Abstract
Objectives
Patients with rheumatoid arthritis (RA) are at increased risk for cardiovascular disease (CVD), although strategies to detect sub-clinical CVD are poorly characterized. The purpose of this study was to assess myocardial function by speckle-tracking echocardiography strain imaging in RA patients without known CVD.
Methods
Eighty-seven RA patients selected from a population-based sample underwent echocardiography. Left (LV) and right ventricular (RV) longitudinal peak systolic strain were measured. A subset of 59 RA patients was compared with 59 age, gender and race-matched subjects with normal echocardiography and no CVD or risk factors.
Results
The mean age of matched RA and normal patients was 55.7±12.1 and 54.5±12.2 years (p=0.42), respectively, and 45 (76%) were female in each group. Global LV (−15.7 ±3.2% versus −18.1 ±2.4%, p<0.001) and RV strain (−17.9 ± 4.7% versus −20.7±2.4%, p<0.001) were reduced in RA patients compared to normal patients. Among all 87 RA patients, the mean disease duration and C-reactive protein at echocardiography were 10.0±6.1 years and 3.5±3.7 mg/L, and 74% were seropositive. Adjusted univariate regression analysis demonstrated a significant correlation between global LV strain and RA health assessment questionnaire disability index (p=0.032), and borderline associations with prior use of oral corticosteroids (p=0.062) and methotrexate (p=0.054) after adjustment for age, gender, blood pressure, body mass index, heart rate and LV mass index.
Conclusions
Global longitudinal LV and RV strain were reduced in RA patients compared with healthy patients. Strain abnormalities correlated with RA disease severity. Strain imaging by echocardiography may detect early myocardial dysfunction in RA.
Keywords: rheumatoid arthritis, cardiovascular disease, strain, speckle-tracking echocardiography
INTRODUCTION
Patients with rheumatoid arthritis (RA) are at increased risk for developing cardiovascular disease (CVD), including a two-fold risk of heart failure (HF).[1-3] RA patients who develop clinical HF often have impaired left ventricular (LV) diastolic function with preserved LV ejection fraction (EF), and may present with relatively fewer signs and symptoms in comparison to HF patients without RA.[4, 5] However, screening RA patients for CVD remains controversial, and the best method for evaluation of subclinical disease remains unclear.
Myocardial strain imaging is an advanced echocardiography modality measuring myocardial deformation during contraction and relaxation.[6] Strain measurement is expressed as percentage change in dimension of an object from one time point to another, and can be performed using a technique called speckle-tracking echocardiography (STE) that utilizes specialized software to track acoustic markers in standard 2-dimensional (2D) gray scale images.[7-9] Strain abnormalities have been characterized for a broad range of CVD,[10] and strain imaging has been shown to be sensitive for detecting impaired systolic function in certain diseases causing clinical HF with preserved LVEF (≥50%).[10-13] Impaired ventricular strain can therefore be utilized as a marker for early and subclinical CVD.
Myocardial strain imaging may be well suited for early detection of CVD in RA. A previous study reported abnormal strain values in RA patients without clinical CVD, however that study included a limited number of patients with a short disease duration.[14] The purpose of this study was to determine if myocardial strain imaging could detect abnormalities of LV and RV systolic function in a larger cohort of RA patients without known CVD, and to determine whether myocardial strain is associated with LV diastolic dysfunction in patients with RA.
METHODS
Study subjects and design
This was a retrospective study of a population-based cohort of residents of Olmsted County, Minnesota that was conducted using resources of the Rochester Epidemiology Project, a population-based medical records linkage system that allows ready access to complete medical records from all community medical providers.[15] An incidence cohort of all residents of Olmsted County, ages ≥18 years who met 1987 American College of Rheumatology classification criteria for RA between January 1, 1980, and December 31, 2007, was previously identified.[16, 17] From this incident cohort we identified eligible RA subjects, specifically those alive and living in Olmsted County. All eligible patients were invited to participate in the study and 278 (66%) of 419 eligible patients agreed. Of these, 64 were excluded from this study (8 with a known history of cardiovascular disease and 58 with indeterminate LV diastolic dysfunction), leaving a total of 212 patients with RA. At study entrance all patients underwent transthoracic echocardiography (TTE) according to previously defined protocols. Patients were included if LVEF was normal by quantitative assessment and RV function was normal by qualitative assessment. To evaluate for possible correlation between strain abnormalities and diastolic dysfunction, 100 patients were selected for strain measurement from the previously assembled cohort of 212 patients according to the degree of diastolic dysfunction present on TTE performed at study entrance. Diastolic dysfunction was categorized as previously described,[18] and patients were selected to include a spectrum of diastolic dysfunction as follows: none (40), mild (40), or moderate to severe (20). Following patient selection, an additional 13 patients were found to have CVD (12 with a history of CAD, 1 with bioprosthetic aortic valve) and were then excluded from the study, leaving 87 patients with RA. A second cohort of patients with normal LV and RV function on clinically indicated TTE and no history of RA, CVD or CVD risk factors was used for comparison. For each patient with RA, a patient with similar age (±5 years) and the same gender and race was randomly chosen from this ‘normal’ cohort. Only 59 RA patients could be matched with normals due the older age of the RA cohort. This study was approved by the Institutional Review Board. All subjects provided written informed consent prior to participation.
