Abstract
Paralysis resulting from spinal cord injury is devastating and persistent. One major reason for the inability of the body to heal this type of injury ensues from the local increase of glial cells leading to the formation of a glial scar, and the upregulation of chondroitin sulfate proteoglycans (CSPGs) at the site of injury through which axons are unable to regenerate. Experimental approaches to overcome this problem have accordingly focused on reducing the inhibitory properties of CSPGs, for example by using chondroitinase to remove the sugar chains and reduce the CSPGs to their core protein constituents, although this step alone does not provide dramatic benefits as a monotherapy. Using in vitro and in vivo approaches, we describe here a potentially synergistic therapeutic opportunity based on tissue plasminogen activator (tPA), an extracellular protease that converts plasminogen (plg) into the active protease plasmin. We show that tPA and plg both bind to the CSPG protein NG2, which functions as a scaffold to accelerate the tPA-driven conversion of plg to plasmin. The binding occurs via the tPA and plg kringle domains to domain 2 of the NG2 CSPG core protein, and is enhanced in some settings after chondroitinase-mediated removal of the NG2 proteoglycan side chains. Once generated, plasmin then degrades NG2, both in an in vitro setting using recombinant protein, and in vivo models of spinal cord injury. Our finding that the tPA and plg binding is in some instances more efficient after exposure of the NG2 proteoglycan to chondroitinase treatment suggests that a combined therapeutic approach employing both chondroitinase and the tPA/plasmin proteolytic system could be of significant benefit in promoting axonal regeneration through glial scars after spinal cord injury.
Keywords: glial scar, chondroitin sulfate proteoglycan, mouse
INTRODUCTION
Tissue plasminogen activator (tPA) is a serine protease that converts the zymogen plasminogen (plg) into the active protease plasmin (Vassalli et al., 1991), and promotes the lysis of blood clots (van Zonneveld et al., 1986). tPA is present in the mammalian central nervous system (CNS) (Carroll et al., 1994; Qian et al., 1993; Sappino et al., 1993; Tsirka et al., 1995) and plays roles in neuronal reorganization and plasticity (Carroll et al., 1994; Krystosek and Seeds, 1981; Qian et al., 1993; Seeds et al., 1995; Tsirka et al., 1995; Wu et al., 2000). tPA is secreted from neuronal growth cones and may promote axonal growth and pathfinding (Krystosek and Seeds, 1981).
Injury to the adult spinal cord results in the formation of a glial scar that acts as a physical and biochemical barrier to axonal regeneration (Davies et al., 1999; Fawcett and Asher, 1999; Jones et al., 2002; Ughrin et al., 2003). In the peripheral nervous system glial scars do not form and injured axons undergo regeneration. This peripheral regeneration is decreased in the absence of tPA, suggesting that proteolytic activity is important for the recovery process (Akassoglou et al., 2000; Neuberger and Cornbrooks, 1989). One of the major growth-inhibitory components of the glial scar is a diverse set of proteoglycans known as chondroitin sulfate proteoglycans (CSPGs) (Jones et al., 2003; Silver and Miller, 2004; Tang et al., 2003), which includes NG2, neurocan, and versican (Dou and Levine, 1994b; Friedlander et al., 1994; Schmalfeldt et al., 2000; Ughrin et al., 2003). CSPGs are composed of a core protein and glycosaminoglycan (GAG) chains; inhibition of axon regeneration after spinal cord injury (SCI) is thought to be mediated primarily by the core protein, but the GAG chains may also contribute (Moon, 2001; Tan et al., 2006; Ughrin et al., 2003). Digestion of GAG chains with the chondroitinase ABC improves axonal regeneration (Bradbury et al., 2002; Moon, 2001; Yick et al., 2000); however, the functional recovery observed in this setting has been attributed to the generation of a more permissive extracellular matrix rather than to the elimination of any direct inhibition mediated by the GAG chains. What biochemical features make this environment more permissive is not known.
NG2 interacts with structural proteins present in the extracellular matrix such as collagens type V and VI (Stallcup et al., 1990; Tillet et al., 1997). Growth factors including bFGF and PDGFAA also bind to NG2, as does plg (Goretzki et al., 1999, 2000). NG2 binding to plg stimulates the generation of plasmin by uPA (Goretzki et al., 1999, 2000). Since tPA is rapidly secreted after CNS injury (Carroll et al., 1994; Krystosek and Seeds, 1981; Qian et al., 1993; Sappino et al., 1993; Tsirka et al., 1995), we investigated whether NG2 binding also renders tPA a more efficient activator of plg. We show here that NG2 binds tPA and plg and accelerates the rate of plasmin generation. At high concentrations of NG2, the binding is promoted by the removal of the GAG chains by chondroitinase ABC. As high levels of proteoglycans quickly accumulate at the site of CNS trauma, it is possible that in vivo chondroitinase treatment after SCI may enhance the interaction between NG2 and the tPA secreted, resulting in locally elevated levels of plasmin and the subsequent breakdown of the growth-inhibitory NG2 core protein in the extracellular matrix.
EXPERIMENTAL PROCEDURE
Animals
Surgical procedures followed the National Institutes of Health guidelines and were approved by the Department of Laboratory Animal Research at the State University of New York (SUNY, Stony Brook, NY). C57BL6 were used as wild-type (wt) mice. Age-matched 25–30 g adult female mice were anesthetized deeply with isoflurane. We adapted to mice a previously described protocol for spinal cord hemisection in the rat (Tan et al., 2006). In brief, a dorsal laminectomy at thoracic level 8 was performed, and the dura matter was removed to expose the spinal cord. Using a 27½-gauge needle, the spinal cord was transected bilaterally to the depth of the central canal. The surgical site was closed with sutures (Ethicon), and the animals were kept warm to recover from anesthesia. After variable lengths of time (1, 3, 7, and 14 days), the animals were perfused with normal saline and the lesioned area of the spinal cord was removed. The lesion epicenters (±1 mm from the injury site) were homogenized in 300-μL ice-cold PBS containing 0.25% TritonX-100 (TX-100). Debris was removed by centrifugation, and total protein content was measured using the Bio-Rad (Hercules, CA) Bradford detergent-compatible (DC) assay.
Amidolytic Assay
tPA activity was assayed as described previously (Andrade-Gordon and Strickland, 1986). Briefly, triplicate samples containing 0.028 μM recombinant tPA (Genentech) were incubated with 0.22 μM plg in a mix containing 0.3 mM S-2251, 0.1M Tris, pH 8.1, 0.1% Tween-80, and 1 mM amiloride. Cleavage of the chromogenic substrate S-2251 by tPA-generated plasmin and the subsequent color change was quantified at 405 nm after 180 min of incubation at 25°C. To accelerate the plasmin generation rate, fibrin (Sigma) was added at different concentrations (1–100 nM) to the incubation. NG2 (300 pM to 100 nM) was added to the mixture to test for enhancement in plasmin generation. The data were analyzed by one-way analysis of variance (ANOVA) with the Bonferroni’s Multiple Comparison Test.
NG2 Domain Specific Fusion Proteins
Amidolytic assays were performed using as substrate NG2 and the individual NG2 domain-specific Fc fusion proteins at a concentration of 3 nM. The full-length NG2 proteins and the fusion proteins were purified from HEK293 cells transfected with the rat cDNA sequences to allow for glycosylation during their biosynthesis. The domain proteins purity and size were assessed using SDS-PAGE and western blot analysis (Ughrin et al., 2003) before and after chondroitinase treatment.
