Abstract
Background
Patients with HIV infection are at increased risk of cardiovascular disease (CVD). Vitamin D insufficiency has been associated with increased CVD risk in non-HIV populations. This study sought to determine the relationship between vitamin D status and markers of CVD and HIV-related factors in HIV-positive patients.
Methods
Patients with HIV infection on antiretroviral therapy and healthy controls were prospectively enrolled. Fasting lipids, glucose, insulin, inflammatory markers (soluble tumour necrosis factor-α receptor I, interleukin-6 and high-sensitivity C-reactive protein) and endothelial markers (soluble intercellular adhesion molecule-1 and soluble vascular cell adhesion molecule-1) were measured. Fasting 25-hydroxyvitamin D (25(OH)D) was measured from stored serum samples. The internal carotid artery and common carotid artery (CCA) intima-media thickness (IMT) were measured in a subset of HIV-positive patients. Baseline cross-sectional data were analysed.
Results
A total of 149 HIV-positive patients (56 with carotid IMT) and 34 controls were included. Controls had higher adjusted mean 25(OH)D levels than HIV-positive patients (P=0.02). In multivariable linear regression among the HIV-positive patients, 25(OH)D was positively associated with CD4+ T-cell restoration after antiretroviral therapy (ΔCD4 = current - nadir CD4+ T-cell; P<0.01), but was not associated with inflammatory or endothelial markers. In multivariable logistic regression, odds of having CCA IMT above the median were more than 10× higher in those with lower 25(OH)D levels (OR=10.62, 95% CI 1.37–82.34; P<0.01).
Conclusions
Vitamin D status in HIV-positive patients was positively associated with improved immune restoration after antiretroviral therapy and negatively associated with CCA IMT. These findings suggest that vitamin D may play a role in HIV-related CVD and in immune reconstitution after antiretroviral therapy.
Introduction
People infected with HIV-1 are at an increased risk of cardiovascular disease (CVD) [1]. The exact aetiology of their increased CVD risk remains incompletely defined and is probably multifactorial, but appears to be partly related to an increased inflammatory state and enhanced endothelial activation. We and others have found that HIV-infected adults have increased levels of proinflammatory cytokines and endothelial activation markers [2–5], which have been associated with CVD in the general population [6–11]. In addition, we demonstrated that the carotid intima-media thickness (IMT), a surrogate marker for atherosclerosis, is associated with levels of proinflammatory cytokines and endothelial activation markers [12]. These observations support a potential role of inflammation and endothelial dysfunction in CVD development in HIV infection.
Vitamin D insufficiency (insufficiency or deficiency often used interchangeably, and defined as serum 25-hydroxyvitamin D [25(OH)D] levels <75 nmol/l [to convert to ng/ml, divide by 2.5]) is associated with the development of CVD in the general population [13]. For example, vitamin D insufficiency is associated with decreased arterial compliance [14] and increased carotid IMT [15]. Strikingly, vitamin D insufficiency is associated with a twofold increased risk of developing an initial cardiovascular event in previously healthy asymptomatic individuals [16]. This association between vitamin D and incident CVD remained significant after adjustment for several confounders associated with CVD including age, sex, systolic blood pressure, diabetes, total to high-density lipoprotein cholesterol ratio, body mass index and tobacco use. Proposed mechanisms of vitamin D’s role in CVD development include its effects on inflammatory cytokines [17], direct effects on the vasculature [18–21] and inhibition of the renin– angiotensin II–aldosterone system [22].
In addition, vitamin D has long been known to play an important role in immune function. Vitamin D receptor expression is found in monocytes, stimulated macrophages, dendritic cells, natural killer cells, T cells and B cells. Additional evidence shows that vitamin D modulates the adaptive immune system as well, through direct effects on T-cell activation and on the phenotype and function of antigen-presenting cells [23–26]. Given vitamin D’s effect on the immune system, it is reasonable to consider a link between HIV-related factors, such as CD4+ T-cell count and inflammation, which then may indirectly also affect an individual’s CVD risk. Few studies have investigated vitamin D status and HIV-related health effects, but a recent study showed that low vitamin D status appears to have a negative effect on HIV disease progression and mortality [27], and another study found a positive relationship between vitamin D dietary intake and CD4+ T-cell counts [28], despite some conflicting data [29].
Vitamin D insufficiency appears to be widespread in HIV-infected adults [30–32], and may contribute to the already increased CVD risk in this population. To date, no study has examined the relationship between 25(OH)D levels and carotid IMT or proinflammatory cytokines and endothelial activation marker levels known to be associated with CVD among HIV-infected individuals. We hypothesized that vitamin D status plays a role in CVD risk in HIV-infected adults by affecting levels of inflammatory markers. Likewise, given vitamin D’s effect on inflammation and the immune system, we also hypothesized that vitamin D status is related to HIV-related factors, such as CD4+ T-cell count.
The primary objectives of this study were to determine the relationship between 25(OH)D and inflammation markers in antiretroviral therapy (ART)-treated, HIV-infected adults; to evaluate the association between 25(OH)D levels and carotid IMT in ART-treated, HIV-infected adults; and to determine whether 25(OH)D levels are correlated with HIV-related factors. A secondary objective was to compare results from the HIV-infected group with a healthy uninfected control group.
Methods
Study design/population
Patients enrolled between 2005 and 2009 in on-going prospective longitudinal cardiovascular and metabolic studies at University Hospitals Case Medical Center/Case Western Reserve University HIV clinic in Cleveland, OH, USA, were considered for inclusion in this current study if they had stored serum samples from their enrolment visit, were ≥18 years of age and had been on a stable ART regimen for at least 24 weeks at the time of enrolment. Exclusion criteria included known CVD, diabetes, current opportunistic infection, and acute or chronic inflammatory condition or medications known to affect inflammation markers. Healthy controls included hospital staff and relatives of HIV-infected patients and staff who self-reported to be free of chronic disease, and were eligible if they had no recent or current infection. Exclusion criteria for controls were the same as for the infected group. All clinical and laboratory assessments for each patient and control, including the 25(OH)D measurement, were collected from a single study visit and blood draw.
