Abstract
Background
Patients with negative TRUS biopsies yet persistently rising PSA values are at risk for occult but significant prostate cancers. The ability of multiparametric MRI and ultrasound (MRI/US) fusion biopsy to detect these occult prostate lesions may make it an effective tool in this challenging scenario.
Methods
Men with one or more negative systematic prostate biopsies participated in this trial. Between March 2007 and November 2011 all men underwent prostate 3T endorectal coil MRI and MRI/US fusion biopsy. In addition, all patients underwent standard 12 core TRUS biopsy in addition to targeted MRI/US fusion biopsy of concerning lesions identified on MRI.
Results
Of the 195 men with previous negative biopsies, 73 (37%) were found to have cancer using the MRI/US fusion platform combined with 12 core TRUS biopsy. High grade cancer (Gleason sum 8+) was discovered in 21 men (11%). All 21 men with high grade disease (100%) were detected with MRI/US fusion targeted biopsy while standard TRUS biopsy missed 12 of these high grade cancers (55%). Upgrading occurred in 28 men (38.9%) as a result of MRI targeting versus standard TRUS biopsy. The diagnostic yield of MRI with guided biopsy was unrelated to the number of previous negative biopsies, and persisted despite increasing number of previous biopsy sessions. On multivariable analysis, only PSAD and MRI suspicion level remained significant predictors of cancer.
Conclusion
Multiparametric MRI in conjunction with a MRI/US fusion biopsy platform is a novel diagnostic tool for detecting prostate cancer and may be ideally suited for patients with negative TRUS biopsies in the face of a persistent clinical suspicion for cancer.
Introduction
Since the advent of PSA screening, the pathologic diagnosis of prostate cancer has been based on the use of unguided systematic trans-rectal ultrasound guided biopsies. It is now well understood that systematic 12-core trans-rectal ultrasound (TRUS) biopsies can under sample apical and anterior areas of prostate, particularly in large glands1. As a result, prostate cancer biopsy diagnosis has traditionally been fraught with poor sensitivity (as low as 53% in autopsy studies) (Haas et al. J Natl Cancer Inst. 2007 Oct 3;99(19):1484-9), raising diagnostic concerns of missed prostate cancers.
One particular challenge is presented by the patient with continued clinical suspicion of prostate cancer (whether based on PSA, PSAV, PSAD, or DRE) after repeated negative biopsies. Different strategies have been employed in this setting to minimize false negative biopsy results including repeat biopsy2-4, the addition of anterior directed biopsy cores5, saturation biopsy templates6,7, and transperineal template guided biopsy8. While all of these strategies add some diagnostic utility, they ultimately remain dependent on random sampling.
Recent advances in multiparametric magnetic resonance imaging (mpMRI) have allowed high quality visualization of the prostate and are aiding in identification of prostate cancer lesions9. We have previously reported on the use of a novel MRI/US fusion biopsy system for the targeted detection of lesions detected on MRI9. In this series, we present our experience in patients with no prior prostate cancer diagnosis with at least one previous negative TRUS biopsy and a continued clinical suspicion of cancer. We aim to show the diagnostic utility of using mpMRI and this novel MRI/US fusion biopsy platform in this challenging clinical scenario.
Methods and Materials
This study was approved by the institutional review board of the National Cancer Institute of the National Institutes of Health. All patient information was protected according to the Health Insurance Portability and Accountability Act (HIPPA). Patients eligible for this study were consented and informed appropriately of the potential harms and benefits. Study enrollment began in March 2007 and continued through November 2011.
