Abstract
Ulcerative colitis (UC) and Crohn disease (CD), collectively referred to as inflammatory bowel disease (IBD), are chronic inflammatory disorders that can affect the gastrointestinal tract of children and adults. Like other autoimmune processes, the cause(s) of these disorders remain unknown but likely involves some interplay between genetic vulnerability and environmental factors. Children, in particular with UC or CD, can present to their primary care providers with similar symptoms, including abdominal pain, diarrhea, weight loss, and bloody stool. Although UC and CD are more predominant in adults, epidemiologic studies have demonstrated that a significant percentage of these patients were diagnosed during childhood. The chronic nature of the inflammatory process observed in these children and the waxing and waning nature of their clinical symptoms can be especially disruptive to their physical, social, and academic development. As such, physicians caring for children must consider these diseases when evaluating patients with compatible symptoms. Recent research efforts have made available a variety of more specific and effective pharmacologic agents and improved endoscopic and radiologic assessment tools to assist clinicians in the diagnosis and interval assessment of their patients with IBD; however, as the level of complexity of these interventions has increased, so too has the need for practitioners to become familiar with a wider array of treatments and the risks and benefits of particular diagnostic testing. Nonetheless, in most cases, and especially when frequent visits to subspecialty referral centers are not geographically feasible, primary care providers can be active participants in the management of their pediatric patients with IBD. The goal of this article is to educate and assist pediatricians and adult gastroenterology physicians caring for children with IBD, and in doing so, help to develop more collaborative care plans between primary care and subspecialty providers.
Keywords: Crohn disease, health supervision, inflammatory bowel disease, preventive care, primary care, ulcerative colitis
Ulcerative colitis (UC) and Crohn disease (CD), collectively referred to as inflammatory bowel disease (IBD), are chronic inflammatory disorders that can affect the gastrointestinal tract of children and adults. Like other autoimmune processes, the cause(s) of these disorders remain unknown but likely involves some interplay between genetic vulnerability and environmental factors. Children, in particular with UC or CD, can present to their primary care providers with similar symptoms, including abdominal pain, diarrhea, weight loss, and bloody stool. Although UC and CD are more predominant in adults, epidemiologic studies have demonstrated that a significant percentage of these patients were diagnosed during childhood. The chronic nature of the inflammatory process observed in these children and the waxing and waning nature of their clinical symptoms can be especially disruptive to their physical, social, and academic development. As such, physicians caring for children must consider these diseases when evaluating patients with compatible symptoms. Recent research efforts have made available a variety of more specific and effective pharmacologic agents and improved endoscopic and radiologic assessment tools to assist clinicians in the diagnosis and interval assessment of their patients with IBD; however, as the level of complexity of these interventions has increased, so too has the need for practitioners to become familiar with a wider array of treatments and the risks and benefits of particular diagnostic testing. Nonetheless, in most cases, and especially when frequent visits to subspecialty referral centers are not geographically feasible, primary care providers can be active participants in the management of their pediatric patients with IBD. The goal of this article is to educate and assist pediatricians and adult gastroenterology physicians caring for children with IBD, and in doing so, help to develop more collaborative care plans between primary care and subspecialty providers.
EPIDEMIOLOGY OF IBD
It is essential that primary care providers recognize and consider the relative risk of IBD in their pediatric patients presenting with symptoms suspicious of either UC or CD. First, the incidence of UC and CD in the general population is on the rise, and some areas report a doubling in the incidence of IBD in the last 20 years (3). It is also important for pediatricians and other providers to recognize that IBD is not uncommon in children, with up to 25% of patients with IBD being diagnosed before age 20 (4). Existing data suggest that the incidence of IBD rises with age. Five in 100,000 children with IBD present at younger than 8 years of age, and this statistic rises to 15/100,000 in children younger than 15 years (5). Where a child lives may also affect the likelihood of them presenting with IBD. Although uncommon in developing nations, data from the United States suggest an incidence of IBD of up to 7/100,000 children/year and a prevalence of up to 20/100,000 (5). Some areas in Canada report incidences of IBD as high as 14/100,000 children per year (6). Providers must also take into account genetics and heredity when considering the possibility of IBD in their patients. Children of Ashkenazi Jewish descent appear to be at especially high risk for developing IBD in general and CD in particular. There may also be a geographic or north-south gradient, with the incidence of IBD falling because populations are located closer to the Equator (7). Although it was originally thought that the prevalence of IBD was higher in white than in either Asian or African American populations, new data suggest that environmental factors may now be playing an “equalizing” role, and clinicians must recognize the rising incidence of IBD in their patients from these previously “protected” populations.
CLINICAL EFFECT OF IBD ON CHILDREN
The chronic nature and waxing and waning clinical course observed in patients with IBD can result in disproportionate morbidity in pediatric populations. As such, primary care providers and gastroenterology physicians must adapt their clinical and pharmacological approach to the developmental age and stage of their patients. They must similarly recognize that, unlike in adult patients with IBD, clinical flares can be especially damaging when they have the potential to affect a child’s linear growth or pubertal development.
Physicians involved in the initial evaluation of children with gastrointestinal symptoms should consider IBD in patients presenting with complaints that are consistent with inflammation in the gastrointestinal tract. In many instances, the clinical appearance of UC and CD can be similar. Diarrhea and abdominal pain are observed in 50% to 90% of patients with both disorders (8). Rectal bleeding is more common in children with UC (50%–90%) than in those presenting with CD (15%–60%). Although fever does not generally discriminate patients with UC from those with CD, other constitutional symptoms including weight loss are generally more common in CD (40%–90%) than in UC (20%–55%). Perianal diseases, including fistulae and anal skin tags, are much more likely to be observed in patients with CD (44%) than those with UC (7%) (8). Because the degree of intestinal inflammation affects appetite, diet selection, and nutrient absorption, patients with IBD and active disease are increasingly susceptible to micronutrient deficiencies with respect to iron, zinc, vitamin B12, folate, vitamin D, and calcium.
The often indolent nature of CD makes it much more likely than UC to affect growth and development in children, and existing data suggest that up to 35% of children with CD, compared with 10% of children with UC, can present with impaired linear growth (9,10). Similarly, impaired growth velocity is observed in 46% of children with CD at diagnosis and only 5% to 10% of patients with UC.
Patients with IBD are at risk for a number of signs and symptoms originating in areas outside the gastrointestinal tract. It remains unclear whether these extraintestinal manifestations of their disease are related to underlying genetics, circulating inflammatory cytokines, bacteria/bacterial products, or the drugs being used to treat their disease. As such, clinicians must watch for elevations in hepatic enzyme levels that could suggest evolving liver diseases, such as drug-related increases in liver function tests, autoimmune hepatitis, or primary sclerosing cholangitis. Dermatologic complications include the painful nodules typically observed on the shins of patients with erythema nodosum and the more ulcerated lesions characteristic of pyoderma gangrenosum. History and physical examination must also assess for ocular findings related to IBD, including uveitis, iritis, and episcleritis. Joint findings can be seen in up to 15% of patients with CD and UC (11). This can range in severity from nonspecific arthralgias, to a migratory nondestructive arthritis typically seen in large joints, to the more severe and debilitating spondyloarthropathies observed most commonly in patients who are HLA-B27 positive. Musculoskeletal conditions that clinicians should consider in children with IBD may also be a result of disease activity or adverse effects of the medications used to treat these disorders, such as the osteopenia, osteoporosis, and avascular necrosis seen in patients treated with corticosteroid therapy. More recent reports have discussed the importance of clinicians considering the hypercoagulable state present in patients with chronic, and especially active, inflammatory diseases. Pediatric patients with IBD are at increased risk for thromboembolic phenomena including sinus and deep venous thromboses (12–14).
The psychosocial effect of IBD on the growing and developing child is considerable. The need for regular and scheduled clinical visits can be logistically difficult for children and parents to reconcile, especially in the context of the increased academic, athletic, and social pressures children face today. The unpredictable and fluctuating clinical course experienced by many children with IBD often affects quality of life and the ability of children to participate fully in school functions, including standardized testing, proms, or competitions (15). Similarly, families of children with IBD often find it difficult to schedule vacations, orchestrate sleep-overs with their friends, or sign their children up for summer camp. For their part, parents must constantly deal with lost work hours and a need to use more personal/vacation time to take care of their children, as well as the financial concerns including whatever costs are not covered by their child’s insurance, including copayments incurred as a result of hospitalization, office visits, or medication purchases. Because parents may be unlikely to discuss financial and personal constraints with their children’s medical providers, it is essential for physicians caring for children with IBD to recognize the broader effect of their patient’s illness on their family when making decisions about laboratory studies, need for hospitalization and testing, and choices of medications.
DIAGNOSING CHILDREN AS HAVING IBD
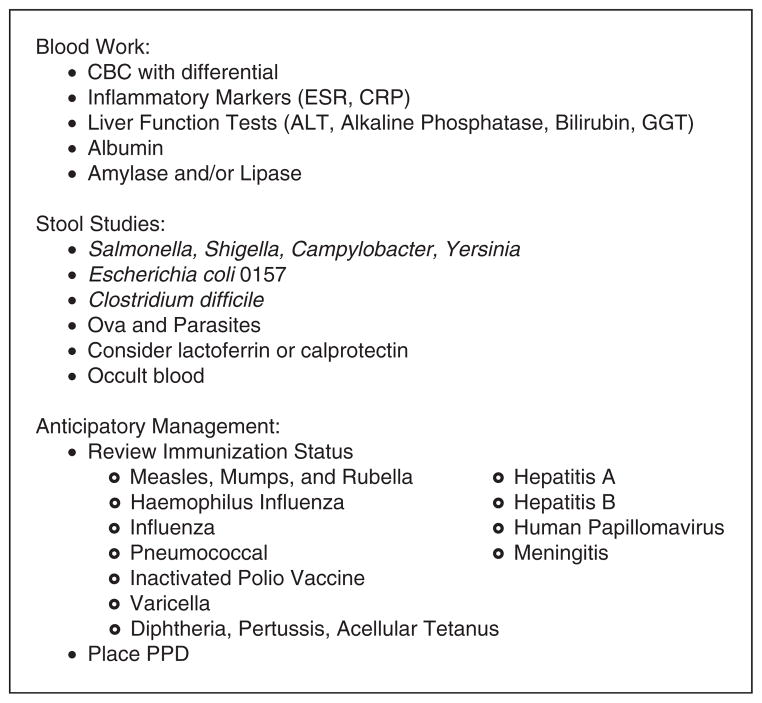
Pediatricians evaluating children with signs and symptoms that they believe could be consistent with either UC or CD are ideally positioned to initiate the workup necessary to expedite subspecialty referral and more definitive testing (Fig. 1). Initial laboratory studies should assess for signs of inflammation, including elevations in white blood cell and platelet counts, erythrocyte sedimentation rate (ESR), or C-reactive protein (CRP). Gastrointestinal blood loss and anemia can be assessed with a hemoglobin or hematocrit, and a mean cell volume (MCV) can help discriminate patients with chronic disease (low MCV) from those with more acute processes (normal MCV), including infection. Serum albumin is often low in patients with newly diagnosed IBD or those experiencing a flare in their condition. Liver function tests (alanine transaminase [ALT], alkaline phosphatase, bilirubin, and γ-glutamyltransferase [GGT]) can be elevated in patients with IBD and may be related to disease activity or an extraintestinal complication, including primary sclerosing cholangitis, autoimmune hepatitis, or biliary tract disease. Children presenting with abdominal pain should be screened for pancreatitis with a serum amylase and/or lipase. Although a number of serologic assays are commercially available to assist physicians in screening their patients for suspected UC or CD, these commercially available serologic assays may fail to detect CD in at least 30% of children with this disorder and may wrongly suggest a diagnosis of IBD that is not supported by subsequent and more definitive (endoscopic study) testing. As such, it may be most prudent for primary care providers to avoid ordering these tests and instead pursue referral and more conclusive specialty testing. Nonetheless, more recent studies suggest that these serologic assays may play a role in providing relevant diagnostic and/or prognostic information after more definitive biochemical, radiologic, and endoscopic information has been collected and reviewed.
FIGURE 1.
Office management of children presenting with suspected IBD. ALT =alanine aminotransferase; CBC = complete blood cell count; CRP = C-reactive protein; ESR = erythrocyte sedimentation rate; GGT = γ-glutamyl-transferase; IPV =inactivated polio vaccine; MMR = measles, mumps, rubella; PPD = purified protein derivative.