The study was reviewed and approved by the University Hospitals Case Medical Center Institutional Review Board. All patients gave written signed consent before participating.
Clinical assessments
Study evaluations for all patients included physical examination, blood pressure, height and weight, waist-to-hip ratio (WHR; with standardized measurements based on procedure recommendations from the Metabolic Study Group of the AIDS Clinical Trials Group), and blood sampling after at least 8 h fasting. Homeostasis model assessment of insulin resistance (HOMA-IR) was calculated using the following formula: HOMA-IR= (fasting insulin (µU/ml) × fasting glucose (mg/dl) / 405 [33]. All patients completed questionnaires in order to obtain their relevant demographic and medical information. In addition, extensive chart review was conducted for HIV-positive patients, including HIV duration, detailed ART history, past and current medical diagnoses, current medications, and nadir CD4+ T-cell count.
Metabolic, inflammatory and cardiovascular tests
Fasting blood was drawn from all patients for real-time measurements of insulin, glucose and lipoprotein profile. We also measured plasma proinflammatory markers, soluble tumour necrosis factor-α receptor I (sTNFR-I), interleukin-6, high-sensitivity C-reactive protein, and endothelial cell adhesion molecules, soluble intercellular adhesion molecule-1, soluble vascular cell adhesion molecule-1 using an enzyme-labelled immunosorbent sandwich assay (Aushon Biosystems, Billerica, MA, USA; and Laboratory for Clinical Biochemistry Research, University of Vermont Pathology, Colchester, VT, USA). Finally, CD4+ T-cell counts and HIV-1 RNA were measured as part of clinical care, and measures closest to the study entry date were used as markers of HIV disease.
Carotid IMT measurements
Carotid ultrasound was performed for carotid IMT measurements on a subset of HIV-infected patients who were also enrolled into an HIV cardiovascular study. All carotid ultrasounds were performed by the same experienced sonographer and read by an experienced radiologist. Both investigators were blinded to patient characteristics. Carotid IMT methods were used as previously described [2]. Briefly, images of the bilateral distal common carotid arteries (CCA) and internal carotid arteries (ICA) were obtained in longitudinal views separately. Images of the near (proximal) and far wall free of plaques (distal) were acquired with a 7–14 MHz AT 1204 linear array transducer (Toshiba American Medical Systems, Tustin, CA, USA) operating at 14 MHz with differential harmonics. Three measurements of the IMT were obtained at the near and far walls of each CCA and ICA. The mean of three measurements at each site (right side and left side) was used as the final measurement of IMT for that site (for both CCA and ICA, the far and near walls had three IMT measurements each, resulting in a total of 12 measurements). The right and left sides were then averaged and reported as a single ICA and CCA measurement. Plaque was defined, measured and graded as a plaque index according to published protocols from the Cardiovascular Health Study [34].
Vitamin D measurements
Stored serum samples were used for 25(OH)D measurements. The serum was stored at −80°C until analysis without prior thawing. All samples were analysed in the same coinvestigator’s laboratory (VT) at Emory University (Atlanta, GA, USA) by experienced personnel (MK). Serum levels of 25(OH)D were assessed using specific ELISA kits (IDS, Ltd, Fountain Hills, AZ, USA). Protocols for each assay were as per the manufacturer’s product manuals. Samples for 25(OH)D were tested in duplicate. Median intra-assay and interassay coefficients of variation were both <12%. Quality control of the 25(OH)D measurements was ensured by participation in the Vitamin D External Quality Assessment Scheme. Laboratory personnel were blinded to all clinical information associated with the serum samples until laboratory analysis.
Statistical methods
Demographics, clinical characteristics and fasting metabolic parameters are described by study group, and HIV-related characteristics are described for HIV-positive patients. Continuous measures are described by medians and IQRs, and nominal variables are described with frequencies and percentages. Transformations were made when necessary to ensure normality assumptions were met. The 25(OH)D levels were tested and adjusted as necessary for season, age, sex and race. A comparison of adjusted 25(OH)D levels between groups was then made by calculating a least-squares mean using general linear models. The level of significance for all comparisons was set at 0.05.
We used Pearson’s χ2 to test for differences in categorical variables and linear models to test for differences in continuous variables. Variables known to influence the association between serum 25(OH)D and markers of cardiovascular function were tested as covariates. These included season, age, race, sex, smoking and body mass index. We also included covariates known to be associated with HIV. These included duration of HIV disease, CD4+ T-cell count and antiretroviral use.
Two multivariable regression models were then constructed to examine the association of serum 25(OH)D with various CVD risk factors and HIV-related variable. First, the association of 25(OH)D with each main outcome of interest was considered in a continuous manner using linear models. Variables were transformed as appropriate to ensure linear assumptions were met. Multivariable linear models were used to examine each main outcome adjusting for other covariates. Linear regression was used to produce least-squared means to examine the adjusted mean 25(OH)D level by HIV status. As more than 20 different associations were examined, a more strict value of P=0.01 was set for significance of the regression models. The model was adjusted for factors known to affect 25(OH)D levels.
After examining variables in a continuous fashion, a second model was created investigating variables of interest in a categorical manner. CCA and ICA IMT levels and the ΔCD4 count (current CD4+ T-cell count at time of evaluation minus nadir CD4+ T-cell count) were dichotomized using the median value for each of these outcomes. Logistic regression was used to examine the association between 25(OH)D as a continuous variable and each dichotomous outcome. The model was adjusted for factors known to affect 25(OH)D levels.
