
| Pregnancy disorders | ||||||||
| Unspecified bleeding | 42,974 | 3.1 (3.0–3.2) | 3.3 (3.2–3.4) | 1.6 (1.4–1.8) | 1.6 (1.4–1.8) | 1.0 (0.9–1.2) | 1.0 (0.9–1.2) | |
| Multiple birth | 39,666 | 12.9 (12.6–13.2) | 11.3 (11.0–11.5) | 1.6 (1.4–1.8) | 1.3 (1.2–1.5) | 0.9 (0.7–1.0) | 0.8 (0.7–0.9) | |
| Chorioamnionitis | 4,177 | 7.5 (7.0–8.1) | 3.8 (3.5–4.1) | 2.0 (1.5–2.7) | 1.3 (1.0–1.8) | 1.2 (0.7–1.8) | 0.9 (0.6–1.5) | |
| Cervical conisation† | 4,750 | 3.1 (2.8–3.3) | 3.0 (2.7–3.3) | 1.1 (0.8–1.5) | 1.1 (0.8–1.4) | 0.4 (0.3–0.8) | 0.5 (0.3–0.9) | |
| Urinary tract infection | 26,691 | 1.1 (1.0–1.1) | 1.0 (0.9–1.0) | 1.2 (1.0–1.4) | 1.0 (0.9–1.2) | 0.9 (0.8–1.1) | 0.9 (0.7–1.1) | |
| Prolonged rupture of membranes | 15,670 | 6.7 (6.5–6.9) | 6.6 (6.3–6.9) | 1.4 (1.1–1.7) | 0.9 (0.8–1.1) | 1.0 (0.8–1.3) | 0.8 (0.6–1.1) | |
| Pre-eclampsia | 50,100 | 4.0 (3.9–4.1) | 2.8 (2.7–2.9) | 1.3 (1.2–1.5) | 1.0 (0.9–1.2) | 1.0 (0.9–1.2) | 0.9 (0.8–1.1) | |
| Placental abruption | 7,700 | 12.8 (12.2–13.4) | 8.3 (7.9–8.7) | 2.3 (1.9–2.8) | 1.6 (1.3–2.0) | 1.0 (0.7–1.4) | 0.9 (0.6–1.3) | |
| Placenta previa | 3,636 | 13.0 (12.1–13.4) | 7.5 (7.0–8.1) | 1.6 (1.1–2.3) | 1.2 (0.8–1.7) | 1.0 (0.6–1.7) | 0.9 (0.5–1.5) | |
| No pregnancy disorder | 1,633,418 | 1 ref. | 1 ref. | 1 ref. | 1 ref. | 1 ref. | 1 ref. | |
| Caesarean section | ||||||||
| Yes | 150,106 | 4.4 (4.3–4.5) | 2.7 (2.7–2.8) | 2.0 (1.9–2.1) | 1.4 (1.3–1.5) | 1.3 (1.2–1.4) | 1.1 (1.0–1.2) | |
| No | 1,610,715 | 1 ref. | 1 ref. | 1 ref. | 1 ref. | 1 ref. | 1 ref. | |
| Maternal age | ||||||||
| < 18 years | 21,405 | 1.7 (1.6–1.7) | 1.5 (1.4–1.6) | 0.6 (0.5–0.8) | 0.7 (0.5–0.9) | 0.8 (0.6–1.0) | 0.8 (0.6–1.1) | |
| 18–39 years | 1,713,892 | 1 ref. | 1 ref. | 1 ref. | 1 ref. | 1 ref. | 1 ref. | |
| ≥ 40 years | 25,434 | 1.4 (1.4–1.5) | 1.3 (1.2–1.3) | 0.6 (0.5–0.8) | 0.6 (0.5–0.7) | 0.9 (0.8–1.1) | 0.9 (0.8–1.1) | |
| Maternal education | ||||||||
| < 11 years | 456,746 | 1.2 (1.2–1.2) | 1.2 (1.1–1.2) | 1.3 (1.2–1.3) | 1.2 (1.2–1.3) | 1.0 (1.0–1.1) | 1.0 (0.9–1.1) | |
| 11–14 years | 802,419 | 1 ref. | 1 ref. | 1 ref. | 1 ref. | 1 ref. | 1 ref. | |