Stool studies are essential in the initial evaluation of any child suspected as having IBD. Given that these tests are typically parent dependent, labor intensive, and time-consuming, there is a premium on providers requesting them as early as possible in the workup. In many cases, stool studies may point away from a chronic inflammatory process and instead identify a pathogen that can be readily addressed with either observation and support (Escherichia coli 0157) or definitive antibiotic therapy (Shigella). Stool should be tested for the presence of occult blood. It is important to note that although the absence of visible or occult blood makes a diagnosis of active colitis unlikely, many children with CD may present without any evidence of GI blood loss. Children with suspected IBD, especially those with hematochezia, should provide a fresh stool sample for assessment of routine pathogens including Salmonella, Shigella, Campylobacter, Yersinia, and Escherichia coli 0157, and Clostridium difficile. Ova and parasite testing can also be considered. Although fecal leukocyte (FL) stain has been used for many years to assist clinician in determining whether or not a patient’s symptoms are more likely to be the result of an inflammatory (FL positive) versus a noninflammatory (FL negative) process, this test relies on the provision of a fresh stool sample, is not quantitative, and is operator dependent. A number of newer assays are now commercially available to detect the presence of intestinal inflammation, as assessed by the presence of white blood cell proteins (lactoferrin and calprotectin) in the stool; however, it is important to note that these studies do not discriminate patients with infectious versus inflammatory processes and the results of parallel stool cultures are essential. Nonetheless, a number of studies have confirmed the utility of fecal lactoferrin and calprotectin measurements to distinguish patients with inflammatory gastrointestinal disease from those with irritable bowel syndrome, and serial measurements of these fecal proteins can be used to assess interval disease activity in patients with IBD (16–20).
The approach to the radiologic assessment of children with suspected IBD must be individualized. Upper gastrointestinal study with small bowel follow-through (SBFT) has long been the mainstay for evaluating mucosal disease in the small intestine and ileum, but these modalities provide limited information about the presence or extent of extramural or mesenteric disease. During the course of the last decade, significant advancements in cross-sectional imaging of the small bowel with computed tomography (CT) and magnetic resonance imaging (MRI) enterography have occurred. Both CT and MRI have a major advantage over fluoroscopic techniques because they provide direct visualization of the extent of bowel wall inflammation and peri-intestinal involvement in multiple imaging planes. This type of information is especially useful to clinicians when there is concern for disease relapse or complications such as abscess, fistula, or obstruction, or when evaluating a patient’s response to medical therapy. Prospective studies in adults have shown improved diagnostic performance of both CT and MRI as compared with SBFT in evaluating extent of small bowel disease (21). Both CT and MRI have equivalent and excellent sensitivity for detecting active small bowel inflammation as compared with ileocolonoscopy (22). MRI has the added advantage of not subjecting patients to ionizing radiation. Pelvic MRI is the best imaging choice for evaluation of the extent of perianal disease including fistulas (23). MRI, especially when used in conjunction with gadolinium enhancement, may provide additional information when clinicians are attempting to discern whether a patient’s IBD is more likely to be CD or UC (24).
Technological advances in abdominal ultrasound (US) have improved spatial resolution for visualization of the bowel and mesentery and permit identification of wall thickening or abdominal and pelvic abscesses more readily in children and adolescents who typically have a small body habitus as compared with adults (25). When compared with MRI, US has the best diagnostic performance when evaluating terminal ileal disease over proximal small bowel or colonic disease (26). Although US examinations tend to be more readily available and are less expensive than MRI, appropriate technique necessitates a focused examination of the entire bowel and a radiologist who is experienced and comfortable with the use of this imaging modality for this particular clinical indication.
Increasing consideration is being given to the use of MRI or US, which do not expose patients to ionizing radiation, especially young children. New data suggest that the lifetime risk of cancer in children undergoing CT scanning may be considerably more than previously thought (27). Radiation resulting from abdominal CT may be especially relevant and could account for up to 50% of radiation-related malignancy (28). This issue is especially relevant to pediatric patients with IBD for 2 reasons. First, children with IBD require diagnostic studies at a time when their bodies are still growing and developing and therefore may be more sensitive to short- and long-term risks of ionizing diagnostic radiation. Second, children with IBD are likely to require repeated imaging during the course of their lives, leaving them vulnerable to accrue a higher lifetime radiation exposure than patients diagnosed with IBD as adults. An abdominal CT study exposes children to the equivalent of multiple individual abdominal radiographs, and carries at least a 2-fold higher radiation dose than SBFT (29). To this end, MRI is increasingly being used for initial diagnosis and follow-up examinations to assess response to medical therapy; however, CT is logistically more available in most centers, provides clinical information more quickly for patients presenting with severe acute symptoms when there is concern about an abdominal abscess or fistula or obstruction, and does not typically require physical or chemical restraint. In general, the choice of which imaging study would be most useful for a particular patient depends on their clinical symptoms, relative cost and availability, and local imaging expertise. As such, primary care providers should work in close collaboration with available subspecialty and radiologic colleagues to choose the most appropriate study to request in their patients with IBD with an objective to keeping acute and long-term radiation exposure to a minimum.
Routine health maintenance issues also take on new relevance in children being evaluated for IBD. Primary care providers should consider placing a purified protein derivative (PPD) to assess a patient’s earlier tuberculosis (TB) exposure, especially if one has not been completed in the last year. Many patients with IBD require long-term immunosuppressive therapy, and initiation of these agents is contraindicated in patients in whom latent TB status has not been assessed by PPD or chest film. It is also important for primary care providers to review their patient’s immunization status early in the IBD evaluation. The administration of live virus vaccines is contraindicated in patients receiving immunosuppressive therapy. As such, documenting (and when necessary completing) a patient’s measles, mumps, and rubella (MMR) and Varicella series may expedite care down the road. It may also be useful to review and document immunization to hepatitis A and B.
MEDICAL MANAGEMENT OF CHILDREN WITH IBD
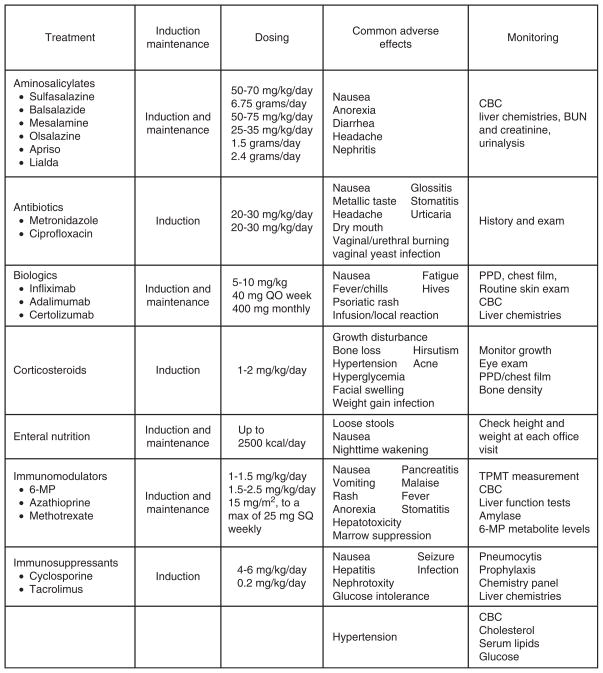
All of the chronic inflammatory diseases are characterized by a waxing and waning clinical course during which patients may go extended periods with relatively little in the way of clinical symptoms. At other times, and often without apparent precipitant, they may experience a worsening of symptoms, often referred to as a disease flare. As such, treatment for children and young adult patients with CD and UC is often thought of as occurring in 2 phases, induction and maintenance, to address these periods of clinical activity and inactivity, respectively (Fig. 2). During the induction phase, clinicians often rely on the use of more potent agents, including corticosteroids, for a relatively short period in an effort to bring a patient’s disease activity under control most expediently. At the same time, consideration should also be given to what medical regimen (typically using agents with less toxicity and potential for adverse effects) will be used to maintain disease remission. Sometimes, medications used for induction can also be continued during the maintenance phase. In all cases, treatment decisions must be individualized to meet the specific clinical needs and predilections of a specific patient and always made after discussing with a patient and their parents variables including potential adverse effects, dosing format (oral vs parenteral vs topical), and formulation (liquid vs tablet vs suspension). The choice of medications used may also depend on the underlying disease (CD vs UC), location of disease (upper vs lower gastrointestinal tract vs both), and a particular patient’s history and clinical severity. In general, medications used for induction in patients with UC include aminosalicylates, steroids, immunosuppressives, and biologics. Maintenance agents for UC include aminosalicylates, immunomodulators, and biologic agents. Induction agents used in CD include antibiotics, steroids, immunomodulators, biologic agents, as well as parenteral and enteral nutrition. Medications used to maintain remission in patients with CD include immunomodulators, antibiotics, aminosalicylates, and biologic agents. Here we present general information about the classes of medications and treatment modalities that clinicians can rely on to treat their children and young adult patients with IBD (30–32). Medication compliance is a major concern in children and adults with IBD and plays a significant role in maintaining remission. It is essential to emphasize this to patients and their parents.
FIGURE 2.
Medical management of inflammatory bowel disease. BUN = blood urea nitrogen; CBC = complete blood cell count; 6-MP = 6-mercaptopurine; PPD = tuberculosis skin test; QO = once per week; SQ = subcutaneously; TPMT = thiopurine methyltransferase.
OFFICE MANAGEMENT OF CHILDREN WITH IBD
The management of patients with IBD must be individualized to meet each patient’s particular needs and clinical course. As such, physicians caring for children with IBD may choose to see a patient in the office more or less frequently, depending upon a given patient’s disease activity, history of compliance, need for safety laboratory testing, and their age and stage of growth and development. In an effort to consolidate recommendations, we have considered office visits for children with IBD to fall within 4 general scenarios including clinically stable patients being maintained on aminosalicylate therapy; clinically stable patients being maintained on immunomodulatory or biologic agents; unscheduled visits for previously stable patients now experiencing a presumed flare in their disease; and primary EBV infection or other intercurrent infections in a patient receiving immunomodulatory or immunosuppressive therapy.
Clinically Stable Patients With Mild UC or CD Being Treated With Aminosalicylate Therapy
Frequency of Office Visits
Every 4 to 12 months.
Height and Weight
Measurement of height, weight, and body mass index (BMI) should be determined at every maintenance visit. Blood pressure should be obtained at least annually in all children with IBD and more frequently in patients receiving renal toxic medications including aminosalicylates and corticosteroids and more potent immunosuppressive therapies (cyclosporine and tacrolimus). Subtle changes in weight gain, linear growth, or growth velocity can be early signs of a clinical relapse. Although the risk of malnutrition and growth failure in patients with IBD cannot be overstated, providers must be aware that children with IBD are also at risk for obesity (33).
History and Physical Examination
General questions about interval symptoms are potentially related to underlying intestinal inflammation, including abdominal pain, stool frequency and the presence or absence of blood, fever, and weight loss. Additional questions should address potential extraintestinal manifestations of IBD including skin lesions (possibly consistent with erythema nodosum and/or pyoderma gangrenosum), changes in skin color or pruritus (suggestive of an evolving liver process such as primary sclerosing cholangitis or autoimmune hepatitis), arthritis and arthralgias, and any eye or visual changes (indicative of episcleritis or uveitis). Avoidance of nonsteroidal agents should be reinforced because these agents may increase the risk of intestinal inflammation. Clinicians must pay attention to the oropharynx, lungs, abdomen, perianal region, and skin. The abdomen should be examined for fullness/masses and tenderness. Although unlikely in well patients, the perianal region should be inspected for occult disease, including tags, fissures, or fistulae. These perineal findings can be observed in approximately 20% of patients with CD (34). Oral aphthous lesions are common and may not be mentioned by patients. Musculoskeletal complaints, including migratory, nondestructive, and large joint arthralgias, should be considered and assessed when clinically indicated. A careful history and pulmonary auscultation should be performed at maintenance visits. Undiagnosed reactive airway disease frequently complicates IBD, and some reports have estimated the frequency of pulmonary function testing abnormalities in patients with IBD to be as high as 20% (33,35–38). It is important to closely examine the skin of all patients with IBD during routine office visits. The risk of nonmelanoma skin cancer in immunosuppressed (largely transplant) patients is well established. There also exists a potential risk of nonmelanoma skin cancer such as squamous and basal cell cancers in patients receiving antitumor necrosis factor (TNF) agents (39,40). Documentation of Tanner staging to ensure appropriate progression through puberty is important. The frequency of this documentation should be tailored to the individual patient’s age and level of disease activity. The inclusion of Tanner staging is perhaps most relevant in children manifesting growth failure. Given the potential for ophthalmologic involvement in patients with IBD, clinicians should inquire about any visual changes at each visit. Although eye examinations can be performed by primary providers in most settings, referral for formal ophthalmologic examination every 1 to 2 years may be more effective for assessing and addressing more subtle ocular and fundoscopic findings (41).
Laboratory Testing
Annual urinalysis and yearly to twice-yearly measurement of serum creatinine is worthwhile in children and young adults receiving aminosalicylate therapy to assess for the infrequent complication of interstitial nephritis leading to renal insufficiency (42). A complete blood cell (CBC) count, as well as measurement of inflammatory markers, such as CRP or ESR, may be useful as indicators of subclinical disease activity. Annual or biannual measurement of aminotransferases, alkaline phosphatase, GGT, and total bilirubin may be useful, given the potential for damage to the liver from the IBD directly (eg, cholangitis or autoimmune hepatitis) or the medications used to treat IBD (drug-induced hepatitis).