Results
Study population
The demographics, clinical, and laboratory characteristics of the two groups, HIV-positive patients and HIV-negative controls, are shown in Table 1. The HIV-positive group had more males, more smokers, higher triglyceride levels, higher biomarker levels and a difference in the season of their blood draw compared with the controls. A total 71% of the HIV-positive patients had physician-reported and/or patient-reported lipoatrophy. Results were compared for patients with and without lipoatrophy, and were similar (data not shown). Therefore, patients were combined and compared with the HIV-uninfected controls as one group.
Table 1.
Clinical characteristics, fasting metabolic parameters, and biomarker levels by study group
| Characteristic | HIV-positive (n=149) | HIV-negative (n=34) |
|---|---|---|
| Age, years | 49 (44–52) | 38 (31–44) |
| Males, n (%)a | 127 (85%) | 21 (62%) |
| Race, n (%) | ||
| Caucasian | 77 (52%) | 24 (71%) |
| African-American | 55 (37%) | 8 (23%) |
| Other | 17 (11%) | 2 (6%) |
| Season of blood draw, n (%)a | ||
| Spring | 41 (27.5%) | 3 (9.0%) |
| Summer | 41 (27.5%) | 18 (53.0%) |
| Fall | 40 (27.0%) | 8 (23.0%) |
| Winter | 27 (18.0%) | 5 (15.0%) |
| Body mass index | 26 (22–28) | 27 (24–29) |
| Current smokers, %b | 73 (49%) | 6 (18%) |
| Systolic blood pressure, mmHg | 120 (110–129) | 120 (110–130) |
| Diastolic blood pressure, mmHg | 80 (75–85) | 79 (72–82) |
| Total cholesterol, mg/dl | 181 (159–216) | 195 (166–215) |
| LDL cholesterol, mg/dl | 109 (83–138) | 122 (86–137) |
| HDL cholesterol, mg/dl | 38 (33–47) | 51 (43–64) |
| Triglycerides, mg/dla | 156 (104–241) | 83 (53–112) |
| HOMA-IR | 2.28 (1.36–3.64) | 0.92 (0.54–1.76) |
| sTNFR-I, pg/mla | 866 (593–1199) | 1,227 (1,087–1,495) |
| hsCRP, µg/mlb | 3.45 (1.03–8.19) | 0.73 (0.41–1.39) |
| IL-6, pg/mlb | 3.7 (2.0–9.5) | 2.3 (1.4–3.2) |
| sICAM-1, ng/ml | 334 (242–491) | 230 (204–266) |
| sVCAM-1, ng/mlb | 690 (442–1198) | 685 (581–808) |
| HIV duration, months | 149 (94–195) | – |
| CD4+ T-cell count, cells/mm3 | 572 (423–745) | – |
| Nadir CD4+ T-cell count, cells/mm3 | 169 (68–271) | – |
| HIV-1 RNA in patients with >50 copies/ml | 108 (73–309) | – |
| HIV-1 RNA <50 copies/ml, n (%) | 122 (82%) | – |
| ΔCD4, cells/mm3 | 389 (220–561) | – |
| NRTI duration, months | 93 (58–124) | – |
| NNRTI duration, months | 20 (0–59) | – |
| PI duration, months | 44 (0–81) | – |
| ICA IMT, mmc | 0.70 (0.55–0.91) | – |
| CCA IMT, mmc | 0.65 (0.55–0.75) | – |
P<0.05.
P<0.001.
n=56.
Data presented as median (IQR) unless otherwise specified. CCA, common carotid artery; ΔCD4, current CD4+ T-cell count at time of evaluation minus nadir CD4+ T-cell count; HDL, high-density lipoprotein; HOMA-IR, homeostasis model assessment of insulin resistance; hsCRP, high-sensitivity C-reactive protein; ICA, internal carotid artery; IL-6, interleukin-6; IMT, intima-media thickness; LDL, low-density lipoprotein; NNRTI, non-nucleoside reverse transcriptase inhibitor; NRTI, nucleoside reverse transcriptase inhibitor; PI, protease inhibitor; sICAM-1, soluble intercellular adhesion molecule-1; sTNFR-I, soluble tumour necrosis factor-α receptor-I; sVCAM-1, soluble vascular cell adhesion molecule-1.
Vitamin D status in HIV-positive individuals and controls
There was a statistically significant difference in adjusted 25(OH)D between the two groups, with healthy controls having a higher level (P=0.02). The adjusted mean (sd) for the controls was 64.6 (1.08) versus 52.1 (1.04) nmol/l for the HIV-positive group. The percentage of patients with adjusted 25(OH)D levels >25 nmol/l, >50 nmol/l and >75 nmol/l for the HIV-positive group were 95%, 54% and 21%, respectively, compared with 100%, 71% and 32%, respectively, for the controls (P=0.16, P=0.06 and P=0.35, respectively).
