Abstract
Background
The effects of gestational nucleoside reverse transcriptase inhibitors (NRTIs) on mitochondrial DNA (mtDNA) are controversial. The effects of mtDNA depletion on mitochondrial function have not been assessed.
Method
In peripheral blood mononuclear cells (PBMCs) from infants born to HIV-infected women and infants born to HIV-1–uninfected women, mtDNA copy numbers were determined by quantitative PCR; nuclear (COXIV)- and mitochondrial (COXII)-encoded polypeptides of the oxidative phosphorylation enzyme cytochrome c-oxidase (COX or complex IV) were quantified by Western blot.
Results
Overall, 86 infants born to HIV-infected women and 50 controls were studied. HIV-infected mothers had a median CD4 count of 506 cells/μL; 59% had HIV RNA ≤ 50 copies/mL. No infant had clinical evidence of mitochondrial disease. The birth weight was lower (p = .016) and the body length higher (p = .002) in the HIV-exposed newborns. Eighty-one HIV-infected women had received gestational NRTIs (median duration 162 days). Median mtDNA copies/PBMC in the HIV-exposed infants were 505 (range, 120–1365) vs. 213 (27–426) in controls (p < .001). COX II/IV ratios were similar in both groups. Although mtDNA levels correlated inversely with maternal lactate, mitochondrial indices did not correlate with maternal CD4+ count, HIV RNA, smoking, or alcohol consumption.
Conclusion
We found elevated mtDNA copy numbers in PBMC of infants born to HIV-infected women, the majority of whom received NRTI-based therapy, when compared to those born to healthy HIV-negative controls, but there was no difference in mtDNA-encoded respiratory chain protein. The clinical consequence of these findings is unknown and requires further investigations.
Keywords: mitochondrial DNA, mitochondrial enzymes, mitochondrial toxicity
The majority of children born to HIV-infected women in developed countries are now perinatally treated with either zidovudine (ZDV) alone or an antiretroviral combination of two or more drugs. Even though this antiretroviral (ARV) prophylaxis has shown overwhelming benefits in reducing vertical HIV transmission, there is still uncertainty about its potential of mitochondrial toxicity.1 In 1999, French pediatricians reported that eight children exposed to ZDV and other nucleoside analogue reverse transcriptase inhibitors (NRTIs) in utero had an increased frequency of symptomatic mitochondrial dysfunction.2 A similar potential for mitochondrial toxicity from in utero exposure to ARV therapy has been suggested by primate studies, in which dose-related fetal abnormalities in mitochondrial structure and function have been reported in skeletal muscle, cardiac muscle, and brain.3,4 Although transient hyperlactatemia is common in infants perinatally exposed to ARV therapy,5,6 only rare cases of symptomatic hyperlactatemia have been reported. In these cases of lactic acidosis, the central nervous system was involved frequently and seems to be particularly vulnerable to mitochondrial insults.2,7,8 In the French cohort, 12 uninfected children perinatally exposed to NRTI had evidence of mitochondrial dysfunction.8 Mitochondrial symptoms manifested as motor abnormalities, seizures, and delay in cognitive development and were often associated with abnormal MRI findings. Biochemical abnormalities in the respiratory chain were identified in 11 of the 12 children; quantitative and qualitative mitochondrial DNA (mtDNA) depletion were not identified however.8 Recently, 20 cases of possible mitochondrial toxicity were reported among 1,037 infants born to HIV-infected mothers within the Pediatric AIDS Clinical Trials Group, but mtDNA investigations were lacking.9
Only a few studies have analyzed mtDNA copy numbers in infants perinatally exposed to ARVs and reported conflicting data. Although some of these studies have documented mtDNA depletion in peripheral blood mononuclear cells (PBMCs),10–12 others have shown no change8,13 or even increased mtDNA copy numbers compared to infants not exposed to HIV or NRTI therapy.14,15 A small study has analyzed the mitochondrial ultrastructure by electron microscopy and has identified significant damage of the organelles in six out of nine NRTI-exposed infants compared to none of seven infants born to HIV-negative women.11 Most of these studies have been small and have not included the assessment of mtDNA impairment on mitochondrial function.
To more closely study the effects of perinatal exposure to NRTIs on mtDNA levels and the expression of mtDNA-encoded respiratory chain subunits in PBMCs of infants born to HIV-1–infected mothers, a substudy of the AIDS Clinical Trials Group (ACTG) Study A5084 was designed. A5084 was a prospective, multicenter, observational study that evaluated the safety and tolerance of ARV in HIV-1–infected pregnant women as measured by metabolic parameters. Infants born to HIV-uninfected healthy mothers served as controls. Our hypothesis was that infants born to HIV-infected mothers would have significantly lower mtDNA levels and respiratory chain subunits in their PBMCs when compared to infants born to HIV-uninfected healthy controls. A secondary objective was to explore a possible association between mitochondrial indices and serum lactate.
METHOD
A5084 was conducted at 28 centers in the United States from 2002 to 2005, and 161 women were enrolled. The study was approved by institutional review boards at all centers, and all study participants provided written informed consent. Written informed consent was also obtained from the father of the fetus, if available.
HIV-infected pregnant women at 20–34 weeks of gestation who had been on either a stable ARV therapy or on no ARV therapy for ≥ 8 weeks were eligible to participate. Women with diabetes, serious bacterial infections, other serious or unstable medical conditions, active drug or alcohol abuse, other obstetrical complications, or carrying a fetus with major congenital abnormalities were not eligible. Laboratory studies on the pregnant women included fasting lactate and liver enzymes. Serum lactate was immediately measured from maternal blood samples collected in a chilled tube without the use of a tourniquet from resting patients. Infants were examined clinically within 48 hours of birth and followed for HIV diagnosis according to US Public Health Service recommendations.16 The protocol instructions specified that PBMCs were to be collected from infants within 48 hours of birth and be stored at –70°C for batched analysis at the end of study. Stored infant PBMC samples that contained at least 3 million cells were used for the analysis of mitochondria.