All patients initially underwent multiparametric magnetic resonance imaging using a 3.0 Telsa MRI scanner (Achieva, Philips Healthcare, Best, The Netherlands) in addition to a 16-channel cardiac surface coil (SENSE, Philips Healthcare) placed over the pelvis with an endorectal coil (BPX-30, Medrad, Pittsburgh, Pennsylvania) as previously described9. The following MRI sequences were also routinely obtained: tri-planar T2-weighted imaging, axial diffusion weighted imaging with apparent diffusion coefficient (ADC) mapping, 3-dimensional point resolved spatially localized spectroscopy, and axial dynamic contrast enhanced MRI. Details of these imaging sequences have been described previously.(Turkbey et al. Radiology. 2011;258:488 –and- Turkbey et al. Radiology. 2010;255:89) The criterion for a positive lesion on T2-weighted and diffusion weighted imaging was a well circumscribed, round-ellipsoid, low signal intensity lesion.(Turkbey et al. Radiology. 2011;258:488) A positive lesion on dynamic contrast enhanced imaging was the presence of foci showing early and intense enhancement, and rapid washout. A positive lesion on spectroscopy was an area where the choline-to-citrate ratio was 3 or more standard deviations above the mean healthy value.( Turkbey et al. Radiology. 2011;258:488). All imaging underwent blinded centralized radiologic evaluation as described previously9. MR imaging sequences were reviewed by our radiologists (PLC, BT) who identified and graded lesions on suspicion for cancer: low (<2), moderate (3) and high suspicion (all 4 sequences positive). MRI data sets were assessed in consensus between two radiologists. Both radiologists were blinded to pre-imaging serum PSA values, prior biopsy status and previous histopathologic findings. Each MRI sequence was evaluated independently and separately.
Patients with lesions suspicious for cancer on MRI were enrolled in a prostate biopsy protocol. All patients undergoing a prostate biopsy were given an antibiotic prophylaxis and a cleansing Fleet enema. Biopsies were performed under local anesthesia with lidocaine jelly and injectable lidocaine for analgesia. All patients first underwent a standard 12-core transrectal ultrasound biopsy. For this biopsy, the operator was blinded to the location of suspicious lesions detected on the MRI. Following TRUS biopsy, patients subsequently underwent a MRI/US fusion guided biopsy of suspicious lesions found on MRI. Briefly, an electromagnetic field generator was placed above the pelvis in order to track the rectal probe real-time during the biopsy and sensors were placed on the transrectal ultrasound probe. Following a 2D sweep of the ultrasound probe thru the prostate, the real-time US image was manually registered to the MRI image, allowing the operator to guide the ultrasound probe to biopsy previously identified suspicious lesions. A full description of this procedure was published recently9. A minimum of two biopsy cores were obtained from each lesion (one in the axial plane, one in the sagittal plane). All biopsies underwent blinded centralized pathologic evaluation by a GU pathologist. Prostate volumes were calculated using MRI segmentation data.
For this retrospective study, patients were included in the analysis if they had undergone at least 1 prior biopsy which did not reveal cancer. All patients with previous biopsy confirmed diagnosis of prostate cancer were excluded. Patients with prior diagnosis of PIN or atypia were included in the analysis. All consecutive patients from initiation of protocol were included in the analysis.
Statistical analysis
Descriptive statistics were used to describe patient demographics including age, PSA, DRE and previous biopsy data. Univariate analysis was performed using T-test for continuous variables and chi-square and Fisher’s exact test for nominal variables. Regarding patients with multiple lesions, MRI suspicion was assigned by the lesion with the highest MRI suspicion level and Gleason score was assigned by the cancer with the highest Gleason score. Multivariable analysis was performed using regression models.Tests were considered significant if p-value was less than 0.05. All tests were two-tailed. Sensitivity of a diagnostic test was calculated as the number of true positive tests divided by the number of those with disease.
Results
Demographics
A total of 195 patients were eligible for this case series based on negative prior prostate biopsies and rising PSA values. The median number of prior biopsies was 2, ranging from 1-9, as presented in Table 1. All patients were found to have at least 1 area of suspicion on MRI imaging. A median of 2 lesions per patient (range 1-7) were identified with some degree of suspicion for prostate cancer on MRI. From these, a minimum of two cores per lesion were sampled, yielding a median of 5 (range 2-14) MRI guided cores taken during MRI/US fusion guided biopsy.