Chondroitinase Treatment
NG2 was treated with 0.025 units of chondroitinase ABC (Seikagaku Corporation) per μg of NG2 protein and incubated at 37°C for 2 h.
NG2 Core Protein Digestion with Proteinase K
NG2 was incubated with Proteinase K (PK; Sigma) to degrade the core protein. PK was incubated with 400 ng (7 nM) NG2 for 15, 30, 60, and 120 min at 55°C. PK activity was heat-inactivated at 75°C for 20 min. Plasmin generation was quantified in triplicate samples. Samples were also electrophoresed and silver stained.
Silver Stain
Samples of NG2 digested with PK were electrophoresed on a 6% Tris-glycine gel. The gel was acid-washed in 5% methanol and 10% acetic acid for 60 min at 25°C, washed in PBS, and incubated for 30 min in 25% glutaraldehyde. The gel was washed in PBS before DTT reduction (1-mg DTT in 200-mL of distilled water), then stained with 0.1% AgNO3 and washed briefly before developing in 3% sodium carbonate and 100-μL 37% formaldehyde. The reaction was terminated in 2.3 M citric acid (10 min).
NG2 Core Protein Digestion by Plasmin
Recombinant NG2 treated with chondroitinase was incubated with tPA, plg, or tPA and plg in 0.1 M Tris-HCl pH 8.0. At different time-points, the samples were analyzed by silver staining. To test ex vivo NG2 degradation, tissue homogenates from tPA−/− mice 14 days after injury were treated with chondroitinase and incubated with tPA, plg, or tPA and plg for 5, 15, and 40 min at room temperature. Samples were separated on 6% SDS-PAGE. NG2 was visualized using rat anti-mouse NG2 (a kind gift from Dr. Jacqueline Trotter, University of Heidelberg) and goat anti-rat HRP secondary antibody (Jackson ImmunoResearch).
Solid-Phase Binding Assay
Assays were performed as described previously (Goretzki et al., 1999). In brief, ELISA plates (Nalge Nunc International) were coated overnight at 4°C with 4 μg/ ml of BSA, tPA (Genentech), plg, or plg kringle domain mutants (American Diagnostica) in 50 mM sodium carbonate pH 9.6. The plates were washed with PBS before blocking with 5% BSA in PBS for 2 h at 25°C, washed with PBS-Tween, and incubated with recombinant NG2, treated with or without chondroitinase overnight at 4°C. Bound NG2 was detected by rabbit anti-NG2 (Chemicon) used at a dilution of 1:500, followed by goat anti-rabbit HRP secondary antibody (Jackson Labs) at a 1:5,000 dilution. Tetramethylbenzidine (TMB) substrate was added after the secondary and incubated for 10 min at 25°C. H2SO4 (0.5 M) was added to quench the reaction. The color change was read at 450 nm. For inhibition studies, tPA or plg were incubated with 100 μM EACA (Sigma) for 2 h at 25°C prior to addition of NG2. The data were analyzed by one-way ANOVA with the Bonferroni’s Multiple Comparison Test.
Spinal Cord Homogenate Analysis
Noninjured spinal cord controls were compared to the lesioned spinal cord homogenates, prepared as above, using a solid-phase binding assay. In brief, 50 μg of spinal cord homogenates were incubated in tPA- or plg-coated 96-well ELISA plates overnight at 4°C. The subsequent primary and secondary antibody detection was the same as described for the solid-phase binding assay. The experiments were repeated in triplicate, totaling six mice for each of the time points evaluated (nontreated, 1, 3, 7, and 14 days after injury).
CHO and Xylosyltransferase-Deficient CHO Cells
CHO cells were obtained from the American Type Culture Collection. Xylosyltransferase-deficient CHO cells were a kind gift from Dr. Jeffrey Esko (University of California, San Diego). Cells were maintained in Ham’s F-12 Nutrient Mixture (Gibco) supplemented with 1% fetal calf serum (FCS) and Penn/Strep in a humidified 37°C/5% CO2 incubator. Media were collected after the cells had grown to confluency. The levels of secreted NG2 were determined using western blot analysis. The Bio-Rad DC assay was used to normalize total protein in the supernatants collected. Conditioned media were tested for their ability to enhance tPA-catalyzed generation of plasmin by amidolytic assay.
CHO and xylo−/− CHO cells were grown to confluency in 96-well tissue-culture plates and incubated with 5 μg of tPA for 2 h at 37°C. The cells were lightly fixed with 4% paraformaldehyde (PFA) at 4°C and blocked in 5% milk/1% normal goat serum in PBS for 1 h. tPA was detected using a rabbit anti-tPA antibody (Molecular Innovations) used at a dilution of 1:1,000 and goat anti-rabbit HRP-conjugated secondary antibody (Jackson Labs) at 1:5,000. TMB was added after the HRP secondary and allowed to incubate for 10 min at 25°C before 0.5 M H2SO4 was added to quench the reaction. The plates were washed 3 times for 5 min with PBS before the addition of the TMB solution. The color change was measured at 450 nm.
NG2 and tPA Coimmunoprecipitation
Spinal cord homogenates were used for the immunoprecipitation (IP) experiments 14 days after injury. Twenty micrograms of total protein was used for total homogenate samples and 200 μg for the IP experiments. Homogenates were spun down at 10,000g for 10 min at 4°C to remove tissue debris. Samples were precleared with protein A/G beads for 2 h at 4°C, and incubated with protein A/G beads and primary antibody (rat anti-mNG2) overnight at 4°C. Beads were pelleted at 1,000g for 1 min, washed in PBS, and resuspended in 1×SDS without β-mercaptoethanol (BME) or boiling for analysis of tPA activity by zymography. Samples were boiled with 1×SDS with BME and probed for NG2 by western blot analysis.
tPA Zymography
Ten percent polyacrylamide-SDS gels were copolymerized with casein and plasminogen. After electrophoresis, the SDS was removed by incubating the gel with 2.5% Triton X-100 for 30 min. The gel was washed with H2O before incubating in 0.1 M Tris pH8.1 overnight at room temperature, stained with coomassie brilliant blue and destained until clear zones of lysis became visible (Siconolfi and Seeds, 2001).
Substrate Preparation for Neurite Outgrowth
Petri dishes (size 60 mm) were coated with 750-μL nitrocellulose dissolved in methanol (5-cm2 nitrocellulose in 12 mL of methanol) and air-dried in the tissue culture hood. Two-microliter droplets of protein solutions (NG2, NG2 proteolyzed by plasmin, NG2 digested with chondroitinase ABC or chondroitinase ABC digested NG2 proteolyzed by plasmin) were spotted onto the nitrocellulose and allowed to air-dry for 10 min. An additional 8-μL droplet of laminin-1 (10 μg/mL) (Sigma) was spotted over the treated or untreated NG2 to form a circular border. NG2 (50 μg/mL) was digested with (0.025 units) chondroitinase ABC for 2 h at 37°C. The proteoglycan was then incubated with plasmin (200 ng) for 4 h at 37°C, and the reaction was terminated by the addition of 200 ng of α2-antiplasmin (Athens Research Technology). The dishes were washed with Neurobasal media with B27 supplement. Cortical neurons were isolated from E16 mice and dissociated by digestion with 0.25% trypsin for 20 min and trituration. Cells were washed with media and plated (50,000 cells/60-mm dish). Cortical neurons were grown in Neurobasal media containing B27 supplement for 5 days and plated at 50,000 cells/60-mm dish. For each substrate condition, neurite length was quantified using Metamorph software. Neurite length was measured from the center of the cell body to the neurite tip. For cells possessing multiple neurites, the longest neurite was measured.