| > 14 years | 488,723 | 0.9 (0.9–1.0) | 0.9 (0.9–0.9) | 1.0 (1.0–1.1) | 0.9 (0.8–0.9) | 1.2 (1.1–1.2) | 1.1 (1.0–1.2) | |
| Paternal education | ||||||||
| < 11 years | 400,884 | 1.2 (1.2–1.2) | 1.1 (1.1–1.2) | 1.2 (1.1–1.3) | 1.2 (1.1–1.3) | 1.0 (0.9–1.0) | 1.0 (0.9–1.0) | |
| 11–14 years | 880,630 | 1 ref. | 1 ref. | 1 ref. | 1 ref. | 1 ref. | 1 ref. | |
| > 14 years | 451,474 | 0.9 (0.9–0.9) | 0.9 (0.9–1.0) | 0.8 (0.8–0.9) | 0.8 (0.8–0.9) | 0.9 (0.9–1.0) | 0.8 (0.8–0.9) | |
| Parity | ||||||||
| 0 | 722,232 | 1 ref. | 1 ref. | 1 ref. | 1 ref. | 1 ref. | 1 ref. | |
| 1 | 611,488 | 0.7 (0.7–0.7) | 0.8 (0.8–0.8) | 1.2 (1.2–1.3) | 1.3 (1.3–1.4) | 1.1 (1.1–1.2) | 1.2 (1.1–1.2) | |
| ≥ 2 | 421,757 | 0.8 (0.8–0.9) | 0.8 (0.8–0.9) | 1.1 (1.1–1.2) | 1.2 (1.2–1.3) | 1.1 (1.0–1.1) | 1.1 (1.1–1.2) | |
| Single mother at birth | ||||||||
| No | 1,586,078 | 1 ref. | 1 ref. | 1 ref. | 1 ref. | 1 ref. | 1 ref. | |
| Yes | 172,237 | 1.4 (1.4–1.4) | 1.2 (1.2–1.3) | 1.5 (1.4–1.6) | 1.5 (1.4–1.6) | 1.3 (1.2–1.4) | 1.4 (1.3–1.6) | |
| Sex | ||||||||
| Female | 856,796 | 0.9 (0.8–0.9) | 0.8 (0.8–0.9) | 0.6 (0.6–0.6) | 0.6 (0.6–0.6) | 1.0 (0.9–1.0) | 1.0 (1.0–1.0) | |
| Male | 904,025 | 1 ref. | 1 ref. | 1 ref. | 1 ref. | 1 ref. | 1 ref. | |
| Immigrant | ||||||||
| Yes | 41,312 | 1.5 (1.5–1.6) | 1.4 (1.4–1.5) | 1.1 (0.9–1.2) | 0.7 (0.6–0.8) | 2.2 (1.9–2.4) | 1.9 (1.7–2.2) | |
| No | 1,719509 | 1 ref. | 1 ref. | 1 ref. | 1 ref. | 1 ref. | 1 ref. | |
OR, odds ratio, CI, confidence interval
Adjusted for all other variables in the table, maternal asthma and atopic dermatitis, and year of birth
Since cervical conization was not recorded before 1987, this variable was analyzed in a sub-cohort, 1987–2001, n = 769,295
Adjusting for potential confounders and year of birth substantially weakened the asthma association with GA, but slightly strengthened the atopic dermatitis association (Table 2). Being born postterm (42–43 weeks’ gestation) was associated with slightly decreased odds for severe asthma and slightly increased odds for severe atopic dermatitis.
Table 2.