Correlation analysis
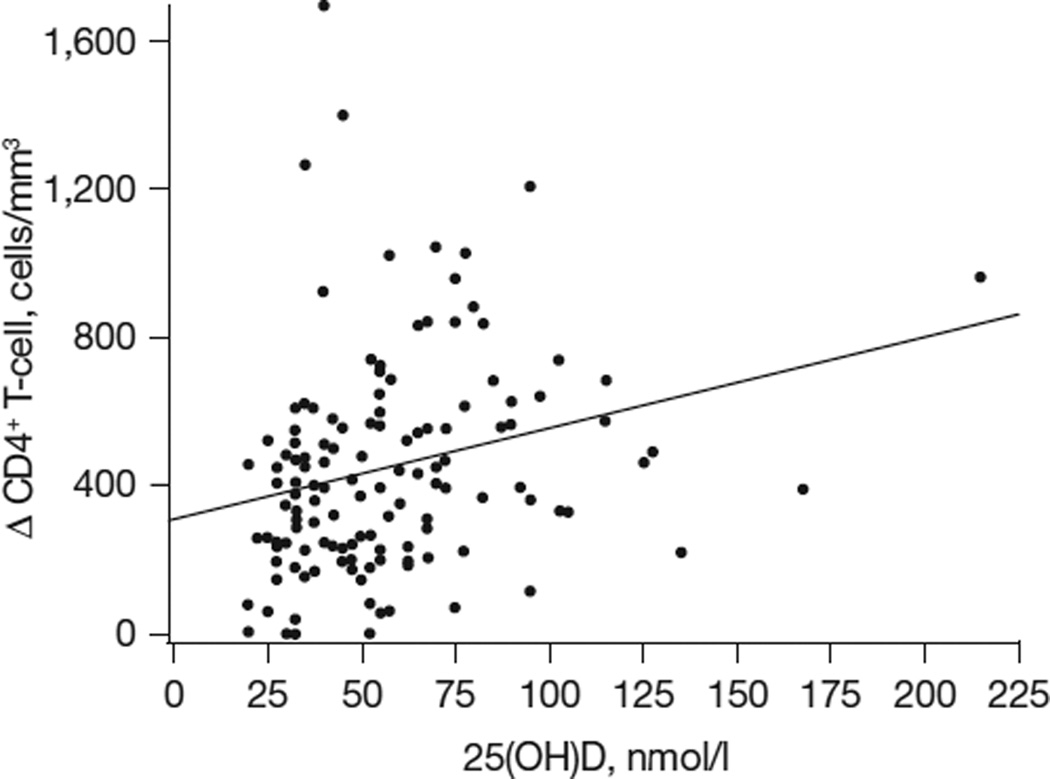
In univariable analysis, 25(OH)D was positively correlated with season (ρ=0.22; P<0.001) for all patients. In the HIV-positive group, 25(OH)D was positively correlated with sTNFR-I (ρ=0.20; P=0.02) and ΔCD4 cell count (ρ=0.20; P=0.04; Figure 1), and negatively correlated with CCA IMT (ρ=-0.30; P<0.01). Variables tested in the HIV-positive group that were not significant included duration of HIV infection, HIV-1 RNA level, current CD4+ T-cell cell count, nadir CD4+ T-cell cell count, cumulative months of ART use, as well as cumulative months of antiretroviral use by class (non-nucleoside reverse transcriptase inhibitors, nucleoside reverse transcriptase inhibitors, and protease inhibitors). Variables tested in both groups that were not significant included HOMA-IR, low-density lipoprotein cholesterol and smoking. None of the biomarkers were significant in either group except as described above.
Figure 1. Correlation between mean 25-hydroxyvitamin D levels and ΔCD4.
In univariate analysis, vitamin D levels were significantly correlated with the degree of immune reconstitution after starting antiretroviral therapy (ρ=0.20; P=0.04). To convert to ng/ml, divide by 2.5. 25(OH)D, 25-hydroxyvitamin D; ΔCD4, current CD4+ T-cell count at time of evaluation minus nadir CD4+ T-cell count.
Regression analyses
Regression models were stratified to account for the differing data structures between groups. Linear regression with standardized regression coefficients was used to compare the association of 25(OH)D by group with key CVD variables and HIV-related factors (Table 2). In the HIV-positive group, ΔCD4 was positively associated with 25(OH)D. For each increase in standard deviation in 25(OH)D, ΔCD4 rose by 0.23 standard deviations.
Table 2.
Multivariable linear regression analysis describing the association between vitamin D and variables of interest by patient group
| β-values (se) | ||
|---|---|---|
| Variable | HIV-positive, ART-treated patients (n=149) | HIV-negative controls (n=34) |
| ΔCD4 | 0.23 (0.09)a | – |
| sICAM-1 | 0.11 (0.08) | −0.47 (0.19)b |
| sTNFR-I | 0.21 (0.08)b | −0.06 (0.15) |
P<0.01.
P<0.05.
Bold text denotes statistical significance; significance was set at P<0.01. Variables tested as main outcomes in this analysis but were not significant for both groups included homeostasis model assessment of insulin resistance, triglycerides, high-density lipoprotein and non-HDL cholesterol, systolic and diastolic blood pressure, soluble vasz cular cell adhesion molecule-1, and high-sensitivity C-reactive protein. Additional variables included but that were not significant for the HIV-positive group included antiretroviral therapy, protease inhibitor and non-nucleoside reverse transcriptase inhibitor duration, and internal carotid artery/common carotid artery intima-media thickness. β values are standardized regression coefficients to ease in comparing associations across various biomarkers and groups. Appropriate transformations were made to account for data structure. Model controls for age, race, sex, body mass index and season. ART, antiretroviral therapy; sICAM-1, soluble intercellular adhesion molecule-1; sTNFR-I, soluble tumour necrosis factor-α receptor-I; ΔCD4, current CD4+ T-cell count at time of evaluation minus nadir CD4+ T-cell count.
Logistic regression was then used to test dichotomized outcomes for carotid IMT and ΔCD4 (Table 3). Variables were dichotomized using the median value for each outcome. The odds of having a CCA IMT above the median were 10 times higher for each standard deviation decrease in the 25(OH)D level.
Table 3.