Table 1.
Patient Demographics
| Median | Range | |
|---|---|---|
| Age (years) | 62 | 37 - 80 |
| Number of prior biopsies | 2 | 2 - 9 |
| PSA (ng/ml) | 9.13 | 0.3 - 103 |
| Prostate volume (ml) | 56 | 16 - 187 |
| PSA density (ng/ml per ml) | 0.156 | 0.019 - 1.675 |
| Lesions of suspicion on MRI | 2 | 1 - 7 |
| MRI guided biopsy cores | 5 | 2 - 14 |
| N | ||
| Race | Caucasian | 148 (75.9%) |
| Black | 36 (18.5%) | |
| Hispanic | 3 (1.5%) | |
| Asian | 8 (4.1%) | |
| Total | 195 (100%) |
Diagnostic yield of MRI fusion platform
Cancer was detected on biopsy (combined standard 12 core TRUS guided and MRI targeted) in 73 of the 195 men (37.4%). High grade cancer (GS 8 or greater) was found in 21 men (10.8%). MRI targeting detected cancer in 56 of the 73 men and found all 21 cases of high grade cancer. Furthermore MRI targeted biopsies upgraded tumors detected on standard TRUS biopsies in 28 men (38.4%). Systematic TRUS biopsy missed 11 of the 21 men with high grade disease (52.3%) (Table 2). A characterization of Gleason score stratification by modality of diagnosis is presented in Table 3. Using MRI targeting, 33 patients were found to have anterior lesions, 12 of whom were found to have high grade disease (36.3%). Of those with cancer discovered in an anterior lesions (N=33), a median of 2 additional lesions of suspicion were seen on MRI (range 0-5). Of those 33 men with cancer detected in anterior lesions, only 8 (24.2%) had cancer detected in MRI suspicious lesions in other non-anterior locations.
Table 2.
Diagnostic yield stratified by standard TRUS vs targeted MRI/US fusion platform
| All cancers | Low Grade (GS 6) |
Intermediate Grade (GS 7) |
High Grade (GS 8+) |
|
|---|---|---|---|---|
| Either modality detected cancer | 73 | 28 | 24 | 21 |
| MRI targeting detected cancer | 56/73 (76.7%) | 16/28 (57.1%) | 19/24 (79.2%) |
21/21 (100%) |
| US only guidance detected cancer | 45/73 (61.6%) | 23/28 (82.1%) | 12/24 (50%) |
10/21 (47.6%) |
| Both methods detected cancer | 28/73 (35.9%) |
11/28 (39.3%) |
7/24 (29.2%) |
10/21 (47.6%) |
| MRI targeting upgraded risk | 28/73 (38.4%) |
5/28 (17.9%) |
12/24 (50%) |
11/21 (52.3%) |
Table 3.