Logistic regression model with odds ratios for asthma and atopic dermatitis according to gestational age among 1,760,821 live births.
| Asthma | Atopic dermatitis | ||||
|---|---|---|---|---|---|
| n = 9,349 | n = 6,930 | ||||
| Gestational age (weeks) |
n | Crude OR 95% CI) |
Adjusted OR* (95% CI) |
Crude OR (95% CI) |
Adjusted OR* (95% CI) |
| 23–31 | 9,512 | 3.63(3.12–4.22) | 2.37 (2.01–2.79) | 0.69 (0.47–1.02) | 0.62 (0.42–0.92) |
| 32–36 | 82,377 | 1.69 (1.56–1.82) | 1.42 (1.30–1.54) | 0.90 (0.79–1.01) | 0.90 (0.80–1.02) |
| 37–41 | 1,439,790 | 1.0 (ref) | 1.0 (ref) | 1.0 (ref) | 1.0 (ref) |
| 42–43 | 229,142 | 0.94 (0.89–1.01) | 0.96 (0.90–1.02) | 1.06 (0.99–1.13) | 1.07 (1.00–1.15) |
OR, odds ratio, CI, confidence interval
Adjusted for pregnancy disorders, caesarean section, maternal asthma and atopic dermatitis, parity, socio-demographic factors, and year of birth
These associations were stable over time, except for the association between preterm birth and asthma in the period 1967–1978. In this period only 640 cases of asthma were recorded in the National Insurance Scheme, while 3,288 cases were registered in the period 1979–1989, and 5,421 cases in the period 1990–2001.
Discussion
In this national cohort, early delivery was associated with increased odds of severe asthma and decreased odds of severe atopic dermatitis. It is possible that the difference in associations for these related diseases may be explained by confounding. After adjustments, the odds ratios for severe asthma within GA categories were reduced by about one third, suggesting the possibility of residual confounding. Confounding seems, however, less likely to explain the association between atopic dermatitis and GA, since the odds ratios were slightly strengthened after adjustments. Thus, the association between GA and atopic dermatitis must be explained in other ways.
Previous studies have shown that prematurity is associated with lower levels of IgE in serum and decreased occurrence of positive skin prick test reactions and allergic rhinitis (15, 16). Combined with the present study, these reports may suggest that prematurity involves exposures that protect for atopic disorders. The biological mechanism of such an effect is not obvious, given that preterm infants are frequently exposed to antibiotic treatment and formula feeding – factors that have been proposed to increase risk for atopic diseases (17, 18). On the other hand, preterm children are early exposed to the extrauterine, microbial environment, and early exposure to microbial diversity may protect from atopy (2).
The fetal immune system is modulated by maternal immune responses (19), and a child’s GA at birth reflects the duration of exposure to the maternal immune system. Particular interest has been given to the T helper cell type 2 predominance in pregnancy, since this imbalance is also found in cases with atopy (20). It has been hypothesized that increased T helper cell type 2 cytokine exposure in utero may enhance risk of atopy (19, 20), but this hypothesis has not yet been convincingly confirmed, and it is uncertain to what extent this might explain atopy risk in preterm and postterm children.
However, a protective effect of preterm birth on atopy cannot explain the association between GA and asthma. Asthma is a heterogenic group of diagnoses, and it has been suggested that only a minority of the cases are atopic (21). In preterm children, asthma might even be less associated with atopy and more with neonatal respiratory complications, reduced airway size, and decreased lung function (22, 23). Preterm children are also more frequently hospitalized for RSV-infections than term children, which may add to the risk of developing asthma (24, 25). Neonatal jaundice is more prevalent among preterm children (26) and has been proposed to be a risk factor for asthma (27). There is also evidence that preterm children have more active respiratory cytokine secretion than term infants (28), which may enhance airway inflammation and predispose to asthma. Use of drugs during pregnancy has been associated with asthma (29), and it is possible that drug use in pregnancy (and their underlying disorders) also may increase risk for preterm birth. Thus, it is possible that increased risk of asthma in preterm children may be related to non-atopic causes of lung disease, and that these causes overshadow a possible protective effect for atopic asthma.