Multivariable logistic regression describing the association between vitamin D as a continuous variable and IMT and immune restoration as dichotomized variables in HIV-positive, ART-treated patients
| Variable | OR | 95% CI | P value |
|---|---|---|---|
| CCA IMT (n=56) | 10.62 | 1.37–82.34 | 0.02 |
| ICA IMT (n=56) | 1.26 | 0.25–6.41 | 0.77 |
| ΔCD4 | 0.54 | 0.24–1.24 | 0.15 |
Bold text denotes statistical significance; significance was set at P<0.01. Model controls for age, race, sex, body mass index, and season. Variables were dichotomized using the median value for each of these outcomes. ART, antiretroviral therapy; CCA, common carotid artery; ICA, internal carotid artery; IMT, intima-media thickness; ΔCD4, current CD4+ T-cellcount at time of evaluation minus nadir CD4+ T-cell count.
Discussion
HIV-positive people are living decades longer than before due to combination ART; however, complications are emerging in this population at rates higher than the general population, including osteoporosis, non-AIDS-defining malignancies and CVD [1,35–37]. Notably, many of the emerging complications related to chronic HIV infection represent disease processes where vitamin D is known to play an important role. This study sought to investigate the relationship between 25(OH)D levels and carotid IMT, cardiac biomarkers and HIV-related factors in HIV-infected, ART-treated patients, in order to determine whether vitamin D status in this population is associated with CVD risk or other HIV-related factors.
The main objective of this study was to evaluate the relationship between 25(OH)D levels and inflammation, cardiovascular markers and HIV-related factors. Strikingly, we show for the first time in HIV a significant association between vitamin D status and CCA IMT in a group of HIV-positive patients on stable antiretroviral treatment. In this group, patients with CCA IMT levels above the median were >10 times more likely to have the lowest levels of 25(OH)D (that is, higher IMT values=higher CVD risk). These data suggest that a high 25(OH)D level may be protective against CVD development in HIV-positive people. Studies in the general population support this finding because 25(OH)D insufficiency is associated with poorer measures of CVD surrogate markers that translate to increased rates of myocardial infarction and stroke [14–16,38]. It is unclear why there was a relationship with CCA IMT and not ICA IMT, but studies that examined only CCA and vitamin D from other conditions showed similar findings for the CCA [39,40]. Likewise, IMT was not significant in the regression analysis when considered as a continuous variable. This may be due to sample size limitation, but deserves further exploration in future studies. In this study, sTNFR-I was associated with 25(OH)D levels in the HIV-positive group in univariate analysis but did not remain significant in multivariable regression analysis. Therefore, further studies are needed to determine whether the association between vitamin D status and carotid IMT may be due to its known effect on inflammation [41,42] or due to a direct effect on vascular endothelium. Studies that have investigated whether vitamin D supplementation affects inflammatory cytokines in other populations have been conflicting [43,44].
Vitamin D has long been known to have an important role in immune function, both innate and acquired [23,25]; however, there are sparse data on the effect of vitamin D on immune function in HIV infection, and studies have focused primarily on 1,25-dihydroxyvitamin D and/or in severely immunosuppressed populations [45,46]. For the first time, we showed that 25(OH)D levels were associated with changes in CD4+ T-cell (current minus nadir CD4+ T-cell count) after starting ART. These results suggest that vitamin D status may play a role in immune function and/or immune restoration after a person is started on ART. The clinical implication of this finding warrants further investigation to see whether vitamin D supplementation given at the same time as initiation of ART would offer a safe and effective means of augmenting the immune restoration response to treatment. In addition, vitamin D optimization could also have implications for improving CD4+ T-cell count in people already virologically suppressed on therapy.
Lastly, when mean 25(OH)D concentrations in the HIV-positive groups were compared with the healthy control group, levels were statistically lower in the HIV-positive group. However, when different concentrations were used to define sufficiency, there was no difference between groups for each cutoff point. Our findings are consistent with other studies that have shown HIV-positive adults have a high prevalence of insufficiency [30–32]; however, studies conflict when evaluating how 25(OH)D levels in HIV-positive people compare with levels in healthy controls [45–47]. Some of the variability among studies can be accounted for by the racial categorization of participants and/or a lack of matching between groups. Even after adjusting 25(OH) levels for season, race, age, and sex, we found a difference between the HIV-positive and control groups. Not unexpectedly, there were significantly more smokers in the HIV-positive group and higher biomarkers levels in the HIV-positive group, which may account for some of the difference seen in our study; however, none of the biomarkers were significant in the multivariable regression analyses. Likewise, smoking was not significant in the univariate analysis and there was no difference between 25(OH)D levels between smokers and nonsmokers (data not shown). Alternatively, it may suggest that HIV infection or antiretroviral therapy influences vitamin D levels, which has been suggested in other studies [48,49]. However, in this study, ART, protease inhibitor and non-nucleoside reverse transcriptase inhibitor duration, as well as HIV duration, were not significant in the multivariable regression analysis. The important point of these findings, however, is that the HIV-positive group in this study had a high prevalence of vitamin D insufficiency, regardless of how they compared with the controls. In a population that is already at an increased risk of CVD, the findings of this study suggest that vitamin D insufficiency is a modifiable risk that deserves further attention.