Controls consisted of infants born from HIV-uninfected healthy, adult women who had no medical condition requiring prescription drugs and no clinical illness. Controls were enrolled from the University Hospitals of Cleveland, Ohio. Infants’ mothers and fathers, when available, signed a written informed consent approved by the local institutional review board.
mtDNA Quantification
Total DNA was extracted from the PBMCs with the QIAamp DNA isolation kit (Qiagen, Hilden, Germany). mtDNA and nuclear DNA (nDNA) copy numbers were determined by quantitative PCR using the ABI 7700 sequence detection system (Applied Biosystems, Foster City, California, USA). We amplified the mtDNA ATP-6 gene between nucleotide positions 8981 and 9061 with the forward primer, 5′-ACCAATAGCCCTGGCCGTAC-3′ and the backward primer 5′-GGTGGCGCTTC-CAATTAGGT-3′. mtDNA was quantified with a FAM-fluorophore labeled probe (5′-6FAMCCTAACCGCTAACATTACTGCAGGCCACCTAMRA-3′). For the detection of nDNA, we selected exon number 8 of the GAPDH-gene between nucleotide positions 4280-4342, using the forward primer 5′-CGGGGCTCTCCAGAACATC-3′ and the backward primer 5′-ATGACCTTGCCCACAGCCT-3′. In this case, we used a VIC-fluorophore labeled probe (5′-VIC-CCCTGCCTCTACTGGCGCTGCCTAMRA-3′).
Each 25 μL reaction contained 25 ng of genomic DNA, 100 nM probe, 200 nM primers, and Taq-man Universal Master Mix (Applied Biosystems). Amplifications of mitochondrial and nuclear products were separately performed in optical 96-well plates (Applied Biosystems). An initial incubation at 50°C for 2 minutes was followed by 10 minutes at 95°C and 40 denaturing steps at 95°C (15 seconds), alternating with combined annealing/extension at 60°C (1 minute). All samples were run in triplicate. Absolute mtDNA and nDNA copy numbers were calculated using serial dilutions of plasmids with known copy numbers.17 The mtDNA copy number was unaffected by the DNA yield per PBMC (U.A. Walker, unpublished observations). The standard deviation of the mtDNA assay in triplicate repeats of different samples was 8%.
Quantification of the mtDNA-Encoded COX II Respiratory Chain Subunit Polypeptides
The subunit II of cytochrome c-oxidase (COXII) is encoded by mtDNA, whereas the subunit IV of cytochrome c-oxidase (COXIV) is encoded by nDNA. COXII was quantified by immunoblot from PBMCs and normalized to the signal of a simultaneously used antibody against COXIV.18,19 The PBMCs were homogenized and sonicated in 20 μL lysis buffer (1% Nonidet P-40, 2mM EDTA, 1 μg/mL Pepstatin A) on ice. Cellular protein was collected from the supernatant after centrifugation (10,000 g for 10 minutes at 4°C) and used for immunoblot. The protein (100 μg) was diluted (1:1) in 10 μL of sample buffer (50 mM Tris-HCl [pH 6.8], 12% glycerol, 4% sodium dodecyl sulphate (SDS), Pepstatin A 1 μg/mL, 0.01% bromophenol blue) and boiled for 3 minutes. Proteins were electro-phoresed on a 11% polyacrylamide gel, containing 0.1% SDS and electroblotted onto nitro-cellulose sheets. The nitro-cellulose sheets were blocked with 10% nonfat dry milk and incubated overnight in blocking buffer containing a 1:100 dilution of both anti-human COX II and anti-human COX IV mouse monoclonal antibodies. Washes, incubation with an alkaline phosphatase-conjugated goat secondary anti-mouse antibody (diluted 1:200), and development with NBT/BCIP were performed according to standard procedures. The blots were also probed with an antibody (Research Diagnostics Inc., Flanders, New Jersey, USA) against glycerol aldehyde phosphate dehydrogenase (GAPDH) in order to compare nDNA-encoded mitochondrial (COXIV) and cytoplasmic (GAPDH) cell components. The intensities of the signals were quantified by scanning densitometry, using Scion-image™ (Scion Corporation, Frederick, Maryland, USA).
Statistical Analyses
From A5084, the study included infants who had enough PBMC stored to allow measurements of at least one of the mitochondrial indices. We anticipated having about 90 available infant samples from HIV-infected women of A5084 and about 50 samples from infants of HIV-negative controls. In addressing the primary objective, such sample size would yield a power of 80% to detect a difference of half the standard deviation between the two infant groups.
Comparisons between groups on categorical or dichotomous data were performed by Fisher's exact tests. For the continuous data (including mtDNA and COX II/IV levels), Wilcoxon rank-sum tests were used for comparison between two groups. The stratified analyses were conducted using the Wilcoxon-Mann-Whitney exact tests.20 The mtDNA values were log10 transformed in the graphic display. Correlations were evaluated with Spearman rank tests. All tests were two sided, and a p value less than .05 was used to determine statistical significance of each test. No adjustments were made for multiple testing. A multivariate analysis was also conducted.
RESULTS
Infant and Maternal Characteristics
Overall, 136 participants were included: 86 infants born to HIV-infected women enrolled in A5084 and 50 infants born to HIV-negative healthy women. These 86 infants from A5084 were the only patients on A5084 who had available stored blood samples and were not randomly selected. All available blood samples were used to measure the mitochondrial assays. We compared the baseline characteristics between the 86 mothers/infants from A5084 and those from A5084 who were not included because of the lack of availability of infants’ samples; the two groups were similar in race/ethnicity, maternal protease inhibitor (PI) receipt, HIV-1 RNA detection, CD4 cell count, smoking status during pregnancy, maternal age, gestational age, infant birth weight and length, cumulative durations of ARV, PI, and any NRTI therapies. However, there was a statistically significant difference in the mothers’ alcohol consumption status during pregnancy (21% in the group included vs. 5% in those not included; p = .019), and maternal cumulative duration of d4T therapy (585 in the included vs. 1,152 days, respectively; p = .022), but not in maternal d4T use during pregnancy. The samples were collected within 2 days after birth for the 50 controls and for 81/86 (94%) of the A5084 infants. For the remaining five A5084 infants’ samples, three were collected within 4 days and one each at 10 and 12 days after birth.
The HIV follow-up test results were insufficient for 8 of the 86 infants born to HIV-infected mothers in A5084, and their infection status deemed indeterminate but very unlikely to be infected by the investigators. The remaining 78 infants were all confirmed HIV negative.