Characterization of Gleason score stratification by modality of diagnosis
| Gleason Stratification | ||||
|---|---|---|---|---|
| Modality of Diagnosis | Low Grade(GS6) | Intermediate Grade (GS7) | High Grade (GS8-10) | Total |
| standard 12 core biopsy only | 12 (85.71%) | 2 (14.29%) | 0 (0%) | 14 |
| MRI guided biopsy only | 5 (26.32%) | 7 (36.84%) | 7 (36.84%) | 19 |
| both standard and MRI guided | 11 (27.5%) | 15 (37.5%) | 14 (35%) | 40 |
| Total | 28 | 24 | 21 | 73 |
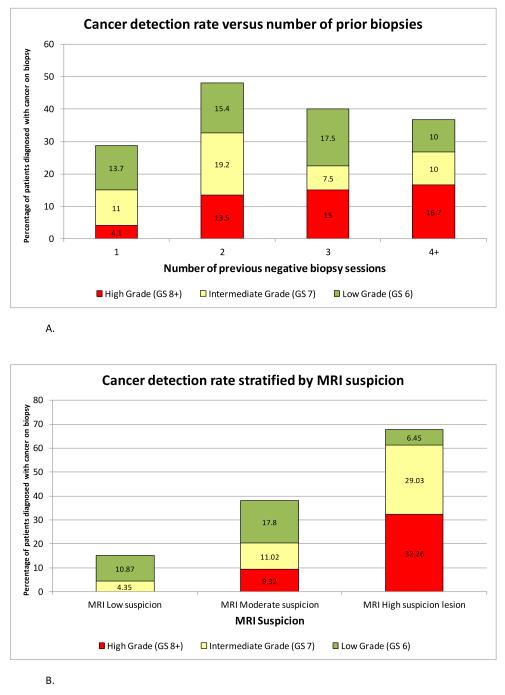
The diagnostic yield of fusion biopsy as stratified by the number of prior biopsies and MRI suspicion is presented in Table 4 and Figure 1. Increased prior number of negative biopsies did not influence the diagnostic yield, especially in the detection of high grade disease. Increasing MRI suspicion was associated with greater diagnostic yield for all cancers (cancer detection rate of 15.22% for low, 38.14% for moderate, and 67.14% for high suspicion lesions). Similarly, MRI suspicion was associated with greater diagnostic yield of high grade disease (high grade cancer detected in 0% of low, 9.32% of moderate, and 32.26% of those with high suspicion lesions).
Table 4.
The diagnostic yield of fusion biopsy as stratified by the number of prior biopsies and MRI suspicion
| Biopsy with cancer | Low grade (GS 6) | Intermediate grade (GS 7) |
High grade (GS8+) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Previous negative biopsy sessions | Total | N | % | N | % | N | % | N | % |
| 1 | 73 | 21/73 | 28.8 | 10/73 | 13.7 | 8/73 | 11.0 | 3/73 | 4.1 |
| 2 | 52 | 25/52 | 48.1 | 8/52 | 15.4 | 10/52 | 19.2 | 7/52 | 13.5 |
| 3 | 40 | 16/40 | 40 | 7/40 | 17.5 | 3/40 | 7.5 | 6/40 | 15.0 |
| 4+ | 30 | 11/30 | 36.7 | 3/30 | 10.0 | 3/30 | 10.0 | 5/30 | 16.7 |
| Biopsy with cancer | Low grade (GS 6) | Intermediate grade (GS 7) |
High grade (GS8+) | ||||||
| MRI Level of Suspicion | Total | N | % | N | % | N | % | N | % |
| MRI Low suspicion | 46 | 7/46 | 15.22 | 5/46 | 10.87 | 2/46 | 4.35 | 0/46 | 0 |
| MRI Moderate suspicion | 118 | 45/118 | 38.14 | 21/118 | 17.8 | 13/118 | 11.02 | 11/118 | 9.32 |
| MRI High supicion lesion | 31 | 21/31 | 67.14 | 2/31 | 6.45 | 9/31 | 29.03 | 10/31 | 32.26 |
Figure 1.
A. Diagnostic yield stratified by number of previous negative biopsies. Diagnostic yield is further broken down into Gleason grade (low grade GS6, intermediate grade GS7, and high grade GS8-10).
B. Diagnostic yield stratified by MRI suspicion level. Diagnostic yield is further broken down into Gleason grade (low grade GS6, intermediate grade GS7, and high grade GS8-10).
