Abstract
The BCR relays extracellular signals and internalizes Ag for processing and presentation. We have previously demonstrated that ligation of the BCR destabilizes Ig-α/Ig-β (Ig-αβ) from μ-H chain (μm). In this study we report that receptor destabilization represents a physical separation of μm from Ig-αβ. Sucrose gradient fractionation localized Ig-αβ to GM1-containing lipid microdomains in the absence of μm. Confocal and electron microscopy studies revealed the colocalization of unsheathed μm with clathrin-coated vesicles. Furthermore, μm failed to associate with clathrin-coated vesicles when receptor destabilization was inhibited, suggesting that unsheathing of μm is required for clathrin-mediated endocytosis. In summary, we found that Ag stimulation physically separates Ig-αβ from μm, facilitating concomitant signal transduction and Ag delivery to the endocytic compartment.
The humoral immune response is initiated upon Ag binding to the BCR. The BCR, composed of membrane Ig non-covalently associated with the Ig-α/Ig-β (Ig-αβ) heterodimer, functions to transduce intracellular signals and accelerate Ag targeting to the endocytic pathway (1–3). These functions are interrelated, because BCR-induced signals promote efficient targeting of Ag into the late endosome through the activation of Syk and B cell linker protein (4, 5). In addition, BCR-mediated signals induce transient formation of Ag-processing compartments and promote acidification of the late endosome (6, 7). These studies demonstrate an important role for BCR-mediated signal transduction in the targeting and processing of Ag in the endocytic pathway.
The initial events that guide entry of Ag-bound receptors to the endocytic pathway remain poorly understood. Early studies suggested that endocytosis occurred via clathrin-coated vesicles (CCVs);3 however, subsequent studies showed that raft-associated BCR and the glycosphingolipid GM1 were targeted to the class II peptide-loading compartments (8, 9). This indicates that GM1-rich lipid rafts may act as platforms for the entry of receptors into the endocytic pathway. Another study linked raft- and clathrin-mediated endocytosis, showing that Lyn phosphorylated clathrin H chain (10). This proposes that receptor-mediated endocytosis occurs through a subset of clathrin that is constitutively associated with lipid rafts. These conclusions were questioned, however, when it was shown that the extent of BCR endocytosis was not affected by the disruption of lipid rafts, demonstrating that the bulk of Ag-bound receptors is internalized through a pathway independent of lipid rafts (11). Furthermore, this study suggested that raft-associated receptors had a different intracellular itinerary than the majority of receptors internalized through CCVs. An independent study showed that although lipid rafts were uniformly distributed across the plasma membrane, few associated with CCVs (12). Although the precise mechanism of BCR-mediated endocytosis remains to be clarified, these studies show that BCR-mediated signal transduction and early endocytic events are interrelated.
We previously showed that BCR-mediated signal transduction destabilized Ig-αβ from μ-H chain (μm), as evidenced by a reduced association of Ig-αβ with μm after BCR ligation (13). However, it remained unclear whether destabilization represented a physical dissociation of the receptor complex, how the destabilized BCR components partitioned on the plasma membrane, and what role BCR destabilization played in targeting Ags to the endocytic pathway. In this study we demonstrate that the BCR complex physically dissociates upon Ag stimulation. Analysis of destabilized receptors showed Ig-αβ in GM1-rich microdomains in the absence of μm. In contrast, Ag-bound μm associated with CCVs in the absence of Ig-αβ. Furthermore, conditions that prevented BCR destabilization also inhibited the association of Ag-bound μm with CCVs. These data show that physical dissociation of the receptor complex allows μm and Ig-αβ to traffic independently on the plasma membrane and promotes clathrin-mediated endocytosis.
Materials and Methods
Cell lines
The K46μ and M12g3r cell lines express nitrophenyl (NP)- or phosphorylcholine (PC)-specific mouse IgM and were maintained as previously described (14).
Abs and reagents
The mAbs b-7-6 (anti-μ), HM79 (anti-Ig-β), anti-Ig-α, anti-Lyn, and anti-Syk have been previously described (13, 15). Primary Abs include goat anti-mouse IgM (Jackson ImmunoResearch Laboratories), mouse anti-clathrin H chain (Ab-1; Oncogene Research Products), mouse anti-phosphotyrosine (Ab-2; Oncogene Science), unconjugated or biotin-conjugated cholera toxin B (List Biological Laboratories), and rabbit anti-cholera toxin (Sigma-Aldrich). Gold (10 nm)-conjugated Ags (NP8BSA and PC10BSA) were prepared as previously described (16). Herbimycin (Calbiochem) was used either at 5 μM for 16 h or at 100 μM for 30 min. At these concentrations of inhibitor, viability remained >95%, Lyn phosphorylation was completely inhibited, and BCR destabilization was inhibited (see Fig. 2A) (13).
FIGURE 2.
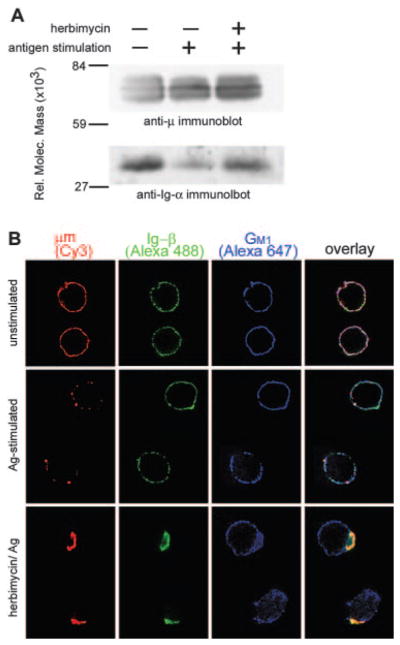
A, BCR ligation induces receptor destabilization. Anti-μ and anti-Ig-α immunoblots of anti-μ immunoprecipitates from M12g3r cells are shown. Cells were unstimulated (lane 1) or stimulated with PC10BSA (1 μg/5 × 106 cells/ml) for 30 min (lane 2) or treated with herbimycin at 100 μM for 30 min, followed by Ag stimulation. Cells were lysed in 1% CHAPS, and the BCR complex was immunoprecipitated with anti-μ. The data are representative of three experiments. B, M12g3r cells were fixed before staining (top panels), stimulated with PC10BSA for 10 min (middle panels), or pretreated with herbimycin (as in A), followed by Ag stimulation (bottom panels). Cells were stained for μm (Cy3), Ig-β (Alexa 488), and GM1 (Alexa 647). Approximately 50% of all cells showed good capping and comparable staining of three fluorochromes. The percent colocalization of Ig-β and μm was quantitated from 19–27 random cells obtained from two to six independent experiments.
