We present spectral domain optical coherence tomography (OCT; Heidelberg Spectralis) imaging of 3 patients with Niemann-Pick disease type B, expanding the range of previously reported anatomic correlates of the ophthalmoscopic macular halo surrounding the “cherry-red spot.” 1
Case 1
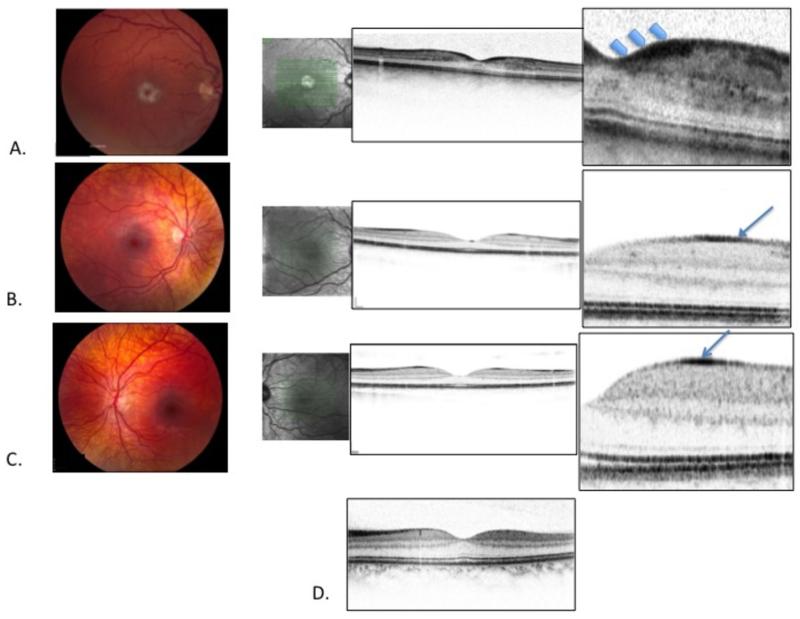
A 34 year-old man with Niemann-Pick disease type B was referred for ophthalmic evaluation. Best-corrected visual acuity was 20/30 OD, 20/25- OS. Dilated fundus examination revealed normal appearing optic nerves, tortuous retinal veins and venous beading. There were striking white macular haloes surrounding the normal red foveal depressions in both eyes (Figure 1, Part A). Spectral domain OCT revealed a layer of hyper-reflective retinal tissue at the shoulders of the foveal depression. The hyper-reflective tissue extended across the foveal floor, but was markedly thinner centrally. (Figure 1, Part A).
Figure 1.
Part A. Fundus photography showing vascular tortuosity and white macular halo with (normal) red center (“cherry-red spot”) in a 34 year–old patient with Niemann-Pick disease. Spectral domain OCT image through the foveal center (confirmed by forward bowing of the ellipsoid line (also referred to as the IS/OS line) and the external limiting membrane) shows hyper-reflective tissue in the retinal ganglion cell layer at the “shoulders” of the foveal depression (arrows), extending across the foveal floor. Hyper-reflective cells are also seen between the photoreceptors and the foveal floor, in the region of the central bouquet.
Part B. Subtle clouding in the foveal region of a 5 year old patient with Niemann-Pick disease. OCT imagery demonstrates hyper-reflective tissue at the retinal surface outside the foveal depression (arrow).
Part C. 10 year-old patient with Niemann-Pick disease with mild perifoveal clouding seen on fundus photography. OCT image demonstrates hyper-reflective tissue at the retinal surface, outside the fovea.
Part D. Spectral domain OCT of a normal 30 year-old for comparison with Part A.
Case 2
A 5 year-old boy with Niemann-Pick disease type B presented for ophthalmic evaluation. Best-corrected visual acuity was 20/30 OD and 20/30+ OS. Dilated fundoscopic examination demonstrated perimacular clouding (Figure 1, Part B). Spectral domain OCT demonstrated an annulus of hyper-reflective tissue at the retinal surface outside the foveal depression.
Case 3
The brother of Case 2 presented for ophthalmic evaluation at age 10. Best-corrected visual acuity was 20/20 OD and 20/20 OS. Subtle clouding of the perimacular region was seen (Figure 1, Part C). Spectral domain OCT demonstrated an annulus of hyper-reflective tissue at the retinal surface outside the foveal depression.