Data collection
At study entrance RA patients completed a questionnaire, provided a blood sample and underwent TTE. The questionnaire included questions pertaining to HF symptoms, CVD risk factors and medication usage. Demographic characteristics were recorded. Presence of the following CVD risk factors was ascertained: smoking (current or prior), diabetes mellitus (based on physician diagnosis and/or documented use of insulin and/or oral hypoglycemic agents), hyperlipidemia (based on elevated fasting lipid levels and/or documented use of lipid-lowering agents), body mass index (BMI, kg/m2) and hypertension (based on physician diagnosis and/or documented use of antihypertensive medications). Data on history of coronary artery disease (CAD) (presence of angina pectoris, myocardial infarction) and prior coronary revascularization (coronary artery bypass graft and/or percutaneous coronary intervention) were gathered by medical record review. For RA patients, rheumatoid factor (RF), anti-citrullinated peptide antibody (ACPA), C-reactive protein (CRP), and B-type natriuretic peptide (BNP) levels were measured. RF testing was performed by nephelometry (latex enhanced assay; Behring Nephelometer II; Dade Behring, Newark, Delaware, USA). ACPA testing was performed by enzyme immunoassay from INOVA Diagnostics (San Diego, California, USA). CRP testing was performed by immunoturbidimetric assay (Roche CRPLX reagent; Roche Diagnostics, Indianapolis, Indiana, USA). BNP testing was performed using the fluorescence immunoassay by Biosite Diagnostics. Medical records were reviewed to collect data on RA disease characteristics from RA incidence date to the time of the study TTE, including erythrocyte sedimentation rate (ESR) at RA incidence, large-joint swelling, joint erosions/destructive changes on radiographs, joint surgeries (i.e., arthroplasty and synovectomy) and presence of rheumatoid nodules. The questionnaire was augmented to obtain the Health Assessment Questionnaire (HAQ) disability score and RA medication usage at the time of TTE, including systemic corticosteroids, disease-modifying anti-rheumatic drugs (DMARDs), biological agents and non-steroidal anti-inflammatory drugs (NSAIDs). Systemic corticosteroid use included either oral or intravenous forms; DMARDs included methotrexate, hydroxychloroquine, sulfasalazine, leflunomide and/or azathioprine; and biological agents included TNFα blockers, anakinra, abatacept and/or rituximab. RA medication usage at the time of TTE was verified by review with the patient. History of RA medication use from the diagnosis of RA to the time of TTE was collected from the medical records. For normal patients without RA, clinical and laboratory data was abstracted from the medical records at the time of TTE. Identical CVD data was collected for RA and normal patient cohorts.
Echocardiography
Two-dimensional and Doppler echocardiograms were performed on all patients in the RA and normal patient cohorts, according to standard guidelines as previously described.[18-20] Frame rate for acquired images was optimized to 40-90 frames/second. Images were acquired from the peak of the R wave, and 3 cardiac cycles were used for analysis. All TTEs were performed by registered diagnostic cardiovascular sonographers and interpreted in the Mayo Clinic Echocardiography Laboratory. The following echocardiographic parameters were measured and/or estimated in each subject: pulmonary artery systolic pressure, left atrial volume index, LV mass, LV mass index, tricuspid valve regurgitant jet velocity, pulsed-wave Doppler examination of mitral inflow peak early filling velocity (E) and atrial contraction velocity (A) (before and during Valsalva maneuver), and tissue Doppler imaging of peak mitral annulus early diastolic velocity (e’), E/A ratio, E/e′ ratio and deceleration time (DT). LV hypertrophy was defined according to standard guidelines.[19] Diastolic dysfunction was categorized as none (normal diastolic function); mild, defined as impaired relaxation without evidence of increased filling pressures; moderate, defined as impaired relaxation associated with moderate elevation of filling pressures or pseudonormal filling; and severe, defined as advanced reduction in compliance or reversible or fixed restrictive filling.[20]
Strain analysis by speckle-tracking echocardiography
All strain measurements were performed by a single investigator (N.M.F.) experienced in strain analysis and blinded to clinical and other echocardiographic patient data. Images selected for strain analysis were exported from a central archive and stored in Digital Imaging and Communications in Medicine (DICOM) format for off-line strain measurement using Syngo Velocity Vector Imaging (VVI) software (version 3.5) (Siemens Medical Solutions, Malvern, Pennsylvania). Transapical imaging window views were selected, including the apical 4-chamber, apical long-axis, and apical 2-chamber views. RV strain was analyzed from the apical 4-chamber view. Peak systolic longitudinal strain was measured in each view. The LV and RV shorten along their longitudinal axis during systolic contraction, therefore strain is expressed as a negative percentage value. Less negative strain or reduced absolute strain values reflect diminished contractile function.
Manual endocardial border tracing of a single frame at end-systole was performed. The periodic displacement of the tracing was automatically tracked in subsequent frames. Tissue velocity was determined by the software according to a shift of the points divided by time between 2D gray scale frames (Figure 1). The software automatically segments the myocardium according to the standard 16-segment model of the LV and 6-segment model of the RV, and calculates a peak systolic segmental and global longitudinal ventricular strain from the velocity.[19] The quality of myocardial tracking was assessed visually, and the process repeated if myocardial tracking was poor.
Figure 1.
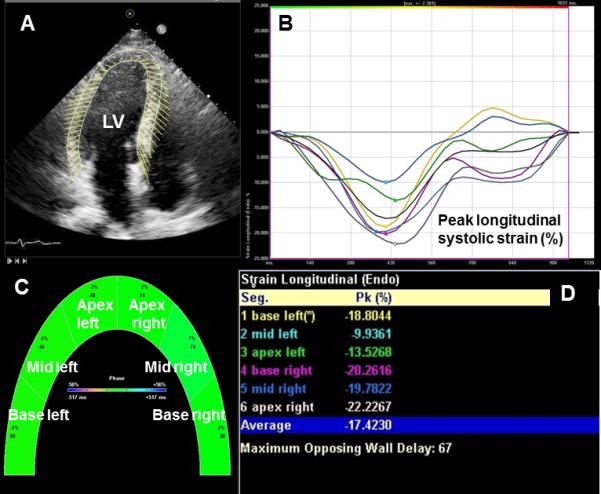
Representative recording with myocardial contour tracing of the left ventricle in the apical 4-chamber view with subsequent velocity vector imaging (A), the software automatically divides the ventricle into 6 segments and displays peak segmental and global longitudinal systolic strain values as strain curves (red arrow) (B), by graphic display (C) and as numerical values (D). LV=left ventricle.