The main limitation to this study is the relatively small sample size, mainly among the controls. However, the main objective of this study was to evaluate HIV-related variables, particularly CVD markers in the HIV-positive population. Ultimately, the inclusion of controls was a secondary objective to offer some comparison of vitamin D status with the HIV-positive patients. Another limitation is that only a subset of patients underwent a carotid ultrasound; however, patients with and without carotid IMT measurements were compared and there were no clinically relevant differences between these two groups (data not shown). Also, it is not clear from our data whether vitamin D plays a direct role in affecting carotid IMT and immune reconstitution, as this cross-sectional study cannot prove causality. It is possible that there are unmeasured HIV confounders, such as HIV progression; however, HIV duration was tested in the logistic regression and CD4+ T-cell count was tested in the univariable analysis, and neither were significant. Likewise, HIV-positive patients who are starting ART may become more active and spend more time outside, thereby increasing their vitamin D levels. These confounders need to be evaluated more completely by evaluating changes in physical activity and sun exposure upon starting ART. Finally, we are assuming that the 25(OH) D level for each patient was similar at the time of their CD4+ T-cell count nadir, since we do not have an actual measurement from this time. However, we feel that this is a reasonable assumption given that the majority of these patients had been longstanding patients at the same clinic and had not changed geographical locations. Nevertheless, this study was primarily explorative, as no studies have previously evaluated vitamin D, CVD and HIV-related factors in this population, and provides a substrate for larger prospective longitudinal studies to confirm and further define these relationships.
To our knowledge, this is the first study to investigate the relationship of vitamin D to CVD risk in the HIV-infected population. Our results show that vitamin D is associated with immune restoration, as well as with carotid IMT, which supports the fact that vitamin D may play a role in both HIV-related CVD and immune reconstitution. These results are novel and not only may offer insight into the pathogenesis of CVD in this population, but may offer a potential safe mechanism for decelerating its development. Likewise, these results suggest that using vitamin D supplementation with ART, especially at the time of ART initiation, may provide an additional way to enhance CD4+ T-cell counts. A randomized placebo-controlled interventional trial is crucial to determine what effect vitamin D may have on surrogate markers of CVD, as well as on immune function and reconstitution, and to determine what vitamin D level is optimal in HIV-positive patients.
Acknowledgements
The study was partly supported by a grant from Bristol-Myers Squibb to GAM, by the Clinical Core of the Case Center for AIDS Research (NIH grant AI36219), by NIH grant K23 AR054334 to VT and by NIH grant T32 DK007298 to MK. The funding agencies have no access to the raw data and no role in the analysis or writing of this manuscript.
GAM serves as a consultant and has received research funding from Bristol-Myers Squibb, GlaxoSmithKline, Gilead, Merck, and Abbott. GAM currently chairs a DSMB for a Pfizer-funded study. ACR has received research funding from Bristol-Myers Squibb, Cubist Pharmaceuticals, and GlaxoSmithKline. CH has received research funding from Bristol-Myers Squibb. VT has received research funding from Genzyme.
Footnotes
Disclosure statement
All other authors declare no competing interests.
References
- 1.Triant VA, Lee H, Hadigan C, Grinspoon SK. Increased acute myocardial infarction rates and cardiovascular risk factors among patients with human immunodeficiency virus disease. J Clin Endocrinol Metab. 2007;92:2506–2512. doi: 10.1210/jc.2006-2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McComsey GA, O’Riordan M, Hazen SL, et al. Increased carotid intima media thickness and cardiac biomarkers in HIV infected children. AIDS. 2007;21:921–927. doi: 10.1097/QAD.0b013e328133f29c. [DOI] [PubMed] [Google Scholar]
- 3.Ross AC, Armentrout R, O’Riordan MA, et al. Endothelial activation markers are linked to HIV status and are independent of antiretroviral therapy and lipoatrophy. J Acquir Immune Defic Syndr. 2008;49:499–506. doi: 10.1097/QAI.0b013e318189a794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Torriani FJ, Komarow L, Parker RA, et al. Endothelial function in human immunodeficiency virus-infected antiretroviral-naive subjects before and after starting potent antiretroviral therapy: the ACTG (AIDS Clinical Trials Group) Study 5152s. J Am Coll Cardiol. 2008;52:569–576. doi: 10.1016/j.jacc.2008.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kuller LH, Tracy R, Belloso W, et al. Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS Med. 2008;5:e203. doi: 10.1371/journal.pmed.0050203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ridker PM, Rifai N, Pfeffer M, Sacks F, Lepage S, Braunwald E. Elevation of tumor necrosis factor-alpha and increased risk of recurrent coronary events after myocardial infarction. Circulation. 2000;101:2149–2153. doi: 10.1161/01.cir.101.18.2149. [DOI] [PubMed] [Google Scholar]