A spectral-domain OCT image from a 30-year old control subject with a clinically unremarkable macula is provided for comparison (Figure 1, part D).
Comment
Niemann-Pick disease is a lysosomal storage disease due to mutations in the sphingomyelin phosphodiesterase I geneSMPD 1. Clinical subtypes A and B are distinguished by the absence or presence, respectively, of residual catalytic activity of the gene product enzyme, and by contrasting patterns of clinical involvement: type A patients develop severe visceral involvement with rapidly progressive neurologic degeneration, usually resulting in death by 3 years of age (OMIM 257200); type B patients manifest a milder syndrome with hepatosplenomegaly, thrombocytopenia, interstitial lung disease, and dyslipidemia with most patients having little or no neurologic involvement (OMIM 607616).
Some Niemann-Pick disease A and B patients manifest “macular haloes” – annular zones of whitening of the inner retina surrounding the foveal depressions. The central foveal zones are ophthalmoscopically uninvolved, giving rise to the traditional, though potentially misleading, clinical description of this lesion as the “cherry-red spot”: the red color of the central fovea is not abnormal – the pathology is in the surrounding extrafoveal portion of the macula. In our experience, macular halos are seen by age 12 months in all patients with Niemann-Pick disease-A, but in only one-third of type B patients 2,3. The macular haloes in Niemann-Pick disease patients rarely exceed three millimeters in outer diameter.
The cellular basis of the macular haloes is currently uncertain. Unprocessed lipid metabolites are thought to accumulate in retinal ganglion cells in NPB as well as in other lipid storage diseases. In the present study retinal sites of hyper-reflectivity in NPB patients included the foveal floor and clivus (Patient 1) and a thin band internal to the ganglion cell layer just outside the foveal depression (patients 2 and 3). A patient similar to patient 1 was described by Kim et al,1 who interpreted their findings to mean that “lipid deposition occurs selectively in the ganglion cell layer.”
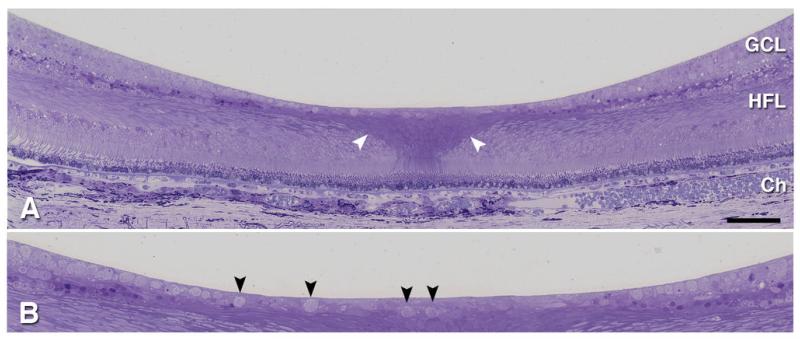
We were initially surprised to observe the hyper-reflectivity as demonstrated by OCT to extend across the foveal floor. We note that the foveal floor is normally populated by a tissue layer including scattered retinal ganglion cells, as shown in Figure 2B, which if affected could contribute to increased reflectivity. These findings should be viewed in context, however. The foveal slope contains the termini of inner plexiform and inner nuclear layers in addition to that of the ganglion cell layer. Further, in patients 2 and 3, it is not the ganglion cell layer that is hyper-reflective but a thin band consistent in location with the nerve fiber layer. Attribution of increased reflectivity exclusively to ganglion cells thus seems unwarranted.
Figure 2.
Histology of the normal human fovea. 1 μm thick epoxy section through retina and choroid of 81 yr old woman, stained with toluidine blue: GCL, ganglion cell layer; HFL, Henle fiber layer; Ch, Choroid. A. Note the darker staining of the central bouquet of cone photoreceptors and Müller cells, sclerad to the foveal floor (white arrowheads). B. Excerpt from panel A, magnified view shows isolated ganglion cells within the foveal floor (black arrowheads). The “ganglion cell layer” consists of ganglion cells, displaced amacrine cells, and intercalated Müller cells.