Intraobserver and interobserver variability was assessed using intraclass correlation coefficients for 15 randomly selected RA patients, where strain analysis was repeated by the same investigator (N.M.F) or performed independently by two separate investigators (N.M.F. and G.L) in a blinded fashion. Variability testing was performed using the same cine imaging loop used for initial strain measurement. Among RA patients, LV longitudinal strain was measured in a total 1,566 segments. There were 50 (3.2%) LV segments from 11 (12.6%) different patients where strain measurement was not feasible due to suboptimal image quality and/or poor myocardial tracking. RV longitudinal strain was measured in a total of 522 segments. There were 36 (6.9%) segments from 16 (18.4%) different patients where strain measurement was not feasible due to poor image quality, including 2 (2.3%) where RV strain could not be measured in any segment. The intraobserver correlation coefficients for LV and RV global strain were excellent, 0.98 and 0.98, respectively. The interobserver correlation coefficients for LV and RV global strain were 0.95 and 0.92, respectively.
Statistical analysis
Continuous variables were expressed as the mean ±standard deviation, and categorical variables as absolute frequencies or relative percentages. Comparisons of characteristics between groups were performed using Wilcoxon rank sum tests and Chi-square tests. Linear regression models adjusted for age, gender, blood pressure, body mass index, heart rate and LV mass index were used to examine associations between global LV strain and RA disease characteristics. Generalized linear models with random effects were used to examine the change between groups in LV and RV strain across ventricular levels (basal, middle and apical). All tests were 2-sided, and a value of ≤ 0.05 was considered statistically significant. Data analysis was performed using SAS version 9.3 statistical software (Cary, NC).
RESULTS
Clinical characteristics
The study population included 87 patients with RA, of whom 59 were matched to normal patients. Baseline characteristics for both cohorts are reported in Table 1. RA related baseline characteristics are reported in Table 2. Mean RA disease duration was 10.0±6.1 years. Other RA baseline characteristics were typical of a general RA patient population.
Table 1.
Baseline demographic and clinical characteristics of RA patients and matched normal patients without RA or cardiovascular disease.
| Parameter | RA (N=59) | Normals (N=59) | p-value |
|---|---|---|---|
| Clinical | |||
| Age (years) | 55.7 ± 12.1 | 54.5 ± 12.2 | 0.42 |
| Female | 45 (76) | 45 (76) | 1.0 |
| Caucasian | 59(100) | 59 (100) | 1.0 |
| BMI (kg/m2) | 29.1 (5.8) | 27.3 (5.8) | 0.037 |
| Smoking history | 26 (44) | 0 (0) | <0.001 |
| Hypertension | 50 (85%) | 0 (0) | <0.001 |
| Hyperlipidemia | 42 (71) | 0 (0) | <0.001 |
| Diabetes mellitus | 8(14) | 0 (0) | 0.003 |
| Heart rate (bpm) | 66.9 ± 8.6 | 70.9 ± 11.4 | 0.08 |
| Systolic blood pressure (mmHg) | 125.8 ± 15.0 | 113.4 ± 15.1 | <0.001 |
| Diastolic blood pressure (mmHg) | 71.4 ± 8.6 | 69.1 ± 7.6 | 0.08 |
| Medications | |||
| Aspirin | 13 (22) | 0 (0) | <0.001 |
| Beta-blockers | 14 (24) | 0 (0) | <0.001 |
| ACEI or ARB | 10 (17) | 0 (0) | 0.001 |
| Statin | 9(15) | 0 (0) | 0.002 |
Continuous data are expressed as mean ±standard deviation, categorical data as N (%).ACEI=angiotensin converting enzyme inhibitor, ARB=angiotension II receptor blockers, BMI=body mass index, bpm=beats per minute, RA=rheumatoid arthritis.
Table 2.
RA disease characteristics among 87 RA patients, and regression analysis for association with left ventricular peak systolic longitudinal strain for RA patients
| Parameter | Value | Adjusted† univariate Coefficient (SE), p |
|---|---|---|
| Clinical | ||
| RA duration (years) | 10.0 ± 6.1 | 0.07 (0.07), 0.30 |
| HAQ disability index | 0.5 ± 0.5 | 1.87 (0.85), 0.032 |
| Large joint swelling | 64 (74) | 0.34 (0.81), 0.67 |
| Foot / ankle swelling | 74 (85) | 0.81 (1.07), 0.45 |
| Prior joint surgery | 19 (22) | 1.30 (0.97), 0.19 |
| Rheumatoid nodules | 39 (45) | 0.96 (0.73), 0.19 |
| Erosions / destructive changes | 47 (54) | 0.97 (0.76), 0.21 |
| Medications | ||
| Methotrexate | ||
| Current | 50 (58) | 0.53 (0.73), 0.47 |
| Ever | 61 (70) | 1.46 (0.75), 0.054 |
| Hydroxychloroquine | ||
| Current | 23 (26) | 0.31 (0.87), 0.72 |
| Ever | 60 (69) | 0.77 (0.81), 0.35 |
| Other DMARD | ||
| Current | 16 (18) | 1.13 (0.94), 0.23 |
| Ever | 37 (42) | 0.81 (0.76), 0.30 |
| Biologic | ||
| Current | 16 (18) | 0.20 (1.05), 0.85 |
| Ever | 19 (22) | 0.40 (0.94), 0.67 |
| Corticosteroid | ||
| Current | 27 (31) | 0.68 (0.85), 0.43 |
| Ever | 74 (85) | 1.84 (0.97), 0.062 |
| Cumulative dose (g) | 5.8 ± 8.7 | 0.05 (0.06), 0.44 |
| Cox-2 inhibitor | ||
| Current | 9 (10) | 1.29 (1.39), 0.36 |
| Ever | 49 (56) | −0.42 (0.74), 0.57 |
| Laboratory | ||
| RF / ACPA positive | 64 (74) | 1.13 (0.80), 0.16 |
| CRP (mg/L) | 3.5 ±3.7 | 0.40* (0.37), 0.28 |
| ESR at RA diagnosis (mm/hr) | 19.4 ± 16.3 | 0.01 (0.03), 0.65 |
| BNP (pg/mL) | 60.9 ± 87.1 | −0.25* (0.47), 0.61 |
Adjusted for age, gender, blood pressure, body mass index, heart rate and LV mass index
log-transformed
Continuous data are expressed as mean ±standard deviation, categorical data as n (%). ACPA=anti-citrullinated peptide antibodies, BNP=brain natriuretic peptide, CRP=c-reactive protein, DMARD=disease modifying anti-rheumatic drug, ESR=erythrocyte sedimentation rate, HAQ=health assessment questionnaire, RA=rheumatoid arthritis, RF=rheumatoid factor.