- 7.Pai JK, Pischon T, Ma J, et al. Inflammatory markers and the risk of coronary heart disease in men and women. N Engl J Med. 2004;351:2599–2610. doi: 10.1056/NEJMoa040967. [DOI] [PubMed] [Google Scholar]
- 8.Ridker PM, Rifai N, Rose L, Buring JE, Cook NR. Comparison of C-reactive protein and low-density lipoprotein cholesterol levels in the prediction of first cardiovascular events. N Engl J Med. 2002;347:1557–1565. doi: 10.1056/NEJMoa021993. [DOI] [PubMed] [Google Scholar]
- 9.Hwang SJ, Ballantyne CM, Sharrett AR, et al. Circulating adhesion molecules VCAM-1, ICAM-1, and E-selectin in carotid atherosclerosis and incident coronary heart disease cases: the Atherosclerosis Risk In Communities (ARIC) study. Circulation. 1997;96:4219–4225. doi: 10.1161/01.cir.96.12.4219. [DOI] [PubMed] [Google Scholar]
- 10.Chung NA, Lydakis C, Belgore F, Li-Saw-Hee FL, Blann AD, Lip GY. Angiogenesis, thrombogenesis, endothelial dysfunction and angiographic severity of coronary artery disease. Heart. 2003;89:1411–1415. doi: 10.1136/heart.89.12.1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davies MJ, Gordon JL, Gearing AJ, et al. The expression of the adhesion molecules ICAM-1, VCAM-1, PECAM, and E-selectin in human atherosclerosis. J Pathol. 1993;171:223–229. doi: 10.1002/path.1711710311. [DOI] [PubMed] [Google Scholar]
- 12.Ross AC, Rizk N, O’Riordan MA, et al. Relationship between inflammatory markers, endothelial activation markers, and carotid intima-media thickness in HIV-infected patients receiving antiretroviral therapy. Clin Infect Dis. 2009;49:1119–1127. doi: 10.1086/605578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Judd SE, Tangpricha V. Vitamin D deficiency and risk for cardiovascular disease. Am J Med Sci. 2009;338:40–44. doi: 10.1097/MAJ.0b013e3181aaee91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.London GM, Guerin AP, Verbeke FH, et al. Mineral metabolism and arterial functions in end-stage renal disease: potential role of 25-hydroxyvitamin D deficiency. J Am Soc Nephrol. 2007;18:613–620. doi: 10.1681/ASN.2006060573. [DOI] [PubMed] [Google Scholar]
- 15.Targher G, Bertolini L, Padovani R, et al. Serum 25-hydroxyvitamin D3 concentrations and carotid artery intima-media thickness among type 2 diabetic patients. Clin Endocrinol (Oxf) 2006;65:593–597. doi: 10.1111/j.1365-2265.2006.02633.x. [DOI] [PubMed] [Google Scholar]
- 16.Wang TJ, Pencina MJ, Booth SL, et al. Vitamin D deficiency and risk of cardiovascular disease. Circulation. 2008;117:503–511. doi: 10.1161/CIRCULATIONAHA.107.706127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schleithoff SS, Zittermann A, Tenderich G, Berthold HK, Stehle P, Koerfer R. Vitamin D supplementation improves cytokine profiles in patients with congestive heart failure: a double-blind, randomized, placebo-controlled trial. Am J Clin Nutr. 2006;83:754–759. doi: 10.1093/ajcn/83.4.754. [see comment]. [DOI] [PubMed] [Google Scholar]
- 18.Rahman A, Hershey S, Ahmed S, Nibbelink K, Simpson RU. Heart extracellular matrix gene expression profile in the vitamin D receptor knockout mice. J Steroid Biochem Mol Biol. 2007;103:416–419. doi: 10.1016/j.jsbmb.2006.12.081. [DOI] [PubMed] [Google Scholar]
- 19.Tishkoff DX, Nibbelink KA, Holmberg KH, Dandu L, Simpson RU. Functional vitamin D receptor (VDR) in the t-tubules of cardiac myocytes: VDR knockout cardiomyocyte contractility. Endocrinology. 2008;149:558–564. doi: 10.1210/en.2007-0805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Simpson RU, Hershey SH, Nibbelink KA. Characterization of heart size and blood pressure in the vitamin D receptor knockout mouse. J Steroid Biochem Mol Biol. 2007;103:521–524. doi: 10.1016/j.jsbmb.2006.12.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.London GM, Guerin AP, Verbeke FH, et al. Mineral metabolism and arterial functions in end-stage renal disease: potential role of 25-hydroxyvitamin D deficiency. J Am Soc Nephrol. 2007;18:613–620. doi: 10.1681/ASN.2006060573. [DOI] [PubMed] [Google Scholar]
- 22.Li YC, Qiao G, Uskokovic M, Xiang W, Zheng W, Kong J. Vitamin D: a negative endocrine regulator of the renin–angiotensin system and blood pressure. J Steroid Biochem Mol Biol. 2004:89–90. 387–392. doi: 10.1016/j.jsbmb.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 23.Kamen DL, Tangpricha V. Vitamin D and molecular actions on the immune system: modulation of innate and autoimmunity. J Mol Med. 2010;88:441–450. doi: 10.1007/s00109-010-0590-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khazai N, Judd SE, Tangpricha V. Calcium and vitamin D: skeletal and extraskeletal health. Curr Rheumatol Rep. 2008;10:110–117. doi: 10.1007/s11926-008-0020-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van Etten E, Stoffels K, Gysemans C, Mathieu C, Overbergh L. Regulation of vitamin D homeostasis: implications for the immune system. Nutr Rev. 2008;66(Suppl 2):S125–S134. doi: 10.1111/j.1753-4887.2008.00096.x. [DOI] [PubMed] [Google Scholar]
- 26.von Essen MR, Kongsbak M, Schjerling P, Olgaard K, Odum N, Geisler C. Vitamin D controls T cell antigen receptor signaling and activation of human T cells. Nat Immunol. 2010;11:344–349. doi: 10.1038/ni.1851. [DOI] [PubMed] [Google Scholar]
- 27.Mehta S, Giovannucci E, Mugusi FM, et al. Vitamin D status of HIV-infected women and its association with HIV disease progression, anemia, and mortality. PLoS ONE. 2010;5:e8770. doi: 10.1371/journal.pone.0008770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.de Luis DA, Bachiller P, Aller R, et al. Nutr Hosp. Vol. 17. Spanish: 2002. Relation among micronutrient intakes with CD4 count in HIV infected patients; pp. 285–289. [PubMed] [Google Scholar]