Noninvasive stool markers of bowel inflammation such as fecal lactoferrin and calprotectin have been demonstrated to correlate with clinical and endoscopic indices of disease activity in patients with CD and UC (20). Furthermore, elevated fecal lactoferrin and calprotectin levels have been shown in temporal proximity to clinical relapse, suggesting a potential of these tests to “predict” relapse (17); however, there are no sufficient data to recommend collection of “surveillance” stool markers during routine office visits. Nonetheless, there is hope that further biomarker research may afford clinicians the opportunity to rely more on noninvasive markers, and less on more invasive testing or radiography, in the interval assessment of their patients with IBD.
Nutritional Assessment
Physicians caring for children with IBD should complete a general dietary and nutritional assessment at each office visit. Energy requirements in this subgroup of patients should be the same as all pediatric patients. All nutritional recommendations should be based on clinical circumstances and individualized to a given patient’s level of activity.
Children with CD, especially those with significant small bowel involvement, are at risk for the development of macro- and micronutrient deficiencies. Chronic occult blood loss can lead to iron deficiency. The anatomic location and severity of a given patient’s disease may also affect discrete absorptive processes and leave them vulnerable to specific micronutrient deficiencies (43). As such, patients with CD involving the small bowel, especially those with extensive disease or resections involving the terminal ileum, should have periodic assessment of serum vitamin B12 and folate levels. Patients with CD and either significant small bowel involvement or a history of small bowel resection are also at increased risk for the development of zinc deficiency (44). Similarly, patients with CD who have undergone terminal ileal resection are at increased risk for impaired absorption of bile acids. These unabsorbed bile acids are free to complex with free fatty acids. The subsequent binding of luminal calcium results in a release and overabundance of free oxalate within the lumen of the intestine and a subsequent significant increase in colonic resorption. Ultimately, increased renal clearance of oxalate can lead to the development of calcium oxalate stones in the kidneys.
Thus, routine nutritional laboratory assessments in children with IBD may include yearly measurements of vitamin B12 and folic acid. Similarly, measurement of CBC as well as serum iron is useful when there is concern for iron deficiency anemia. If there is a history of significant small bowel disease or the alkaline phosphatase is low in a particular patient, zinc deficiency should be assessed. As noted above, clinicians should be suspicious about the development of nephrolithiasis in children with a history of significant small bowel disease, surgical resection, or a positive family history. When clinically indicated, measurement of urine creatinine, electrolytes, calcium, magnesium, uric acid, citrate, oxalate, volume, and pH should be obtained.
Bone Health in Children With IBD
Recent data underscore the need for physicians caring for children with IBD to pay particular attention to assessing and ensuring adequate vitamin D and calcium intake by their patients. Significant deficits in bone mass have been observed in 10% to 40% of children presenting with IBD, and these deficits appear to be more pronounced in patients with CD relative to those with UC (45,46). This deficit may affect the attainment of peak bone mass, which is the most important determinant of long-term skeletal health (including the potential risk of fracture), as well as linear growth (45,46). A combination of nutritional deficiencies, physical inactivity, inflammatory cytokines, skeletal muscle mass deficits, and glucocorticoids can affect adversely bone formation in children with IBD.
Dual x-ray absorptiometry (DXA) is the most readily available tool to measure bone mass. The x-ray dose, although measurable, is extremely low (about 1/20th of a chest x-ray). When interpreted for chronological age, DXA can underestimate bone mineral density (BMD) in children with delays in growth and maturation, which are common findings in children with IBD (47). To adjust for this, BMD is expressed as a z score, which measures the deviation of the patient’s BMD from the normal mean for age and sex. Children with growth stunting should have their BMD further adjusted for skeletal age as determined by radiologic assessment, height z score, and Tanner staging. The International Society of Clinical Densitometry recommends that children with IBD have a DXA scan of the total body (minus skull) and lumbar spine at diagnosis (48). The scanner should use appropriate pediatric software to avoid underestimating bone mass. When indicated, repeat DXA studies should be performed in the same scanner and in no sooner than 6-month intervals to assess for response to a bone-active intervention or the effect(s) of anti-inflammatory therapy on bone mass or body composition (48). It should be noted that low BMD z scores should never be the single reason to institute bone-active therapy (eg, calcitonin, bisphosphonates) in children. In addition, the terms “osteopenia” and “osteoporosis,” which are appropriate for postmenopausal women, should not be used to describe bone mineral deficits in children. Instead, terms such as “significant reduction in bone mass compared with children of the same age and sex” are favored.
Children with IBD who have a low BMI, severe inflammatory activity, and low serum albumin are at particular risk for low bone mass. Children with significant bone mineral mass deficits (z score <−2 at any site) should be evaluated further including bone age, serum calcium, phosphorus, magnesium, blood urea nitrogen, creatinine, parathyroid hormone (PTH), ionized calcium, tissue transglutaminase IgA, and measurement of serum 25 hydroxy-vitamin D (25-OH D) and 1,25 dihydroxyvitamin D levels. Children and young adults with low BMD and/or a history of fracture may benefit from a referral to a pediatric endocrinologist for consideration of bone-active agents.
Vitamin D and Calcium Supplementation
The role played by vitamin D in maintaining normal intestinal calcium absorption is well established; however, recent epidemiologic and laboratory findings suggest that vitamin D also plays important roles in the regulation of cellular proliferation and differentiation, modulation of the immune system, and maintenance of blood pressure (49). Epidemiological studies further suggest that the falling geographic incidence (north-to-south) observed for many autoimmune diseases, including IBD, may be explained, at least in part by sun exposure and the prevalence of vitamin D deficiency. Vitamin D deficiency is especially common in children with IBD who live in northern latitudes caused by limited exposure to unfiltered UVB light. Sunscreen applied properly markedly reduces vitamin D synthesis in the skin, so clinicians should be aware that even children in sunny climates can be vitamin D insufficient. Dark-skinned individuals are especially at risk for vitamin D deficiency because melanin is an effective UVB radiation blocker.
Serum 25-OH D levels required to prevent rickets are reasonably well established (≥12.5 ng/mL) (49). The Institute of Medicine defined vitamin D deficiency as a serum 25-OH D of <20 ng/mL, which is associated with inadequate bone mineralization; however, the serum levels of 25-OH D necessary to affect positively the extraskeletal effects of this agent remain undefined, and more research is needed in this important area. Based on studies looking at suppression of PTH in adults, the optimal serum 25-OH D is ≥30 ng/mL; however, there is no serum 25-OH D level in children that has been associated with maximal suppression of PTH. In addition, the relation between serum 25-OH D and PTH levels can be influenced by calcium intake.
Vitamin D status (serum 25-OH D) should be checked at least once yearly in the spring, when levels are lowest, and supplements should be prescribed accordingly. Physicians caring for children with IBD should also routinely perform a dietary assessment, paying especially close attention to the consumption of vitamin D–containing foods including dairy products, fortified cereals, oily fish, and certain fungi (eg, shiitake mushrooms) (Table 1) (50). Given the eclectic dietary predilections observed in children and adolescents, some patients may require vitamin D supplementation in addition to a multivitamin to achieve and maintain adequate serum vitamin D levels (Tables 2 and 3). The Institute of Medicine has recently published dietary reference intakes for calcium and vitamin D (Table 4) (4) and the American Academy of Pediatrics guidelines for the prevention and treatment of vitamin D (Tables 1–4), which can be applied to children with IBD (50).
TABLE 1.
Vitamin D content of foods (50)
| Food | Vitamin D content, IU |
|---|---|
| Cow’s milk | 3–40/L |
| Fortified milk/infant formulas | 400/L |
| Fortified orange juice/soy milk/rice milk | 400/L |
| Butter | 35/100 g |
| Margarine, fortified | 60/tablespoon |
| Yogurt (normal, lowfat, or nonfat) | 89/100 g |
| Cheddar cheese | 12/100 g |
| Parmesan cheese | 28/100 g |
| Swiss cheese | 44/100 g |
| Cereal fortified | 40/serving |
| Tofu fortified (1/5 block) | 120 |
| Fresh shitake mushrooms | 100/100 g |
| Dried shitake mushrooms (nonradiated) | 1660/100 g |
| Egg yolk | 20–25 per yolk |
| Shrimp | 152/100 g |
| Calf liver | 15–50/100 g |
| Canned tuna/sardines/salmon/ mackerel in oil | 224–332/100 g |
| Canned pink salmon with bones in oil | 624/100 g |
| Cooked salmon/mackerel | 345–360/100 g |
| Atlantic mackerel (raw) | 360/100 g |
| Atlantic herring (raw) | 1628/100 g |
| Smoked herring | 120/100 g |
| Pickled herring | 680/100 g |
| Codfish (raw) | 44/100 g |
| Cod liver oil | 175/g; 1360/tablespoon |
TABLE 2.
Available vitamin D, calcium, and phosphorus preparations (50)
| Vitamin D and its analogs |
| Vitamin D2 (ergocalciferol): available in 3 forms |
| 200-μg/mL (8000 IU/mL) solution in propylene glycol solution |
| 1250-μg (50,000 IU) gel caps |
| 625- and 1250-μg (25,000- and 50,000 IU) tablets have been available |
| Trade names: Calciferol, Drisdol, most children’s chewable multivitamins including Flintstones and Garfield, prenatal and women’s multivitamins |
| Vitamin D3 (cholecalciferol): may be 3 times as potent as vitamin D2 |
| Trade names: Delta-D, Poly-Vi-Sol |
| 1.0 μg of vitamin D = 40 IU; 1.0 mg of vitamin D = 40,000 IU |
| Calcium preparations |
| Calcium gluconate: 10% injection, preservative-free solution, 100 mg/mL; elemental calcium 9 mg/mL |
| Calcium chloride: 10% injection, preservative-free solution, 100 mg/mL; elemental calcium 27.2 mg/mL |
| Calcium carbonate: oral suspension 1250 mg/5 mL (elemental calcium 500 mg/5 mL); chewable tablets (400-mg elemental calcium per gram of calcium carbonate); trade names: Tums, Viactiv, Caltrate, OsCal, and others |
| Calcium glubionate: oral solution 1800 mg/5 mL (elemental calcium 115 mg/5 mL); trade name: Calcionate |
| Tribasic calcium phosphate: caplet containing 600 mg of calcium and 280 mg of phosphorus (390 mg of elemental calcium per gram of tribasic calcium phosphate); Trade names: Posture |
TABLE 3.
Treatment of vitamin D–deficiency rickets: vitamin D and calcium supplementation and monitoring of therapy (50)
| Vitamin D (ergocalciferol or vitamin D) |
| Double-dose vitamin D: 800 IU (20 μg)/day × 3–4 mo; or |
| Pharmacological doses of vitamin D: 1000–10,000 IU (25–125 μg)/day × 8–12 wk depending on the age of the child, then maintain at 400–1000 IU (10–25 μg)/day; or |
| Stoss therapy: 100,000–600,000 IU (~2.5–15.0 mg) of vitamin D orally (over 1–5 days), then maintain at 400–1000 IU (10–25 μg) of vitamin D per day, or 50,000 IU (1.25 mg) of vitamin D2 weekly for 8 wk orally (teenagers and adults) |
| Calcium |
| 30–75 mg · kg−1 · day−1 of elemental calcium in 3 divided doses (start at a higher dose, and wean down to the lower end of the range over 2–4 wk) |
| Monitoring of therapy |
| At 1 mo: calcium, phosphorus, Alk phos. |
| At 3 mo: calcium, phosphorus, magnesium, Alk phos, PTH, 25(OH)-D, urine calcium/creatinine ratio (frequency depends on severity of rickets and hypocalcemia); recheck radiologic findings in 3 mo |
| At 1 y and annually: 25(OH)-D |
Alk phos = alkaline phosphatase; 25(OH)-D = 25-hydroxyvitanmin D; PTH = parathyroid hormone. Adapted from Levine M, Zapalowski C, Kappy M. Disorders of calcium, phosphate, parathyroid hormone and vitamin D metabolism. In: Kappy MS, Allen DB, Geffner ME, eds. Principles and Practice of Pediatric Endocrinology. Springfield, IL: Charles C Thomas; 2005:741.
TABLE 4.
Dietary reference intakes for calcium and vitamin D
| Calcium
| |||
|---|---|---|---|
| Life stage group | Estimated average requirement, mg/day | Recommended dietary allowance, mg/day | Upper level intake, mg/day |
| 0–6 mo | — | — | 1000 |
| 6–12 m | — | — | 1500 |
| 1–3 y | 500 | 700 | 2500 |
| 4–8 y | 800 | 1000 | 2500 |
| 9–13 y | 1100 | 1300 | 3000 |
| 14–18 y | 1100 | 1300 | 3000 |
| 19–30 y | 800 | 1000 | 2500 |
| Vitamin D
| |||
|---|---|---|---|
| Life stage group | Estimated average requirement, IU/day | Recommended dietary allowance, IU/day | Upper level intake, IU/day |
| 0–6 mo | – | – | 1000 |
| 6–12 mo | – | – | 1500 |
| 1–3 y | 400 | 600 | 2500 |
| 4–8 y | 400 | 600 | 3000 |
| 9–13 y | 400 | 600 | 4000 |
| 14–18 y | 400 | 600 | 4000 |
| 19–30 y | 400 | 600 | 4000 |
Reprinted with permission from the National Academies Press, Copyright 2010, National Academy of Sciences.