Echocardiographic characteristics
Echocardiographic characteristics are reported in Table 3. As per study entrance criteria, the mean LVEF was normal in both RA and normal patient cohorts, and there was significantly more diastolic dysfunction present among RA patients. There was no significant difference in LV mass index or the prevalence of LV hypertrophy between RA and normal patients (p=0.08 and p=0.12, respectively).
Table 3.
Echocardiographic and myocardial strain characteristics of RA patients and matched normal patients without RA or cardiovascular disease.
| Parameter | RA (N=59) | Normals (N=59) | p-value |
|---|---|---|---|
| LV end-diastolic diameter (mm) | 46.6 ± 4.7 | 45.7 ± 4.2 | 0.96 |
| LV end-systolic diameter (mm) | 28.7 ± 4.4 | 30.0 ± 3.0 | 0.09 |
| LV EF (%) | 63.7 ± 4.9 | 62.5 ± 3.7 | 0.08 |
| Left atrial volume index (mL/m2) | 25.4 ± 5.0 | 24.6 ± 3.4 | 0.31 |
| Estimated RVSP (mmHg) | 28.5 ± 5.3 | 26.9 ± 4.5 | 0.09 |
| LV mass index (g/m2) | 81.4 ± 14.2 | 77.1 ± 11.9 | 0.08 |
| LV hypertrophy | 4 (7) | 0 (0) | 0.12 |
| Longitudinal strain (%) | |||
| Global LV strain | −15.7 ± 3.2 | −18.1 ± 2.4 | <0.001 |
| LV levels | |||
| Basal LV | −17.2 ± 4.2 | −18.9 ± 2.7 | 0.002 |
| Middle LV | −15.4 ± 3.0 | −18.6 ± 2.5 | <0.001 |
| Apical LV | −14.3 ± 4.2 | −16.7 ± 2.8 | <0.001 |
| LV walls | |||
| Anteroseptum | −15.2 ± 4.8 | −18.7 ± 3.6 | <0.001 |
| Inferoseptum | −16.2 ± 4.4 | −17.8 ± 3.0 | 0.003 |
| Inferior | −16.9 ± 5.4 | −17.4 ± 3.3 | 0.37 |
| Posterior | −15.4 ± 5.1 | −18.8 ± 3.7 | <0.001 |
| Lateral | −15.5 ± 4.2 | −17.9 ± 3.1 | <0.001 |
| Anterior | −15.4 ± 5.1 | −17.8 ± 3.1 | 0.005 |
| Global RV strain | −17.9 ± 4.7 | −20.7 ± 2.4 | <0.001 |
| RV levels | |||
| Basal RV | −21.2 ± 6.8 | −24.8 ± 3.6 | 0.001 |
| Middle RV | −17.3 ± 5.2 | −20.6 ± 3.2 | <0.001 |
| Apical RV | −15.2 ± 6.8 | −16.6 ± 3.9 | 0.025 |
| RV walls | |||
| Septum | −16.1 ± 4.1 | −19.1 ± 3.2 | <0.001 |
| Free wall | −19.8 ± 7.0 | −22.2 ± 3.6 | 0.009 |
Continuous data are expressed as mean ±standard deviation, categorical data as n (%). A=mitral inflow late diastolic peak Doppler velocity (m/sec), E=mitral inflow early diastolic peak Doppler velocity (m/sec), e'=early diastolic mitral annular tissue Doppler velocity (m/sec), EF=ejection fraction, LV=left ventricle, RV=right ventricle, RA=rheumatoid arthritis, RVSP=right ventricular systolic pressure.
Ventricular strain
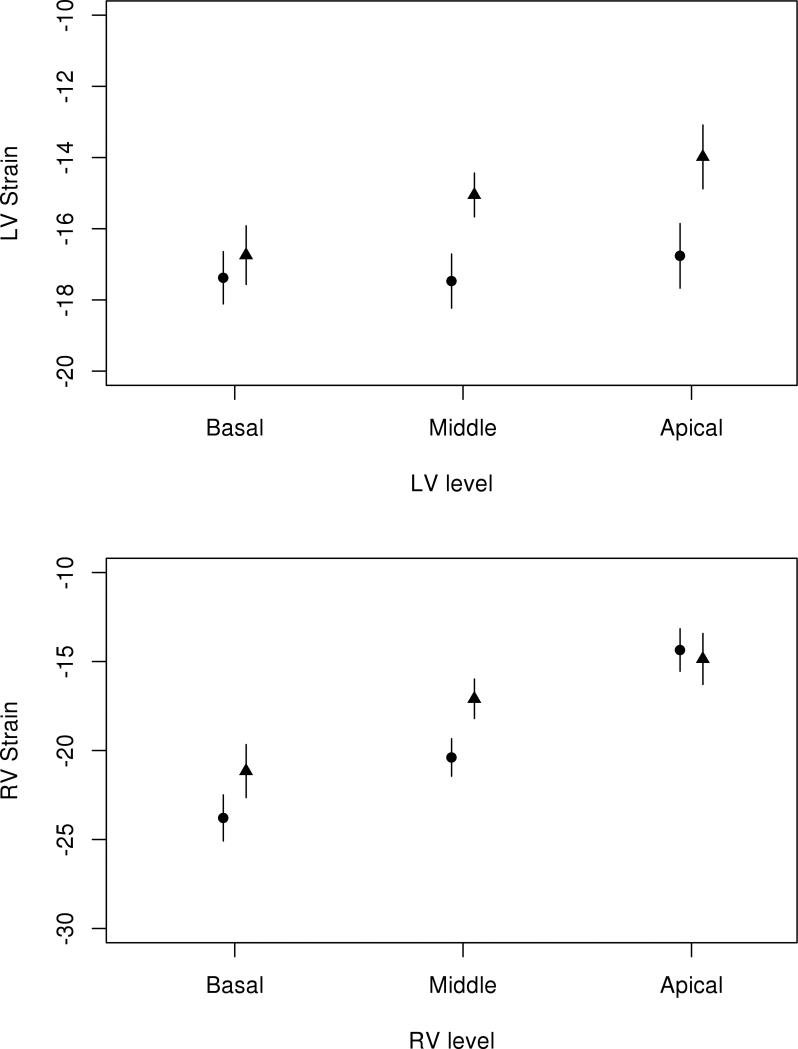
LV and RV strain values for the RA and normal patient cohorts are presented in Table 3. Patients with RA had significantly worse (less negative or reduced absolute value) global LV peak longitudinal systolic strain compared to normal patients (−15.7±3.2% and −18.1±2.4%, respectively, p<0.001). This difference persisted after adjustment for age, gender, blood pressure, body mass index, heart rate and LV mass index (p<0.001). In addition, this difference persisted when normal patients were compared to the subset of RA patients with normal LV diastolic function (p<0.001) with the same list of adjustors. At each LV level (basal, middle and apical), strain values were significantly worse among RA patients than normal patients (p=0.002, p<0.001 and p<0.001, respectively). Among RA patients there was a gradient of worsening strain values from base to apex (p<0.001), a trend also noted among normal patients (p<0.001). This gradient was similar among both groups (interaction p=0.22; Figure 2). All LV walls other than the inferior wall had significantly worse strain than normal patients (Table 4). Among normal patients, the anteroseptum and posterior LV walls had the greatest absolute value (most negative) strain, while these walls had the worst strain for RA patients (p<0.001 for both).