Prediction of biopsy outcome
Results of univariate analysis are presented in Table 5. Neither race nor number of prior negative biopsies nor DRE findings were found to be statistically associated with a positive biopsy result on fusion MRI biopsy (either all cancers or those of high grade). Age was not found to be higher in those with cancer overall, but was found to be higher in those with diagnosis of high grade disease (67.71 vs. 61.11 y, p = 0.0001). In those with diagnosis of cancer, prostate volume was significantly smaller (54.51 vs. 71.64 ml, p=0.0006), PSA was significantly higher (18.73 vs. 11.28 ng/ml, p = 0.0005), and PSAD was significantly greater (0.3843 vs. 0.1639 ng/ml/mL, p<0.0001). This association was consistent in those with a diagnosis of high grade disease, with smaller prostate volume (46.43 vs. 67.49 mL, p < 0.0001), higher PSA (27.37 vs. 12.46 ng/ml, p = 0.0071), and greater PSAD (0.6342 vs. 0.1985 ng/ml/mL). MRI suspicion level was significantly correlated with biopsy detection of both all cancers and high grade cancers. Those with cancer detected on biopsy had significantly higher MRI suspicion (9.59% low suspicion vs 30.97% low suspicion, p = 0.0004). This was also noted in those diagnosed with high grade cancers in (0% with low suspicion vs 26.44% with low suspicion, p = 0.0048). A multivariable logistic regression model including age, race, number of previous negative biopsies, MRI suspicion level, PSA, prostate volume, and PSAD was constructed to predict biopsy outcome. In this analysis, only PSAD (p = 0.0026) and MRI suspicion level (p = 0.0334) remained significant predictors of biopsy outcome of cancer.
Table 5.
Univariate analysis of biopsy outcome.
| All cancers | High risk disease (GS 8+) | |||||
|---|---|---|---|---|---|---|
| Negative | Positive | p value | Negative | Positive | p value | |
| Mean Age | 61.15y | 62.96y | 0.1086 | 61.11y | 67.71y | 0.0001 |
| Black Race | 15.57% | 23.29% | 0.1875 | 18.39% | 19.05% | 1.0000 |
| Abnormal DRE | 10.66% | 15.07% | 0.3759 | 10.92% | 23.81% | 0.1487 |
| Mean #prior bxs | 2.15 | 2.34 | 0.3119 | 2.17 | 2.67 | 0.0958 |
| Mean PSA (ng/dL) | 11.28 | 18.73 | 0.0005 | 12.46 | 27.37 | <0.0001 |
| Prostate volume (mL) | 71.64 | 54.51 | 0.0006 | 67.49 | 46.43 | 0.0071 |
| PSAD (ng/dL/mL) | 0.1639 | 0.3843 | <0.0001 | 0.1985 | 0.6342 | <0.0001 |
| MRI Low Suspicion | 31.97% | 9.59% | 0.0004 | 26.44% | 0% | 0.0048 |
| MRI not Low supicion | 68.03% | 90.41% | 0.0004 | 73.56% | 100.00% | 0.0004 |
Using PSAD and MRI suspicion level as a threshold for biopsy
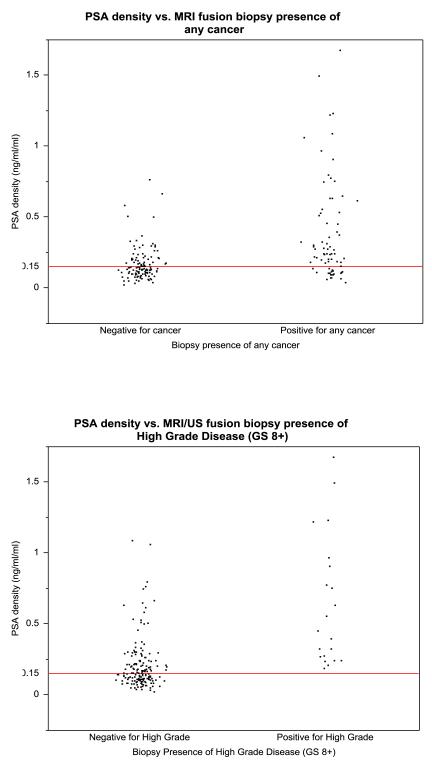
In Figure 2, we present the PSAD stratified by biopsy result. When we applied the threshold PSAD of 0.15 ng/ml/ml, the reported threshold for very low risk cancer by NCCN guidelines, (NCCN Prostate cancer guidelines, Version 4.2011) no high grade cancers were found in patients below this value. Specifically, the minimum PSAD in those with high grade cancer was 0.1851 ng/ml/ml). If we applied this 0.15 ng/ml/ml PSAD threshold and chose not to biopsy those who fell below this level, we could potentially prevent unnecessary biopsies without impacting cancer detection. Applying this PSAD threshold, 94 men would have avoided biopsy. In this setting, diagnostic yield for high grade disease would have improved from 10.82% to 21.0%, while not missing any high grade cancers (i.e. 100% sensitivity for high grade cancers). Regarding the detection of all cancers, 21 of those 94 men would not have had their cancer detected (15 men with GS6 and 6 men with GS7).