Adequate calcium intake is also critical to ensure normal bone mineralization. There is no evidence that recommending routine avoidance of dairy products will decrease mucosal inflammation or clinical outcome in patients with IBD; however, a combination of either dietary habits and/or comorbidities, including lactose intolerance, may make it difficult for some patients to meet the higher calcium requirements observed during adolescence (Table 4). Although calcium-fortified juices and lactose-free beverages are helpful for some patients, supplements are often necessary. Multiple calcium supplements are available, and some preparations combine calcium with vitamin D.
Assessing Mental Health in Children With IBD
The burden of chronic diseases, and IBD in particular, is characterized by a waxing and waning clinical course that can affect disproportionately pediatric patients who must by necessity constantly reconcile effects of their disease with an array of competing developmental, social, academic, and athletic demands. As such, it is important for providers to recognize that although CD and UC are clearly not manifestations of underlying psychosomatic disorders, pediatric patients with IBD are at increased risk for the development of depression, anxiety, social isolation, altered self-image, family conflict, medical adherence problems, and school absences (51). Screening studies suggest that up to 25% of adolescents with IBD may display symptoms of depression, and 97% of these patients would have gone unrecognized if not specifically queried (52). Although children with IBD in remission or with mild disease appear to have psychosocial functioning similar to healthy children, several pediatric studies have found that adolescents with active IBD are more likely to express greater psychosocial difficulty (53–56). Depression in particular has been correlated with pain, diarrhea, weight loss, and elevated ESR in children with IBD (57). Additional studies are necessary to better understand the role played by life stressors (starting school, examination, graduation) on disease course in children with IBD.
Appropriate screening and treatment of depressive symptoms and dysfunction in children with IBD can have a significant effect on their clinical outcome and health-related quality of life (57). Present studies are evaluating how much improved overall psychosocial functioning can positively affect medication compliance, a major prognostic predictor of clinical outcome in patients with IBD.
Providers with concerns about persistent changes in mood, appetite, or levels of social, athletic, and academic functioning in their pediatric patients can use the Children’s Depression Inventory (CDI) as a screening tool. This metric is the mostly widely used and reliable measure of depressive symptoms and impaired social functioning in children ranging from 6 to 17 years of age (58). A parallel metric (CDI-P) is also available to assess parental data. The CDI is available for purchase online, takes approximately 5 minutes to complete, and can be readily administered and scored (0–54) without the assistance of a mental health professional. In general, child or parental CDI scores ≥10 should prompt referral to a mental health professional. There are other measures of psychosocial or emotional distress available in the public domain. In general, clinicians caring for children and adolescents with IBD should make it a practice to ask about any significant changes in mood, behavior, and performance as part of the medical visit.
Psychosocial interventions are available and have been shown to improve IBD-related quality of life and level of functioning. Cognitive behavioral therapy has the most empirical support in improving psychosocial functioning in adults with IBD (59–62). Psychotropic medications, including serotonin and dopamine reuptake inhibitors, have been reported to reduce anxiety and improve depressive symptoms in adults with IBD (63–65); however, there are no clear data on which agents are most effective and under which circumstances, particularly in the pediatric population, in which long-term developmental sequelae of psychotropic medications have yet to be determined. In addition, clinicians must be aware of any potential interactions between psychotropic medications and the agents being used to treat a child’s underlying IBD.
Referral for cognitive behavioral therapy has been shown to be especially effective in improving depressive symptoms and functioning in children with IBD (66). This problem-oriented therapy is readily adapted to children and works to identify and modify maladaptive behaviors and thought processes. Medical hypnosis has also shown promising effects on improving quality of life and inflammation in adults with IBD (67). Given that children are generally more receptive to hypnosis, this treatment modality clearly holds promise for addressing emotional symptoms in pediatric patients with IBD. Patients and their families may also benefit from participation in support groups or Web-based interactive and narrative therapies (15). Sites including www.experiencejournal.com, www.ccfa.org, www.ibdsf.com, www.starlight.org, and www.myibd.org provide patients and their parents with an opportunity to relate their own experiences. In many cases, this can lead to improved feelings of patient satisfaction, an improved ability to integrate potentially difficult emotional experiences, and an overall improved level of familial communication and collective adaptation (68,69). Efforts to increase children’s overall social support network can also have a positive effect on children with IBD. Attendance at a summer IBD camp improved overall quality of life and participation in monthly support groups increased emotional and functional outcomes in female adolescents (70,71).
In many cases, physicians may need to advocate for the establishment of a 504 Plan for their patients with IBD. This is a process that federally mandates all public schools to provide any child with a chronic illness or disability that necessitates their missing school because of their condition or its treatment, with an individual educational plan to enable them to catch up academically and without penalty. The plan can be individualized but may include provisions for discrete bathroom access, tutoring for missed classes, and extra time to make up examinations or assignments without penalty. Although not similarly mandated, private schools will often cooperate with an articulated individual educational plan when accompanied by a letter from physicians requesting such accommodations. Brochures are available from the Crohn’s and Colitis Foundation of America (CCFA), which specifically use language and terminology helpful for teachers and others to understand IBD in pediatric patients. In addition, 504 Plans are also a way of educating teachers and school officials so that they can better understand what accommodations may be necessary to support their students with IBD. In summary, it is important for pediatric providers and parents to advocate for their patients to get the services that they need to ensure their academic success, despite the limitations posed by chronic diseases like IBD.
Immunizations
The present immunization guidelines for patients with IBD were set forth by a panel of experts formed by the CCFA in 2004 (72). The committee recommended that patients with IBD be immunized with inactivated vaccines. This includes diphtheria, pertussis, acellular tetanus, HBV, Haemophilus influenza, inactivated polio vaccine, influenza, pneumococcus, and hepatitis A virus vaccinations in early childhood, as well as immunizations against human papilloma virus and meningococcal diseases during school age and adolescence. Ideally, an effort should be made to immunize pediatric patients with IBD with any live viral vaccines (including Varicella and MMR) they may require before the need arises for them to receive immunosuppressive therapy. Special consideration should be made regarding the Varicella vaccine. If parents cannot recall a history of natural infection, Varicella vaccine should be administered to children. If the patient and/or parents are unsure, Varicella antibody titers should be checked (73,74). If patients do not have a history of Varicella infection or Varicella immunization, they should be vaccinated before starting immunosuppressive therapy, which is defined by treatment with high-dose systemic corticosteroids (≥2 mg · kg−1 · day−1 of prednisone or its equivalent or ≥20 mg/day of prednisone or its equivalent for 14 days or more), cyclosporine or tacrolimus, immunomodulatory agents, or biologic therapy. It is recommended that patients wait at least 1 month after discontinuing corticosteroids before immunization with Varicella vaccine (75).
Patients should also be tested for latent hepatitis B or seronegativity (HBsAb negative) because treatment with anti-TNF agents has been reported to result in HBV reactivation (74). Providers should consider checking hepatitis A and hepatitis C status as well.
Reproductive Health Counseling
Although fertility is less of an immediate concern to most children with IBD, the issue is more pressing to parents and adolescents. Previous studies have suggested that fertility among patients with UC is generally no different than the general population, with the major exception being those patients with UC that have undergone ileal-pouch anal anastomosis (76,77). Fecundity rates in this group of postcolectomy patients have been estimated in a large systematic review to be approximately 26%, in comparison with 12% observed in patients with UC who did not undergo surgery (78). The literature has been more conflicting with respect to the subject of fertility in patients with CD. It is now generally accepted that among patients with CD that are in remission and without earlier surgery, fertility rates are similar between patients and control subjects (79).
Genetic Counseling
Hereditability of IBD is a common concern for both patients and the parents of children with IBD. CD appears to confer a higher risk of development of IBD in first-degree relatives including offspring when compared with UC (80,81). Present data suggest the overall risk of IBD developing in the offspring of affected parents to be 5 to 10 times that of the general population. To use numbers patients and their families can readily understand, if the overall risk of developing IBD in the general population is roughly 0.5%, the most liberal risk estimation for a sibling or child of a patient with IBD to develop the disorder would be approximately 5%. To further put this risk into perspective, the overall likelihood of developing colon cancer in the United States is approximately 6% and breast cancer in women is 12% (82).
Cancer Screening
Other than an assessment for skin cancer during routine physical examinations, colon cancer screening is the only test that physicians caring for children and young adults should consider. Existing data document a real and substantial increase in the risk of developing colon cancer among patients with either UC or CD involving a substantial portion of the colon (83–88). As such, patients with IBD should undergo screening colonoscopic examinations with surveillance biopsies every 1 to 2 years, beginning approximately 7 to 10 years after their initial diagnosis (89). Patients with concomitant primary sclerosing cholangitis are at further risk for the development of cholangiocarcinoma and colon cancer. As such, these patients should begin a screening colonoscopic program, with studies performed every 1 to 2 years, beginning at the time of diagnosis (89).
Patients in Clinical Remission and Being Treated With Immunosuppressive Therapy
Patients are characterized as being receiving immunosuppressive therapy if they are taking corticosteroids (≥20 mg daily or ≥2 mg/kg if <10 kg body weight), thiopurines, methotrexate, or any anti-TNF agent.
Frequency of Office Visits
Every 3 to 6 months.
Height/Weight
Height, weight, and BMI should be determined at every maintenance visit.
Physical Examination
In addition to the examination features noted above, clinicians must pay special attention to complications related to immunosuppressive therapy. Note should be made of any lymphadenopathy present in the neck, axillae, and groin. Similarly, assessment for hepatosplenomegaly is particularly important in the examination of any patient receiving ongoing immunosuppressive therapy. Clinicians should inquire about and inspect the skin for the development of any new or changing lesions. In addition to the evaluation for skin malignancy, physicians should be aware of the development of psoriasis-like plaques on patients taking anti-TNF agents (90,91).
Laboratory Testing in Patients Receiving Immunomodulatory Therapy
Similar recommendations can be made for those patients with mild disease being treated with aminosalicylates. In addition, close monitoring of CBC and hepatic transaminase levels is recommended even in patients who are clinically stable on thiopurine therapy. The frequency of laboratory testing in these patients may be tailored to the particular patient. Patients recently beginning thiopurine therapy may require weekly, biweekly, or monthly safety laboratory testing. In patients who have been clinically and biochemically stable for some time, the frequency of safety laboratory testing may be extended to every 3 to 4 months; however, it is important to note that even patients who have displayed stable laboratory studies for extended periods of time can develop unexpected leukopenia while receiving thiopurine therapy (92,93). There is less clinical experience on which to base recommendations for laboratory surveillance testing in children with IBD being treated with methotrexate. When used in the treatment of psoriasis, it is recommended that clinicians obtain a CBC and aspartate aminotransferase, ALT, and alkaline phosphatase levels biweekly for several months and then every 2 to 3 months thereafter (94). If one excludes the use of methotrexate in high-risk patients such as those with steatohepatitis and diabetes, surveillance of liver function tests is sufficient to monitor for hepatic complications of long-term methotrexate use (93).
Laboratory Testing in Patients Receiving Anti-TNF Therapy
Leukopenia or liver test abnormalities have rarely been reported in association with treatment with anti-TNF therapy. Nonetheless, it is advisable to monitor CBC levels in these patients approximately every 3 to 6 months.
There is a risk of anti-TNF therapy activating latent TB (95). As such, all children should be screened for TB with PPD (preferably with an anergy panel) and/or chest film before starting anti-TNF therapy. There are presently no subsequent guidelines with respect to yearly monitoring for tuberculosis in patients receiving anti-TNF therapy; however, because the greatest risk for developing tuberculosis in patients receiving anti-TNF agents is likely at the outset of therapy, yearly screening, particularly in low-risk populations, is not likely to be of significant benefit or cost-effective. When there is particular clinical concern for TB in a patient receiving anti-TNF therapy, it is important for clinicians to recognize that existing data suggest that patients with IBD receiving immunosuppressive therapy have an unacceptably high rate of anergy to standard skin testing (96). Therefore, the use of newer T cell–based assays including QuantiFERON-TB (Cellestis, Valencia, CA) and T-SPOT-TB (Oxford Immunotec, Abingdon, UK) that display improved sensitivity and do not appear to be complicated by Bacille Calmette-Guerin positivity are likely to provide clinicians with more reliable data (97). Other infections that may occur in pediatric patients treated with anti-TNF medications include histoplasmosis and listeriosis.
Immunizations
Children being treated with immunosuppressive agents should receive all of the scheduled nonlive vaccines as noted above and as outlined by the American Academy of Pediatrics (98). A panel of experts convened by the CCFA in 2004 recommended that patients with IBD who are receiving immunosuppressive therapy should not be immunized with live vaccines (including the Rotaviral, MMR, Varicella, and intranasal influenza vaccines), given the possibility of vaccine-associated disease (72).