Figure 2.
Myocardial strain values by level (basal, middle and apical) of the left ventricle (LV, top) and right ventricle (RV, bottom) among RA patients (triangles) and normal patients without RA or cardiovascular disease (circles). RA=rheumatoid arthritis.
Table 4.
Peak systolic longitudinal ventricular strain values for RA patients according to levels of diastolic dysfunction.
| Longitudinal Strain (%) | Normal diastolic function (N=35) | Mild diastolic dysfunction (N=36) | Moderate/Severe diastolic dysfunction (N=16) | p-value |
|---|---|---|---|---|
| Global LV | −15.4 ± 3.2 | −14.5 ± 2.8 | −16.6 ± 3.2 | 0.07 |
| LV levels | ||||
| Basal LV | −17.3 ± 4.3 | −15.5 ± 3.0 | −18.3 ± 4.2 | 0.039 |
| Middle LV | −15.2 ± 2.9 | −14.4 ± 2.9 | −16.2 ± 2.9 | 0.23 |
| Apical LV | −13.7 ± 4.1 | −13.6 ± 4.3 | −15.5 ± 4.3 | 0.31 |
| Global RV | −17.6 ± 4.3 | −16.6 ± 4.4 | −20.5 ± 5.8 | 0.07 |
| RV levels | ||||
| Basal RV | −21.7 ± 5.5 | −19.1 ± 8.1 | −24.6 ± 6.7 | 0.10 |
| Middle RV | −16.1 ± 5.7 | −17.0 ± 4.8 | −19.5 ± 5.0 | 0.18 |
| Apical RV | −15.0 ± 7.8 | −13.5 ± 4.6 | −17.4 ± 8.3 | 0.40 |
Data are expressed as mean ±standard deviation, categorical data as n (%). LV=left ventricle, RA=rheumatoid arthritis, RV=right ventricle.
RA patients had significantly worse global RV peak longitudinal systolic strain compared to normal patients (−17.9±4.7% and −20.7±2.4%, respectively, p<0.001) (Table 3). This difference persisted after adjustment for age, gender, blood pressure, body mass index, heart rate and LV mass index (p=0.019). This trend was also present at each RV level. While RV strain declined from base to apex in all patients (p<0.001), the gradient was somewhat less pronounced in RA patients (interaction p=0.062; Figure 2). The RV free wall had greater magnitude of strain than the septum for both RA and normal patients, and RA patients had worse strain values for both the free wall and septum.
Among the 87 RA patients, 35 had normal diastolic function, 36 had mild diastolic dysfunction and 16 had moderate/severe diastolic dysfunction (Table 4). LV strain differed somewhat between these groups (−15.4±3.2% in normal, −14.5±2.8% in mild and −16.6±3.2% in moderate/severe; p=0.07). This difference did not persist after adjustment for age, gender and blood pressure (p=0.29). Similarly, RV strain differed somewhat between these groups (−17.6±4.3% in normal, −16.6±4.4% in mild and −20.5±5.8% in moderate/severe; p=0.07). This difference was not significantly altered by adjustment for age, gender and blood pressure (p=0.09).
Results of linear regression among all 87 RA patients revealed modest or no associations between LV strain and traditional CVD risk factors such as hypertension (coefficient: 1.71, standard error [SE]: 1.04, p=0.10), history of smoking (coefficient: 0.20, SE: 0.66, p=0.76), hyperlipidemia (coefficient: 1.64, SE: 0.79, p=0.052) and diabetes mellitus (coefficient: 1.87, SE: 1.02, p=0.07) adjusted for age and gender. A significant association between LV strain and HAQ disability index (p=0.032), and borderline associations with prior use of corticosteroids(p=0.062) and methotrexate (p=0.054) (Table 2) were found after adjusting for age, gender, blood pressure, body mass index, heart rate and LV mass index. Significant associations between acute phase reactants (CRP and ESR) were noted when we adjusted for age and gender alone, but these associations were attenuated following additional adjustment for blood pressure, body mass index, heart rate and LV mass index. No significant associations between RV strain and RA disease characteristics were found (data not shown).