RESULTS
tPA and plg Interact with NG2 and Neurocan Resulting in Enhanced Plasmin Generation
The ability of NG2 to bind the zymogen plg and urokinase (Goretzki et al., 1999, 2000) led us to ask whether tPA, the other activator of the plasmin system, also interacts with NG2. Binding was examined using a solid-phase assay, with the interaction between NG2 and plg serving as a positive control. NG2 was found to bind to tPA (Fig. 1A) and plg (Fig. 1C) in a dose-dependent manner. Treatment of NG2 with chondroitinase ABC altered the binding of NG2 to tPA, and to a lesser extent, plg. At low concentrations (5 nM) of NG2, chondroitinase treatment reduced the binding of NG2 to tPA and plg, whereas at higher concentrations, greater binding was observed (P < 0.001).
Fig. 1.
NG2 binds to tPA and plasminogen through their kringle domains. Binding of full-length NG2 to immobilized tPA (A) or plg (C) was measured at different concentrations (5–30 nM) in the presence or absence of pretreatment with chondroitinase (Chon). The amounts of bound NG2 were quantified using a solid-phase binding assay. Addition of 100 μM EACA was added to the reaction to evaluate the contribution of the kringle domains of tPA (B) and plg (D) to the binding of NG2. In the four rightmost columns, individual plg kringle domains (K4 or K1-3) were used to generate the solid-phase substrate. Data represent three separate experiments, each performed with duplicate samples. Error bars represent S.E.M where #P < 0.001, *P < 0.01, **P < 0.001 by ANOVA followed by Bonferroni’s post hoc test.
Binding of tPA and plg to other proteins is frequently mediated via their lysine-rich kringle domains. Plg has been reported to bind to NG2 using this mechanism (Goretzki et al., 2000). The compound -aminocaproic acid (EACA), a derivative of the amino acid lysine, binds to the kringle domains of plg and blocks its binding to fibrin and subsequent plasmin activation. We employed EACA to determine if the tPA kringle domains mediate tPA’s interaction with NG2, using the plg:NG2 interaction as a positive control. The addition of EACA inhibited the binding of tPA and plg to NG2, indicating that tPA uses a similar mechanism to bind to NG2, and both tPA and NG2 did so independently of whether the samples had been subjected to chondroitinase treatment (Figs. 1B,D). To confirm the role of the kringle domains in the interaction, plg mutants containing only selected subsets of the multiple kringle domains present in the protein were tested using a solid-phase binding assay. Kringle domains 4 and 1–3 were independently found to mediate partial NG2 binding (P < 0.001) (Fig. 1D).
To explore the biological consequences of the interaction between NG2, plg and tPA, we measured plasmin activity. The activation of purified plg by purified tPA is actually very inefficient, and detectably measurable levels of activation at physiologically relevant concentrations occur only when scaffolding proteins, such as fibrin, are present to concentrate the tPA and plg onto a surface of restricted dimensionality (Hoylaerts et al., 1982). As shown in Fig. 2A, a concentration of 10 nM fibrin triggers detectable activation of plg by tPA, and half-maximal activation is observed at a concentration of 30 nM. Higher fibrin concentrations (10 and 30 μM) indicated that the reaction had reached a plateau (data not shown). NG2 similarly provided scaffolding support to tPA-catalyzed activation of plg; and in fact, NG2 was found to be 10-fold more potent than fibrin for half-maximal activation. However, and in contrast to what was observed for fibrin, increasing the amount of NG2 beyond the optimal concentration (10 nM) led to less efficient and finally no activation of plg by tPA. The basis for this inhibition is unknown. Pretreatment of the NG2 with chondroitinase right-shifted its dose-response curve by approximately threefold at all concentrations. As a consequence, chondroitinase pretreatment inhibited the NG2 scaffolding enhancement at low NG2 concentrations (1–10 nM), and facilitated it at high concentrations (30 nM to 3 μM). This result extends the finding in Fig. 1, where chondroitinase pretreatment of NG2 inhibited its binding to tPA and plg at low concentrations (5 nM) and facilitated it at high concentrations (30 nM).
Fig. 2.
NG2 accelerates the rate of plasmin generation. (A) tPA-catalyzed plasmin generation was measured using an amidolytic assay. Increasing molar concentrations of NG2 (■), NG2 pretreated with Chondroitinase (▼), or fibrin (○) were incubated with tPA, plg, and amiloride, followed by the addition of the chromogenic substrate S-2251. (B) NG2 was incubated with tPA (0.028 μM), and plg and plasmin generation was quantified. Additional tPA (0.28 μM) was added to some samples as shown. Error bars represent S.E.M where #P < 0.05 and **P < 0.01 by ANOVA followed by Bonferroni’s post hoc test.
Increasing the amount of tPA incubated with an inhibitory amount of NG2 (50 nM) rescued the tPA-catalyzed activation of plg (Fig. 2B). This result indicates that the inhibition observed does not derive by nonspecific inhibition of enzymatic reaction, but rather that a tight stoichiometric relationship among the participating molecules may be required for productive interactions. This finding suggests that the mechanisms of scaffolding for NG2 and fibrin differ, but the basis for the difference is unclear.
Specific NG2 Domains Accelerate Plasmin Generation
NG2 is a large multidomain CSPG, and like many other CSPGs, each structural domain has different functional properties (Fang et al., 1999; Stallcup et al., 1990; Tillet et al., 1997; Ughrin et al., 2003). To determine which domains of NG2 contribute to the activation of plasmin generation, we used different domain-specific NG2 fusion proteins. The ability of these domains to enhance plasmin generation was tested before and after the addition of chondroitinase (Fig. 3A). The amount of plasmin generated by the NG2 fusion proteins was normalized over the tPA/plg control and represented as fold-increase over control. We found that domains 2 and the combination 1 + 2 were able to promote plasmin generation (2.9 and 2.0-fold increases in activity, respectively), whereas domains 1 and 3 were unable to enhance the rate of tPA-catalyzed plasmin formation above that of the tPA/plg baseline control, a result that is distinct than previous findings using uPA to activate plg (Goretzki et al., 2000). When the interaction between uPA and NG2 was evaluated as control, enhanced rate of plasmin generation was observed when full length recombinant NG2 or domain 3 were used, but not in the presence of domain 2 (data not shown). Although domain 2-containing fusion proteins were most effective for the tPA-mediated activation of plg, it is important to note that none of the individual domain-specific fusion proteins were able to enhance plasmin generation to the level of the full length NG2. Therefore, the interactions of tPA and plg require domain 2 of the NG2 core protein, but other regions within the NG2 core protein may cooperate with domain 2 to enhance the rate of plasmin generation.
Fig. 3.
NG2 domain-specific fusion proteins exhibit differences in plasmin generation. Individual NG2 domain-Fc fusion proteins were used at 3 nM in a tPA-catalyzed plasmin generation assay without (A) or with (B) chondroitinase pretreatment. The activities observed were normalized over the nonenhanced tPA/plasminogen control to enable presentation as fold-increases. The expression levels and integrity of the recombinant fusion proteins were confirmed by western blot analysis (data not shown). Error bars represent S.E.M.