Table 1 presents the characteristics of all study participants, and Table 2 details the HIV-related characteristics of the HIV-exposed group. There were more Caucasians (40% vs. 16%) and fewer Hispanics (4% vs. 21%) in the HIV-unexposed group compared to the HIV-exposed group (p = .0027 for racial differences between groups). The African American race representation was comparable in the two groups. There were fewer vaginal births than Cesarean deliveries in the HIV-positive group compared to the control group, but the difference was not statistically significant. The median gestational age was 38.6 weeks and the median mother's age was 26 years for all study participants, without significant differences between the HIV-positive participants and their HIV-negative counterparts. Compared to controls, the birth weight was lower in the HIV-exposed newborns (median 3072 vs. 3319 g) and the body length higher (49 vs. 47 cm; p = .02 and .002, respectively). The mother's body mass index (BMI) at delivery was similar in the HIV-positive and -negative groups. Overall, 41% of the 86 HIV-infected women from A5084 had detectable HIV-1 RNA (>50 copies/mL, equivalent to 1.7 in log10), with a maximum of 4.8 log10 copies. The median (range) CD4+ cell count was 506 (86–1159) cells/μL. Smoking data were available for 77 HIV-positive women, of whom 34% indicated smoking during pregnancy. Alcohol consumption data were available for 73 HIV-infected women, of whom 21% indicated any alcohol consumption during pregnancy.
Table 1.
Infant and maternal characteristics for all study participants
| Characteristics | Total (N = 136) | Maternal HIV status |
p | |
|---|---|---|---|---|
| HIV− (n = 50) | HIV+ (n = 86) | |||
| Race/ethnicity | ||||
| Caucasian | 34 (25%) | 20 (40%) | 14 (16%) | .0027 |
| African American | 74 (54%) | 27 (54%) | 47 (55%) | |
| Hispanic | 20 (15%) | 2 (4%) | 18 (21%) | |
| Asian | 4 (3%) | 1 (2%) | 3 (3%) | |
| American Indian | 3 (2%) | 0 (0%) | 3 (3%) | |
| Other/unknown | 1 (1%) | 0 (0%) | 1 (1%) | |
| Maternal age, years | 26 (16–44) | 26 (18–37) | 28 (16–44) | .30 |
| Delivery mode | ||||
| C-section | 63 (46%) | 19 (38%) | 44 (51%) | .15 |
| Vaginal | 72 (53%) | 31 (62%) | 41 (48%) | |
| Unknown | 1 (1%) | 0 (0%) | 1 (1%) | |
| Gestational age, weeks | 39 (32–42) | 39 (35–41) | 38 (32–42) | .087 |
| Birth weight, g | 3142 (1680–5045) | 3319 (2291–4382) | 3072 (1680–5045) | .016 |
| Birth length, cm | 48 (40–56) | 47 (40–52) | 49 (41–56)a | .002 |
| Maternal BMI at delivery, kg/m2 | 33 (20–50) | 32.5 (20–47) | 33 (21–50)b | .33 |
| Maternal lactate, mmol/L | 1.0 (0.3–7.0)a | |||
Note: Values are stated as number (%) or median (range). BMI = body mass index.
N = 85.
N = 60.
Table 2.
HIV-related infant and maternal characteristics for the HIV-exposed infants (n = 86)
| Characteristics | HIV-exposed infants |
|---|---|
| NRTI exposure during pregnancy | |
| ZDV | 75 (87%) |
| 3TC | 70 (81%) |
| ABC | 17 (20%) |
| d4t | 7 (8%) |
| ddI | 6 (7%) |
| None | 5 (6%) |
| PI exposure during pregnancy | 41 (48%) |
| Maternal CD4 count, cells/μL | 506 (42–1159) |
| Maternal HIV-1 RNA, copies/mL | 1.7 (1.7–4.8) |
| Maternal HIV-1 RNA ≤ 50 copies/mL | 59% |
Note: Values are stated as number (%) or median (range). NRTI = nucleoside reverse transcriptase inhibitor; ZDV = zidovudine; 3TC = lamivudine; ABC = abacavir; d4T = stavudine; ddI = didanosine; PI = protease inhibitor.
Venous lactate was available from 85 of the HIV-infected women. The median maternal serum lactate level was 1.0 (range, 0.3–7.0) mmol/L. Lactate was >2 mmol/L in only four women. None of the women, including those with lactate >2 mmol/L, had any symptoms consistent with mitochondrial toxicity, and none required changes or discontinuation of ARV therapy.
In terms of blood chemistry results of the HIV-exposed infants, the highest grade for alanine aminotransferase (ALT), blood urea nitrogen (BUN), and creatinine was grade 1. For creatine kinase (CK), there were 30 participants who had grade 1, six had grade 2, and two each had grades 3 and 4. The median platelet count was 291/mL (range, 127–508) in the HIV-exposed group. The signs and symptoms of all infants with grade >1 laboratory abnormalities were reviewed, but no infant had further indications of mitochondrial disease.8
During the gestational period, the median duration of NRTI was 162 days, and the median gestational ZDV, d4T, ddI, lamivudine (3TC), and abacavir (ABC) treatment durations were 158, 153, 185, 158, and 166 days, respectively. There were seven women who received d4T during pregnancy and six who received ddI. None of the women were exposed to ddC or concurrently to d4T and ddI. The majority of women received ZDV (75 women) and 3TC (70 women).
The past ARV history was also reviewed from all HIV-infected women; 81 had received NRTI therapy at some point prior to study entry. The median cumulative ARV duration was 180 days, and the median duration of any NRTI exposure was 177 days. There were 77 women who had a history of ZDV use. The median times of cumulative exposure to ZDV, stavudine (d4T), and didanosine (ddI) were 172, 585, and 284 days, respectively. The median duration of cumulative PI treatment was 277 days for the 43 women reporting PI exposure.
The ARV medications given to infants born to HIV-positive mothers at birth consisted of mostly ZDV, which was administered to all except for one who received ddI and 3TC only. Such data were missing for three infants. The majority of infants (n = 71) were placed on ZDV monotherapy. No medications were administered to infants born to HIV-negative mothers.