DISCUSSION
The principle finding of this study is that ventricular longitudinal systolic strain measured using STE in RA patients without known CVD is reduced in comparison with an age, gender and race matched population of patients with normal ventricular function and no history of CVD. Strain was impaired for both the LV and RV in RA patients. Strain values in RA patients were associated with markers of RA disease severity such as HAQ disability score and the need for DMARD treatment. Traditional CVD risk factors such as hypertension, smoking, hyperlipidemia and diabetes did not predict abnormal strain values in this RA population, suggesting the strain impairment was more likely caused by their RA. Furthermore, systolic strain impairment did not correlate with diastolic dysfunction as measured by echocardiography, and was impaired in RA patients with no or only mild diastolic dysfunction, suggesting that strain may be a more sensitive non-invasive tool for detecting mechanical LV dysfunction in this population. Strain imaging was both feasible and reproducible. These findings add to the growing body of evidence that suggest an intrinsic cardiomyopathy may be present in some RA patients despite a normal LVEF, and that myocardial strain imaging may be an effective technique for detecting subclinical disease. Importantly, ours is the first study to measure RV strain in RA, and the finding of impaired RV strain further illustrates the global nature of cardiac impairment in RA.
CVD represents a significant source of morbidity and mortality among RA patients, accounting for up to 50% of all deaths.[21, 22] This risk is independent of traditional CVD risk factors or the presence of CAD.[1, 18] The considerable disease burden highlights the importance of early recognition of patients at risk for developing clinical HF, which could facilitate better prognostic assessment and potentially earlier therapeutic intervention to improve outcomes. While it has been demonstrated that RA patients have a higher prevalence of LV diastolic dysfunction as measured by echocardiography compared with age and gender matched subjects without RA,[23-32] the mechanism underlying this observation remains unclear.[18] Plasma markers such as B-type natriuretic peptide (BNP) have been shown to be less effective for CVD screening in RA.[5] These reports indicate a need for better strategies for evaluating RA patients for subclinical CVD. In addition, the presence and extent of RV dysfunction in RA is unknown.
The mechanism of CVD in RA remains an area of ongoing research. There is considerable evidence suggesting that chronic inflammation contributes to impaired endothelial and microcirculatory function, leading to the early onset of atherosclerosis in RA patients.[33-35] This perfusion impairment may lead to both systolic and diastolic dysfunction. Furthermore, prolonged inflammation may lead to oxidative stress, myocyte dysfunction,[34, 36] and to a cytokine-induced increase in fibroblast activity causing myocardial collagen deposition and interstitial fibrosis.[1] These processes may be potentially mitigated by early RA treatment if an appropriate patient selection tool is recognized. Strain imaging has proven to be a valuable non-invasive tool for identifying subclinical CVD in other forms of HF,[11] and the findings from this study suggest the same may be true for RA.
Our study is the largest to evaluate the myocardial strain imaging characteristics of RA patients without known CVD. Previous studies have demonstrated strain impairment in RA patients. Ikonomidis et al. studied 46 RA patients without CVD and compared them with 23 healthy controls.[37] Significant differences in LV systolic longitudinal, circumferential and radial strain between RA patients and controls were observed (circumferential strain measures the relative rotation of the LV around its short-axis during contraction, while radial strain measures LV short-axis thickening). Their study also measured LV strain differences between RA patients following treatment with the interleukin-1 inhibitor anakinra versus those treated with prednisolone for 30 days. There was greater improvement in LV strain after treatment with anakinra, suggesting strain abnormalities in RA may be responsive to treatment. A more recent study found significant differences in segmental LV longitudinal and radial strain values between 22 RA patients with a mean disease duration of 34±4 months and without a history of CVD compared to healthy control subjects.[14]
Meune et al. compared 27 RA patients without CVD with control subjects and found a significant difference in diastolic strain rate, but not systolic strain rate.[26] No RA variables were significantly correlated with LV strain rate. These findings contrast ours and other studies in which systolic strain was significantly different between RA patients and controls, and strain values did correlate with RA disease variables. The reason for these discrepant findings may be the smaller number of patients included in the Meune study limiting their statistical power to demonstrate differences, the measurement of strain rate (the rate of myocardial deformation change) rather than strain, and that strain rate was measured using tissue-Doppler imaging rather than STE. Doppler-based strain measurement has more technical limitations than STE, including a higher imaging frame rate requirement and insonation angle dependency, whereas STE is more automated and is the preferred strain imaging modality for current research and clinical practice.[7-9]
The difference in absolute strain values observed between RA and normal patients in our study was modest and would likely not lead to symptomatic CVD. This was not surprising as all RA patients in our study with known CVD were excluded. Rather, the difference in biventricular strain values between RA and normal patients may be more valuable for screening purposes to detect preclinical or early CVD in RA. It has also been demonstrated that certain forms of myocardial disease have a characteristic pattern of ventricular strain impairment, including reduced LV septal wall strain in patients with hypertrophic cardiomyopathy,[12] and reduced basal LV strain in patients with infiltrative and hypertension induced cardiomyopathies.[11, 38, 39] In our study apical strain values were most impaired in RA patients, in comparison with basal or middle values for both the LV and RV. This finding suggests a pattern of strain reduction that could help distinguish RA from other common causes of myocardial disease. Even though a significant proportion of RA patients in our cohort also had hypertension, the presence of this pattern further suggests their strain reduction was caused by RA, as opposed to other risk factors for ventricular dysfunction. Additional research is needed to characterize regional patterns of abnormal strain in RA compared to other myocardial diseases with preserved LVEF.