Isolation of detergent-insoluble microdomains
K46μ cells (20–30 × 106) were stimulated with NP8BSA (1 μg/10 × 106 cells/ml) for the indicated times, resuspended to a final concentration of 108 cells/ml in ice-cold MES buffer containing 0.1% Triton X-100, and incubated for 30 min on ice. Cells were homogenized (Dounce; Kontes), and lysates were mixed with 80% sucrose/MES to a final concentration of 40% sucrose. Samples were overlaid with MES/30% sucrose, followed by MES/5% sucrose, and then spun for 20 h at 37,000 rpm. Fractions from the gradient were separated on 10% SDS-PAGE, then immunoblotted.
Immunofluorescence staining
M12g3r cells were stimulated with PC10BSA (1 μg/ml) for 5–10 min, then stained with goat anti-mouse IgM and Cy3-anti-goat IgG. Cells were spun onto poly-D-lysine (Sigma-Aldrich)-coated coverslips, fixed with 3% paraformaldehyde, and stained with biotinylated anti-Ig-β, followed by streptavidin-Alexa 488 or streptavidin-Alexa 647. To assess the association of μm or Ig-β with lipid rafts, cells were additionally stained for GM1 with cholera toxin B, rabbit anti-cholera toxin B, and anti-rabbit IgG-Alexa 647. To assess the association of receptor component with clathrin, cells were permeabilized with 100% ice-cold methanol, then stained with mouse anti-clathrin and anti-mouse IgG-Alexa 488. Samples were observed using the Zeiss Axioplan 2 fluorescence microscope and were digitally deconvolved using SlideBook (Intelligent Imaging Innovation). The percent colocalization among μm, Ig-β, and clathrin was determined by statistical analysis of a masked area of the image.
Plasma membrane sheets and transmission electron microscopy
Cells grown on poly-D-lysine-coated coverslips were stimulated with gold-conjugated Ags for the indicated times. Plasma membrane sheets were prepared as described previously (16, 17). Unstimulated cells were fixed with paraformaldehyde before staining. Samples were examined using the Zeiss EM 10C, and images were obtained at a magnification of ×25,000. The criteria used to assess the association of μm and Ig-αβ was established using membranes from the IgM/α chimeric cell line. On these membranes, the average distance between 10 nm (μm) and 5 nm (Ig-α) gold particles was 11 ± 2 nm (±SEM). To be conservative, 10- and 5-nm gold particles separated by >30 nm were considered dissociated. The criteria used to claim the dissociation of μm from Ig-αβ was established by measuring the actual distance from 10 nm gold particles (μm) to the nearest 5 nm gold particles (Ig-α), and data are presented as the mean ± SEM. The association of receptor components with CCVs was defined by the presence of μm or Ig-α in vesicles that stained specifically for clathrin and exhibited a prototypic polyhedral structure.
Analysis of BCR endocytosis
The kinetics of internalization of PC10BSA-HRP was determined using a colorimetric assay that quantitates HRP activity (18). Briefly, M12g3r cells were either not treated or treated with herbimycin (5 μM) for 16 h at 37°C, then labeled with PC10BSA-HRP (1 μM) for 30 min on ice. After washing, cells were incubated at 37°C to allow receptor internalization. At each corresponding time point, internalization was stopped with ice-cold buffer. The HRP activity remaining on cell surface was measured by adding substrate o-phenyenediamine-2HCl (Sigma-Aldrich) and H2O2, and data were read at OD492.
Results
Ag stimulation localizes Ig-αβ to lipid microdomains in the absence of μm
Ag ligation of the BCR destabilizes Ig-αβ from μm, as evidenced by the inability to coprecipitate stoichiometric amounts of Ig-αβ with μm (13). Given the dual role of BCR in signal transduction and receptor-mediated endocytosis, we reasoned that receptor destabilization might provide a mechanism for Ag to rapidly enter the endocytic pathway while simultaneously allowing Ig-αβ to sustain signal transduction. This predicts that BCR destabilization represents a physical separation of μm from Ig-αβ, thereby allowing independent trafficking on the plasma membrane.
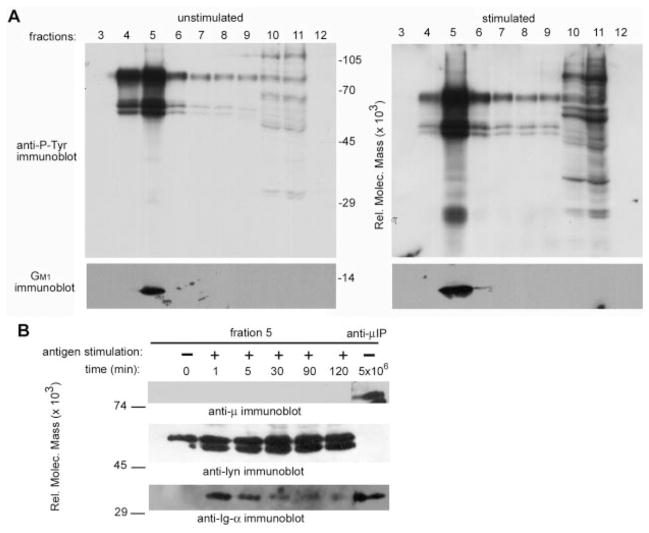
Ag ligation of the BCR was previously shown to induce translocation of the receptor complex to lipid rafts (9). Although this study showed both Ig-α and μm in lipid rafts, it was unclear whether the levels of μm relative to Ig-α were stoichiometric. To determine whether BCR destabilization was evident in lipid microdomains, we fractionated unstimulated and Ag-stimulated cells on a sucrose gradient (Fig. 1A). Simultaneously we immunoprecipitated the BCR complex from unstimulated cells (Fig. 1B, lane 7) to define the stoichiometry of sheathed receptors, thus providing a means to accurately determine immunoblot exposure times. Ag-stimulated K46μ cells showed induced protein tyrosine phosphorylation within detergent-insoluble (fraction 5) and -soluble fractions (fractions 10 and 11; Fig. 1A). Immunoblot analysis revealed that fraction 5 contained lipid microdomains, as evidenced by the presence of GM1 and Lyn (Fig. 1A, bottom panel, and Fig. 1B, middle panel). In addition, fraction 5 showed the translocation of Ig-α, but not μm, within 1 min of Ag stimulation (Fig. 1B, top and bottom panels). The amount of Ig-α recovered from fraction 5 was similar to the amount immunoprecipitated from 5 × 106 unstimulated cells (Fig. 1B; anti-Ig-α blot, lane 2 compared with lane 7). However, the amount of μm in fraction 5 was undetectable compared with the amount expected from 5 × 106 sheathed receptors (Fig. 1B, anti-μ blot, lanes 2–6 compared with lane 7). The data show Ig-α in lipid microdomains in the absence of μm, suggesting that BCR destabilization represents a physical separation of the receptor complex.