Figure 2.
Scatter plots representing PSA density stratified by MRI/US fusion biopsy results for all cancers (left) and high grade cancers (right). No high grade cancers were found below the 0.15ng/ml/ml threshold used in the 2011 NCCN guidelines for very low risk cancer.
Repeat MRI fusion biopsy in those with prior negative result
Of the 122 men with negative results on MRI fusion biopsy, a total of 10 underwent subsequent fusion biopsy. Seven of these men had negative results on subsequent biopsy. The other 3 men had disease detected on second fusion biopsy. High grade disease was noted in 1 of these 3 patients. We have previously reported that MRI/US fusion has a known spatial accuracy of 2.4 mm as assessed in phantom models (Xu S, Kruecker J, Turkbey B et al: Real-time MRI-TRUS fusion for guidance of targeted prostate biopsies. Comput Aided Surg 2008; 13: 255). As such, it is possible to miss targets, especially those which are small in size. Of note, none of the patients found to have cancer on subsequent fusion biopsy had low MRI suspicion. Diagnosis in all three patients on subsequent biopsy was made by MRI targeting only and was missed by standard biopsy. We add that it is our practice to repeat fusion biopsy in those patients with high MRI suspicion lesions with negative results.
Discussion
This series of patients demonstrates the well understood limitations of traditional random TRUS biopsy sampling strategies, namely, missed clinically significant prostate cancers. We demonstrate in this group of highly screened men that MP-MRI fusion biopsy was able to identify 37.5% who harbored cancer, nearly a third of whom harbored high grade clinically significant lesions.
This study suggests that MRI will be particularly useful in identifying lesions harboring high grade tumors in men with previous negative biopsies. Studies on the utility of MRI for cancer detection in men with prior negative biopsies have been previously reported. Hoeks et al. recently published similar favorable diagnostic results using MRI-guided biopsies in patients with one or more prior negative biopsy, with a cancer detection rate of 41%10. While our fusion platform has some similarity to the technique described by this group, substantial differences exist. Our fusion guided biopsy was conducted in an outpatient setting building on the common urological skill set of TRUS biopsy. This is in contrast to the Hoeks et al. study, which utilized an in-gantry MRI biopsy platform10. While our overall detection rates are similar, we believe the convenience of an outpatient biopsy platform will ultimately be more feasible for the application of MRI in prostate cancer detection.