Limitations
This was a retrospective, single-center study with a modest patient sample size. Thus our findings require confirmation in larger prospective studies before routine clinical utilization of strain imaging can be recommended in RA. Although our study excluded patients with known CVD, RA patients with risk factors for CVD such as hypertension or diabetes were included. The presence of those risk factors may have influenced the strain values of those RA patients, although these risk factors were not or only modestly associated with LV strain values. Strain values of RA patients were compared with those of normal patients without a history of CVD and with normal ventricular function on clinically indicated TTE, rather than a prospectively recruited cohort of normal subjects, which may be a potential source of bias. Previous studies have measured other myocardial vectors of strain deformation such as circumferential or radial strain, in addition to longitudinal strain. We chose to measure longitudinal strain only because it has been demonstrated to be the most reproducible when measured using STE and the most sensitive for detecting early stages of myocardial disease, and therefore is the most frequently applied in clinical practice.[40] Longitudinal strain is predominantly influenced by the longitudinally oriented endocardial muscle fibres of the LV, which are commonly the first affected by global myopathic processes. Circumferential LV strain may in fact remain preserved in patients with heart failure with preserved LVEF, even at advanced stages.[41] Further research examining serial changes in all LV myocardial contractile vectors over time are needed to determine the impact of RA disease progression on ventricular mechanical performance. RV myocardial fibres are predominantly longitudinally oriented, and longitudinal strain is the principle technique used to assess RV systolic function using STE. Circumferential and radial RV strain is highly technically challenging and not routinely performed in clinical or research applications.
Conclusion
RA patients without a history of CVD have impaired LV and RV systolic longitudinal strain as measured using STE in comparison to matched patients with normal cardiac function and without RA, CVD or CVD risk factors. Strain abnormalities were associated with markers of RA disease severity. These findings suggest subclinical myocardial disease may be present in RA patients prior to the development of symptomatic CVD. Strain imaging may represent an effective tool for detection of subclinical CVD, and identifying RA patients at increased risk for developing HF.
Acknowledgements
None declared.
Funding Sources: This work was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under Award Number R01AR46849, and by the National Institute on Aging of the National Institutes of Health under Award Number R01AG034676. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
Footnotes
Competing Interest: None declared.
REFERENCES
- 1.Maradit-Kremers H, Nicola PJ, Crowson CS, et al. Cardiovascular death in rheumatoid arthritis: a population-based study. Arthritis Rheum. 2005;52:722–732. doi: 10.1002/art.20878. [DOI] [PubMed] [Google Scholar]
- 2.Nicola PJ, Maradit-Kremers H, Roger VL, et al. The risk of congestive heart failure in rheumatoid arthritis: a population-based study over 46 years. Arthritis Rheum. 2005;52:412–420. doi: 10.1002/art.20855. [DOI] [PubMed] [Google Scholar]
- 3.Nicola PJ, Crowson CS, Maradit-Kremers H, et al. Contribution of congestive heart failure and ischemic heart disease to excess mortality in rheumatoid arthritis. Arthritis Rheum. 2006;54:60–67. doi: 10.1002/art.21560. [DOI] [PubMed] [Google Scholar]
- 4.Davis JM, 3rd, Roger VL, Crowson CS, et al. The presentation and outcome of heart failure in patients with rheumatoid arthritis differs from that in the general population. Arthritis Rheum. 2008;58:2603–2611. doi: 10.1002/art.23798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crowson CS, Myasoedova E, Davis JM, et al. Use of B-type natriuretic peptide as a screening tool for left ventricular diastolic dysfunction in rheumatoid arthritis patients without clinical cardiovascular disease. Arthritis Care Res. 2011;63:729–734. doi: 10.1002/acr.20425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Amundsen BH, Helle-Valle T, Edvardsen T, et al. Noninvasive myocardial strain measurement by speckle tracking echocardiography: validation against sonomicrometry and tagged magnetic resonance imaging. J Am Coll Card. 2006;47:789–793. doi: 10.1016/j.jacc.2005.10.040. [DOI] [PubMed] [Google Scholar]
- 7.Leitman M, Lysyansky P, Sidenko S, et al. Two-dimensional strain-a novel software for real-time quantitative echocardiographic assessment of myocardial function. J Am Soc Echocardiogr. 2004;17:1021–1029. doi: 10.1016/j.echo.2004.06.019. [DOI] [PubMed] [Google Scholar]
- 8.Reisner SA, Lysyansky P, Agmon Y, et al. Global longitudinal strain: a novel index of left ventricular systolic function. J Am Soc Echocardiogr. 2004;17:630–633. doi: 10.1016/j.echo.2004.02.011. [DOI] [PubMed] [Google Scholar]
- 9.Langeland S, D'Hooge J, Wouters PF, et al. Experimental validation of a new ultrasound method for the simultaneous assessment of radial and longitudinal myocardial deformation independent of insonation angle. Circulation. 2005;112:2157–2162. doi: 10.1161/CIRCULATIONAHA.105.554006. [DOI] [PubMed] [Google Scholar]
- 10.Marwick TH. Measurement of strain and strain rate by echocardiography: ready for prime time? J Am Coll Card. 2006;47:1313–1327. doi: 10.1016/j.jacc.2005.11.063. [DOI] [PubMed] [Google Scholar]
- 11.Bellavia D, Pellikka PA, Abraham TP, et al. Evidence of impaired left ventricular systolic function by Doppler myocardial imaging in patients with systemic amyloidosis and no evidence of cardiac involvement by standard two-dimensional and Doppler echocardiography. Am J Card. 2008;101:1039–1045. doi: 10.1016/j.amjcard.2007.11.047. [DOI] [PubMed] [Google Scholar]