FIGURE 1.
Destabilized Ig-αβ resides in lipid microdomains in the absence of μm. A, Cell lyates from unstimulated and Ag (NP8BSA)-stimulated (7 min) K46μ cells were separated on sucrose gradients and immunoblotted for tyrosine-phosphorylated proteins (upper panel) and GM1 (lower panel). B, Anti-μ, anti-Lyn, and anti-Ig-α immunoblots from GM1-containing lipid microdomains (fraction 5). Lane 1, Unstimulated; lanes 2–6, NP8BSA-stimulated cells; lane 7, anti-μ immunoprecipitate from unstimulated, unfractionated K46μ cells. The data presented are from a single experiment, representative of five performed.
Ig-β fails to cocap with μm after Ag stimulation
To assess whether physical dissociation was evident in live cells, we examined unstimulated and Ag-stimulated B cells for their membrane distribution of Ig-β and μm after anti-μ-induced capping. We reasoned that if Ag ligation induced a physical separation of the receptor complex, subsequent capping of μm would leave 50–75% of Ig-αβ outside the cap (13). The analysis was performed on M12g3r cells, because they fail to express surface Ig-αβ in the absence of transfected μm (19). Within 30 min of Ag stimulation, M12g3r cells showed a loss of coprecipitated Ig-αβ; treating cells with the Src kinase inhibitor, herbimycin, prevented this effect (Fig. 2A). This suggests that BCR-mediated signals regulate receptor destabilization, consistent with previous findings (13). Immunofluorescence staining of unstimulated cells showed a 40% colocalization of μm and Ig-β (Fig. 2B, top panels). In addition, lipid microdomains stained for GM1 were evenly distributed on membrane. After Ag stimulation the amount of Ig-β that colocalized with μm was reduced to only 14% (Fig. 2B, middle panels). This observation was not due to an unexpected consequence of Ab-induced capping, because anti-μ maintained the colocalization of μm and Ig-β when cells were treated with herbimycin to inhibit destabilization (Fig. 2B, bottom panels). In addition, inhibiting kinase activation induced μm and Ig-β to cocap independently of GM1 aggregation. This suggests that receptor destabilization occurs outside lipid microdomains. Because the lipid microdomains did not visibly aggregate upon Ag stimulation, the capped μm/Ig-αβ complexes (herbimycin treatment) colocalized with some of these regions (Fig. 2B, bottom panels). Therefore, we cannot exclude the possibility that association of the BCR with a small fraction of lipid microdomains is sufficient to induce the dissociation of 50–75% of Ig-αβ from μm during BCR destabilization. Nonetheless, the 3-fold decrease in the colocalization of Ig-β and μm after Ag stimulation supports the idea that BCR destabilization represents a physical separation of μm from Ig-αβ.
Destabilization of the BCR complex represents a physical dissociation of Ig-αβ from μm
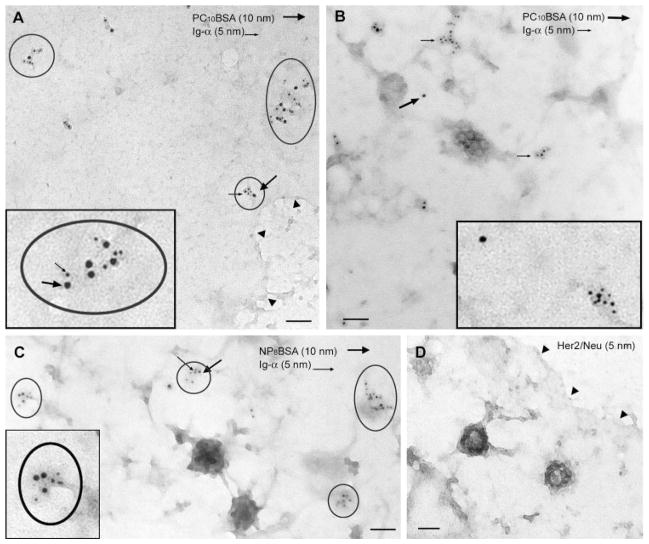
The data strongly suggest that Ig-αβ dissociated from μm after Ag stimulation. However, we could not formally exclude the possibility that Ig-αβ was re-expressed outside the anti-μ-induced cap during stimulation and staining. To address this, we assessed the location of Ig-αβ relative to μm immediately after stimulation using native membrane sheets viewed by transmission electron microscopy. This method afforded greater resolution than confocal microscopy and, unlike ultrathin sectioning, allowed the analysis of large pieces of native membrane (16, 17). In addition, it allowed a more accurate assessment of the kinetics of destabilization. Native membranes prepared from M12g3r cells that were fixed before the addition of gold-conjugated Ag exhibited colocalization of 10 nm (gold-conjugated Ag) and 5 nm (Ig-α) gold particles, confirming the association of μm and Ig-α in unstimulated cells (Fig. 3A and Table I). The percent colocalization was determined by the frequency of 10 nm particles that associated with 5 nm particles (see Materials and Methods for the criteria of assessment). Of the 3830 PC10BSA-gold particles (10 nm) counted, 46% were associated with Ig-α-gold particles (5 nm), with an average distance of 18 ± 2 nm separating small and large gold particles. In contrast, cells stimulated for 5 min showed 7% colocalization, with an average distance of 218 ± 5 nm separating the receptor components (Fig. 3B and Table I). The 6-fold decrease in the association of μm with Ig-α and the large increase in the distance separating the receptor components confirm a physical dissociation of the BCR complex within 5 min of receptor ligation.
FIGURE 3.
Ag stimulation induces a physical dissociation of μm from Ig-αβ. Unstimulated M12g3r cells were fixed before μm staining (A) or were stimulated with PC10BSA-gold (10 nm; large arrows) for 5 min before the preparation of native membranes, then stained for Ig-α (5 nm; small arrows; B). C, Cells expressing chimeric BCR were stimulated with NP8BSA-gold (10 nm; large arrows) for 5 min before the preparation of native membrane then stained for Ig-α (5 nm; small arrows). D, Cells were stained with unrelated isotype-matched Ab, Her2/Neu (5 nm), after preparing native membrane sheets. Circles depict clusters of colocalized μm (10 nm) and Ig-α (5 nm). Insets, Higher magnification of association/dissociation of μm and Ig-α. The large and small arrows represent large and small gold particles. Arrowheads in A and D depict the membrane edge. Bar = 100 nm.
Table I.