Mitochondrial Analysis
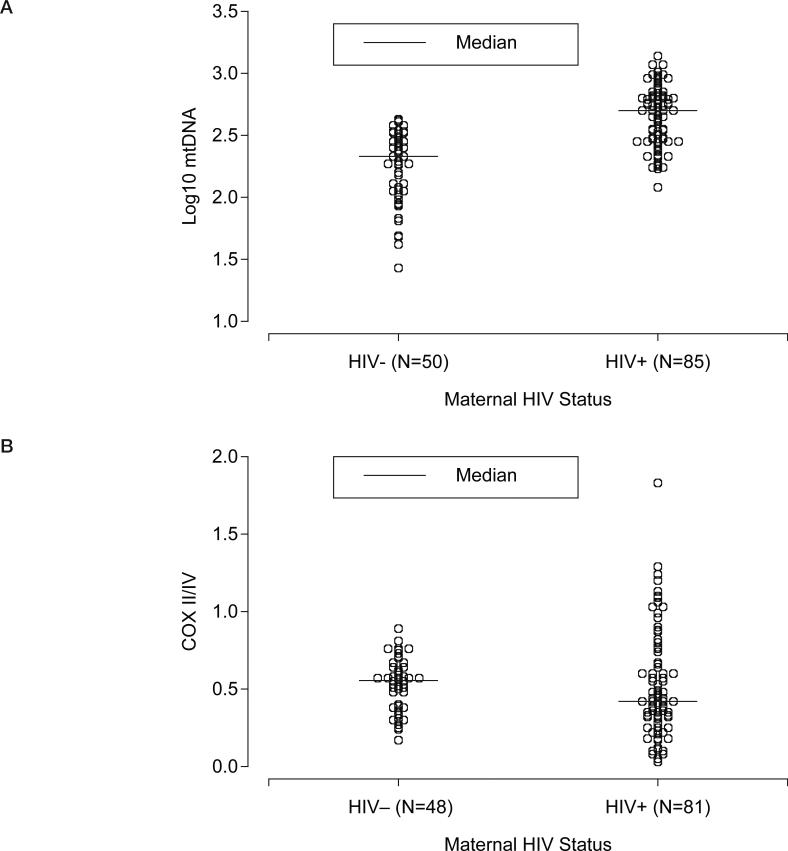
The mtDNA results were available for 135 participants (85 HIV exposed and 50 controls) and COX II/IV ratio results for 129 participants (81 in HIV exposed and 48 controls). The median mtDNA level (Figure 1A) was significantly higher in the infants born to HIV-infected mothers compared to the HIV-negative controls (2.7 log10 = 505 [range, 120–1365] vs. 2.3 log10 = 213 [27–426] copies/cell; p < .001). The COX II/IV levels (Figure 1B) were similar in the two groups (0.4 [0–1.8] vs. 0.6 [0.2–0.9] in the HIV-exposed and controls, respectively; p =.16). The results also remained virtually unchanged when we excluded the two infants in which the PBMCs were collected beyond 4 days of life. The COXIV/GADPH levels were also similar in the two groups (0.068 [0.036–0.097] vs. 0.064 [0.040–0.097] in the HIV-exposed and controls, respectively; p = .43).
Figure 1.
Log10 mitochondrial DNA (mtDNA) and COX II/IV ratios by maternal HIV status.
There were only five HIV-infected mothers who were not receiving NRTI as part of their perinatal treatment. The median (range) mtDNA copies were somewhat higher in the infants born to the women not exposed to NRTI during pregnancy (n = 5) compared to those born to HIV-infected women who had received NRTI (n = 80; 660 [462–928] vs. 499 [120–1365] copies/cell), although this result was not statistically significant (p = .087).
We also considered the effect of in utero exposure to specific NRTIs. Table 3 shows that the mtDNA levels and COXII/IV ratios in those infants who were born to HIV-positive mothers and prenatally exposed to ZDV+3TC, d4T, and either d4T or ddI (d-drugs) did not differ from those infants who did not receive the respective NRTIs. The COX II/IV ratio was higher in the infants born to women exposed to d4T compared to those who were not (1.0 [0.4–1.2] vs. 0.4 [0–1.8]; p = .025), whereas the mtDNA levels were not statistically different (282 [208–907] vs. 499 [120–1,365]; p = .55).
Table 3.
Mitochondrial indices in HIV-exposed infants by in utero ARV exposure
| Exposure in utero to respective ARV in the HIV-exposed infants |
|||||
|---|---|---|---|---|---|
| Yes |
No |
||||
| Type of ARV | n | Median Q1, Q3 | n | Median Q1, Q3 | p |
| mtDNA (copies/PBMCs) | |||||
| d4T | 7 | 282 215, 733 |
73 | 499 298, 647 |
.55 |
| ZDV+3TC | 62 | 532 298, 652 |
18 | 333 240, 647 |
.25 |
| Any d-drug | 12 | 328 248, 685 |
68 | 517 298, 650 |
.51 |
| Any NRTI | 80 | 499 283, 650 |
5 | 660 576, 830 |
.087 |
| PI | 41 | 498 298, 702 |
44 | 517 291, 642 |
.95 |
| COX II/IV ratio | |||||
| d4T | 5 | 1.03 (0.42–1.24) 0.57, 1.13 |
71 | 0.42 (0.03–1.83) 0.25, 0.66 |
.025* |
| ZDV+3TC | 60 | 0.43 (0.05–1.83) 0.30, 0.62 |
16 | 0.50 (0.03–1.24) 0.28, 0.87 |
.60 |
| Any d-drug | 12 | 0.50 (0.10–1.24) 0.32, 1.03 |
68 | 0.43 (0.03–1.83) 0.28, 0.66 |
.46 |
| Any NRTI | 76 | 0.43 (0.03–1.83) 0.30, 0.71 |
5 | 0.42 (0.18–0.76) 0.32, 0.48 |
.69 |
| PI | 39 | 0.44 (0.03–1.83) 0.28, 0.87 |
42 | 0.42 (0.05–1.20) 0.32, 0.64 |
.40 |
Note: ARV = antiretroviral; PBMCs = peripheral blood mononuclear cells; d4T = stavudine; ZDV = zidovudine; 3TC = lamivudine; d-drugs = d4T and/or ddI (didanosine); NRTI = nucleoside analogue reverse transcriptase inhibitors; PI = protease inhibitor.
There was no difference in the infants’ mtDNA copy numbers and COX II/IV ratios by maternal smoking or alcohol consumption in the HIV-infected women (data not shown).