Our assessment of confounding by perinatal and socio-demographic factors showed both differences and similarities for the two diseases. Both diseases were associated with parity, single mother at birth, and caesarean section, while girls and children born by mothers with low or high age were protected from asthma, but not from atopic dermatitis. Parental education was inversely associated with asthma (which may reflect parents’ smoking habits, for which we had no data), but not with atopic dermatitis. It may be criticized that all factors were adjusted for in the analyses of preterm birth and disease in offspring, but the estimates were minimally changed after adjusting only for factors that were significantly associated with preterm birth and the two diseases.
While the association of preterm birth with asthma is well established in the literature (4), reports on preterm birth and atopic dermatitis are inconsistent (6–9). This may be due to different definitions or measures of outcome, but may also be due to small sample size. The present study provides large numbers and minimal loss-to-follow up, and no previous study has been able to assess such a wide set of pregnancy disorders as risk factors. There are also several important limitations. The National Insurance Scheme only grants benefits to individuals with severe disease, resulting in a much lower prevalence of asthma and atopic dermatitis than compared with a Norwegian questionnaire-based survey (29). A particularly low prevalence of asthma was found in the period 1967–1978, possibly because of other disease definition. With severe asthma and atopic dermatitis as outcome, a caveat to the results is that risk factors for severe disease may differ from those of mild and moderate disease. The information in the insurance registry did not allow us to discern between current or previous disease, and we did not have data on when the cases were diagnosed. There has not been a validation of asthma or atopic dermatitis registered in the National Insurance Scheme, but all cases have been examined by a physician and only severe disease qualifies for benefits. It is thus possible that specificity of asthma and atopic dermatitis is higher in the present study than in studies based on questionnaires or prescriptions. Residual confounding has been mentioned as a possible source of bias. Smoking during pregnancy – a possible confounder for the link between preterm birth and asthma – was not possible to adjust for, since this information was not recorded in the Medical Birth Registry in Norway before 1998. Another limitation is the unavailability of data on postnatal exposures.
Extremely preterm children are often hospitalized for several weeks, while most late preterm children are not admitted to a neonatal unit. It is possible that parents who are in close contact with the health care system over a long period obtain better knowledge of possible insurance benefits for their child than those who are not. This may result in a higher proportion of applicants for these benefits among preterm children than for children born late preterm or after 36 weeks’ gestation. This possible selection bias may influence the association between preterm birth and severe asthma in the present study, but it cannot explain the association between preterm birth and severe atopic dermatitis. However, it is possible that atopic dermatitis is considered a minor problem compared with other disabilities that preterm children may have. For this reason, parents with preterm children may be less prone to apply for benefits based on atopic dermatitis. This may represent another type of selection bias.
In sum, our results confirm that preterm birth is associated with increased risk for severe asthma and suggest that preterm birth is associated with decreased risk for severe atopic dermatitis. Further studies are needed to assess whether there is a generalized reduced risk of atopic diseases by preterm birth, and if so, to identify the biological background for this association. The perinatal period has been postulated to be a window of opportunities in preventing atopic disorders (2), and identification of protective factors may lead to novel strategies.
Acknowledgments
The study was funded by the Western Norwegian Regional Health Authority and by the Intramural Research Program of the NIH, National Institute of Environmental Health Sciences.