Assessment of subcellular localization of μm, Ig-α, and CCVs on native membrane sheets in M12g3r cellsa
| IgM/α Chimera
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Time (minutes) | 0 | 2 | 5 | 5 | Time (minutes) | 0 | 2 | 5 | 10 | 20 |
| Total PC10BSA-gold particlesb | 3830 | 3525 | 3249 | 2543 | Total PC10BSA-goldc | 1750 | 1833 | 3212 | 1995 | 2064 |
| (NP8BSA-gold) | PC10BSA-gold particles colocalized with CCVs (%) | 14 | 55 | 47 | 45 | 54 | ||||
| PC10BSA-gold particles colocalized with Ig-α-gold particles (%) | 46 | 7 | 7 | 47 | Total Ig-α gold particlesd | 987 | 7556 | 2812 | 1283 | 2147 |
| Ig-α-gold particles colocalized with CCVs (%) | 20 | 16 | 23 | 13 | 11 |
Cells were either fixed prior to μm staining (t = 0) or stimulated with PC10BSA-gold (10 nm) or unconjugated Ag. Native membrane sheets were prepared and stained on the intracellular side for clathrin and Ig-α. Membrane localization of gold particles was analyzed by TEM at ×25,000.
Gold particles (10 nm) were counted from at least 55 different native membrane sheets obtained from 8–10 different experiments.
Gold particles (10 nm) were counted from 14–39 different native membrane sheets obtained from 2–7 different experiments.
Gold particles (12 nm) were counted from 18–76 different native membrane sheets obtained from 3–12 different experiments.
To determine whether the low frequency of μm that associated with Ig-μ in resting cells (46%) reflected the limitations of staining the BCR complex or constitutive dissociation of resting receptors, we assessed the frequency of μm/Ig-α association in cells expressing a chimeric BCR. The chimeric receptor contained an NP-specific extracellular domain fused to an Ig-α cytoplasmic tail (IgM/α) and was predicted to show 100% colocalization of μm and Ig-α (20). However, only 47% of chimeric μm colocalized with Ig-α (Fig. 3C and Table I). Thus, the relatively low frequency of μm/Ig-α association in unstimulated cells reflected the inability to label the components of the BCR at saturating levels. This finding is consistent with the confocal microscopy study, in which 40% of μm colocalized with Ig-β. Nonetheless, the 6-fold decrease in the colocalization of μm with Ig-α clearly indicates a dissociation of the receptor complex after ligation. The Ig-α staining was specific, in that an unrelated, isotype-matched Ab failed to stain membranes (Fig. 3D).
Ag-bound μm associates with CCVs after BCR ligation
Previous studies suggested that endocytosis of Ag-bound receptors occurred through CCVs (8). To assess whether receptor destabilization played a role in this process, we viewed intact cells using confocal microscopy for the distribution of μm, Ig-β, and clathrin after Ag stimulation. We chose a time point of 5–10 min based on the electron microscopy study (Fig. 3, A and B). Images of z-axis planes from unstimulated cells revealed that μm and Ig-β were evenly distributed on the plasma membrane, whereas clathrin was distributed throughout the cytoplasm, with a slightly higher concentration at the membrane (Fig. 4, upper panels). In unstimulated cells, only 5% of μm and 3% of Ig-β colocalized with CCVs. After Ag stimulation, this pattern changed dramatically, such that 68% of μm became clathrin associated in large foci at the membrane (Fig. 4, lower panels). However, the majority of Ig-β remained distributed over the plasma membrane, with only 2% of Ig-β associating with CCVs, similar to the findings in Fig. 2B (middle panels). These data indicate that Ag stimulation promotes the association of μm with CCVs, but not the association of Ig-β.
FIGURE 4.
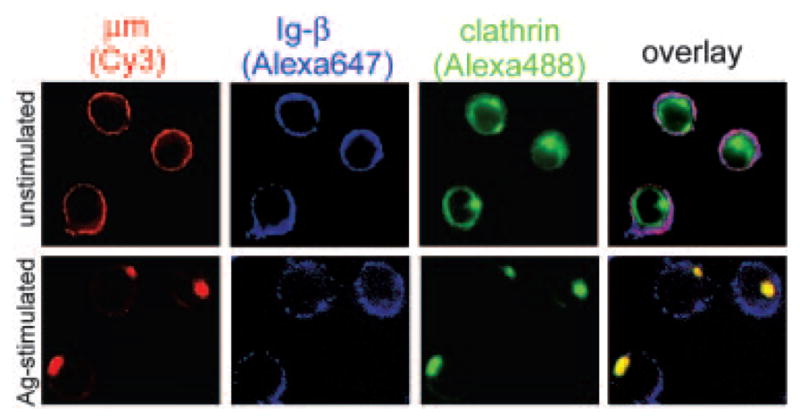
Ag stimulation colocalizes μm with CCVs. Cells were fixed before staining (upper panels) or were stimulated with PC10BSA for 10 min (lower panels), then stained for μm (Cy3), Ig-β (Alexa 647), and clathrin (Alexa 488). The percent colocalization of μm or Ig-β with CCVs was quantitated from 15 cells obtained from three independent experiments. Approximately 50% of all cells showed good capping and comparable staining of all three fluorochromes. Of these, 80% exhibited caps that contained μm and clathrin.
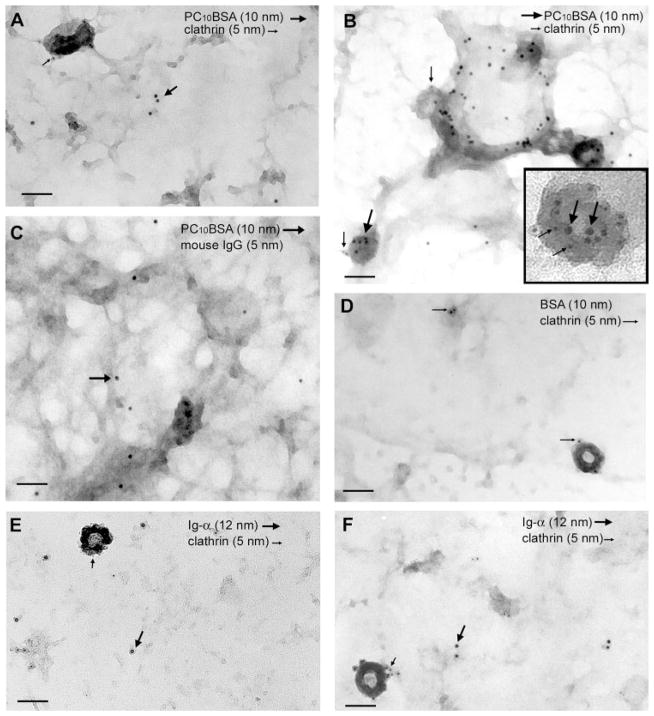
To further assess the distribution of μm, Ig-αβ, and clathrin in native membranes, we analyzed native membrane sheets by transmission electron microscopy. As shown in Fig. 5A and Table I, 14% of PC10BSA-gold (10 nm) resided within CCVs (5 nm) in native membranes from resting cells. Within 5 min of BCR ligation, the distribution of μm changed dramatically, revealing a 4-fold increase in the association of PC10BSA-gold (10 nm) with clathrin (5 nm) (Fig. 5B and Table I). The staining for clathrin H chain and Ag was specific, because a gold-labeled, isotype-matched, polyclonal Ab and gold-conjugated BSA failed to stain the membrane (Fig. 5, C and D). These data show that Ag-bound μm associates with CCVs coincident with destabilization of the BCR complex.