The approach to Varicella-naïve patients with IBD being treated with immunosuppressive agents is less clear. The increased morbidity and mortality observed in older children and adults experiencing a primary Varicella infection are considerable and even more significant in children receiving immunosuppressive therapy. These concerns should be taken into account upon discussion of the risk–benefit ratio for Varicella vaccination in children receiving immunosuppressive therapy.
There are no studies that examine the safety and immune response to Varicella vaccine in patients with IBD receiving immunosuppressive therapy; however, one case series of 6 children with IBD who received Varicella vaccine demonstrated that this vaccine was tolerated, and all but 1 patient developed immunity after immunization (99). When clinically indicated, the Centers for Disease Control and Prevention has recommended Varicella zoster vaccine for patients with IBD taking azathioprine (≤3 mg · kg−1 · day−1) and 6-MP (≤1.5 mg · kg−1 · day−1) (100); however, primary providers should consult with infectious disease specialists before making any decisions about the use of Varicella zoster vaccines in patients with IBD being treated with immunosuppressive therapy. If immunocompromised patients do not have immunity against Varicella and experience a significant exposure to Varicella, then VariZIG or acyclovir should be given. It is recommended that VariZIG be administered as soon as possible and within 96 hours of exposure. If >96 hours have elapsed since exposure, or VariZIG is unavailable, then some experts may suggest giving acyclovir within 7 to 10 days of the initial exposure. If immunocompromised patients acquire varicella infection, then intravenous acyclovir is recommended (75).
Patients being treated with immunosuppressive agents should receive annual influenza immunization, and when available, combined influenza/swine flu vaccines. Intranasal flu vaccine contains an attenuated live virus and should be avoided in patients receiving immunomodulatory therapy. When patients are known or suspected to be infected with influenza, physicians may opt to hold immunosuppressive therapy until a patient is clinically improving and their infection resolves. Patients with IBD, regardless of their medication status, should be treated with antiviral medications (including Tamiflu) when clinically indicated (101).
Before beginning anti-TNF therapy, providers should consider screening patients for hepatitis A, B, and C. Children found to be seronegative should be vaccinated for HBV and hepatitis A virus.
Cancer Screening
Anti-TNF and thiopurine therapies have been associated with a small but increased risk of non-Hodgkin lymphoma (NHL). Recent data demonstrate a risk among patients taking both medications together to be approximately 3-fold the general population or 1/1600 patient-years (102–108). There are similar estimates of risk in patients receiving thiopurine therapies alone, and thus at this point it is unclear how much the anti-TNF therapy contributes to this risk. More recently, there has been increased concern about the incidence of hepatosplenic T-cell lymphoma in young patients (typically boys) receiving concomitant immunomodulatory and biological therapy (106,107). Although the especially poor prognosis of this form of lymphoma is understandably of particular concern to patients and their parents, it is important to note that it is difficult to estimate the true relative risk because the number of patients being treated with combined immunomodulatory and biological therapy worldwide is not presently known. Furthermore, there are no validated modalities in place to allow clinicians to either screen for hepatosplenic T-cell lymphoma or determine which patients may be at increased risk.
Unscheduled Visits for a Presumed Disease Flare in Previously Stable Patients
Assessing Patients With Symptoms of Colitis
The most important initial consideration in a child with IBD who is presenting to their primary care provider with signs and symptoms of active IBD (including abdominal pain, diarrhea, rectal bleeding, and fever) is discerning whether the clinical presentation is a manifestation of their underlying IBD or the result of an alternate diagnosis. In many cases, when infection is present, an increase in symptoms may be readily addressed with a course of antibiotics, thereby precluding the need for further invasive testing. Specifically, complications presenting in a fashion mimicking IBD would be the colitis observed in patients with Clostridium difficile or more unusual infections that form pseudomembranes such as Klebsiella oxytoca (109). Cytomegalovirus colitis is always a consideration in patients with IBD being treated with immunosuppressive therapy (110). Rarely, patients with colitis may suffer from an allergic reaction to aminosalicylates, manifesting as abdominal cramping or diarrhea, and thereby mimicking a flare in the patient’s IBD. Thus, a fresh stool sample should be collected for all of the patients with established colitis presenting with a suspected flare in their disease. Relevant stool studies include Clostridium difficile and routine pathogens including Salmonella, Shigella, Yersinia, Campylobacter, and E coli 0157. When clinical suspicion is high, such as in the context of recent antibiotic therapy, it may be useful to collect several stool samples to measure C difficile toxin. The sensitivity of identifying toxin in the stool increases from 54% to 92% if 4 samples, instead of a single stool sample, are assayed (111). C difficile polymerase chain reaction has been found to be a more sensitive test of C difficile infection. Measurement of quantitative fecal lactoferrin or calprotectin may also be useful to determine the nature and severity of a patient’s presentation, especially when previously levels are available for comparison. Finally, clinicians must also be aware that young patients with IBD are at risk for the development of comorbidities, including irritable bowel syndrome. In these cases, an increase in gastrointestinal symptoms may be more indicative of an underlying functional disorder that will likely be unresponsive to the addition to, or escalation of, a patient’s anti-inflammatory or immunosuppressive regimen (112,113).
A number of disease activity indices have been developed to assist providers in assessing their patient’s clinical status in a concise and standardized fashion. These indices are in the most widespread use in clinical trials; however, the pediatric UC activity index has been validated for use in clinical practice (Table 5). This noninvasive index accurately assesses disease activity, responds to changes in clinical course, and may find increasing use in the assessment of children with UC who have been unresponsive to changes in therapeutic regimens and already found to be negative for infectious causes (114,115).
Table 5.
Pediatric Ulcerative Colitis Activity Index
| Item | Points |
|---|---|
| Abdominal pain | |
| No pain | 0 |
| Pain can be ignored | 5 |
| Pain cannot be ignored | 10 |
| Rectal bleeding | |
| None | 0 |
| Small amount only, in <50% of stools | 10 |
| Small amount with most stool | 20 |
| Large amount (>50% of the stool content) | 30 |
| Stool consistency of most stools | |
| Formed | 0 |
| Partially formed | 5 |
| Completely unformed | 10 |
| No. stools/24 hours | |
| 0–2 | 0 |
| 3–5 | 5 |
| 6–8 | 10 |
| >8 | 15 |
| Nocturnal stools (any episode causing waking) | |
| No | 0 |
| Yes | 10 |
| Activity level | |
| No limitation of activity | 0 |
| Occasional limitation of activity | 5 |
| Severe restricted activity | 10 |
| Sum of Pediatric Ulcerative Colitis Activity | |
| Index (0–85) | |
| Disease activity: <10: no disease; 10–34: mild activity; 35–64: moderate activity; ≥65: severe activity | |
Reprinted with permission from Elsevier (Gastroenterology. 2007; 133:423–32).
Laboratory testing can be useful to determine disease activity and for assessing nutritional parameters in acutely ill patients. This may include CBC with differential, ESR, CRP, and liver function tests (ALT, alkaline phosphatase, bilirubin, GGT, and albumin). Amylase and lipase may also be useful when patients present with abdominal pain or if they are being treated with agents that can affect the pancreas (especially azathioprine and 6-mercaptopurine). Serum iron studies, including saturation, total iron-binding capacity, and ferritin, may reflect whether blood loss due to rectal bleeding has been acute or chronic; however, it is important to note that ferritin is an acute-phase reactant and thus may be normal or elevated even in the presence of inflammation and iron deficiency. For this reason, ferritin levels up to 50 ng/mL may still be consistent with iron deficiency, and iron-binding capacity may not be increased due to associated protein-losing enteropathy. A chemistry panel will assist in the assessment of hydration status and renal function.
Patients with persistent or worsening symptoms, especially in the context of a negative infectious workup, should be referred to their primary gastroenterologist. Depending on the presentation and earlier clinical history, patients may benefit from procto-sigmoidoscopy in the acute setting. The endoscopic and histologic appearance of their mucosa can be used to assess the location and severity of inflammation and to assess for infectious processes including cytomegalovirus colitis.
Assessing Patients With Symptoms of Small Bowel Disease
The evaluation of patients presenting with symptoms potentially related to a recurrence of terminal ileal or small bowel CD is more complicated. Obtaining stool studies as noted above may be useful when the patient’s symptoms could be consistent with an intercurrent infectious process. Laboratory testing, including CBC and inflammatory markers, can similarly provide useful clinical information to the evaluating provider. Colonoscopy with ileoscopy is generally the most sensitive and specific test for assessing disease activity in patients with established terminal ileal CD; however, this necessitates a 1- to 2-day clean-out regimen, is expensive and invasive, and is not always feasible. As noted above, fecal biomarkers may reflect disease activity in patients with IBD, but they are nonspecific and their elevation does not discriminate between patients with primary mucosal inflammation (IBD) and those with an acute bacterial infection. In efforts to develop alternative and less invasive technologies for use in the interval assessment of these patients, investigators have studied the use of wireless capsule endoscopy (WCE). Although the images provided during these studies can be useful clinically, the studies are limited by the inability to either control the view of the capsule or obtain diagnostic mucosal biopsies. In addition, retention of the capsule within the small intestine, often necessitating surgical exploration and enterotomy, has been well reported (116). Therefore, WCE is not generally recommended in the acute assessment of small bowel disease. CT enterography has been shown to be more sensitive than either WCE or traditional fluoroscopic SBFT for identifying small intestinal disease in patients with CD (117). CT examination also enables the physician to assess for evidence of complications of CD, including perforation and structuring; however, in recognition of concerns related to the risks of ionizing radiation in young patients, many physicians are turning more often to MR enterography with comparable results (22). Abdominal US is another radiation-sparing diagnostic modality that can be used in the place of CT imaging to assess acutely for abscess or thickened bowel loops suggestive of active small bowel CD (118,119).
Ensuring Treatment Compliance
It is important to review the medication history of patients presenting with an increase in their symptoms of IBD. Non-compliance, particularly in young patients, is a frequent cause for disease flare (120–123). As such, educating patients and their parents about appropriate dosing may not only address the present flare but also decrease the likelihood of further complications with age. Providers should also review the doses of medications being administered. In many cases, children with stable disease may have outgrown their dose of aminosalicylates or immunomodulator therapy. It may also be useful to obtain and review metabolite levels (6-thioguanine and 6-methylmercaptopurine) in patients being treated with mercaptopurine and azathioprine. In many cases, simplifying a patient’s medication regimen, including once- or at most twice-daily dosing, when possible, may improve rates of adherence considerably. Conversion to injectable medications (methotrexate or infliximab) may be beneficial in patients in whom attempts to improve adherence to daily oral medications are not successful.
Primary EBV Infection in the Patient on Immunosuppressive Therapy
Epstein-Barr virus (EBV) infection occurs frequently in children and young adults. Most cases of primary infection resolve without complication and may often go clinically unrecognized. The most concerning complications of EBV infection in patients with IBD, including the development of EBV-related lymphoma or EBV-associated hemophagocytic lymphohistiocytosis (EBV-HLH), occur primarily in those being treated with immunomodulatory or biological therapy. Both of these clinical entities are EBV-related, more probable in the context of primary infection, and are more likely to be experienced by an immunocompromised host.
Most of the thiopurine-associated cases of NHL are EBV related (105). As such, it is important to counsel our patients and their parents about the potential for EBV-related lymphoma when considering instituting immunomodulatory therapy.
Epidemiologic data with respect to EBV-related complications in patients with IBD are not robust. Nonetheless, existing data suggest that adult patients with IBD who are being treated with either thiopurine monotherapy or concomitant dual immunotherapy with anti-TNF agents experience an increased risk of developing NHL in the range of approximately 1/1600 patient-years (108). HLH is far less common, but can be far more serious and is also related to primary EBV infection. Acute infection with EBV (acute infectious mononucleosis) typically results in fever, swollen lymph nodes, pharyngitis, and splenomegaly. Patients with acute mononucleosis who subsequently develop cytopenia, hyperferritinemia, hypertrigly-ceridemia, and hypofibrinogenemia should be referred immediately for hospitalization and urgent evaluation for HLH in consultation with hematology and infectious disease colleagues (124). Present management strategies for EBV-HLH include plasmapheresis, corticosteroids, etopside, or calcineurin inhibitors (124).
Careful consideration of the management of immunosuppressive therapy for IBD in a patient with acute EBV infection is recommended. Once the diagnosis of acute infectious mononucleosis has been established, one strategy is to hold immunosuppressive therapy and follow for cytopenia and hyperferritinemia at least weekly, depending upon the clinical course of the patient. Physicians may choose to check antibodies to viral capsid antigen (VCA) and the EBV nuclear antigen (EBNA). Immunoglobulin M (IgM) antibody to VCA reflects acute infection and typically disappears within 4 to 6 weeks. In contrast, IgG antibody to VCA develops in the first few weeks and persists for life. IgG antibody to EBNA develops within 2 to 4 months of acute infection and also persists for life. Ideally, immunosuppression should be held until IgG VCA/EBNA is positive and the IgM to VCA is undetectable. Alternatively, if the patient is clinically recovered from infectious mononucleosis and begins to develop IBD-related symptoms, one can check EBV by polymerase chain reaction. If the copy number is low, it is reasonable to resume therapy for IBD.