Table 4 outlines the results of the correlation analysis. In the HIV-exposed group, an inverse correlation was found between the infant PBMC mtDNA copy numbers and the maternal lactate levels (Spearman correlation coefficient –0.27, p = .014). The mtDNA copy numbers/PBMC of the infants born to the four HIV-infected women with elevated lactate levels (2.1, 2.5, 3.1, and 7.0 mmol/L) were 964, 352, 636, and 552 copies, respectively. No correlation was found between COX II/IV ratios and lactate levels. There were also no significant correlations between both mitochondrial indices and maternal CD4+ cell count or HIV-RNA levels. In addition, there was no correlation between mtDNA level and platelet count (Spearman correlation coefficient = –0.032; p = .79).
Table 4.
Correlations between mitochondrial markers and clinical indices
| N | Spearman correlation coefficient | p | |
|---|---|---|---|
| HIV-positive mothers | |||
| mtDNA with lactate | 84 | –0.27 | .014 |
| COX II/IV ratio with lactate | 80 | 0.08 | .51 |
| mtDNA with HIV RNA | 34 | 0.06 | .75 |
| COX II/IV ratio with HIV RNA | 34 | –0.05 | .77 |
| mtDNA with CD4 | 84 | 0.02 | .86 |
| COX II/IV ratio with CD4 | 80 | 0.05 | .67 |
| mtDNA with COX II/IV ratio | 80 | 0.04 | .73 |
| mtDNA with gestational age | 85 | –0.15 | .17 |
| HIV-negative mothers | |||
| mtDNA with COX II/IV ratio | 48 | 0.65 | <.0001 |
| mtDNA with gestational age | 50 | 0.0002 | .99 |
Note: mtDNA = mitochondrial DNA.
The mtDNA copy numbers correlated with the COX II/IV ratios in the HIV-unexposed group (Spearman correlation coefficient 0.65, p < .0001) but not in the HIV-exposed group. There were no significant correlations between both mitochondrial markers and gestational age.
The following variables were considered for stratified analyses: race, gestational age at delivery, birth weight, delivery mode, maternal age, birth length, and maternal BMI. Of these, race, gestational age, birth weight, and length were found to be different between the HIV-exposed and unexposed groups, so they were assessed for possible confounding. When stratified by race (whites vs. non-whites), HIV status was again still statistically significant (p < .0001) for mtDNA but not for COX II/IV (p = .30). When stratified by infant weight (<2500 vs. ≥2500 g), HIV status was still statistically significant (p < .0001) for mtDNA but not for COX II/IV (p = .099). The results are similar when stratified by infant length (<48 vs. ≥48 cm; p < .0001 for mtDNA but p = .22 for COX II/IV) or by gestation age (<37 vs. ≥37 weeks; p < .0001 for mtDNA but p = .082 for COX II/IV). Therefore, the HIV/ARV effect was statistically significant for mtDNA but not for COX II/IV, even after accounting for the differences between the HIV-exposed and -unexposed groups in race, infant weight and length, and gestation age.
DISCUSSION
Our study did not identify mtDNA depletion in PBMCs of infants exposed to HIV-infected mothers, most of whom were on NRTI-based therapy, and in contrast found higher mtDNA levels when compared to HIV-unexposed controls. Our study was the first to also systematically examine the expression of mtDNA-encoded respiratory chain subunits in the PBMCs of these infants. In accord with the mtDNA data, we did not identify substantial differences in COXII/IV ratios between the two groups. By measuring COXIV/GAPDH, we were able to show that the lack of difference in COXII/IV between the groups is the result of the absence of change in the mtDNA-encoded COXII and is not due to regulation of COXIV.
The study standard deviation of the log10-transformed mtDNA values was 0.3, hence there was 80% power to detect a mean difference of about 0.15. The observed difference between the two infant group means was 0.50 in log10: 2.7 in the HIV-positive group and 2.2 in the HIV-negative controls (Figure 1A). The study standard deviation of the COX II/IV ratio was also about 0.3, and the observed difference between group means was 0.01 (0.52 and 0.53 in the HIV-positive and HIV-negative groups, respectively; Figure 1B).
In developed countries, it is becoming exceedingly difficult to find HIV-infected pregnant women who are not receiving ARV therapy. This is why the vast majority of our HIV-infected pregnant women were receiving ARV therapy, and thus we were unable to dissect the relative effect of the exposure to HIV versus that of ARVs on the study results.
Given the propensity of NRTIs to induce mtDNA depletion in a variety of human tissues,21–23 our findings are at first glance surprising, although they are consistent with the results of two recent studies.14,15 However, it should be noted that ZDV unlike the dideoxynucleosides did not cause mtDNA depletion in proliferating cells such as lymphocytes in vitro,24 whereas in contrast a reduction of mtDNA copy numbers was induced by the same agent in functionally postmitotic tissues (adipose tissue and skeletal muscle).21–23 These findings are in keeping with the observation that ZDV is only a weak polymerase-gamma inhibitor and that ZDV inhibits mainly thymidine kinase type 2, which specifically in postmitotic tissues is the enzyme responsible for the de novo synthesis of intramitochondrial pyrimidine building blocks required for mtDNA synthesis.25
In vitro data also indicate that the mtDNA content of mononuclear blood cells is heavily influenced by the proliferation status of the cells.26 In lymphocytes, for example, mtDNA levels are upregulated by more than 3-fold upon mitotic stimulation. This mechanism may also operate in HIV-infected patients and thus participate in the higher mtDNA copy numbers observed in infants born to HIV-positive mothers. Another possibility for our finding is that mtDNA levels could be upregulated in an effort to compensate for an organellar damage that is unrelated to the mtDNA replication, transcription, or translation machinery.27–31 Such an insult to the mitochondria may be represented by reactive oxygen species that are known to be generated by ZDV.32 It is also conceivable that mitochondrial toxicity to maternal tissues could induce a compensatory and more efficient mitochondrial biogenesis in fetal tissues.
The assessment of the effects of individual NRTIs on mitochondrial indices should be interpreted with caution. For example, there were only 10 mothers who were not on ZDV. Under a normal distribution, such breakdown would have an 83% power to detect 1 SD difference between the ZDV and non-ZDV regimen, but only a 31% power to detect a difference of half the standard deviation between these groups. With a more uneven breakdown (such as the ddI analysis where only five women were on ddI-based regimens), the power to detect the drug effect is lower.