FIGURE 5.
BCR ligation induces the association of Ag-μm, but not Ig-α, with clathrin-coated vesicles. Cells were fixed, then stained with PC10BSA-gold (10 nm; large arrows) and clathrin (5 nm; small arrows; A) or stimulated for 5 min before preparation and staining of native membranes (B). Inset, Higher magnification of μm association with CCVs. The large and small arrows represent some of the large and small gold particles. C, Cells were prepared as described in A and then stained with an isotype-matched, polyclonal mouse IgG Ab (5 nm). Arrows depict 10 nm gold particles. D, Cells were stimulated with BSA-gold (10 nm) for 5 min before the preparation of native membranes and then stained for clathrin (5 nm). Arrows depict 5 nm particles staining CCVs. Cells were fixed and then stained for Ig-α (12 nm; large arrows) and clathrin (5 nm; small arrows; E) or stimulated with PC10BSA for 5 min before preparation and staining of native membranes (F). The data are representative of 5–10 experiments. Bar = 100 nm.
Ig-α fails to translocate into CCVs after BCR ligation
Collectively, the data demonstrate a physical separation of Ig-αβ from μm after Ag ligation of the BCR. The findings that Ag-bound μm associates with CCVs whereas Ig-α resides in lipid microdomains suggest that μm associates with clathrin in the absence of Ig-αβ. To determine whether Ag stimulation induced comparable recruitment of Ig-α into CCVs, we stained native membranes for clathrin (5 nm) and Ig-α (12 nm). In resting cells, 20% of Ig-α associated with CCVs (Fig. 5E and Table I). However, there was no increased association of Ig-α with CCVs after Ag stimulation (Fig. 5F and Table I). These findings were not due to the inability of the anti-Ig-α Ab to recognize phosphorylated protein, because this Ab immunoprecipitated phosphorylated Ig-α (15). In addition, the density of Ig-α staining in membranes from unstimulated cells compared with Ag-stimulated cells did not change. The failure to colocalize was not due to the inability of 12 nm gold particles (Ig-α) to enter the clathrin lattice, because reversing the gold particle size did not change the results (data not shown). This suggests that although some Ig-α constitutively colocalizes with clathrin, Ag ligation of the BCR does not induce additional association.
Unsheathed μm preferentially associates with CCVs
The selective association of μm, but not Ig-α, with CCVs suggests that destabilization may play a role in entry of μm into CCVs. However, the low basal association of Ig-α with CCVs, regardless of Ag stimulation, raised the possibility that a small pool of BCR entered CCVs as an intact complex. To resolve this, we assessed the frequency at which μm and Ig-α colocalized within the same CCVs. CCVs containing PC10BSA-gold (10 nm) particles were identified by their prototypic polyhedral structure. In resting cells, 10% of the PC10BSA-gold (10 nm) particles found within CCVs were associated with Ig-α (5 nm) (Fig. 6A). After Ag stimulation, there was a 4-fold increase in the association of μm with CCVs (Table I); however, only 3–4% of the μm was associated with Ig-α (Fig. 6A). This indicates that unsheathed μm preferentially enters CCVs.
FIGURE 6.
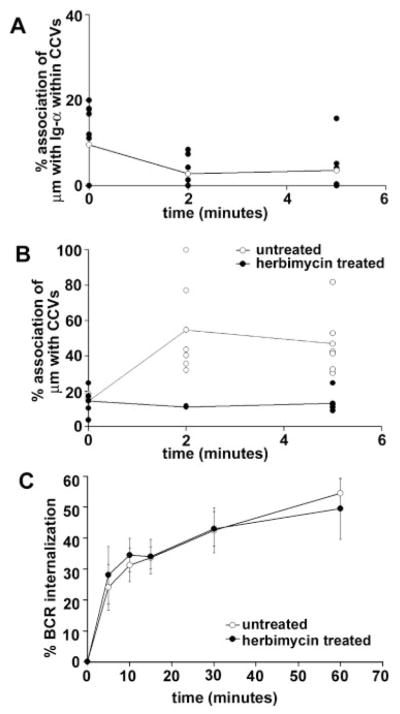
BCR destabilization facilitates the translocation of Ag-bound μm to CCVs. A, Native membranes from M12g3r cells were fixed before μm staining (0 min) or stimulated with PC10BSA-gold (10 nm) for 2 and 5 min, then stained for Ig-α (5 nm). The number of PC10BSA-gold particles enumerated within CCVs was 419 (0 min), 946 (2 min), and 1039 (5 min), obtained from 55–83 membrane sheets. B, M12g3r cells were either untreated (○) or pretreated with herbimycin (●) before stimulation with PC10BSA-gold. The data are depicted as individual experiments, with the average percent association of μm with CCVs represented by the line. The numbers of PC10BSA-gold particles counted for herbimycin-treated cells were 1750 (0 min), 1163 (2 min), and 2470 (5 min) from 20–39 membrane sheets. C, Receptor internalization of PC10BSA-HRP was compared in untreated M12g3r cells (○) and cells pretreated with herbimycin (●) at 5 μM for 16 h. The data are from four experiments.
Destabilization of Ig-αβ is required for μm association with CCVs
To assess the role of BCR destabilization in Ag entry to CCVs, we treated cells with a kinase inhibitor to prevent BCR destabilization (Fig. 2A) (13). We reasoned that if BCR destabilization were required for clathrin-mediated endocytosis, inhibiting destabilization would diminish the translocation of μm to CCVs. As shown in Fig. 6B, μm failed to associate with CCVs in herbimycin-treated cells, suggesting that BCR destabilization facilitates the movement of Ag-bound μm to CCVs. It remained possible that the lack of μm association with CCVs was simply due to a failure of receptor internalization. To directly assess whether BCRs clear the membrane in the absence of kinase activation, we monitored the clearance of HRP-tagged Ag in untreated and herbimycin-treated M12g3r cells. As shown in Fig. 6C, receptor internalization in untreated or herbimycin-treated cells occurred with comparable kinetics and magnitude. Furthermore, herbimycin treatment did not affect receptor clearance, as detected by flow cytometry (data not shown). Collectively, conditions that prevented BCR destabilization also inhibited the association of μm with CCVs, suggesting that receptor destabilization is required for the entry of Ag into the endocytic pathway.