To evaluate the role of GAG chains in promoting plasmin generation, the domain-specific NG2 fusion proteins were chondroitinase-treated (Fig. 3B). Domains 1 and 3 do not contain functional sites for GAG attachment (Nishiyama et al., 1991; Stallcup and Dahlin-Huppe, 2001). Accordingly, chondroitinase treatment of these domains resulted in no increase in activation. Chondroitinase treatment of NG2 domains 1 + 2 and NG2 domain 2 decreased their facilitation of plasmin generation (Fig. 3B). Since the domains were used at a concentration of 3 nM, this outcome is consistent with the results presented in Figs. 1 and 2, wherein chondroitinase inhibited NG2 facilitation of plasmin generation at concentrations less than 10 nM. Thus, low concentrations of NG2-associated GAG chains may not be inhibitory to the tPA-catalyzed generation of plasmin, and the chondroitin-sensitive effect resides in domain 2 of the core protein.
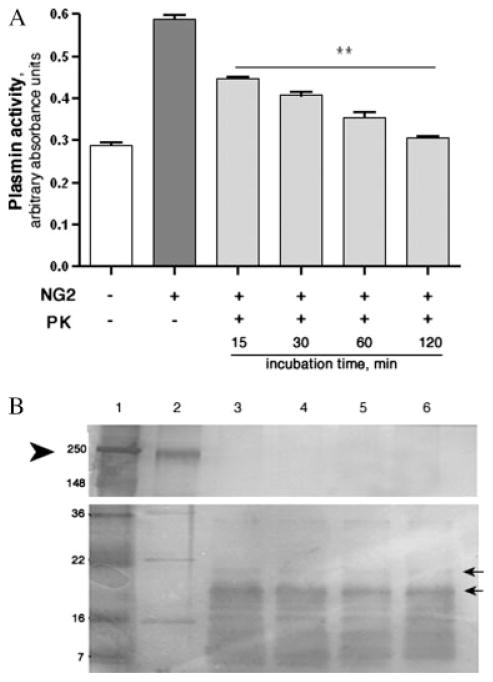
Plasmin Generation Decreases as NG2 is Degraded
To examine whether the NG2 core protein is required to accelerate the formation of plasmin, PK was employed to degrade it prior to the plasmin activity assay. Incubation of NG2 with PK abolished the ability of NG2 to accelerate tPA-catalyzed plasminogen activation in a time-dependent manner (Fig. 4A). Silver staining was used to detect NG2 breakdown products (Fig. 4B). Fifteen minutes of PK digestion eliminated the full-length NG2 core protein, suggesting that some of the breakdown products visibly formed continued to promote the plg activation, until their eventual degradation as well. This finding indicates that the core protein is required for plasminogen activation, either for direct interaction with tPA and plg, or for providing a base for the structural organization of GAG chains.
Fig. 4.
Degradation of the NG2 core protein results in loss of plasmin generation. PK was incubated with NG2 for different amounts of time. (A) A tPA-catalyzed plasmin generation assay was subsequently used to measure plasmin activity. The control reactions [tPA/plg alone (+NG2, −PK), and NG2 core protein (−NG2, −PK)] were allowed to proceed for 120 min. (B) silver staining was used to analyze NG2 breakdown products after PK digestion: lane 1: ladder, lane 2: non-treated NG2, lanes 3–6: 15, 30, 60, and 120 min of PK treatment, respectively. Arrowhead points to full-length NG2 (lane 2) and arrows point to some of the detectable degradation products (lanes 3–6). Molecular weight standards are also indicated. Error bars represent S.E.M where **P < 0.001 by ANOVA followed by Bonferroni’s post hoc test.
The Role of GAG Chains in tPA-Catalyzed Plasmin Generation
To assess the role of the GAG chains in modulating the binding ability of NG2 to tPA and plg, we took advantage of a strain of modified CHO cells that lack xylosyltransferase activity. These cells are unable to transfer the first sugar in glycosaminoglycan synthesis, and consequently do not produce glycosaminoglycans (Esko et al., 1985). Although our interests lie in the role of NG2 in the context of the glial scar and the CNS, other cell types and tissues in mammals also express NG2, such as endothelial cells and vascular smooth muscle cells (Grako, 1995). NG2 is also expressed in the embryonic heart by cardiomyocytes (Ozerdem et al., 2001), and a variety of tumor cells and angiogenic tumor vasculature (Burg et al., 1999). Using western blot analysis, we confirmed that wt cells and xylosyltransferase-deficient CHO cells produce and secrete comparable amounts of NG2 (Fig. 5A). Wt CHO cells and xylosyltransferase-deficient CHO cells also both express NG2 on the cell surface (Fig. 5B).
Fig. 5.
Glycosaminoglycan chains do not affect tPA-catalyzed plasmin generation. (A) Western blot analysis of NG2 secretion by wt and xylotransferase−/− CHO cells. Conditioned media (CM) from wt CHO cells was treated with chondroitinase to remove GAG chains before the western blot analysis, whereas the xylo−/− CHO CM was electrophoresed without prior chondroitinase treatment. Chondroitinase-treated recombinant purified NG2 was electrophoresed in parallel as a loading control. (B) wt CHO cells and xylo−/− CHO cells were immunostained for membrane-bound NG2 (red). Nuclei were stained using DAPI (blue). (C) wt CHO CM and xylotransferase−/− CM were scored for their ability to enhance plasmin generation in the presence or absence of tPA and plg. Nonconditioned medium was used as a negative control. (D) Binding assays with wt cells or Xylo−/− CHO cells in the presence or absence of tPA. Error bars represent S.E.M where **P < 0.001, #P < 0.05 by ANOVA followed by Bonferroni’s post hoc test. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
We compared the ability of conditioned medium from wild-type CHO cells and xylosyltransferase−/− CHO cells to enhance the tPA-catalyzed conversion of plg into plasmin. Both conditioned media facilitated the catalysis compared to the tPA/plg control, and did so at comparable levels (P > 0.05) (Fig. 5C). These data suggest that the core NG2 protein suffices to facilitate the tPA-mediated plg conversion.
Finally, we examined the wild-type and mutant cell lines using the binding assay approach to determine if the presence of GAG chains affected the recruitment of tPA to the cell surface. A significant (P < 0.001) increase was observed in the amount of tPA that bound to xylosyltransferase−/− cells in comparison to wild-type cells (Fig. 5D). This result is consistent with the in vitro binding assays, and demonstrates that removal of the GAG chains from the NG2 core protein increases the binding of tPA in the context of cell surface-expressed NG2, suggesting that the NG2 is present there at relatively high functional concentrations (i.e., >10 nM).
tPA-Activated Plasmin Degrades NG2
Plasmin can cleave and degrade many types of substrates including amyloid-beta aggregates (Tucker et al., 2000) and phosphacan (Wu et al., 2000). Phosphacan can act as a growth-inhibitory CSPG and becomes increased at sites of spinal cord injury (Jones et al., 2003). We examined whether the tPA/plg proteolytic system can degrade NG2. Incubation of recombinant NG2 with just tPA or plg did not lead to cleavage (Fig. 6A). However, the combination of tPA and plg together generated plasmin, which then performed a time-dependent degradation of the NG2.
Fig. 6.