Our results are in contrast with other studies showing lower mtDNA levels in infants exposed to HIV/ARV.10–12 It is important to mention that several methodological differences exist between each of these studies and ours. Whereas Shiramizu12 used fresh cord blood mononuclear cells to measure mtDNA levels, Poirier10 used frozen cord blood leukocytes for mtDNA quantitation, and Divi11 used frozen cord blood mononuclear cells. Similar to our study, Shiramizu et al. reported mtDNA levels in copies/cell, whereas Poirier and Divi reported results as a ratio of gene copy numbers relative to a nuclear gene. Also, the timing of blood sampling is important and notably different; Shiramizu and Divi used cord blood, but we used infants’ PBMCs drawn within 4 days of life, and Poirier used infants’ blood leukocytes drawn at 1 and 2 years of life. More studies are needed to assess the ideal tissue to sample and the effect of timing of samples on mtDNA levels. The mothers of the HIV-exposed infants in our study were under good virologic control, whereas those included in Divi study had much higher HIV-1 RNA (mean 7.4 log). Other studies did not provide details on maternal HIV-1 RNA. The effect of maternal HIV-1 RNA on infants’ mtDNA levels is unclear.
Other study limitations may also include center-specific variations in the methodology of PBMC isolation and platelet contamination. Such variations may have confounded our results,33 although all PBMCs (including those of the HIV-unexposed control group) were collected in ACTG-certified laboratories and followed similar strict process ing techniques. In addition, the platelet count in the HIV-exposed infants was within the normal range of that of “normal” healthy neonates, which makes it unlikely that the platelet contamination could have confounded the results in our study groups. Also, intraday assays of the COXII/COXIV ratio have a relatively high standard deviation (about 30% of the mean in triplicates) compared to mtDNA measurements (less than 10% of the mean in triplicates [U.A. Walker, unpublished observations]). Another potential limitation is that the timing of PBMC isolation slightly differed between the HIV-exposed group and the HIV-uninfected group. Although the sites were instructed to draw the PBMCs from HIV-exposed infants within the first 48 hours of life, they did not completely adhere to the protocol. To ensure that results were not biased by the infants whose blood was drawn later in life, we repeated the analysis while excluding the two infants in whom blood was drawn after the first 4 days of life; the results were unchanged. To our knowledge, no studies have assessed normal changes in mtDNA levels in the first few days of life, thus we are unable to comment on the potential confounding effect of this slight variation in timing of the blood sampling between the two study groups. Because there were only five participants with samples drawn after the first 48 hours of life, such effect could, at worst, be minimal in our study.
Although the issue of potential mitochondrial toxicity in infants born to HIV-infected mothers is an important one, it should not deter from NRTI-based efforts to decrease vertical transmission.
It is important though for pediatricians who follow these infants to remember that mitochondrial toxicity may not manifest until later in life, at 12–24 months of age, and may lead to nonspecific symptoms that may go misdiagnosed.
Taken together, our data suggest that mtDNA results from PBMCs may not be a clinically useful biomarker in detecting a possible mitochondrial risk in accord with similar data from adults.34 Nonetheless, mtDNA levels were abnormal in HIV-exposed infants.
In summary, we found elevated mtDNA copy numbers in PBMCs of infants born to HIV-infected women, when compared to those born of healthy HIV-negative controls, but there was no difference in mtDNA-encoded respiratory chain protein. Because the vast majority of the pregnant women were receiving ARV therapy, it is unclear to what extent the results were influenced by the exposure to HIV infection itself versus the exposure to the ARV therapy. The clinical consequence of these findings is unknown and requires further investigations.
ACKNOWLEDGMENTS
This work was supported by the AIDS Clinical Trials Group of the National Institute for Allergy and Infectious Diseases (UO1 AI069501, AI38558, and AI068636) and the Case Western Reserve University Center for AIDS Research AI36219.
The following sites and individuals participated in conducting A5084: Stephen Spector MD, and Kim Norris, BSN, University of California San Diego Child and Adolescent HIV Program, San Diego, CA; John Stoneman, BSN, and Teresa Spitz, BSN, Washington University, St. Louis, MO (grant AI25903); Michele Acker, ARNP, and Deb Goldman, ARNP, Children's Hospital Regional Medical Center/University of Washington, Seattle, WA (grant AI27664); Dianne Allen, BSN, and Tamika Watson, BSN, Children's Hospital of Michigan, Ann Arbor, MI; Eleanor Jimenez, MD, and Maria del Pilar Thurin, City Hospital at San Juan, PR; Daniel Gebhardt, BA, and Patricia Walton, BSN, Case Western Reserve University, Cleveland, OH (grant AI25879); Kim Whitely, RN, and Deborah Young, RN, MetroHealth Medical Center, Cleveland, OH (grant RR000080); Katherine Knapp, MD, Jill Utech, MSN, Edwin Thorpe, MD, and Nina Sublette, FNP, St. Jude Children's Research Hospital and Regional Medical Center, Memphis, TN; Deborah McMahon, MD, and Carol Oriss, BSN, University of Pittsburgh, PA; Arlene Bardeguez, MD, and Charmane Calilap-Bernardo, RN, University of Medicine and Dentistry, Newark, NJ (grant AI25883); Douglas Watson, MD, and Kimberly Klipner, BSN, University of Maryland, Baltimore, MD; Maura Laverty, RN, and Janet Forcht, RN, New York University/ New York City Health and Hospital Corporation at Bellevue Hospital, New York, NY (grant RR00096 and AI27665); Vicki Bailey, RN, and Therese Remus, RN, Vanderbilt University, TN; Mindy Katz, MD, and Mary Ann Hennessy, RN, Jacobi Medical Center, Bronx, NY; Ana Melendez and LaShonda Spencer, Los Angeles County Medical Center/University of Southern California, Los Angeles, CA (grant RR00043); Marilyn J. Crain, MD, and Robert F. Pass, MD, University of Alabama, Birmingham, AL; Keith Henry, MD, and Bette Bordenave, RN, Hennepin County Medical Center/University of Minnesota, Minneapolis, MN (grant AI27661); Beverly E. Sha, MD, and Joan A. Swiatek, APN, Rush University Medical Center, Chicago, IL (grant AI25915); Gwen Scott, MD, and Amanda Cotter, MD, University of Miami, FL; Paul Sax, MD, and Jon Gothing, ACRN, Brigham and Women's Hospital, Boston, MA; Kenneth Fife, MD, and Janet Hernadez, RN, Indiana University, Bloomington, IN (grant AI25859); Erica Walsh, BS, and Cris Milne, CNP, University of Hawaii, Honolulu, HI (grant AI34853); Daniel Johnson, MD, and Dominika Kowalski, RN, Sinai Children's Hospital, Chicago, IL: Sohail Rana, MD, and Jhoanna C. Roa, Howard University, Washington, DC; Katherine Luzuriaga, MD, and Sharon Cormier, RN, University of Massachusetts Medical Center, Worcester, MA (grant AI32907 and AI42845); Barbara Stechenberg, MD, and Eileen Theroux, RN, Baystate Medical Center, Springfield, MA; Richard Reichman, MD, and Carol Greisberger, RN, University of Rochester, NY (grant RR00044); Joan Riddle, RN, Duke University, Durham, NC; Sharon Kohrs, ACRN, and Sharon Mitchell, University of Cincinnati, OH (grant AI25897); and Carol Salbenblatt, MSN, and Adriana Weinberg, MD, University of Colorado, Denver, CO (grant RR00069).