Discussion
Our results show that receptor cross-linking induces a physical separation of Ig-αβ from Ag-bound μm. Destabilized μm associates with CCVs, whereas Ig-α resides in lipid microdomains. These findings do not simply reflect the movement of different pools of sheathed BCR to two locations, rather, they indicate independent trafficking, because Ig-α is rarely found in CCVs (Fig. 5, E and F, and Table I), and insignificant amounts of μm are found in lipid microdomains (Fig. 1B). Collectively, the data support a model in which equilibrium exists between Ig-αβ-sheathed receptors and unsheathed receptors. In the absence of Ag, sheathed receptors predominate, although some unsheathed receptors constitutively recycle through CCVs (Table I; 0 min). Upon Ag ligation, BCR-mediated signal transduction triggers receptor dissociation, shifting the equilibrium toward a predominance of unsheathed receptors and the association of Ag-bound μm with CCV. Another possible interpretation is that BCR ligation stabilizes dissociated receptors. In confocal and electron microscopy, the frequency of μm that colocalizes with Ig-αβ in resting cells was low (40 and 46%, respectively). However, staining of chimeric receptors revealed a similar colocalization frequency (47%). This indicated an inability to label the components of the BCR at saturating levels, rather than a large pool of dissociated receptors. Consistent with this interpretation, the frequency of μm associated with CCV in resting cells was only 14%. Thus, we interpret these data as indicating that BCR ligation induces receptor dissociation, rather than stabilizes dissociated receptors.
The location of BCR destabilization remains unclear. One possibility is that the BCR dissociates on the plasma membrane before μm and Ig-αβ move to CCVs or lipid microdomains. Alternatively, the BCR could translocate to lipid microdomains, then dissociate. The finding that sheathed receptors (herbimycin treated) mainly cocapped outside of GM1-rich lipid microdomains (Fig. 2B, bottom panels) suggests that BCR destabilization occurs before the movement of receptors to lipid microdomains. However, additional analysis is needed to confirm the location of destabilization. Nonetheless, the data indicate that receptor dissociation precedes the targeting of μm to CCVs. A direct role for BCR destabilization in targeting of μm to CCVs is suggested by two findings. First, inhibition of BCR destabilization prevents the association of μm with CCVs (Fig. 6B). Second, in the absence of receptor ligation, the basal association of μm with clathrin occurs in the absence of Ig-α (Fig. 6A).
A physical dissociation of the BCR complex was first suggested by the presence of Ig-α in lipid microdomains in the absence of μm (Fig. 1B). These findings contrast with a previous report claiming translocation of intact BCR complexes into lipid rafts (9). We immunoprecipitated sheathed BCR from unstimulated cells as a means of quantitating the amounts of μm relative to Ig-α in lipid microdomains. Establishing this stoichiometry was not shown in the earlier study; thus, a small amount of μm in lipid rafts may have been amplified by a long immunoblot exposure time. Consistent with this idea, we found that long exposures revealed a small amount of μm in lipid microdomains (J.-H. Kim and B. Vilen, unpublished observations). We attempted to confirm the association of Ig-αβ with lipid microdomains by staining raft-associated molecules on native membranes. However, we were unable to identify aggregates of linker for activation of B cells (LAB), Lyn, or GM1 after Ag stimulation. This is similar to another study showing that the location of aggregated FcεR1 in native membranes does not correlate with the distribution of previously described raft-associated molecules (21).
Clathrin-mediated endocytosis is facilitated by the interaction of clathrin-adaptor molecules with a receptor-encoded endocytic motif (22). The structural resemblance of the Ig-αβ ITAMs to a canonical tyrosine-based YXXØ motifs (X, any amino acid; Ø, bulky hydrophobic residue) suggested their roles as endocytic motifs (23–26). Our data show that μm preferentially enters CCVs in the absence of Ig-αβ (Fig. 6A and Table I), suggesting that an alternative mechanism is responsible. This is consistent with studies showing that μm attaches to the cytoskeleton in the absence of Ig-αβ, and that signaling-competent BCR mutants fail to process and present Ag despite the presence of intact Ig-αβ (27, 28). Other possible mechanisms to promote the entry of μm into CCVs may include ubiquitination or phosphorylation of μm (29, 30). These modifications may induce receptor destabilization and simultaneously facilitate the association of Ag-bound μm with CCVs. Alternatively, dissociation of Ig-αβ from μm may expose a non-canonical endocytosis motif that associates with an adapter molecule.
Our data indicate that inhibiting the activation of Src family kinases prevents the association of μm with CCVs coincident with inhibiting BCR destabilization (Fig. 6B and 2A). Others have shown that the Src kinase, Lyn, is responsible for the phosphorylation of clathrin H chain and the endocytosis of BCR through raft-associated clathrin (10). Collectively, the data suggest a model in which BCR-mediated signal transduction functions to dissociate μm from Ig-αβ, thus promoting association of μm with CCV, and to phosphorylate clathrin H chain, possibly preparing CCVs to receive BCR-bound Ag. Currently, we cannot distinguish whether receptor destabilization or clathrin phosphorylation initiates the association of Ag-μm with CCVs, if the events occur simultaneously, or if clathrin phosphorylation is affected at our concentration of herbimycin. However, despite the lack of kinase activation, receptor internalization occurred normally (Fig. 6C). This indicates that in the absence of clathrin association, the sheathed receptor complexes are internalized through routes other than CCVs. Consistent with this idea, lipid rafts and actin cytoskeleton have been implicated in receptor clearance (8–11, 23). In addition, previous studies have shown that clathrin-mediated internalization is not solely responsible for the clearance of receptors from the cell surface (31–34). However, this does not refute the importance of clathrin-mediated endocytosis, because productive processing of Ag internalized in the absence of kinase activation is unlikely. Others have shown that kinase activation is required for clathrin H chain phosphorylation and BCR endocytosis through lipid rafts (10). In addition, kinase activation is required for late endosome acidification and for the destabilization of the BCR (7, 13). Thus, the fate of receptors internalized in the absence of kinase activation may be degradation (35). It is important to note that previous studies showed that Lyn deficiency impeded BCR internalization, whereas our data show normal receptor clearance in the presence of Src kinase inhibitors (10, 36). This discrepancy may reflect the different ligands used to cross-link the BCR. Our study used bona fide Ag, whereas the previous study used polyvalent cross-linkers, such as F(ab′)2 of anti-μ, which have been reported to slow the rate of BCR endocytosis (37). Importantly, our data are consistent with studies showing that BCR mutants lacking the ability to transduce signals remain competent to internalize receptors (28, 38, 39).