Parenteral and Enteral Nutrition in the Management of Children With IBD
Parenteral nutrition is indicated for the management of children with CD or UC with malnutrition (Table 6) who have not tolerated an adequate trial of enteral nutrition or in selected cases for patients with CD with severe fistulizing disease to provide bowel rest.
Table 6.
Nutritional and growth assessment
| Status | Definition |
|---|---|
| Nutritional status at risk | Weight percentile changed lower by 1 isobar or Weight stable (no gain) or 1%–9% loss (involuntary) Weight <10th percentile for age (Adjust for prednisone treatment) |
| Nutritional failure | Weight percentile changed lower by 2 isobars or Weight loss 310% Weight <3rd percentile for age (Adjust for prednisone treatment) |
| Nutritional status satisfactory | Not at risk or failure |
| Growth status at risk | Height percentile changed lower by 1 isobar or Height velocity <10th percentile or Height percentile <10th percentile |
| Growth failure | Height percentile changed lower by 2 isobars or Height velocity <3rd percentile or Height percentile <3rd percentile |
| Growth satisfactory | Not at risk or failure |
Exclusive enteral nutrition can be used as primary therapy in children with mild to moderate CD for induction of remission and has been shown to display comparable efficacy compared with children treated with corticosteroids (125). Children are typically treated with a polymeric formula such as Ensure, Pediasure, or Boost, with a goal of providing them with at least 120% of the RDA for protein and energy (Table 7). The use of higher energy (1.5 calories/mL formula) may be better tolerated because it will reduce the daily goal volume that a patient must consume. Children displaying significant malnutrition at the start of therapy should be monitored for refeeding (including daily or every other measurement of serum chemistries, calcium, magnesium, and phosphorus) after the initiation of feeds.
Table 7.
Energy requirements for pediatric patients
| Age | Average energy needs each day |
|---|---|
| 0–5 mo | 650 |
| 5–12 mo | 850 |
| 1–3 y | 1300 |
| 4–6 y | 1800 |
| 7–10 y | 2000 |
| Boys | |
| 11–14 y | 2500 |
| 15–18 y | 3000 |
| Girls | |
| 11–14 y | 2200 |
| 15–18 y | 2200 |
Adapted from Recommended Dietary Allowances, 10th ed. Washington, DC: National Academy Press; 1989.
Induction in response to primary nutritional therapy typically takes about 6 weeks, during which children are permitted their enteral formula, supplemented with clear liquids. Some children may find it difficult to take in sufficient quantities of enteral formula by mouth. In these cases, formula can be delivered via continuous overnight nasogastric tube feeds. Mild bloating and morning diarrhea are relatively common. A maintenance medication is required to maintain remission once the volume of the enteral feeds is reduced after the first 6 weeks and a regular diet is resumed. Supplemental enteral nutrition is indicated in all of the children with CD or UC with malnutrition or linear growth failure.
SUMMARY
IBDs, including CD and UC, are increasingly common in children and adolescents. Recent basic and translational research efforts have greatly increased our understanding of the genetics of these disorders, as well as better ways to diagnose and follow clinical disease activity. There remains a clear need for subspecialty input in the management of these children. Nonetheless, primary care providers are ideally positioned to provide expedient IBD care, especially when specialty referral is limited by either availability or geography. As such, efforts directed at the development of a collaborative treatment model will be cost-effective and likely best suited to optimize the care of these children and their families.
Acknowledgments
P.A.R. receives research support from TechLab. L.A.D. receives grant funding from NIH and CCFA. E.S. receives grant funding from NIMH/NIH, payment for lectures as a CME speaker for Scripps, and gave a lecture funded by Merck. P.S. attended advisory board meetings for Merck/Janssen, received payment for lectures from Janssen and AstraZeneca, and travel/CME expenses paid by Merck/Janssen. G.T.W. receives payment for lectures from Centocor, and UCB, and his institution receives grant funding from Centocor, UCB, and Abbott. W.A.F. receives consultancy fees from Genentech, Shire, and Centocor, and grant funding from NIH, and the Leona Helmsley Foundation.
Footnotes
F.A.S., Y.L., and L.M.S. report no conflicts of interest.
References
- 1.Deleted in proof.
- 2.Deleted in proof.
- 3.Armitage E, Drummond H, Ghosh S, et al. Incidence of juvenile-onset Crohn’s disease in Scotland. Lancet. 1999;353:1496–7. doi: 10.1016/S0140-6736(99)00333-5. [DOI] [PubMed] [Google Scholar]
- 4.Turunen P, Kolho KL, Auvinen A, et al. Incidence of inflammatory bowel disease in Finnish children 1987–2003. Inflamm Bowel Dis. 2006;12:677–83. doi: 10.1097/00054725-200608000-00002. [DOI] [PubMed] [Google Scholar]
- 5.Kugathasan S, Judd RH, Hoffmann RG, et al. Epidemiologic and clinical characteristics of children with newly diagnosed inflammatory bowel disease in Wiscons: a statewide population-based study. J Pediatr. 2003;143:525–31. doi: 10.1067/s0022-3476(03)00444-x. [DOI] [PubMed] [Google Scholar]
- 6.Grieci T, Butter A. The incidence of inflammatory bowel disease in the pediatric population of Southwestern Ontario. J Pediatr Surg. 2009;44:977–80. doi: 10.1016/j.jpedsurg.2009.01.038. [DOI] [PubMed] [Google Scholar]
- 7.Bernstein CN, Blanchard JF, Rawsthorne P, et al. Epidemiology of Crohn’s disease and ulcerative colitis in a central Canadian province: a population-based study. Am J Epidemiol. 1999;149:916–24. doi: 10.1093/oxfordjournals.aje.a009735. [DOI] [PubMed] [Google Scholar]
- 8.Mamula P, Markowitz JE, Baldassano RN. Inflammatory bowel disease in early childhood and adolescence: special considerations. Gastroenterol Clin North Am. 2003;32:967–95. doi: 10.1016/s0889-8553(03)00046-3. [DOI] [PubMed] [Google Scholar]
- 9.Heuschkel R, Salvestrini C, Beattie RM, et al. Guidelines for the management of growth failure in childhood inflammatory bowel disease. Inflamm Bowel Dis. 2008;14:839–49. doi: 10.1002/ibd.20378. [DOI] [PubMed] [Google Scholar]
- 10.Kim SC, Ferry GD. Inflammatory bowel diseases in pediatric and adolescent patients: clinical, therapeutic, and psychosocial considerations. Gastroenterology. 2004;126:1550–60. doi: 10.1053/j.gastro.2004.03.022. [DOI] [PubMed] [Google Scholar]
- 11.Palm O, Moum B, Jahnsen J, et al. The prevalence and incidence of peripheral arthritis in patients with inflammatory bowel disease, a prospective population-based study (the IBSEN study) Rheumatology (Oxford) 2001;40:1256–61. doi: 10.1093/rheumatology/40.11.1256. [DOI] [PubMed] [Google Scholar]
- 12.Barclay AR, Keightley JM, Horrocks I, et al. Cerebral thromboembolic events in pediatric patients with inflammatory bowel disease. Inflamm Bowel Dis. 2010;16:677–83. doi: 10.1002/ibd.21113. [DOI] [PubMed] [Google Scholar]
- 13.Standridge S, de los Reyes E. Inflammatory bowel disease and cerebrovascular arterial and venous thromboembolic events in 4 pediatric patients: a case series and review of the literature. J Child Neurol. 2008;23:59–66. doi: 10.1177/0883073807308706. [DOI] [PubMed] [Google Scholar]
- 14.Rahhal RM, Pashankar DS, Bishop WP. Ulcerative colitis complicated by ischemic colitis and Budd Chiari syndrome. J Pediatr Gastroenterol Nutr. 2005;40:94–7. doi: 10.1097/00005176-200501000-00018. [DOI] [PubMed] [Google Scholar]
- 15.Karwowski CA, Keljo D, Szigethy E. Strategies to improve quality of life in adolescents with inflammatory bowel disease. Inflamm Bowel Dis. 2009;15:1755–64. doi: 10.1002/ibd.20919. [DOI] [PubMed] [Google Scholar]
- 16.Turner D, Leach ST, Mack D, et al. Faecal calprotectin, lactoferrin, M2-pyruvate kinase and S100A12 in severe ulcerative colitis: a prospective multicentre comparison of predicting outcomes and monitoring response. Gut. 2010;59:1207–12. doi: 10.1136/gut.2010.211755. [DOI] [PubMed] [Google Scholar]
- 17.Gisbert JP, Bermejo F, Perez-Calle JL, et al. Fecal calprotectin and lactoferrin for the prediction of inflammatory bowel disease relapse. Inflamm Bowel Dis. 2009;15:1190–8. doi: 10.1002/ibd.20933. [DOI] [PubMed] [Google Scholar]
- 18.Joishy M, Davies I, Ahmed M, et al. Fecal calprotectin and lactoferrin as noninvasive markers of pediatric inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 2009;48:48–54. doi: 10.1097/MPG.0b013e31816533d3. [DOI] [PubMed] [Google Scholar]
- 19.Sipponen T, Karkkainen P, Savilahti E, et al. Correlation of faecal calprotectin and lactoferrin with an endoscopic score for Crohn’s disease and histological findings. Aliment Pharmacol Ther. 2008;28:1221–9. doi: 10.1111/j.1365-2036.2008.03835.x. [DOI] [PubMed] [Google Scholar]
- 20.Schoepfer AM, Trummler M, Seeholzer P, et al. Accuracy of four fecal assays in the diagnosis of colitis. Dis Colon Rectum. 2007;50:1697–706. doi: 10.1007/s10350-007-0303-9. [DOI] [PubMed] [Google Scholar]
- 21.Lee SS, Kim AY, Yang SK, et al. Crohn disease of the small bowel: comparison of CT enterography, MR enterography, and small-bowel follow-through as diagnostic techniques. Radiology. 2009;251:751–61. doi: 10.1148/radiol.2513081184. [DOI] [PubMed] [Google Scholar]
- 22.Siddiki HA, Fidler JL, Fletcher JG, et al. Prospective comparison of state-of-the-art MR enterography and CT enterography in small-bowel Crohn’s disease. AJR Am J Roentgenol. 2009;193:113–21. doi: 10.2214/AJR.08.2027. [DOI] [PubMed] [Google Scholar]
- 23.Essary B, Kim J, Anupindi S, et al. Pelvic MRI in children with Crohn disease and suspected perianal involvement. Pediatr Radiol. 2007;37:201–8. doi: 10.1007/s00247-006-0372-2. [DOI] [PubMed] [Google Scholar]
- 24.Darbari A, Sena L, Argani P, et al. Gadolinium-enhanced magnetic resonance imaging: a useful radiological tool in diagnosing pediatric IBD. Inflamm Bowel Dis. 2004;10:67–72. doi: 10.1097/00054725-200403000-00001. [DOI] [PubMed] [Google Scholar]
- 25.Alison M, Kheniche A, Azoulay R, et al. Ultrasonography of Crohn disease in children. Pediatr Radiol. 2007;37:1071–82. doi: 10.1007/s00247-007-0559-1. [DOI] [PubMed] [Google Scholar]
- 26.Potthast S, Rieber A, Von Tirpitz C, et al. Ultrasound and magnetic resonance imaging in Crohn’s disease: a comparison. Eur Radiol. 2002;12:1416–22. doi: 10.1007/s00330-001-1191-3. [DOI] [PubMed] [Google Scholar]
- 27.Brenner DJ, Hall EJ. Computed tomography–an increasing source of radiation exposure. N Engl J Med. 2007;357:2277–84. doi: 10.1056/NEJMra072149. [DOI] [PubMed] [Google Scholar]
- 28.Berrington de Gonzalez A, Mahesh M, Kim KP, et al. Projected cancer risks from computed tomographic scans performed in the United States in 2007. Arch Intern Med. 2009;169:2071–7. doi: 10.1001/archinternmed.2009.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gaca AM, Jaffe TA, Delaney S, et al. Radiation doses from small-bowel follow-through and abdomen/pelvis MDCT in pediatric Crohn disease. Pediatr Radiol. 2008;38:285–91. doi: 10.1007/s00247-007-0702-z. [DOI] [PubMed] [Google Scholar]