In vitro and ex vivo degradation of NG2 by plasmin. (A) Plasmin [generated by tPA’s (20 ng) action on plg (400 ng)] was incubated with recombinant NG2, as indicated. Degradation products were visualized by silver staining. NG2 runs at molecular weights of 250 and 148 kDa (arrows). NG2 degradation by plasmin was quantified by densitometry and plotted as mean intensity. MW: molecular weight markers in the first lane. This experiment was repeated three times. (B) Injured spinal cord homogenates (in two separate experiments) were incubated with tPA (20 ng) and plg (100 ng) and analyzed by SDS-PAGE. NG2 was detected by western blot analysis using a mouse anti-NG2 antibody. Amounts of the remaining full-length NG2 at the different time points were quantified by densitometry and plotted as mean intensity.
The ability of plasmin to degrade NG2 present at the lesioned epicenter 14 days after SCI was next examined. We found that neither tPA nor plasminogen alone degraded NG2, but the combination of tPA and plasminogen was highly effective (Fig. 6B). These data indicate that plasmin generated by tPA is able to degrade recombinant and the native NG2 that is biosynthesized and deposited in glial scars after SCI.
Plasmin-Mediated NG2 Degradation Allows for Enhanced Neurite Outgrowth
To investigate whether the degradation of NG2 by plasmin would result in facilitation of neurite outgrowth, cortical neurons were plated onto different substrates. The four experimental conditions used were NG2 alone, NG2 pre-incubated with plasmin, NG2 treated with Chondroitinase ABC, and finally NG2 that had been treated with Chondroitinase and incubated with plasmin. The incubation mixes were spotted onto nitrocellulose-coated plates and laminin was added (Dou and Levine, 1994; Wu et al., 2000). Neurons attached and extended neurites (see Fig. 7) over the laminin-coated region (mean length of neurites was 179.88 μm). The NG2/laminin coated region displayed little cell attachment and was inhibitory to neurite outgrowth (mean length of neurites was 76.9 μm). Although neurite outgrowth was reduced in comparison to the preferred laminin substrate, in the plasmin proteolyzed NG2/laminin coated area more cell attachment was evident and the inhibitory properties of NG2 were significantly diminished via the degradation of the proteoglycan (mean length of neurites: 113.08 μm). When NG2 was incubated with chondroitinase there was an increase in cell attachment and a minor increase in neurite length (mean length was 87.37 μm). Furthermore, NG2 incubated with both plasmin and chondroitinase prior to coating the plates had a similar effect as plasmin proteolyzed NG2, displaying significantly more cell attachment and increase in neurite length (mean length of 108.84 μm). The substrate of plasmin proteolyzed NG2 also resulted in a significant increase in the number of neurites in comparison to neurites grown on untreated NG2 (P < 0.05) (data not shown). These results suggest that when plasmin degrades NG2 it can reduce the proteoglycan’s inhibitory effects and create a more permissible environment for neurite outgrowth.
Fig. 7.
Plasmin-mediated NG2 degradation and neurite outgrowth. Cortical neurons were plated onto nitrocellulose-coated dishes containing (A) NG2; (B) Chondroitinase ABC digested NG2; (C) Plasmin proteolyzed NG2; (D) NG2 treated with both chondroitinase ABC and plasmin or (E) laminin. Cultures were photographed after 5 days of growth. Phase-contrast images of cortical neuronal cells are shown. (F) Quantification of neurite length was performed using Metamorph software. Statistical analysis was performed using paired, two-tailed Student’s t-test. Scale bar denotes 100 μm.
tPA and plg Bind Native NG2 from Injured Spinal Cord Homogenates
To confirm that native NG2 generated at sites of injury can interact with tPA and plg, injured spinal cord homogenates were utilized as a source for NG2 in the solid-phase binding assay. NG2 is quickly upregulated after injury to the spinal cord, peaking in level of expression at 7 days in mice, and persisting at slightly elevated levels for more than 6 months (Camand et al., 2004; Jones et al., 2002; Tang et al., 2003). SCIs were performed as described, and the mice sacrificed at successive time points after injury. NG2 binding to the tPA and plg solid-phase substrates increased over time, with a small but significant increase seen at Day 1 (Fig. 8A (P < 0.01); Fig. 8B, (P < 0.05)), and a peak of binding observed at Day 7 postinjury (P < 0.001). These findings illustrate that tPA and plg are not only able to bind to native NG2, but also NG2 that is secreted or shed from cells present in the lesion area after injury to the spinal cord.
Fig. 8.
tPA and plasminogen bind the NG2 present in injured spinal cord. tPA (A) and plasminogen (B) were incubated with injured spinal cord homogenates in a solid-phase binding assay and adherent NG2 was detected. Data represent three separate experiments for each time point (n = 2). (C) Recombinant NG2 and tPA were used to generate a binding curve. (D) NG2 was immunoprecipitated from injured spinal cord homogenates, and analyzed using SDS-PAGE zymography to assess the presence of active tPA in the immunoprecipitated complex. tPA (64 kDa; black arrow on the right side of the gel). Beads alone (beads) or beads incubated with preimmune serum (Beads + serum) were used as negative controls. TH, total homogenates; IP, immunoprecipitated material. The lower band of activity is most likely uPA. In the presence of the inhibitor amiloride, the band disappears. (E) NG2 immunoprecipitated material was analyzed by western blot using a rat anti-mouse NG2 antibody. NG2 Ctr, NG2 detected in total homogenates; NG2 IP, NG2 detected in immunoprecipitated material. Beads alone (beads) or beads incubated with preimmune serum (Beads + serum) were used as negative controls. (F) Representative western blot showing the upregulation of NG2 in Day 7 SCI lysates from wild-type (wt) or tPA−/− animals (using the rat anti-mouse NG2 antibody) compared to noninjured control levels. Arrows point to NG2 bands. Error bars represent S.E.M where *P < 0.05; **P < 0.001 by ANOVA followed by Bonferroni’s post hoc test. (G, H) Quantification of NG2 levels from western blots of wt and tPA−/− not-injured animals, and animals 7 days after SCI (n = 3 experiments with groups varying between 4 and 6 animals). Results were plotted as ratios of NG2 levels in each sample over those in not injured animals for each genotype and analyzed by ANOVA followed by Bonferroni’s post hoc test (P = 0.061). Similar trends were evident 5 and 14 days post SCI. Samples were normalized for total protein levels using plasminogen levels as control protein (data not shown).
To determine if the nanomolar amounts of NG2 used in the in vitro binding assays (Fig. 1A) were physiologically relevant, we generated a dose-response binding curve for recombinant NG2 binding to the solid-phase tPA and plg substrates (Fig. 8C). The binding curve suggested that secreted/shed NG2 is present in the injured spinal cord homogenates at a concentration above 30 nM. This result suggests that the 30 nM amount of NG2 used in our experiments was physiologically relevant, and that the concentration of NG2 at the injury site is within a range that could allow for enhanced tPA-catalyzed conversion of plg into plasmin, and sufficiently high that chondroitinase might improve the efficiency of activation of plasmin, as inferred from Fig. 2.