Rather, involvement of foveal Müller cells could account for the overall pattern of reflectivity in all 3 patients. Müller radial glia intercalate among the retinal neurons, spanning all the layers. Müller cells in the foveal center exhibit unusual histochemical and morphologic properties (Figure 2A) that may render them particularly vulnerable to the effects of sphingomyelin phosphodiesterase mutation. Those associated with the central bouquet of foveal cones sweep laterally within inner retina, in concert with the laterally displaced neurons. Müller cell involvement in Nieman-Pick type B was also previously inferred from fundus appearance4. Analysis of a Nieman-Pick type B mouse model indicates that neurons are certainly affected, as cerebellar Purkinje cells disappear, but glial involvement has not yet been directly investigated5. The cellular locus of sphingomyelinase deficiency in Nieman-Pick type B may prove an important consideration for the choice of promoters in gene therapy trials.
The correlation between macular haloes and the clinical severity of Niemann-Pick disease is imperfect. OCT imaging may further clarify the nature of the disease process, as the retina remains the only tissue that can be directly observed in the living subject accumulating abnormal metabolites in Niemann-Pick disease and other lysosomal storage diseases.
Acknowledgements
The OCT images in Figure 1 Part A were graciously provided by Dr. Martin Pearlman of East Lansing, MI.
This work was supported in part by an unrestricted grant to the Department of Ophthalmology, Mount Sinai School of Medicine, from Research to Prevent Blindness, Inc.; by NIH grant EY06109 and unrestricted funds to the Department of Ophthalmology at the University of Alabama at Birmingham from Research to Prevent Blindness, Inc., and the EyeSight Foundation of Alabama; and by grant 5 MO1 RR00071 to the Mount Sinai General Clinical Research Center from the National Center for Research Resources, National Institutes of Health
Contributor Information
Danielle S. Rudich, Department of Ophthalmology, Mount Sinai Medical Center, New York, NY.
Christine A. Curcio, Department of Ophthalmology, University of Alabama at Birmingham, Birmingham, Alabama
Melissa Wasserstein, Department of Pediatrics, Genetics and Genomic Sciences, Mount Sinai Medical Center, New York, NY
Scott E. Brodie, Department of Ophthalmology, Mount Sinai Medical Center, New York, NY
References
- 1.Kim C, Jeong J, Yu HG. Diagnostic and predictive methods for a Niemann-Pick. disease type B patient with ocular involvement. J In herit Metab Dis. 2010 Oct;33(5):633–4. doi: 10.1007/s10545-010-9153-z. [DOI] [PubMed] [Google Scholar]
- 2.McGovern MM, Wasserstein MP, Aron A, Desnick RJ, Schuchman EH, Brodie SE. Ocular Manifestations of Niemann-Pick Disease Type B. Ophthalmology. 2004;111:1424–1427. doi: 10.1016/j.ophtha.2003.10.034. [DOI] [PubMed] [Google Scholar]
- 3.McGovern MM, Aron A, Brodie SE, Desnick RJ, Wasserstein MP. Natural history of type A Niemann-Pick disease: possible endpoints for therapeutic trials. Neurology. 2006;66:228–232. doi: 10.1212/01.wnl.0000194208.08904.0c. [DOI] [PubMed] [Google Scholar]
- 4.Lowe D, Martin F, Sarks J. Ocular manifestations of adult Niemann-Pick disease: a case report. Australian and New Zealand Journal of Ophthalmology. 1986;14:41–47. doi: 10.1111/j.1442-9071.1986.tb00006.x. PMID 3964479. [DOI] [PubMed] [Google Scholar]
- 5.Marathe S, Miranda SR, Devlin C, Johns A, Kuriakose G, Williams KJ, Schuchman EH, Tabas I. Creation of a mouse model for non-neurological (type B) Niemann-Pick disease by stable, low level expression of lysosomal sphingomyelinase in the absence of secretory sphingomyelinase: relationship between brain intra-lysosomal enzyme activity and central nervous system function. Hum Mol Genet. 2000;9:1967–1976. doi: 10.1093/hmg/9.13.1967. PMID 10942425. [DOI] [PubMed] [Google Scholar]