The immune response requires efficient Ag processing and presentation, such that pathogen-derived peptides can be efficiently presented to T cells. Our data extend previous findings to address the biophysical nature of Ag-induced receptor destabilization and to show that receptor destabilization promotes entry of Ag-μm complexes into CCVs while maintaining a pool of Ig-αβ within the GM1-containing lipid microdomains. The rapid association of Ag-bound μm with CCV allows Ag to rapidly enter the endocytic pathway, while the association of unsheathed Ig-αβ with lipid microdomains allows sustained signal transduction and/or provides a pool of Ig-αβ to prime the recycling pool of MHC class II molecules (19).
Acknowledgments
We thank Steve Clarke and Jim Drake for helpful discussions and critical review of the manuscript. We acknowledge the generosity of the Lineberger Comprehensive Cancer Center EM Core Facility and the technical advice of Tony Perdue.
Footnotes
This work was supported by a National Institute of Allergy and Infectious Diseases Research Scholar Development Award (to B.V.).
Abbreviations used in this paper: CCV, clathrin-coated vesicle; μm, μ-H chain; NP, nitrophenyl; PC, phosphorylcholine.
Disclosures
The authors have no financial conflict of interest.
References
- 1.Matsuuchi L, Gold MR. New views of BCR structure and organization. Curr Opin Immunol. 2001;13:270–277. doi: 10.1016/s0952-7915(00)00215-6. [DOI] [PubMed] [Google Scholar]
- 2.Clark MR, Massenburg D, Zhang M, Siemasko K. Molecular mechanisms of B cell antigen receptor trafficking. Ann NY Acad Sci. 2003;987:26–37. doi: 10.1111/j.1749-6632.2003.tb06030.x. [DOI] [PubMed] [Google Scholar]
- 3.Cheng PC, Steele CR, Gu L, Song W, Pierce SK. MHC class II antigen processing in B cells: accelerated intracellular targeting of antigens. J Immunol. 1999;162:7171–7180. [PubMed] [Google Scholar]
- 4.Lankar D, Briken V, Adler K, Weiser P, Cassard S, Blank U, Viguier M, Bonnerot C. Syk tyrosine kinase and B cell antigen receptor (BCR) immunoglobulin-α subunit determine BCR-mediated major histocompatibility complex class II-restricted antigen presentation. J Exp Med. 1998;188:819–831. doi: 10.1084/jem.188.5.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Siemasko K, Skaggs BJ, Kabak S, Williamson E, Brown BK, Song W, Clark MR. Receptor-facilitated antigen presentation requires the recruitment of B cell linker protein to Igα. J Immunol. 2002;168:2127–2138. doi: 10.4049/jimmunol.168.5.2127. [DOI] [PubMed] [Google Scholar]
- 6.Lankar D, Vincent-Schneider H, Briken V, Yokozeki T, Raposo G, Bonnerot C. Dynamics of major histocompatibility complex class II compartments during B cell receptor-mediated cell activation. J Exp Med. 2002;195:461–472. doi: 10.1084/jem.20011543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Siemasko K, Eisfelder BJ, Williamson E, Kabak S, Clark MR. Cutting edge: signals from the B lymphocyte antigen receptor regulate MHC class II containing late endosomes. J Immunol. 1998;160:5203–5208. [PubMed] [Google Scholar]
- 8.Salisbury JL, Condeelis JS, Maihle NJ, Satir P. Receptor-mediated endocytosis by clathrin-coated vesicles: evidence for a dynamic pathway. Cold Spring Harbor Symp Quant Biol. 1982;46:733–741. doi: 10.1101/sqb.1982.046.01.070. [DOI] [PubMed] [Google Scholar]
- 9.Cheng PC, Dykstra ML, Mitchell RN, Pierce SK. A role for lipid rafts in B cell antigen receptor signaling and antigen targeting. J Exp Med. 1999;190:1549–1560. doi: 10.1084/jem.190.11.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stoddart A, Dykstra ML, Brown BK, Song W, Pierce SK, Brodsky FM. Lipid rafts unite signaling cascades with clathrin to regulate BCR internalization. Immunity. 2002;17:451–462. doi: 10.1016/s1074-7613(02)00416-8. [DOI] [PubMed] [Google Scholar]
- 11.Putnam MA, Moquin AE, Merrihew M, Outcalt C, Sorge E, Caballero A, Gondre-Lewis TA, Drake JR. Lipid raft-independent B cell receptor-mediated antigen internalization and intracellular trafficking. J Immunol. 2003;170:905–912. doi: 10.4049/jimmunol.170.2.905. [DOI] [PubMed] [Google Scholar]
- 12.Nichols BJ. GM1-containing lipid rafts are depleted within clathrin-coated pits. Curr Biol. 2003;13:686–690. doi: 10.1016/s0960-9822(03)00209-4. [DOI] [PubMed] [Google Scholar]
- 13.Vilen BJ, Nakamura T, Cambier JC. Antigen-stimulated dissociation of BCR mIg from Ig-α/Ig-β: implications for receptor desensitization. Immunity. 1999;10:239–248. doi: 10.1016/s1074-7613(00)80024-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim KJ, Kanellopoulos-Langevin C, Merwin RM, Sachs DH, Asofsky R. Establishment and characterization of BALB/c lymphoma lines with B cell properties. J Immunol. 1979;122:549–554. [PubMed] [Google Scholar]
- 15.Vilen BJ, Famiglietti SJ, Carbone AM, Kay BK, Cambier JC. B cell antigen receptor desensitization: disruption of receptor coupling to tyrosine kinase activation. J Immunol. 1997;159:231–243. [PMC free article] [PubMed] [Google Scholar]
- 16.Wilson BS, Pfeiffer JR, Oliver JM. Observing FcεRI signaling from the inside of the mast cell membrane. J Cell Biol. 2000;149:1131–1142. doi: 10.1083/jcb.149.5.1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wilson BS, Pfeiffer JR, Surviladze Z, Gaudet EA, Oliver JM. High resolution mapping of mast cell membranes reveals primary and secondary domains of FcεRI and LAT. J Cell Biol. 2001;154:645–658. doi: 10.1083/jcb.200104049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Drake JR, Repasky EA, Bankert RB. Endocytosis of antigen, anti-idiotype, and anti-immunoglobulin antibodies and receptor re-expression by murine B cells. J Immunol. 1989;143:1768–1776. [PubMed] [Google Scholar]