To assess whether the interaction between tPA and NG2 occurs in vivo, an immunoprecipitation experiment was performed. SCI homogenates obtained 7 days after injury were analyzed by zymography and shown to produce a clear zone of lysis at the same molecular weight as tPA (Fig. 8D). The homogenates were then incubated with a rat antibody against mouse NG2 and protein A/G beads, and again analyzed for tPA activity by zymography. The results of the NG2 immunoprecipitation showed a clear zone of lysis at the same molecular weight as tPA, suggesting that tPA coimmunoprecipitated with NG2 (Fig. 8D). To confirm the specificity of the anti-NG2 antibody, we analyzed the samples by western blot analysis and probed for NG2 (Fig. 8E). As expected, we observed an immunoreactive band of the correct size for NG2 in the SCI total homogenate and the immunoprecipitated samples. Western blot analysis confirmed that NG2 was indeed upregulated in lesion epicenter lysates obtained 7 days post SCI (Figs. 8F,G), as previously reported in the literature (Camand et al., 2004; Jones et al., 2002; Tang et al., 2003). Finally, although spinal cord lysates from wild-type and tPA-deficient mice express NG2 at similar levels when uninjured (Figs. 8F,G), the upregulation of NG2 during injury is greater in tPA−/− animals, presumably reflecting decreased degradation of the CSPG.
DISCUSSION
Chondroitin sulfate proteoglycans (CSPGs) are upregulated after injury to the CNS, being produced by reactive astrocytes that form the glial scar (McKeon et al., 1999). NG2 is a critical inhibitory proteoglycan that is produced by oligodendrocyte precursor cells (OPCs) and microglia/macrophages after trauma to the spinal cord (Levine, 1994; Jones et al., 2002). The increase in inhibitory proteoglycans after spinal cord injury has been identified as an important component of the physical barrier that blocks functional recovery after spinal cord injury.
Recent work has focused on ways to use extracellular proteolysis as a means to decrease the inhibitory nature of the glial scar in hopes of fostering a more permissive environment that would better support axonal regeneration. Matrix metalloproteinase-2 can degrade CSPGs and create a less extensive astrocytic scar after SCI (Hsu et al., 2006). Plasmin generation can regulate the outgrowth of the axons of granule cells (mossy fibers) in the mouse hippocampus after seizures by degrading the proteoglycan substrate phosphacan (Wu et al., 2000). The infusion of decorin, a leucine-rich proteoglycan, into a spinal cord injury site reduces inflammation, the astroglial limitans and the inhibitory proteoglycans, NG2 and neurocan, and results in axonal regeneration through the glial scar (Davies et al., 2004). Further analysis showed that decorin promotes CSPG degradation through an increase in plasmin (Davies et al., 2006).
We have demonstrated that NG2 has a similar function as that of the scaffolding protein fibrin during fibrinolysis, in that it enhances tPA-catalyzed conversion of plg into plasmin by binding both molecules and bringing the tPA and plg into close proximity. Our data also suggest, however, that tPA and plg bind to NG2 in an interaction that appears different than the one promoted by fibrin. At elevated NG2 concentrations, the nature of the binding appears to inhibit the binding and plasmin activation, which is not the case for fibrin (Fig. 2A). Removal of the GAG chains from NG2 by chondroitinase treatment right-shifts the binding reaction and consequently the plasmin activation efficiency. These results suggest that at high levels of NG2, increased activation of plasmin and hence better clearance of NG2 can be achieved using chondroitinase. We also show that the amounts of NG2 present in homogenates of injured spinal cords appear to rise to a sufficiently high level (>30 nM), such that it is likely that chondroitinase treatment would be beneficial in a therapeutic context.
We show that tPA, like plg, is able to bind NG2 through its kringle domains. This binding to NG2 appears to be mediated through domain 2, not domain 3, as has been reported for interactions between uPA and NG2 (Goretzki et al., 2000). NG2 can bind other extracellular matrix molecules and cationic growth factors through domain 2 (Goretzki et al., 1999). NG2 may be able to adopt different conformations depending on whether it is an integral membrane protein, or if it has been shed or secreted from the cell surface. As an integral membrane protein, NG2 domains 1 and 2 are exposed and could bind tPA and plg. In the shed or secreted form of NG2, domains 1 and 3 are thought to be accessible, once NG2 binds collagen through domain 2 (Ughrin et al., 2003). It is possible that tPA or plg could also be potential binding partners via the exposed domain 2 of the NG2 core protein, once it has been shed or secreted. These hypotheses are supported by our findings that tPA and plg can bind to a physiologically relevant level of NG2 present in tissue homogenates from injured spinal cords. A physical interaction between tPA and NG2 is further supported by the fact that tPA coimmunoprecipitates with NG2 in injured spinal cord homogenates.
How does this binding work? Our experiments indicate that the kinetics of binding are not linear. It is possible that more than one binding site exists on tPA for NG2, and the binding sites potentially have different affinity for NG2. One can envision that while binding at one site enhances tPA activity, binding at the other site at higher concentrations of NG2 may induce conformational changes on the tPA “backbone” or make the active site inaccessible, resulting in decreases in tPA activity. Alternatively, tPA and plg might both bind to NG2 in an interaction that appears less transient than the one with fibrin. At higher NG2 concentrations, such binding to tPA and plg might cause a physical separation of tPA from plg if they bound to different proteoglycan molecules, and fail to come into sufficient proximity to allow plasmin to become activated. Since incubation with chondroitinase shifted the plasmin activation curve to the right, it is conceivable that at low NG2 concentrations, removal of the GAG chains results in loss of electrostatic interactions between the lysine-rich kringle domains of tPA and plg and the negatively charged GAG chains. The most efficient binding of tPA or plg to NG2 occurred at elevated molar concentrations of NG2 after chondroitinase treatment in the in vitro binding assay, suggesting that the removal of GAG chains from NG2 may decrease steric hindrance resulting in more efficient binding. It is also possible that the removal of GAG chains reveals higher affinity binding sites.
In the CNS, tPA plays a role in neuronal plasticity, learning and memory, and the remodeling of neuronal connections (Carroll et al., 1994; Krystosek and Seeds, 1981; Qian et al., 1993; Seeds et al., 1995; Tsirka et al., 1995; Wu et al., 2000). However, within the injured CNS, we have shown that tPA-catalyzed conversion of plg into the broad specificity protease plasmin is capable of degrading the inhibitory CSPG NG2. Plasmin can also degrade other CSPGs, including phosphacan (Davies et al., 2006; Wu et al., 2000) and neurocan (Davies et al., 2006). Microglia proliferate after spinal cord injury (Popovich et al., 1997) and are activated by tPA that has been released by injured neurons, establishing a cascade that initiates the further release of tPA (Siao et al., 2003) and plg (Davies et al., 2006). Activated microglia may create an environment where the components of the tPA/plasmin proteolytic cascade are able to breakdown different inhibitory proteoglycans that are upregulated and deposited after injury.
In vivo removal of GAG chains by the addition of chondroitinase ABC leads to improved locomotor recovery after SCI (Bradbury et al., 2002; Moon, 2001; Yick et al., 2000). However, the precise reason why the removal of GAG chains enhances functional recovery has not been studied. It has been postulated that the removal of GAG chains results in an environment more permissive to growing axons. Our findings show that the beneficial effects of chondroitinase treatment may be due in part, to the enhancement of the tPA/plasmin proteolytic cascade. Chondroitinase treatment may allow for the availability of more binding sites for tPA and plg on the core protein of proteoglycans. Such availability may allow proteoglycans to act as scaffolding proteins that enhance the rate of plasmin generation, and possibly the subsequent degradation of the inhibitory extracellular matrix created after SCI. Both the in vitro and in vivo data suggest that components of the tPA/plasmin proteolytic cascade are interacting with neurocan and NG2 at the site of SCI. Because the interaction between NG2, tPA and plg enhances the rate of activity of the broad-spectrum protease plasmin, it is possible that the locomotor improvement seen in mice and rats after chondroitinase treatment is a result of the degradation of inhibitory proteoglycans by the tPA/plasmin system.