- 30.Caprilli R, Gassull MA, Escher JC, et al. European evidence based consensus on the diagnosis and management of Crohn’s disease: special situations. Gut. 2006;55 (suppl 1):i36–58. doi: 10.1136/gut.2005.081950c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Escher JC, Taminiau JA, Nieuwenhuis EE, et al. Treatment of inflammatory bowel disease in childhood: best available evidence. Inflamm Bowel Dis. 2003;9:34–58. doi: 10.1097/00054725-200301000-00006. [DOI] [PubMed] [Google Scholar]
- 32.Travis SP, Stange EF, Lemann M, et al. European evidence based consensus on the diagnosis and management of Crohn’s disease: current management. Gut. 2006;55 (suppl 1):i16–35. doi: 10.1136/gut.2005.081950b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kugathasan S, Nebel J, Skelton JA, et al. Body mass index in children with newly diagnosed inflammatory bowel disease: observations from two multicenter North American inception cohorts. J Pediatr. 2007;151:523–7. doi: 10.1016/j.jpeds.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 34.Schwartz DA, Loftus EV, Jr, Tremaine WJ, et al. The natural history of fistulizing Crohn’s disease in Olmsted County, Minnesota. Gastroenterology. 2002;122:875–80. doi: 10.1053/gast.2002.32362. [DOI] [PubMed] [Google Scholar]
- 35.Ceyhan BB, Karakurt S, Cevik H, et al. Bronchial hyperreactivity and allergic status in inflammatory bowel disease. Respiration. 2003;70:60–6. doi: 10.1159/000068407. [DOI] [PubMed] [Google Scholar]
- 36.Mansi A, Cucchiara S, Greco L, et al. Bronchial hyperresponsiveness in children and adolescents with Crohn’s disease. Am J Respir Crit Care Med. 2000;161:1051–4. doi: 10.1164/ajrccm.161.3.9906013. [DOI] [PubMed] [Google Scholar]
- 37.Raj AA, Birring SS, Green R, et al. Prevalence of inflammatory bowel disease in patients with airways disease. Respir Med. 2008;102:780–5. doi: 10.1016/j.rmed.2007.08.014. [DOI] [PubMed] [Google Scholar]
- 38.Bernstein CN, Wajda A, Blanchard JF. The clustering of other chronic inflammatory diseases in inflammatory bowel disease: a population-based study. Gastroenterology. 2005;129:827–36. doi: 10.1053/j.gastro.2005.06.021. [DOI] [PubMed] [Google Scholar]
- 39.Maddox JS, Soltani K. Risk of nonmelanoma skin cancer with azathioprine use. Inflamm Bowel Dis. 2008;14:1425–31. doi: 10.1002/ibd.20444. [DOI] [PubMed] [Google Scholar]
- 40.Bongartz T, Sutton AJ, Sweeting MJ, et al. Anti-TNF antibody therapy in rheumatoid arthritis and the risk of serious infections and malignancies: systematic review and meta-analysis of rare harmful effects in randomized controlled trials. JAMA. 2006;295:2275–85. doi: 10.1001/jama.295.19.2275. [DOI] [PubMed] [Google Scholar]
- 41.Mintz R, Feller ER, Bahr RL, et al. Ocular manifestations of inflammatory bowel disease. Inflamm Bowel Dis. 2004;10:135–9. doi: 10.1097/00054725-200403000-00012. [DOI] [PubMed] [Google Scholar]
- 42.de Jong DJ, Tielen J, Habraken CM, et al. 5-Aminosalicylates and effects on renal function in patients with Crohn’s disease. Inflamm Bowel Dis. 2005;11:972–6. doi: 10.1097/01.mib.0000185402.65288.19. [DOI] [PubMed] [Google Scholar]
- 43.Yakut M, Ustun Y, Kabacam G, et al. Serum vitamin B(12) and folate status in patients with inflammatory bowel diseases. Eur J Intern Med. 2010;21:320–3. doi: 10.1016/j.ejim.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 44.Nishi Y, Lifshitz F, Bayne MA, et al. Zinc status and its relation to growth retardation in children with chronic inflammatory bowel disease. Am J Clin Nutr. 1980;33:2613–21. doi: 10.1093/ajcn/33.12.2613. [DOI] [PubMed] [Google Scholar]
- 45.Dubner SE, Shults J, Baldassano RN, et al. Longitudinal assessment of bone density and structure in an incident cohort of children with Crohn’s disease. Gastroenterology. 2009;136:123–30. doi: 10.1053/j.gastro.2008.09.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sylvester FA, Wyzga N, Hyams JS, et al. Natural history of bone metabolism and bone mineral density in children with inflammatory bowel disease. Inflamm Bowel Dis. 2007;13:42–50. doi: 10.1002/ibd.20006. [DOI] [PubMed] [Google Scholar]
- 47.Zemel BS, Leonard MB, Kelly A, et al. Height adjustment in assessing dual energy x-ray absorptiometry measurements of bone mass and density in children. J Clin Endocrinol Metab. 2010;95:1265–73. doi: 10.1210/jc.2009-2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lewiecki EM, Gordon CM, Baim S, et al. International Society for Clinical Densitometry 2007 Adult and Pediatric Official Positions. Bone. 2008;43:1115–21. doi: 10.1016/j.bone.2008.08.106. [DOI] [PubMed] [Google Scholar]
- 49.Holick MF. Vitamin D: evolutionary, physiological and health perspectives. Curr Drug Targets. 2011;12:4–18. doi: 10.2174/138945011793591635. [DOI] [PubMed] [Google Scholar]
- 50.Misra M, Pacaud D, Petryk A, et al. Vitamin D deficiency in children and its management: review of current knowledge and recommendations. Pediatrics. 2008;122:398–417. doi: 10.1542/peds.2007-1894. [DOI] [PubMed] [Google Scholar]
- 51.Szigethy E, McLafferty L, Goyal A. Inflammatory bowel disease. Child Adolesc Psychiatr Clin N Am. 2010;19:301–18. doi: 10.1016/j.chc.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 52.Szigethy E, Levy-Warren A, Whitton S, et al. Depressive symptoms and inflammatory bowel disease in children and adolescents: a cross-sectional study. J Pediatr Gastroenterol Nutr. 2004;39:395–403. doi: 10.1097/00005176-200410000-00017. [DOI] [PubMed] [Google Scholar]
- 53.Mackner LM, Crandall WV. Long-term psychosocial outcomes reported by children and adolescents with inflammatory bowel disease. Am J Gastroenterol. 2005;100:1386–92. doi: 10.1111/j.1572-0241.2005.41428.x. [DOI] [PubMed] [Google Scholar]
- 54.Nicholas DB, Otley A, Smith C, et al. Challenges and strategies of children and adolescents with inflammatory bowel disease: a qualitative examination. Health Qual Life Outcomes. 2007;5:28. doi: 10.1186/1477-7525-5-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Otley AR, Griffiths AM, Hale S, et al. Health-related quality of life in the first year after a diagnosis of pediatric inflammatory bowel disease. Inflamm Bowel Dis. 2006;12:684–91. doi: 10.1097/00054725-200608000-00003. [DOI] [PubMed] [Google Scholar]
- 56.Perrin JM, Kuhlthau K, Chughtai A, et al. Measuring quality of life in pediatric patients with inflammatory bowel disease: psychometric and clinical characteristics. J Pediatr Gastroenterol Nutr. 2008;46:164–71. doi: 10.1097/MPG.0b013e31812f7f4e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Karwowski CA, Keljo D, Szigethy E. Strategies to improve quality of life in adolescents with inflammatory bowel disease. Inflamm Bowel Dis. 2009;15:1755–64. doi: 10.1002/ibd.20919. [DOI] [PubMed] [Google Scholar]
- 58.Sitarenios GM, Kovacs M. Use of the Children’s Depression Inventory. In: Maruish ME, editor. Psychological Testing for Treatment Planning and Outcomes Assessment. 2. Mahwah, NJ: Lawrence Erlbaum Associates; 1999. pp. 267–98. [Google Scholar]
- 59.Garcia-Vega E, Fernandez-Rodriguez C. A stress management programme for Crohn’s disease. Behav Res Ther. 2004;42:367–83. doi: 10.1016/S0005-7967(03)00146-3. [DOI] [PubMed] [Google Scholar]
- 60.Milne B, Joachim G, Niedhardt J. A stress management programme for inflammatory bowel disease patients. J Adv Nurs. 1986;11:561–7. doi: 10.1111/j.1365-2648.1986.tb01288.x. [DOI] [PubMed] [Google Scholar]
- 61.Mussell M, Bocker U, Nagel N, et al. Reducing psychological distress in patients with inflammatory bowel disease by cognitive-behavioural treatment: exploratory study of effectiveness. Scand J Gastroenterol. 2003;38:755–62. doi: 10.1080/00365520310003110. [DOI] [PubMed] [Google Scholar]
- 62.Wahed M, Corser M, Goodhand JR, et al. Does psychological counseling alter the natural history of inflammatory bowel disease? Inflamm Bowel Dis. 2010;16:664–9. doi: 10.1002/ibd.21098. [DOI] [PubMed] [Google Scholar]
- 63.Kane S, Altschuler EL, Kast RE. Crohn’s disease remission on bupropion. Gastroenterology. 2003;125:1290. doi: 10.1016/j.gastro.2003.02.004. [DOI] [PubMed] [Google Scholar]
- 64.Kast RE, Altschuler EL. Bone density loss in Crohn’s disease: role of TNF and potential for prevention by bupropion. Gut. 2004;53:1056. [PMC free article] [PubMed] [Google Scholar]
- 65.Mikocka-Walus AA, Turnbull DA, Moulding NT, et al. Controversies surrounding the comorbidity of depression and anxiety in inflammatory bowel disease patients: a literature review. Inflamm Bowel Dis. 2007;13:225–34. doi: 10.1002/ibd.20062. [DOI] [PubMed] [Google Scholar]
- 66.Szigethy E, Kenney E, Carpenter J, et al. Cognitive-behavioral therapy for adolescents with inflammatory bowel disease and subsyndromal depression. J Am Acad Child Adolesc Psychiatry. 2007;46:1290–8. doi: 10.1097/chi.0b013e3180f6341f. [DOI] [PubMed] [Google Scholar]
- 67.Mawdsley JE, Jenkins DG, Macey MG, et al. The effect of hypnosis on systemic and rectal mucosal measures of inflammation in ulcerative colitis. Am J Gastroenterol. 2008;103:1460–9. doi: 10.1111/j.1572-0241.2008.01845.x. [DOI] [PubMed] [Google Scholar]
- 68.Bers MU, Gonzalez-Heydrich J, Demaso DR. Use of a computer-based application in a pediatric hemodialysis unit: a pilot study. J Am Acad Child Adolesc Psychiatry. 2003;42:493–6. doi: 10.1097/01.CHI.0000046810.95464.68. [DOI] [PubMed] [Google Scholar]
- 69.Demaso DR, Marcus NE, Kinnamon C, et al. Depression experience journal: a computer-based intervention for families facing childhood depression. J Am Acad Child Adolesc Psychiatry. 2006;45:158–65. doi: 10.1097/01.chi.0000190353.98570.fe. [DOI] [PubMed] [Google Scholar]
- 70.Shepanski MA, Hurd LB, Culton K, et al. Health-related quality of life improves in children and adolescents with inflammatory bowel disease after attending a camp sponsored by the Crohn’s and Colitis Foundation of America. Inflamm Bowel Dis. 2005;11:164–70. doi: 10.1097/00054725-200502000-00010. [DOI] [PubMed] [Google Scholar]
- 71.Szigethy E, Hardy D, Craig AE, et al. Girls connect: effects of a support group for teenage girls with inflammatory bowel disease and their mothers. Inflamm Bowel Dis. 2009;15:1127–8. doi: 10.1002/ibd.20775. [DOI] [PubMed] [Google Scholar]
- 72.Sands BE, Cuffari C, Katz J, et al. Guidelines for immunizations in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2004;10:677–92. doi: 10.1097/00054725-200409000-00028. [DOI] [PubMed] [Google Scholar]
- 73.Melmed GY, Ippoliti AF, Papadakis KA, et al. Patients with inflammatory bowel disease are at risk for vaccine-preventable illnesses. Am J Gastroenterol. 2006;101:1834–40. doi: 10.1111/j.1572-0241.2006.00646.x. [DOI] [PubMed] [Google Scholar]
- 74.Shale MJ, Seow CH, Coffin CS, et al. Review article: chronic viral infection in the anti-tumour necrosis factor therapy era in inflammatory bowel disease. Aliment Pharmacol Ther. 2010;31:20–34. doi: 10.1111/j.1365-2036.2009.04112.x. [DOI] [PubMed] [Google Scholar]
- 75.Pickering L, Baker C, Kimberlin D, et al., editors. Red Book: 2009 Report of the Committee on Infectious Diseases. 28. Elk Grove Village, IL: American Academy of Pediatrics; 2009. Varicella-zoster infections. [Google Scholar]
- 76.Olsen KO, Joelsson M, Laurberg S, et al. Fertility after ileal pouch-anal anastomosis in women with ulcerative colitis. Br J Surg. 1999;86:493–5. doi: 10.1046/j.1365-2168.1999.01076.x. [DOI] [PubMed] [Google Scholar]
- 77.Olsen KO, Juul S, Bulow S, et al. Female fecundity before and after operation for familial adenomatous polyposis. Br J Surg. 2003;90:227–31. doi: 10.1002/bjs.4082. [DOI] [PubMed] [Google Scholar]