- 19.Lang P, Stolpa JC, Freiberg BA, Crawford F, Kappler J, Kupfer A, Cambier JC. TCR-induced transmembrane signaling by peptide/MHC class II via associated Ig-α/β dimers. Science. 2001;291:1537–1540. doi: 10.1126/science.291.5508.1537. [DOI] [PubMed] [Google Scholar]
- 20.Williams GT, Peaker CJ, Patel KJ, Neuberger MS. The α/β sheath and its cytoplasmic tyrosines are required for signaling by the B-cell antigen receptor but not for capping or for serine/threonine-kinase recruitment. Proc Natl Acad Sci USA. 1994;91:474–478. doi: 10.1073/pnas.91.2.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wilson BS, Steinberg SL, Liederman K, Pfeiffer JR, Surviladze Z, Zhang J, Samelson LE, Yang LH, Kotula PG, Oliver JM. Markers for detergent-resistant lipid rafts occupy distinct and dynamic domains in native membranes. Mol Biol Cell. 2004;15:2580–2592. doi: 10.1091/mbc.E03-08-0574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Collins BM, McCoy AJ, Kent HM, Evans PR, Owen DJ. Molecular architecture and functional model of the endocytic AP2 complex. Cell. 2002;109:523–535. doi: 10.1016/s0092-8674(02)00735-3. [DOI] [PubMed] [Google Scholar]
- 23.Bonnerot C, Lankar D, Hanau D, Spehner D, Davoust J, Salamero J, Fridman WH. Role of B cell receptor Igα and Igβ subunits in MHC class II-restricted antigen presentation. Immunity. 1995;3:335–347. doi: 10.1016/1074-7613(95)90118-3. [DOI] [PubMed] [Google Scholar]
- 24.Patel KJ, Neuberger MS. Antigen presentation by the B cell antigen receptor is driven by the α/β sheath and occurs independently of its cytoplasmic tyrosines. Cell. 1993;74:939–946. doi: 10.1016/0092-8674(93)90473-4. [DOI] [PubMed] [Google Scholar]
- 25.Siemasko K, Eisfelder BJ, Stebbins C, Kabak S, Sant AJ, Song W, Clark MR. Igα and Igβ are required for efficient trafficking to late endosomes and to enhance antigen presentation. J Immunol. 1999;162:6518–6525. [PubMed] [Google Scholar]
- 26.Owen DJ. Linking endocytic cargo to clathrin: structural and functional insights into coated vesicle formation. Biochem Soc Trans. 2004;32:1–14. doi: 10.1042/bst0320001. [DOI] [PubMed] [Google Scholar]
- 27.Hartwig JH, Jugloff LS, De Groot NJ, Grupp SA, Jongstra-Bilen J. The ligand-induced membrane IgM association with the cytoskeletal matrix of B cells is not mediated through the Igαβa heterodimer. J Immunol. 1995;155:3769–3779. [PubMed] [Google Scholar]
- 28.Mitchell RN, Barnes KA, Grupp SA, Sanchez M, Misulovin Z, Nussenzweig MC, Abbas AK. Intracellular targeting of antigens internalized by membrane immunoglobulin in B lymphocytes. J Exp Med. 1995;181:1705–1714. doi: 10.1084/jem.181.5.1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hicke L, Dunn R. Regulation of membrane protein transport by ubiquitin and ubiquitin-binding proteins. Annu Rev Cell Dev Biol. 2003;19:141–172. doi: 10.1146/annurev.cellbio.19.110701.154617. [DOI] [PubMed] [Google Scholar]
- 30.Marchese A, Benovic JL. Ubiquitination of G-protein-coupled receptors. Methods Mol Biol. 2004;259:299–305. doi: 10.1385/1-59259-754-8:299. [DOI] [PubMed] [Google Scholar]
- 31.Nashar TO, Betteridge ZE, Mitchell RN. Evidence for a role of ganglioside GM1 in antigen presentation: binding enhances presentation of Escherichia coli enterotoxin B subunit (EtxB) to CD4+ T cells. Int Immunol. 2001;13:541–551. doi: 10.1093/intimm/13.4.541. [DOI] [PubMed] [Google Scholar]
- 32.Wettey FR, Hawkins SF, Stewart A, Luzio JP, Howard JC, Jackson AP. Controlled elimination of clathrin heavy-chain expression in DT40 lymphocytes. Science. 2002;297:1521–1525. doi: 10.1126/science.1074222. [DOI] [PubMed] [Google Scholar]
- 33.Riezman H, Munn A, Geli MI, Hicke L. Actin-, myosin- and ubiquitin-dependent endocytosis. Experientia. 1996;52:1033–1041. doi: 10.1007/BF01952099. [DOI] [PubMed] [Google Scholar]
- 34.Di Guglielmo GM, Le Roy C, Goodfellow AF, Wrana JL. Distinct endocytic pathways regulate TGF-β receptor signalling and turnover. Nat Cell Biol. 2003;5:410–421. doi: 10.1038/ncb975. [DOI] [PubMed] [Google Scholar]
- 35.Wagle NM, Kim JH, Pierce SK. Signaling through the B cell antigen receptor regulates discrete steps in the antigen processing pathway. Cell Immunol. 1998;184:1–11. doi: 10.1006/cimm.1998.1264. [DOI] [PubMed] [Google Scholar]
- 36.Ma H, Yankee TM, Hu J, Asai DJ, Harrison ML, Geahlen RL. Visualization of Syk-antigen receptor interactions using green fluorescent protein: differential roles for Syk and Lyn in the regulation of receptor capping and internalization. J Immunol. 2001;166:1507–1516. doi: 10.4049/jimmunol.166.3.1507. [DOI] [PubMed] [Google Scholar]
- 37.Thyagarajan R, Arunkumar N, Song W. Polyvalent antigens stabilize B cell antigen receptor surface signaling microdomains. J Immunol. 2003;170:6099–6106. doi: 10.4049/jimmunol.170.12.6099. [DOI] [PubMed] [Google Scholar]
- 38.Shaw AC, Mitchell RN, Weaver YK, Campos-Torres J, Abbas AK, Leder P. Mutations of immunoglobulin transmembrane and cytoplasmic domains: effects on intracellular signaling and antigen presentation. Cell. 1990;63:381–392. doi: 10.1016/0092-8674(90)90171-a. [DOI] [PubMed] [Google Scholar]
- 39.Mitchell RN, Shaw AC, Weaver YK, Leder P, Abbas AK. Cytoplasmic tail deletion converts membrane immunoglobulin to a phosphatidylinositol-linked form lacking signaling and efficient antigen internalization functions. J Biol Chem. 1991;266:8856–8860. [PubMed] [Google Scholar]