Acknowledgments
Grant sponsor: NIH; Grant numbers: 2R0INS042168-05 to SET; 5R01NS021198-19 to JML; Grant sponsor: NYSCIRP IDEA; Grant number: C018605; Grant sponsor: CART; Grant number: C020929.
The authors thank members of the Tsirka and Levine laboratories and Dr. Michael Frohman for critical reading of the manuscript.
Abbreviations
- CSPG
chondroitin sulfate proteoglycans
- SCI
spinal cord injury
- tPA
tissue plasminogen activator
References
- Akassoglou K, Kombrinck KW, Degen JL, Strickland S. Tissue plasminogen activator-mediated fibrinolysis protects against axonal degeneration and demyelination after sciatic nerve injury. J Cell Biol. 2000;149:1157–1166. doi: 10.1083/jcb.149.5.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrade-Gordon P, Strickland S. Interaction of heparin with plasminogen activators and plasminogen: Effects on the activation of plasminogen. Biochemistry. 1986;25:4033–4040. doi: 10.1021/bi00362a007. [DOI] [PubMed] [Google Scholar]
- Bradbury EJ, Moon LD, Popat RJ, King VR, Bennett GS, Patel PN, Fawcett JW, McMahon SB. Chondroitinase ABC promotes functional recovery after spinal cord injury. Nature. 2002;416:636–640. doi: 10.1038/416636a. [DOI] [PubMed] [Google Scholar]
- Burg M, Pasqualinia W, Arap W, Ruoslahti E, Stallcup W. NG2 proteoglycan-binding peptides target tumor neovasculature. Cancer Res. 1999;59:2869–2874. [PubMed] [Google Scholar]
- Camand E, Morel MP, Faissner A, Sotelo C, Dusart I. Long-term changes in the molecular composition of the glial scar and progressive increase of serotoninergic fibre sprouting after hemisection of the mouse spinal cord. Eur J Neurosci. 2004;20:1161–1176. doi: 10.1111/j.1460-9568.2004.03558.x. [DOI] [PubMed] [Google Scholar]
- Carroll PM, Tsirka SE, Richards WG, Frohman MA, Strickland S. The mouse tissue plasminogen activator gene 5′ flanking region directs appropriate expression in development and a seizure-enhanced response in the CNS. Development. 1994;120:3173–3183. doi: 10.1242/dev.120.11.3173. [DOI] [PubMed] [Google Scholar]
- Davies JE, Tang X, Bournat JC, Davies SJ. Decorin promotes plasminogen/plasmin expression within acute spinal cord injuries and by adult microglia in vitro. J Neurotrauma. 2006;23:397–408. doi: 10.1089/neu.2006.23.397. [DOI] [PubMed] [Google Scholar]
- Davies JE, Tang X, Denning JW, Archibald SJ, Davies SJ. Decorin suppresses neurocan, brevican, phosphacan and NG2 expression and promotes axon growth across adult rat spinal cord injuries. Eur J Neurosci. 2004;19:1226–1242. doi: 10.1111/j.1460-9568.2004.03184.x. [DOI] [PubMed] [Google Scholar]
- Davies SJ, Goucher DR, Doller C, Silver J. Robust regeneration of adult sensory axons in degenerating white matter of the adult rat spinal cord. J Neurosci. 1999;19:5810–5822. doi: 10.1523/JNEUROSCI.19-14-05810.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dou CL, Levine JM. Inhibition of neurite growth by the NG2 chondroitin sulfate proteoglycan. J Neurosci. 1994b;14:7616–7628. doi: 10.1523/JNEUROSCI.14-12-07616.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esko JD, Stewart TE, Taylor WH. Animal cell mutants defective in glycosaminoglycan biosynthesis. Proc Natl Acad Sci USA. 1985;82:3197–3201. doi: 10.1073/pnas.82.10.3197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang X, Burg MA, Barritt D, Dahlin-Huppe K, Nishiyama A, Stallcup WB. Cytoskeletal reorganization induced by engagement of the NG2 proteoglycan leads to cell spreading and migration. Mol Biol Cell. 1999;10:3373–3387. doi: 10.1091/mbc.10.10.3373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fawcett JW, Asher RA. The glial scar and central nervous system repair. Brain Res Bull. 1999;49:377–391. doi: 10.1016/s0361-9230(99)00072-6. [DOI] [PubMed] [Google Scholar]
- Friedlander DR, Milev P, Karthikeyan L, Margolis RK, Margolis RU, Grumet M. The neuronal chondroitin sulfate proteoglycan neurocan binds to the neural cell adhesion molecules Ng-CAM/L1/NILE and N-CAM, and inhibits neuronal adhesion and neurite outgrowth. J Cell Biol. 1994;125:669–680. doi: 10.1083/jcb.125.3.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goretzki L, Burg MA, Grako KA, Stallcup WB. High-affinity binding of basic fibroblast growth factor and platelet-derived growth factor-AA to the core protein of the NG2 proteoglycan. J Biol Chem. 1999;274:16831–16837. doi: 10.1074/jbc.274.24.16831. [DOI] [PubMed] [Google Scholar]
- Goretzki L, Lombardo CR, Stallcup WB. Binding of the NG2 proteoglycan to kringle domains modulates the functional properties of angiostatin and plasmin(ogen) J Biol Chem. 2000;275:28625–28633. doi: 10.1074/jbc.M002290200. [DOI] [PubMed] [Google Scholar]
- Grako KA, Stallcup WB. Participation of the NG2 proteoglycan in rat aortic smooth muscle cell response to platelet-derived growth factor. Exp Cell Res. 1995;221:231–240. doi: 10.1006/excr.1995.1371. [DOI] [PubMed] [Google Scholar]
- Hoylaerts M, Rijken DC, Lijnen HR, Collen D. Kinetics of the activation of plasminogen by human tissue plasminogen activator. Role of fibrin. J Biol Chem. 1982;257:2912–2919. [PubMed] [Google Scholar]
- Hsu JY, McKeon R, Goussev S, Werb Z, Lee JU, Trivedi A, Noble-Haeusslein LJ. Matrix metalloproteinase-2 facilitates wound healing events that promote functional recovery after spinal cord injury. J Neurosci. 2006;26:9841–9850. doi: 10.1523/JNEUROSCI.1993-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones LL, Margolis RU, Tuszynski MH. The chondroitin sulfate proteoglycans neurocan, brevican, phosphacan, and versican are differentially regulated following spinal cord injury. Exp Neurol. 2003;182:399–411. doi: 10.1016/s0014-4886(03)00087-6. [DOI] [PubMed] [Google Scholar]
- Jones LL, Yamaguchi Y, Stallcup WB, Tuszynski MH. NG2 is a major chondroitin sulfate proteoglycan produced after spinal cord injury and is expressed by macrophages and oligodendrocyte progenitors. J Neurosci. 2002;22:2792–2803. doi: 10.1523/JNEUROSCI.22-07-02792.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krystosek A, Seeds NW. Plasminogen activator release at the neuronal growth cone. Science. 1981;213:1532–1534. doi: 10.1126/science.7197054. [DOI] [PubMed] [Google Scholar]