References
- 1.van der Hulst AE, Klip H, Brand PL. Risk of developing asthma in young children with atopic eczema: a systematic review. J Allergy Clin Immunol. 2007;120:565–569. doi: 10.1016/j.jaci.2007.05.042. [DOI] [PubMed] [Google Scholar]
- 2.Garn H, Renz H. Epidemiological and immunological evidence for the hygiene hypothesis. Immunobiology. 2007;212:441–452. doi: 10.1016/j.imbio.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 3.Algert CS, Bowen JR, Lain SL, Allen HD, Vivian-Taylor JM, Roberts CL. Pregnancy exposures and risk of childhood asthma admission in a population birth cohort. Pediatr Allergy Immunol. 2011;22:836–842. doi: 10.1111/j.1399-3038.2011.01206.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jaakkola JJ, Ahmed P, Ieromnimon A, et al. Preterm delivery and asthma: a systematic review and meta-analysis. J Allergy Clin Immunol. 2006;118:823–830. doi: 10.1016/j.jaci.2006.06.043. [DOI] [PubMed] [Google Scholar]
- 5.Moster D, Lie RT, Markestad T. Long-term medical and social consequences of preterm birth. N Engl J Med. 2008;359:262–273. doi: 10.1056/NEJMoa0706475. [DOI] [PubMed] [Google Scholar]
- 6.Govaere E, Van Gysel D, Verhamme KM, Doli E, Oranje AP, De Baets F. The prevalence, characteristics of and risk factors for eczema in Belgian schoolchildren. Pediatr Dermatol. 2009;26:129–138. doi: 10.1111/j.1525-1470.2008.00801.x. [DOI] [PubMed] [Google Scholar]
- 7.Kvenshagen B, Jacobsen M, Halvorsen R. Atopic dermatitis in premature and term children. Arch Dis Child. 2009;94:202–205. doi: 10.1136/adc.2008.142869. [DOI] [PubMed] [Google Scholar]
- 8.Linneberg A, Simonsen JB, Petersen J, Stensballe LG, Benn CS. Differential effects of risk factors on infant wheeze and atopic dermatitis emphasize a different etiology. J Allergy Clin Immunol. 2006;117:184–189. doi: 10.1016/j.jaci.2005.09.042. [DOI] [PubMed] [Google Scholar]
- 9.Olesen AB, Ellingsen AR, Olesen H, Juul S, Thestrup-Pedersen K. Atopic dermatitis and birth factors: historical follow up by record linkage. BMJ. 1997;314:1003–1008. doi: 10.1136/bmj.314.7086.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Norwegian Institute of Public Health. Medical Birth Registry of Norway. 2013 http://www.fhi.no/eway/default.aspx?pid=240&trg=Main_6664&Main_6664=6898:0:25,7840:1:0:0:::0:0.
- 11.Statistics Norway. 2013 http://www.ssb.no/en/
- 12.The Norwegian Labour and Welfare Administration. Membership in The National Insurance Scheme. 2013 http://www.nav.no/English/Membership+in+The+National+Insurance+Scheme.
- 13.Norwegian ministry of labour. Survey: The Norwegian Social Insurance Scheme. 2010 http://www.regjeringen.no/upload/AD/publikasjoner/veiledninger_brosjyrer/2010/DNT_2010_eng.pdf.