Interestingly, the diagnostic yield of MRI in this study was not dependent on number of prior biopsies, in stark contrast to results seen with repeat random biopsy 2,3,11 as well as repeat saturation templates12. Zaytoun et al. described their experience with a repeat saturation template but noted that due to the previously reported decreased diagnostic utility for biopsy after multiple negative biopsies, decided only to include patients with 1 prior negative biopsy in their study6. On the contrary, we believe that the maintenance of diagnostic yield in detecting cancer after multiple prior negative biopsies on our platform confirms that the localization of tumor with MRI and subsequent targeting of tumor with MRI/US fusion is a true departure from stochastic processes seen in repeated random biopsy strategies. This is especially evident in the case of lesions in the anterior prostate which are more likely missed by the standard random template, as made evident by the fact that nearly half of the patients in our series were found to have cancer localized anteriorly (33 of 73 men). Non-targeted strategies to reach these anterior lesions have been less fruitful, with Chon and colleagues reporting on a series of 111 men with previous negative biopsy whose repeat extended template biopsies were supplemented with 6 additional anteriorly directed cores5. This non-targeted strategy to sample the anterior gland uniquely discovered only 2 cancers (2%).
Another technique utilized to overcome under sampling of non-targeted TRUS prostate biopsy is transperineal template based prostate biopsy. This has been reported to have excellent diagnostic yield and results in thorough sampling of areas of the anterior prostate8,13. However, this technique requires general anesthesia and is consequently associated with higher rate of complications (approximately 10% rate of urinary retention) as well as increased cost. On the other hand, MRI/US fusion biopsy utilizes urologist’s existing skill sets, avoids general anesthesia, and is able to be delivered comfortably using local anesthesia to an awake patient in an outpatient office based setting.
As prostate cancer diagnostic techniques advance, concerns rise that additional diagnostic yield may be associated with the discovery of additional small, low grade, and clinically insignificant lesions. This series emphasizes that previous negative non-targeted biopsy does not rule out the absence of high grade, clinically significant lesions, especially in those with continued clinical suspicion (rising PSA and elevated PSAD). We previously reported that mpMRI was most sensitive at detecting for tumors larger than 5 mm in diameter as well as for those with Gleason scores 8 or greater14. With this performance, MRI targeting tends to focus on clinically relevant lesions, a fact emphasized by the relatively high diagnostic yield of high grade lesions in this group (11%). Indeed, the primary predictive factor indicating the detection of cancer was PSAD and was unrelated to previous sampling. Thus, PSAD could be incorporated into future trials in order to further improve performance characteristics, preventing unnecessary biopsies while maintaining sensitivity in the detection of cancer.
A number of weaknesses exist in the analysis of this series. As our center is a specialty referral center, the very act of primary urologist referral in the face of negative biopsy suggests some clinical suspicion of cancer, resulting in selection bias. The true “denominator” of those men with negative results and who were not referred cannot be assessed. It is likely that a number of factors contribute to this clinical suspicion (including PSA, PSAV, PSAD, DRE, age specific PSA thresholds, and patient anxiety). The applicability of this data to the larger screening population will require more testing. Formal randomized prospective trial, as always, is the best methodology to clarify this issue.
Conclusion
MR/US fusion biopsy specifically targets clinically suspicious cancers throughout the prostate, including the anterior gland. This biopsy technique delivers a significant diagnostic yield in patients with prior negative TRUS biopsies and is not degraded by the number of previous biopsy sessions. Therefore, in contrast to non-targeted diagnostic strategies, we believe that MR/US fusion biopsy is ideally suited for those men with persistent clinical suspicion of cancer but negative biopsy.