REFERENCES
- 1.Venhoff N, Walker UA. Mitochondrial disease in the off-spring as a result of antiretroviral therapy. Expert Opin Drug Saf. 2006;5:373–381. doi: 10.1517/14740338.5.3.373. [DOI] [PubMed] [Google Scholar]
- 2.Blanche S, Tardieu M, Rustin P, Slama A, Barret B, Firtion G, et al. Persistent mitochondrial dysfunction and perinatal exposure to antiretroviral nucleoside analogues. Lancet. 1999;354:1084–1089. doi: 10.1016/S0140-6736(99)07219-0. [DOI] [PubMed] [Google Scholar]
- 3.Gerschenson M, Erhart SW, Paik CY, St Claire MC, Nagashima K, Skopets B, et al. Fetal mitochondrial heart and skeletal muscle damage in Erythrocebus patas monkeys exposed in utero to 3′-azido-3′-deoxythymidine. AIDS Res Hum Retroviruses. 2000;16:635–644. doi: 10.1089/088922200308864. [DOI] [PubMed] [Google Scholar]
- 4.Ewings EL, Gerschenson M, St Claire MC, Nagashima K, Skopets B, Harbaugh SW, et al. Genotoxic and functional consequences of transplacental zidovudine exposure in fetal monkey brain mitochondria. J Acquir Immune Defic Syndr. 2000;24:100–105. doi: 10.1097/00126334-200006010-00003. [DOI] [PubMed] [Google Scholar]
- 5.Ekouevi DK, Toure R, Becquet R, Viho I, Sakarovitch C, Rouet F, et al. for the Agence Nationale de Recherches sur le SIDA 1201/1202 Ditrame Plus Study Goup Serum lactate levels in infants exposed peripartum to antiretroviral agents to prevent mother-to-child transmission of HIV. Pediatrics. 2006;118:e1071–1077. doi: 10.1542/peds.2006-0371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Noguera A, Fortuny C, Munoz-Almagro C, Sanchez E, Vilaseca MA, Artuch R, et al. Hyperlactatemia in human immunodeficiency virus-uninfected infants who are exposed to antiretrovirals. Pediatrics. 2004;114:e598–603. doi: 10.1542/peds.2004-0955. [DOI] [PubMed] [Google Scholar]
- 7.Tovo PA, Chiapello N, Gabiano C, Zeviani M, Spada M. Zidovudine administration during pregnancy and mitochondrial disease in the offspring. Antivir Ther. 2005;10:697–699. [PubMed] [Google Scholar]
- 8.Barret B, Tardieu M, Rustin P, Lacroix C, Chabrol B, Desguerre I, et al. for the French Perinatal Cohort Study Group Persistent mitochondrial dysfunction in HIV-1-exposed but uninfected infants: clinical screening in a large prospective cohort. AIDS. 2003;17:1769–1785. doi: 10.1097/00002030-200308150-00006. [DOI] [PubMed] [Google Scholar]
- 9.Brogly SB, Ylitalo N, Mofenson L, Oleske J, Van Dyke R, Crain M, et al. In utero nucleoside reverse transcriptase inhibitor exposure and signs of possible mitochondrial dys-function in HIV-uninfected children. AIDS. 2007;21:929–938. doi: 10.1097/QAD.0b013e3280d5a786. [DOI] [PubMed] [Google Scholar]
- 10.Poirier MC, Divi RL, Al-Harthi L, et al. Long term mitochondrial toxicity in HIV uninfected infants born to HIV infected mothers. J Acquir Immune Defic Syndr. 2003;33:175–183. doi: 10.1097/00126334-200306010-00010. [DOI] [PubMed] [Google Scholar]
- 11.Divi RL, Walker VE, Wade NA, et al. Mitochondrial damage and mtDNA depletion in cord blood and umbilical cord from infants exposed in utero to Combivir. AIDS. 2004;18:1013–1021. doi: 10.1097/00002030-200404300-00009. [DOI] [PubMed] [Google Scholar]
- 12.Shiramizu B, Shikuma KM, Kamemoto L, Gerschenson M, Erdem G, Pinti M, et al. Placenta and cord blood mitochondrial DNA toxicity in HIV-infected women receiving nucleo-side reverse transcriptase inhibitors during pregnancy. J Acquir Immune Defic Syndr. 2003;32:370–374. doi: 10.1097/00126334-200304010-00004. [DOI] [PubMed] [Google Scholar]
- 13.Vigano A, Bianchi R, Schneider L, Cafarelli L, Tornaghi R, Pinti M, et al. Lack of hyperlactatemia and impaired mitochondrial DNA content in CD4+ cells of HIV-uninfected infants exposed to perinatal antiretroviral therapy.. Presented at: 11th Conference on Retroviruses and Opportunistic Infections; San Francisco, CA. February 8–11, 2004. [Google Scholar]
- 14.Aldrovandi G, Moye J, Chu C, Meyer W, Ha B, Handelsman E, et al. the Women and Infants Transmission Study Group Mitochondrial DNA content of peripheral blood mononuclear cells in uninfected infants born to HIV-infected women with or without ART exposure in the Women and Infants Transmission Study.. Program and abstracts of the 13th Conference on Retroviruses and Opportunistic Infections; Denver, Colorado, USA. February 5–8, 2006; Poster 695. [Google Scholar]
- 15.Cote H, Forbes J, Bitnun A, Raboud J, Money D, Maan E, et al. Mitochondrial DNA and mtRNA levels during perinatal ART exposure in HIV-uninfected infants born to HIV-infected mothers.. Program and abstracts of the 14th Conference on Retroviruses and Opportunistic Infections; Los Angeles, California, USA. February 25–28, 2007; Poster 714. [Google Scholar]
- 16.Guidelines for the use of antiretroviral agents in pediatric infection (updated October 26, 2006) Available at: http://www.aidsinfo.nih.gov.