- 29.Arpadi SM, McMahon D, Abrams EJ, et al. Effect of bimonthly supplementation with oral cholecalciferol on serum 25-hydroxyvitamin D concentrations in HIV-infected children and adolescents. Pediatrics. 2009;123:e121–e126. doi: 10.1542/peds.2008-0176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mueller NJ, Fux CA, Ledergerber B, et al. High prevalence of severe vitamin D deficiency in combined antiretroviral therapy-naive and successfully treated Swiss HIV patients. AIDS. 2010;24:1127–1134. doi: 10.1097/QAD.0b013e328337b161. [DOI] [PubMed] [Google Scholar]
- 31.Rodríguez M, Daniels B, Gunawardene S, Robbins GK. High frequency of vitamin D deficiency in ambulatory HIV-positive patients. AIDS Res Hum Retroviruses. 2009;25:9–14. doi: 10.1089/aid.2008.0183. [DOI] [PubMed] [Google Scholar]
- 32.Van Den Bout-Van Den Beukel CJ, Fievez L, Michels M, et al. Vitamin D deficiency among HIV type 1-infected individuals in the Netherlands: effects of antiretroviral therapy. AIDS Res Hum Retroviruses. 2008;24:1375–1382. doi: 10.1089/aid.2008.0058. [DOI] [PubMed] [Google Scholar]
- 33.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 34.O’Leary DH, Polak JF, Wolfson SK, Jr, et al. Use of sonography to evaluate carotid atherosclerosis in the elderly. The Cardiovascular Health Study. CHS Collaborative Research Group. Stroke. 1991;22:1155–1163. doi: 10.1161/01.str.22.9.1155. [DOI] [PubMed] [Google Scholar]
- 35.Martínez E, Milinkovic A, Buira E, et al. Incidence and causes of death in HIV-infected persons receiving highly active antiretroviral therapy compared with estimates for the general population of similar age and from the same geographical area. HIV Med. 2007;8:251–258. doi: 10.1111/j.1468-1293.2007.00468.x. [DOI] [PubMed] [Google Scholar]
- 36.Obel N, Thomsen HF, Kronborg G, et al. Ischemic heart disease in HIV-infected and HIV-uninfected individuals: a population-based cohort study. Clin Infect Dis. 2007;44:1625–1631. doi: 10.1086/518285. [DOI] [PubMed] [Google Scholar]
- 37.Brown TT, Qaqish RB. Antiretroviral therapy and the prevalence of osteopenia and osteoporosis: a meta-analytic review. AIDS. 2006;20:2165–2174. doi: 10.1097/QAD.0b013e32801022eb. [see comment]. [DOI] [PubMed] [Google Scholar]
- 38.Giovannucci E. Vitamin D status and cancer incidence and mortality. Adv Exp Med Biol. 2008;624:31–42. doi: 10.1007/978-0-387-77574-6_3. [DOI] [PubMed] [Google Scholar]
- 39.Targher G, Bertolini L, Padovani R, et al. Serum 25-hydroxyvitamin D3 concentrations and carotid artery intima-media thickness among type 2 diabetic patients. Clin Endocrinol (Oxf) 2006;65:593–597. doi: 10.1111/j.1365-2265.2006.02633.x. [DOI] [PubMed] [Google Scholar]
- 40.Kraśniak A, Drozdz M, Pasowicz M, et al. Factors involved in vascular calcification and atherosclerosis in maintenance haemodialysis patients. Nephrol Dial Transplant. 2007;22:515–521. doi: 10.1093/ndt/gfl564. [DOI] [PubMed] [Google Scholar]
- 41.Schleithoff SS, Zittermann A, Tenderich G, Berthold HK, Stehle P, Koerfer R. Vitamin D supplementation improves cytokine profiles in patients with congestive heart failure: a double-blind, randomized, placebo-controlled trial. Am J Clin Nutr. 2006;83:754–759. doi: 10.1093/ajcn/83.4.754. [DOI] [PubMed] [Google Scholar]
- 42.Shea MK, Booth SL, Massaro JM, et al. Vitamin K and vitamin D status: associations with inflammatory markers in the Framingham Offspring Study. Am J Epidemiol. 2008;167:313–320. doi: 10.1093/aje/kwm306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jorde R, Sneve M, Torjesen PA, Figenschau Y, Gøransson LG, Omdal R. No effect of supplementation with cholecalciferol on cytokines and markers of inflammation in overweight and obese subjects. Cytokine. 2010;50:175–180. doi: 10.1016/j.cyto.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 44.Stubbs JR, Idiculla A, Slusser J, Menard R, Quarles LD. Cholecalciferol supplementation alters calcitriol-responsive monocyte proteins and decreases inflammatory cytokines in ESRD. J Am Soc Nephrol. 2010;21:353–361. doi: 10.1681/ASN.2009040451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jaeger P, Otto S, Speck RF, et al. Altered parathyroid gland function in severely immunocompromised patients infected with human immunodeficiency virus. J Clin Endocrinol Metab. 1994;79:1701–1705. doi: 10.1210/jcem.79.6.7989478. [DOI] [PubMed] [Google Scholar]
- 46.Haug C, Muller F, Aukrust P, Froland SS. Subnormal serum concentration of 1,25-vitamin D in human immunodeficiency virus infection: correlation with degree of immune deficiency and survival. J Infect Dis. 1994;169:889–893. doi: 10.1093/infdis/169.4.889. [DOI] [PubMed] [Google Scholar]
- 47.Teichmann J, Stephan E, Lange U, et al. Osteopenia in HIV-infected women prior to highly active antiretroviral therapy. J Infect. 2003;46:221–227. doi: 10.1053/jinf.2002.1109. [DOI] [PubMed] [Google Scholar]
- 48.Brown TT, McComsey GA. Association between initiation of antiretroviral therapy with efavirenz and decreases in 25-hydroxyvitamin D. Antivir Ther. 2010;15:425–429. doi: 10.3851/IMP1502. [DOI] [PubMed] [Google Scholar]
- 49.Cozzolino M, Vidal M, Arcidiacono MV, Tebas P, Yarasheski KE, Dusso AS. HIV-protease inhibitors impair vitamin D bioactivation to 1,25-dihydroxyvitamin D. AIDS. 2003;17:513–520. doi: 10.1097/00002030-200303070-00006. [DOI] [PubMed] [Google Scholar]