Acknowledgements
This research was supported [in part] by the Intramural Research Program of the National Cancer Institute, NIH
References
- 1.Presti JC., Jr Repeat prostate biopsy--when, where, and how. Urol Oncol. 2009 May-Jun;27(3):312–314. doi: 10.1016/j.urolonc.2008.10.029. [DOI] [PubMed] [Google Scholar]
- 2.Roehl KA, Antenor JA, Catalona WJ. Serial biopsy results in prostate cancer screening study. J Urol. 2002 Jun;167(6):2435–2439. [PubMed] [Google Scholar]
- 3.Yanke B, Gonen M, Scardino P, Kattan M. Validation of a Nomogram for Predicting Positive Repeat Biopsy for Prostate Cancer. The Journal of Urology. 2005;173(2):421–424. doi: 10.1097/01.ju.0000150522.82760.00. [DOI] [PubMed] [Google Scholar]
- 4.Keetch DW, McMurtry JM, Smith DS, Andriole GL, Catalona WJ. Prostate specific antigen density versus prostate specific antigen slope as predictors of prostate cancer in men with initially negative prostatic biopsies. J Urol. 1996 Aug;156(2 Pt 1):428–431. doi: 10.1097/00005392-199608000-00025. [DOI] [PubMed] [Google Scholar]
- 5.Chon CH, Lai FC, McNeal JE, Presti JC., Jr Use of extended systematic sampling in patients with a prior negative prostate needle biopsy. J Urol. 2002 Jun;167(6):2457–2460. [PubMed] [Google Scholar]
- 6.Zaytoun OM, Moussa AS, Gao T, Fareed K, Jones JS. Office Based Transrectal Saturation Biopsy Improves Prostate Cancer Detection Compared to Extended Biopsy in the Repeat Biopsy Population. The Journal of Urology. 2011;186(3):850–854. doi: 10.1016/j.juro.2011.04.069. [DOI] [PubMed] [Google Scholar]
- 7.Stewart CS, Leibovich BC, Weaver AL, Lieber MM. Prostate cancer diagnosis using a saturation needle biopsy technique after previous negative sextant biopsies. J Urol. 2001 Jul;166(1):86–91. [PubMed] [Google Scholar]
- 8.Pinkstaff DM, Igel TC, Petrou SP, Broderick GA, Wehle MJ, Young PR. Systematic transperineal ultrasound-guided template biopsy of the prostate: Three-year experience. Urology. 2005;65(4):735–739. doi: 10.1016/j.urology.2004.10.067. [DOI] [PubMed] [Google Scholar]
- 9.Pinto PA, Chung PH, Rastinehad AR, et al. Magnetic resonance imaging/ultrasound fusion guided prostate biopsy improves cancer detection following transrectal ultrasound biopsy and correlates with multiparametric magnetic resonance imaging. J Urol. 2011 Oct;186(4):1281–1285. doi: 10.1016/j.juro.2011.05.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoeks CM, Schouten MG, Bomers JG, et al. Three-Tesla Magnetic Resonance-Guided Prostate Biopsy in Men With Increased Prostate-Specific Antigen and Repeated, Negative, Random, Systematic, Transrectal Ultrasound Biopsies: Detection of Clinically Significant Prostate Cancers. Eur Urol. 2012 Feb 1; doi: 10.1016/j.eururo.2012.01.047. [DOI] [PubMed] [Google Scholar]
- 11.Keetch DW, Catalona WJ, Smith DS. Serial prostatic biopsies in men with persistently elevated serum prostate specific antigen values. J Urol. 1994 Jun;151(6):1571–1574. doi: 10.1016/s0022-5347(17)35304-1. [DOI] [PubMed] [Google Scholar]
- 12.Rabets JC, Jones JS, Patel A, Zippe CD. Prostate Cancer Detection with Office Based Saturation Biopsy in a Repeat Biopsy Population. The Journal of Urology. 2004;172(1):94–97. doi: 10.1097/01.ju.0000132134.10470.75. [DOI] [PubMed] [Google Scholar]
- 13.Bott SRJ, Henderson A, Halls JE, Montgomery BSI, Laing R, Langley SEM. Extensive transperineal template biopsies of prostate: Modified technique and results. Urology. 2006;68(5):1037–1041. doi: 10.1016/j.urology.2006.05.033. [DOI] [PubMed] [Google Scholar]
- 14.Turkbey B, Mani H, Shah V, et al. Multiparametric 3T prostate magnetic resonance imaging to detect cancer: histopathological correlation using prostatectomy specimens processed in customized magnetic resonance imaging based molds. The Journal of Urology. 2011 Nov;186(5):1818–1824. doi: 10.1016/j.juro.2011.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]