- 78.Cornish JA, Tan E, Teare J, et al. The effect of restorative proctoco-lectomy on sexual function, urinary function, fertility, pregnancy and delivery: a systematic review. Dis Colon Rectum. 2007;50:1128–38. doi: 10.1007/s10350-007-0240-7. [DOI] [PubMed] [Google Scholar]
- 79.Khosla R, Willoughby CP, Jewell DP. Crohn’s disease and pregnancy. Gut. 1984;25:52–6. doi: 10.1136/gut.25.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Orholm M, Fonager K, Sorensen HT. Risk of ulcerative colitis and Crohn’s disease among offspring of patients with chronic inflammatory bowel disease. Am J Gastroenterol. 1999;94:3236–8. doi: 10.1111/j.1572-0241.1999.01526.x. [DOI] [PubMed] [Google Scholar]
- 81.Peeters M, Geypens B, Claus D, et al. Clustering of increased small intestinal permeability in families with Crohn’s disease. Gastroenterology. 1997;113:802–7. doi: 10.1016/s0016-5085(97)70174-4. [DOI] [PubMed] [Google Scholar]
- 82.National Cancer Institute. Surveillance Epidemiology and End Results, in SEER. National Cancer Institute; http://seer.cancer.gov/csr/1975_2007/browse_csr.php. [Google Scholar]
- 83.Ekbom A. Risk of cancer in ulcerative colitis. J Gastrointest Surg. 1998;2:312–3. doi: 10.1016/s1091-255x(98)80067-x. [DOI] [PubMed] [Google Scholar]
- 84.Ekbom A, Helmick C, Zack M, et al. Ulcerative colitis and colorectal cancer. A population-based study. N Engl J Med. 1990;323:1228–33. doi: 10.1056/NEJM199011013231802. [DOI] [PubMed] [Google Scholar]
- 85.Fireman Z, Grossman A, Lilos P, et al. Intestinal cancer in patients with Crohn’s disease. A population study in central Israel. Scand J Gastroenterol. 1989;24:346–50. doi: 10.3109/00365528909093058. [DOI] [PubMed] [Google Scholar]
- 86.Gillen CD, Andrews HA, Prior P, et al. Crohn’s disease and colorectal cancer. Gut. 1994;35:651–5. doi: 10.1136/gut.35.5.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Jess T, Gamborg M, Matzen P, et al. Increased risk of intestinal cancer in Crohn’s disease: a meta-analysis of population-based cohort studies. Am J Gastroenterol. 2005;100:2724–9. doi: 10.1111/j.1572-0241.2005.00287.x. [DOI] [PubMed] [Google Scholar]
- 88.Jess T, Loftus EV, Jr, Velayos FS, et al. Risk of intestinal cancer in inflammatory bowel disease: a population-based study from olmsted county, Minnesota. Gastroenterology. 2006;130:1039–46. doi: 10.1053/j.gastro.2005.12.037. [DOI] [PubMed] [Google Scholar]
- 89.Rubenstein JH, Waljee AK, Jeter JM, et al. Cost effectiveness of ulcerative colitis surveillance in the setting of 5-aminosalicylates. Am J Gastroenterol. 2009;104:2222–32. doi: 10.1038/ajg.2009.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cohen JD, Bournerias I, Buffard V, et al. Psoriasis induced by tumor necrosis factor-alpha antagonist therapy: a case series. J Rheumatol. 2007;34:380–5. [PubMed] [Google Scholar]
- 91.Passarini B, Infusino SD, Barbieri E, et al. Cutaneous manifestations in inflammatory bowel diseases: eight cases of psoriasis induced by anti-tumor-necrosis-factor antibody therapy. Dermatology. 2007;215:295–300. doi: 10.1159/000107622. [DOI] [PubMed] [Google Scholar]
- 92.Markowitz JF. Therapeutic efficacy and safety of 6-mercaptopurine and azathioprine in patients with Crohn’s disease. Rev Gastroenterol Disord. 2003;3:S23–9. [PubMed] [Google Scholar]
- 93.Salliot C, van der Heijde D. Long-term safety of methotrexate mono-therapy in patients with rheumatoid arthritis: a systematic literature research. Ann Rheum Dis. 2009;68:1100–4. doi: 10.1136/ard.2008.093690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kalb RE, Strober B, Weinstein G, et al. Methotrexate and psoriasis: 2009 National Psoriasis Foundation Consensus Conference. J Am Acad Dermatol. 2009;60:824–37. doi: 10.1016/j.jaad.2008.11.906. [DOI] [PubMed] [Google Scholar]
- 95.Theis VS, Rhodes JM. Review article: minimizing tuberculosis during anti-tumour necrosis factor-alpha treatment of inflammatory bowel disease. Aliment Pharmacol Ther. 2008;27:19–30. doi: 10.1111/j.1365-2036.2007.03553.x. [DOI] [PubMed] [Google Scholar]
- 96.Mow WS, Abreu-Martin MT, Papadakis KA, et al. High incidence of anergy in inflammatory bowel disease patients limits the usefulness of PPD screening before infliximab therapy. Clin Gastroenterol Hepatol. 2004;2:309–13. doi: 10.1016/s1542-3565(04)00060-6. [DOI] [PubMed] [Google Scholar]
- 97.Schoepfer AM, Flogerzi B, Fallegger S, et al. Comparison of inter-feron-gamma release assay versus tuberculin skin test for tuberculosis screening in inflammatory bowel disease. Am J Gastroenterol. 2008;103:2799–806. doi: 10.1111/j.1572-0241.2008.02050.x. [DOI] [PubMed] [Google Scholar]
- 98.American Academy of Pediatrics. [Accessed May 24, 2012];Immunization, 2012 Schedules. http://www.aap.org/immunization/IZSchedule.html.
- 99.Lu Y, Bousvaros A. Varicella vaccination in children with inflammatory bowel disease receiving immunosuppressive therapy. J Pediatr Gastroenterol Nutr. 2010;50:562–5. doi: 10.1097/MPG.0b013e3181bab351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Harpaz R, Ortega-Sanchez IR, Seward JF. Prevention of herpes zoster: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2008;57:1–30. [PubMed] [Google Scholar]
- 101.Pickering L, Baker C, Kimberlin D, et al., editors. Red Book: 2009 Report of the Committee on Infectious Diseases. 28. Elk Grove Village, IL: American Academy of Pediatrics; 2009. Influenza; pp. 400–12. [Google Scholar]
- 102.Askling J, Brandt L, Lapidus A, et al. Risk of haematopoietic cancer in patients with inflammatory bowel disease. Gut. 2005;54:617–22. doi: 10.1136/gut.2004.051771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bernstein CN, Blanchard JF, Kliewer E, et al. Cancer risk in patients with inflammatory bowel disease: a population-based study. Cancer. 2001;91:854–62. doi: 10.1002/1097-0142(20010215)91:4<854::aid-cncr1073>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 104.Jess T, Winther KV, Munkholm P, et al. Intestinal and extra-intestinal cancer in Crohn’s disease: follow-up of a population-based cohort in Co-penhagen County, Denmark. Aliment Pharmacol Ther. 2004;19:287–93. doi: 10.1111/j.1365-2036.2004.01858.x. [DOI] [PubMed] [Google Scholar]
- 105.Loftus EV, Jr, Tremaine WJ, Habermann TM, et al. Risk of lymphoma in inflammatory bowel disease. Am J Gastroenterol. 2000;95:2308–12. doi: 10.1111/j.1572-0241.2000.02316.x. [DOI] [PubMed] [Google Scholar]
- 106.Rosh JR, Gross T, Mamula P, et al. Hepatosplenic T-cell lymphoma in adolescents and young adults with Crohn’s disease: a cautionary tale? Inflamm Bowel Dis. 2007;13:1024–30. doi: 10.1002/ibd.20169. [DOI] [PubMed] [Google Scholar]
- 107.Thayu M, Markowitz JE, Mamula P, et al. Hepatosplenic T-cell lymphoma in an adolescent patient after immunomodulator and biologic therapy for Crohn disease. J Pediatr Gastroenterol Nutr. 2005;40:220–2. doi: 10.1097/00005176-200502000-00026. [DOI] [PubMed] [Google Scholar]
- 108.Siegel CA, Marden SM, Persing SM, et al. Risk of lymphoma associated with combination anti-tumor necrosis factor and immunomodulator therapy for the treatment of Crohn’s disease: a meta-analysis. Clin Gastroenterol Hepatol. 2009;7:874–81. doi: 10.1016/j.cgh.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sweetser S, Schroeder KW, Pardi DS. Pseudomembranous colitis secondary to Klebsiella oxytoca. Am J Gastroenterol. 2009;104:2366–8. doi: 10.1038/ajg.2009.289. [DOI] [PubMed] [Google Scholar]
- 110.Kandiel A, Lashner B. Cytomegalovirus colitis complicating inflammatory bowel disease. Am J Gastroenterol. 2006;101:2857–65. doi: 10.1111/j.1572-0241.2006.00869.x. [DOI] [PubMed] [Google Scholar]
- 111.Issa M, Ananthakrishnan AN, Binion DG. Clostridium difficile and inflammatory bowel disease. Inflamm Bowel Dis. 2008;14:1432–42. doi: 10.1002/ibd.20500. [DOI] [PubMed] [Google Scholar]
- 112.Long MD, Drossman DA. Inflammatory bowel disease, irritable bowel syndrome, or what? A challenge to the functional-organic dichotomy. Am J Gastroenterol. 2010;105:1796–8. doi: 10.1038/ajg.2010.162. [DOI] [PubMed] [Google Scholar]
- 113.Keohane J, O’Mahony C, O’Mahony L, et al. Irritable bowel syndrome-type symptoms in patients with inflammatory bowel disease: a real association or reflection of occult inflammation? Am J Gastroenterol. 2010;105:1788. doi: 10.1038/ajg.2010.156. [DOI] [PubMed] [Google Scholar]
- 114.Turner D, Hyams J, Markowitz J, et al. Appraisal of the pediatric ulcerative colitis activity index (PUCAI) Inflamm Bowel Dis. 2009;15:1218–23. doi: 10.1002/ibd.20867. [DOI] [PubMed] [Google Scholar]
- 115.Turner D, Seow CH, Greenberg GR, et al. A systematic prospective comparison of noninvasive disease activity indices in ulcerative colitis. Clin Gastroenterol Hepatol. 2009;1:1. doi: 10.1016/j.cgh.2009.06.024. [DOI] [PubMed] [Google Scholar]
- 116.Atay O, Mahajan L, Kay M, et al. Risk of capsule endoscope retention in pediatric patients: a large single-center experience and review of the literature. J Pediatr Gastroenterol Nutr. 2009;49:196–201. doi: 10.1097/MPG.0b013e3181926b01. [DOI] [PubMed] [Google Scholar]
- 117.Solem CA, Loftus EV, Jr, Fletcher JG, et al. Small-bowel imaging in Crohn’s disease: a prospective, blinded, 4-way comparison trial. Gastrointest Endosc. 2008;68:255–66. doi: 10.1016/j.gie.2008.02.017. [DOI] [PubMed] [Google Scholar]
- 118.Neye H, Ensberg D, Rauh P, et al. Impact of high-resolution trans-abdominal ultrasound in the diagnosis of complications of Crohn’s disease. Scand J Gastroenterol. 2010;45:690–5. doi: 10.3109/00365521003710190. [DOI] [PubMed] [Google Scholar]
- 119.Ripolles T, Martinez MJ, Paredes JM, et al. Crohn disease: correlation of findings at contrast-enhanced US with severity at endoscopy. Radiology. 2009;253:241–8. doi: 10.1148/radiol.2531082269. [DOI] [PubMed] [Google Scholar]
- 120.Kane S, Dixon L. Adherence rates with infliximab therapy in Crohn’s disease. Aliment Pharmacol Ther. 2006;24:1099–103. doi: 10.1111/j.1365-2036.2006.03092.x. [DOI] [PubMed] [Google Scholar]
- 121.Kane S, Shaya F. Medication non-adherence is associated with increased medical health care costs. Dig Dis Sci. 2008;53:1020–4. doi: 10.1007/s10620-007-9968-0. [DOI] [PubMed] [Google Scholar]
- 122.Kane SV. Systematic review: adherence issues in the treatment of ulcerative colitis. Aliment Pharmacol Ther. 2006;23:577–85. doi: 10.1111/j.1365-2036.2006.02809.x. [DOI] [PubMed] [Google Scholar]
- 123.Kane SV, Accortt NA, Magowan S, et al. Predictors of persistence with 5-aminosalicylic acid therapy for ulcerative colitis. Aliment Pharmacol Ther. 2009;29:855–62. doi: 10.1111/j.1365-2036.2009.03941.x. [DOI] [PubMed] [Google Scholar]
- 124.Imashuku S. Systemic type Epstein-Barr virus-related lymphoproli-ferative diseases in children and young adults: challenges for pediatric hemato-oncologists and infectious disease specialists. Pediatr Hematol Oncol. 2007;24:563–8. doi: 10.1080/08880010701640499. [DOI] [PubMed] [Google Scholar]
- 125.Borrelli O, Cordischi L, Cirulli M, et al. Polymeric diet alone versus corticosteroids in the treatment of active pediatric Crohn’s disease: a randomized controlled open-label trial. ClinfGastroenterol Hepatol. 2006;4:744–53. doi: 10.1016/j.cgh.2006.03.010. [DOI] [PubMed] [Google Scholar]