- Levine JM. Increased expression of the NG2 chondroitin-sulfate proteoglycan after brain injury. J Neurosci. 1994;14:4716–4730. doi: 10.1523/JNEUROSCI.14-08-04716.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeon RJ, Jurynec MJ, Buck CR. The chondroitin sulfate proteoglycans neurocan and phosphacan are expressed by reactive astrocytes in the chronic CNS glial scar. J Neurosci. 1999;19:10778–10788. doi: 10.1523/JNEUROSCI.19-24-10778.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon LD. Regeneration of CNS axons back o their target following treatment of adult rat brain with chondroitinase ABC. Nature Neurosci. 2001;4:465–466. doi: 10.1038/87415. [DOI] [PubMed] [Google Scholar]
- Neuberger TJ, Cornbrooks CJ. Transient modulation of Schwann cell antigens after peripheral nerve transection and subsequent regeneration. J Neurocytol. 1989;18:695–710. doi: 10.1007/BF01187088. [DOI] [PubMed] [Google Scholar]
- Nishiyama A, Dahlin KJ, Prince JT, Johnstone SR, Stallcup WB. The primary structure of NG2, a novel membrane-spanning proteoglycan. J Cell Biol. 1991;114:359–371. doi: 10.1083/jcb.114.2.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozerdem GK, Dahlin-Huppe K, Monosov E, Stallcup WB. NG2 proteoglycan is expressed exclusively by mural cells during vascular morphogenesis. Dev Dyn. 2001;222:218–227. doi: 10.1002/dvdy.1200. [DOI] [PubMed] [Google Scholar]
- Popovich PG, Wei P, Stokes BT. Cellular inflammatory response after spinal cord injury in Sprague-Dawley and Lewis rats. J Comp Neurol. 1997;377:443–464. doi: 10.1002/(sici)1096-9861(19970120)377:3<443::aid-cne10>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Qian Z, Gilbert ME, Colicos MA, Kandel ER, Kuhl D. Tissue-plasminogen activator is induced as an immediate-early gene during seizure, kindling and long-term potentiation. Nature. 1993;361:453–457. doi: 10.1038/361453a0. [DOI] [PubMed] [Google Scholar]
- Sappino AP, Madani R, Huarte J, Belin D, Kiss JZ, Wohlwend A, Vassalli JD. Extracellular proteolysis in the adult murine brain. J Clin Invest. 1993;92:679–685. doi: 10.1172/JCI116637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmalfeldt M, Bandtlow CE, Dours-Zimmermann MT, Winterhalter KH, Zimmermann DR. Brain derived versican V2 is a potent inhibitor of axonal growth. J Cell Sci. 2000;113(Part 5):807–816. doi: 10.1242/jcs.113.5.807. [DOI] [PubMed] [Google Scholar]
- Seeds NW, Williams BL, Bickford PC. Tissue plasminogen activator induction in Purkinje neurons after cerebellar motor learning. Science. 1995;270:1992–1994. doi: 10.1126/science.270.5244.1992. [DOI] [PubMed] [Google Scholar]
- Siao CJ, Fernandez SR, Tsirka SE. Cell type-specific roles for tissue plasminogen activator released by neurons or microglia after excitotoxic injury. J Neurosci. 2003;23:3234–3242. doi: 10.1523/JNEUROSCI.23-08-03234.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siconolfi LB, Seeds NW. Mice lacking tPA, uPA, or plasminogen genes showed delayed functional recovery after sciatic nerve crush. J Neurosci. 2001;21:4348–4355. doi: 10.1523/JNEUROSCI.21-12-04348.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver J, Miller JH. Regeneration beyond the glial scar. Nat Rev Neurosci. 2004;5:146–156. doi: 10.1038/nrn1326. [DOI] [PubMed] [Google Scholar]
- Stallcup WB, Dahlin K, Healy P. Interaction of the NG2 chondroitin sulfate proteoglycan with type VI collagen. J Cell Biol. 1990;111:3177–3188. doi: 10.1083/jcb.111.6.3177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stallcup WB, Dahlin-Huppe K. Chondroitin sulfate and cytoplasmic domain-dependent membrane targeting of the NG2 proteoglycan promotes retraction fiber formation and cell polarization. J Cell Sci. 2001;114:2315–2325. doi: 10.1242/jcs.114.12.2315. [DOI] [PubMed] [Google Scholar]
- Tan AM, Colletti M, Rorai AT, Skene JH, Levine JM. Antibodies against the NG2 proteoglycan promote the regeneration of sensory axons within the dorsal columns of the spinal cord. J Neurosci. 2006;26:4729–4739. doi: 10.1523/JNEUROSCI.3900-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang X, Davies JE, Davies SJ. Changes in distribution, cell associations, and protein expression levels of NG2, neurocan, phosphacan, brevican, versican V2, and tenascin-C during acute to chronic maturation of spinal cord scar tissue. J Neurosci Res. 2003;71:427–444. doi: 10.1002/jnr.10523. [DOI] [PubMed] [Google Scholar]
- Tillet E, Ruggiero F, Nishiyama A, Stallcup WB. The membrane-spanning proteoglycan NG2 binds to collagens V, VI through the central nonglobular domain of its core protein. J Biol Chem. 1997;272:10769–10776. doi: 10.1074/jbc.272.16.10769. [DOI] [PubMed] [Google Scholar]
- Tsirka SE, Gualandris A, Amaral DG, Strickland S. Excitotoxin-induced neuronal degeneration and seizure are mediated by tissue plasminogen activator. Nature. 1995;377:340–344. doi: 10.1038/377340a0. [DOI] [PubMed] [Google Scholar]
- Tucker HM, Kihiko M, Caldwell JN, Wright S, Kawarabayashi T, Price D, Walker D, Scheff S, McGillis JP, Rydel RE, Estus S. The plasmin system is induced by and degrades amyloid-beta aggregates. J Neurosci. 2000;20:3937–3946. doi: 10.1523/JNEUROSCI.20-11-03937.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ughrin YM, Chen ZJ, Levine JM. Multiple regions of the NG2 proteoglycan inhibit neurite growth and induce growth cone collapse. J Neurosci. 2003;23:175–186. doi: 10.1523/JNEUROSCI.23-01-00175.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Zonneveld AJ, Veerman H, MacDonald ME, van Mourik JA, Pannekoek H. Structure and function of human tissue-type plasminogen activator (t-PA) J Cell Biochem. 1986;32:169–178. doi: 10.1002/jcb.240320302. [DOI] [PubMed] [Google Scholar]
- Vassalli JD, Sappino AP, Belin D. The plasminogen activator/ plasmin system. J Clin Invest. 1991;88:1067–1072. doi: 10.1172/JCI115405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu YP, Siao CJ, Lu W, Sung TC, Frohman MA, Milev P, Bugge TH, Degen JL, Levine JM, Margolis RU, Tsirka SE. The tissue plasminogen activator (tPA)/plasmin extracellular proteolytic system regulates seizure-induced hippocampal mossy fiber outgrowth through a proteoglycan substrate. J Cell Biol. 2000;148:1295–1304. doi: 10.1083/jcb.148.6.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yick LW, Wu W, So KF, Yip HK, Shum DK. Chondroitinase ABC promotes axonal regeneration of Clarke’s neurons after spinal cord injury. Neuroreport. 2000;11:1063–1067. doi: 10.1097/00001756-200004070-00032. [DOI] [PubMed] [Google Scholar]