- 14.Skjaerven R, Gjessing HK, Bakketeig LS. Birthweight by gestational age in Norway. Acta Obstet Gynecol Scand. 2000;79:440–449. [PubMed] [Google Scholar]
- 15.Pekkanen J, Xu B, Jarvelin MR. Gestational age and occurrence of atopy at age 31-a prospective birth cohort study in Finland. Clin Exp Allergy. 2001;31:95–102. [PubMed] [Google Scholar]
- 16.Siltanen M, Wehkalampi K, Hovi P, et al. Preterm birth reduces the incidence of atopy in adulthood. J Allergy Clin Immunol. 2011;127:935–942. doi: 10.1016/j.jaci.2010.12.1107. [DOI] [PubMed] [Google Scholar]
- 17.Greer FR, Sicherer SH, Burks AW. Effects of early nutritional interventions on the development of atopic disease in infants and children: the role of maternal dietary restriction, breastfeeding, timing of introduction of complementary foods, and hydrolyzed formulas. Pediatrics. 2008;121:183–191. doi: 10.1542/peds.2007-3022. [DOI] [PubMed] [Google Scholar]
- 18.Risnes KR, Belanger K, Murk W, Bracken MB. Antibiotic exposure by 6 months and asthma and allergy at 6 years: Findings in a cohort of 1,401 US children. Am J Epidemiol. 2011;173:310–318. doi: 10.1093/aje/kwq400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Herberth G, Hinz D, Roder S, et al. Maternal immune status in pregnancy is related to offspring's immune responses and atopy risk. Allergy. 2011;66:1065–1074. doi: 10.1111/j.1398-9995.2011.02587.x. [DOI] [PubMed] [Google Scholar]
- 20.Kim JH, Kim KH, Woo HY, Shim JY. Maternal cytokine production during pregnancy and the development of childhood wheezing and allergic disease in offspring three years of age. J Asthma. 2008;45:948–952. doi: 10.1080/02770900802419676. [DOI] [PubMed] [Google Scholar]
- 21.Pearce N, Pekkanen J, Beasley R. How much asthma is really attributable to atopy? Thorax. 1999;54:268–272. doi: 10.1136/thx.54.3.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Doyle LW. Respiratory function at age 8–9 years in extremely low birthweight/very preterm children born in Victoria in 1991–1992. Pediatr Pulmonol. 2006;41:570–576. doi: 10.1002/ppul.20412. [DOI] [PubMed] [Google Scholar]
- 23.Halvorsen T, Skadberg BT, Eide GE, Roksund O, Aksnes L, Oymar K. Characteristics of asthma and airway hyper-responsiveness after premature birth. Pediatr Allergy Immunol. 2005;16:487–494. doi: 10.1111/j.1399-3038.2005.00314.x. [DOI] [PubMed] [Google Scholar]
- 24.Bacharier LB, Cohen R, Schweiger T, et al. Determinants of asthma after severe respiratory syncytial virus bronchiolitis. J Allergy Clin Immunol. 2012;130:91–100. doi: 10.1016/j.jaci.2012.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boyce TG, Mellen BG, Mitchel EF, Jr, Wright PF, Griffin MR. Rates of hospitalization for respiratory syncytial virus infection among children in medicaid. J Pediatr. 2000;137:865–870. doi: 10.1067/mpd.2000.110531. [DOI] [PubMed] [Google Scholar]
- 26.Billing BH, Cole PG, Lathe GH. Increased plasma bilirubin in newborn infants in relation to birth weight. BMJ. 1954;2:1263–1265. doi: 10.1136/bmj.2.4899.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ku MS, Sun HL, Sheu JN, Lee HS, Yang SF, Lue KH. Neonatal jaundice is a risk factor for childhood asthma: a retrospective cohort study. Pediatr Allergy Immunol. 2012;23:623–628. doi: 10.1111/j.1399-3038.2012.01345.x. [DOI] [PubMed] [Google Scholar]
- 28.Matias V, San Feliciano L, Fernandez JE, et al. Host and environmental factors influencing respiratory secretion of pro-wheezing biomarkers in preterm children. Pediatr Allergy Immunol. 2012;23:441–447. doi: 10.1111/j.1399-3038.2012.01269.x. [DOI] [PubMed] [Google Scholar]
- 29.Källén B, Finnström O, Nygren KG, Otterblad Olausson P. Maternal drug use during pregnancy and asthma risk among children. Pediatr Allergy Immunol. 2013;24:28–32. doi: 10.1111/pai.12034. [DOI] [PubMed] [Google Scholar]
- 30.Hansen TE, Evjenth B, Holt J. Increasing prevalence of asthma, allergic rhinoconjunctivitis and eczema among schoolchildren: three surveys during the period 1985–2008. Acta paediatrica. 2013;102:47–52. doi: 10.1111/apa.12030. [DOI] [PubMed] [Google Scholar]