- 17.Hammond EL, Sayer D, Nolan D, et al. Assessment of precision and concordance of quantitative mitochondrial DNA assays: a collaborative international quality assurance study. J Clin Virol. 2003;27:97–110. doi: 10.1016/s1386-6532(02)00134-8. [DOI] [PubMed] [Google Scholar]
- 18.Capaldi RA, Marusich MF, Taanman JW. Mammalian cytochrome-c oxidase: characterization of enzyme and immunological detection of subunits in tissue extracts and whole cells. Methods Enzymol. 1995;260:117–132. doi: 10.1016/0076-6879(95)60134-1. [DOI] [PubMed] [Google Scholar]
- 19.Walker UA, Setzer B, Venhoff N. Increased long-term mitochondrial toxicity in combinations of nucleoside analogue reverse-transcriptase inhibitors. AIDS. 2002;16:2165–2173. doi: 10.1097/00002030-200211080-00009. [DOI] [PubMed] [Google Scholar]
- 20.Groggel DJ, Skillings JH. Distribution-free tests for main effects in multifactor designs. Am Statistician. 1986;40:99–102. [Google Scholar]
- 21.Arnaudo E, Dalakas MC, Shanske S, Moraes CT, DiMauro S, Schon EA. Depletion of muscle mitochondrial DNA in AIDS patients with zidovudine-induced myopathy. Lancet. 1991;337:508–510. doi: 10.1016/0140-6736(91)91294-5. [DOI] [PubMed] [Google Scholar]
- 22.McComsey GA, Paulsen DM, Lonergan TJ, Hessenthaler SM, Hoppel CL, Williams VC, et al. Improvements in lipoatrophy, mitochondrial DNA content and adipose tissue apoptosis levels after replacement of stavudine with either abacavir or zidovudine. AIDS. 2005;19:15–23. doi: 10.1097/00002030-200501030-00002. [DOI] [PubMed] [Google Scholar]
- 23.Walker UA, Bäuerle J, Laguno M, Murillas J, Mauss S, Schmutz G, et al. Depletion of mitochondrial DNA in liver under antiretroviral therapy with didanosine, stavudine, or zalcitabine. Hepatology. 2004;39:311–317. doi: 10.1002/hep.20074. [DOI] [PubMed] [Google Scholar]
- 24.Setzer B, Schlesier M, Thomas AK, Walker UA. Mitochondrial toxicity of nucleoside analogues in primary human lymphocytes. Antivir Ther. 2005;10:327–334. [PubMed] [Google Scholar]
- 25.Lynx M, Bentley AT, McKee EE. 3′-Azido-3′-deoxythymi-dine (AZT) inhibits thymidine phosphorylation in isolated rat liver mitochondria: a possible mechanism of AZT hepatotoxicity. Biochem Pharmacol. 2006;71:1342–1348. doi: 10.1016/j.bcp.2006.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Setzer B, Schlesier M, Walker UA. Effects of didanosine-related depletion of mtDNA in human T lymphocytes. J Infect Dis. 2005;191:848–855. doi: 10.1086/427655. [DOI] [PubMed] [Google Scholar]
- 27.Masini A, Scotti C, Calligaro A, Cazzalini O, Stivala LA, Bianchi L, et al. Zidovudine-induced experimental myopathy: dual mechanism of mitochondrial damage. J Neurol Sci. 1999;166:131–140. doi: 10.1016/s0022-510x(99)00126-4. [DOI] [PubMed] [Google Scholar]
- 28.Lund KC, Wallace KB. Mitochondrial toxicity of 3′-azido-3′deoxythymidine (AZT) in the absence of mitochondrial DNA (mtDNA) depletion.. Mitochondrion; Presented at the Mitochondrial Medicine Meeting; Pittsburgh, PA. August 4–7, 2004; 2004. p. 11. Abstract 19. [Google Scholar]
- 29.Barile M, Valenti D, Hobbes GA. Mechanism of toxicity of 3′-azido-3′ deoxythymidine. Its interaction with adenylate kinase. Biochem Pharmacol. 1997;53:913–920. doi: 10.1016/0006-2952(94)90564-9. [DOI] [PubMed] [Google Scholar]
- 30.Lee HC, Yin PH, Lu CY, Chi CW, Wei YH. Increase of mitochondria and mitochondrial DNA in response to oxidative stress in human cells. Biochem J. 2000;348:425–432. [PMC free article] [PubMed] [Google Scholar]
- 31.Barrientos A, Casademont J, Cardellach F, et al. Qualitative and quantitative changes in skeletal muscle mtDNA and expression of mitochondrial-encoded genes in the human aging process. Biochem Mol Med. 1997;62:165–171. doi: 10.1006/bmme.1997.2647. [DOI] [PubMed] [Google Scholar]
- 32.de la Asuncion JG, del Olmo ML, Sastre J, Millan A, Pellin A, Pallardo FV, Vina J. AZT treatment induces molecular and ultrastructural oxidative damage to muscle mitochondria: prevention by antioxidant vitamins. J Clin Invest. 1998;102(1):4–9. doi: 10.1172/JCI1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cossarizza A. Tests for mitochondrial function and DNA: potentials and pitfalls. Curr Opin Infect Dis. 2003;16:5–10. doi: 10.1097/00001432-200302000-00002. [DOI] [PubMed] [Google Scholar]
- 34.Cossarizza A, Riva A, Pinti M, Ammannato S, Fedeli P, Mussini C, Esposito R, Galli M. Increased mitochondrial DNA content in peripheral blood lymphocytes from HIV-infected patients with lipodystrophy. Antivir Ther. 2003;8:315–321. [PubMed] [Google Scholar]