- 12.Yang H, Sun JP, Lever HM, et al. Use of strain imaging in detecting segmental dysfunction in patients with hypertrophic cardiomyopathy. J Am Soc Echocardiogr. 2003;16:233–239. doi: 10.1067/mje.2003.60. [DOI] [PubMed] [Google Scholar]
- 13.Liu YW, Tsai WC, Su CT, et al. Evidence of left ventricular systolic dysfunction detected by automated function imaging in patients with heart failure and preserved left ventricular ejection fraction. J Card Fail. 2009;15:782–789. doi: 10.1016/j.cardfail.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 14.Sitia S, Tomasoni L, Cicala S, et al. Detection of preclinical impairment of myocardial function in rheumatoid arthritis patients with short disease duration by speckle tracking echocardiography. Int J Cardiol. 2012;160:8–14. doi: 10.1016/j.ijcard.2011.03.012. [DOI] [PubMed] [Google Scholar]
- 15.Maradit Kremers H, Crowson CS, Gabriel SE. Rochester Epidemiology Project: a unique resource for research in the rheumatic diseases. Rheum Dis Clin North Am. 2004;30:819–834. doi: 10.1016/j.rdc.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 16.Myasoedova E, Crowson CS, Kremers HM, et al. Is the incidence of rheumatoid arthritis rising?: results from Olmsted County, Minnesota, 1955-2007. Arthritis Rheum. 2010;62:1576–1582. doi: 10.1002/art.27425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arnett FC, Edworthy SM, Bloch DA, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 18.Liang KP, Myasoedova E, Crowson CS, et al. Increased prevalence of diastolic dysfunction in rheumatoid arthritis. Ann Rheum Dis. 2010;69:1665–1670. doi: 10.1136/ard.2009.124362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lang RM, Bierig M, Devereux RB, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–1463. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 20.Nagueh SF, Appleton CP, Gillebert TC, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. J Am Soc Echocardiogr. 2009;22:107–133. doi: 10.1016/j.echo.2008.11.023. [DOI] [PubMed] [Google Scholar]
- 21.Naz SM, Symmons DP. Mortality in established rheumatoid arthritis. Best Pract Res Clin Rheumatol. 2007;21:871–883. doi: 10.1016/j.berh.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 22.del Rincon ID, Williams K, Stern MP, et al. High incidence of cardiovascular events in a rheumatoid arthritis cohort not explained by traditional cardiac risk factors. Arthritis Rheum. 2001;44:2737–2745. doi: 10.1002/1529-0131(200112)44:12<2737::AID-ART460>3.0.CO;2-%23. [DOI] [PubMed] [Google Scholar]
- 23.Yazici D, Tokay S, Aydin S, et al. Echocardiographic evaluation of cardiac diastolic function in patients with rheumatoid arthritis: 5 years of follow-up. Clin Rheumatol. 2008;27:647–650. doi: 10.1007/s10067-007-0820-x. [DOI] [PubMed] [Google Scholar]
- 24.Wislowska M, Jaszczyk B, Kochmanski M, et al. Diastolic heart function in RA patients. Rheumatol Int. 2008;28:513–519. doi: 10.1007/s00296-007-0473-8. [DOI] [PubMed] [Google Scholar]
- 25.Udayakumar N, Venkatesan S, Rajendiran C. Diastolic function abnormalities in rheumatoid arthritis: relation with duration of disease. Singapore Med J. 2007;48(6):537–542. [PubMed] [Google Scholar]
- 26.Meune C, Wahbi K, Assous N, et al. Myocardial dysfunction in rheumatoid arthritis: a controlled tissue-Doppler echocardiography study. J Rheumatol. 2007;34:2005–2009. [PubMed] [Google Scholar]
- 27.Rexhepaj N, Bajraktari G, Berisha I, et al. Left and right ventricular diastolic functions in patients with rheumatoid arthritis without clinically evident cardiovascular disease. Int J Clin Pract. 2006;60:683–688. doi: 10.1111/j.1368-5031.2006.00746.x. [DOI] [PubMed] [Google Scholar]
- 28.Arslan S, Bozkurt E, Sari RA, et al. Diastolic function abnormalities in active rheumatoid arthritis evaluation by conventional Doppler and tissue Doppler: relation with duration of disease. Clin Rheumatol. 2006;25:294–299. doi: 10.1007/s10067-005-0014-3. [DOI] [PubMed] [Google Scholar]
- 29.Gonzalez-Gay MA, Gonzalez-Juanatey C, Martin J. The increased risk of ventricular diastolic dysfunction and congestive heart failure in patients with rheumatoid arthritis is independent of the duration of the disease. Semin Arthritis Rheum. 2005;35:132–133. doi: 10.1016/j.semarthrit.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 30.Alpaslan M, Onrat E, Evcik D. Doppler echocardiographic evaluation of ventricular function in patients with rheumatoid arthritis. Clin Rheumatol. 2003;22:84–88. doi: 10.1007/s10067-002-0677-y. [DOI] [PubMed] [Google Scholar]
- 31.Di Franco M, Paradiso M, Mammarella A, et al. Diastolic function abnormalities in rheumatoid arthritis. Evaluation By echo Doppler transmitral flow and pulmonary venous flow: relation with duration of disease. Ann Rheum Dis. 2000;59:227–229. doi: 10.1136/ard.59.3.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Corrao S, Salli L, Arnone S, et al. Echo-Doppler left ventricular filling abnormalities in patients with rheumatoid arthritis without clinically evident cardiovascular disease. Eur J Clin Invest. 1996;26:293–297. doi: 10.1046/j.1365-2362.1996.133284.x. [DOI] [PubMed] [Google Scholar]
- 33.Folsom AR, Aleksic N, Catellier D, et al. C-reactive protein and incident coronary heart disease in the Atherosclerosis Risk In Communities (ARIC) study. Am Heart J. 2002;144:233–238. doi: 10.1067/mhj.2002.124054. [DOI] [PubMed] [Google Scholar]
- 34.Ikonomidis I, Lekakis JP, Nikolaou M, et al. Inhibition of interleukin-1 by anakinra improves vascular and left ventricular function in patients with rheumatoid arthritis. Circulation. 2008;117:2662–2669. doi: 10.1161/CIRCULATIONAHA.107.731877. [DOI] [PubMed] [Google Scholar]
- 35.Ciftci O, Yilmaz S, Topcu S, et al. Impaired coronary microvascular function and increased intima-media thickness in rheumatoid arthritis. Atherosclerosis. 2008;198:332–337. doi: 10.1016/j.atherosclerosis.2007.11.013. [DOI] [PubMed] [Google Scholar]
- 36.Ikonomidis I, Athanassopoulos G, Lekakis J, et al. Myocardial ischemia induces interleukin-6 and tissue factor production in patients with coronary artery disease: a dobutamine stress echocardiography study. Circulation. 2005;112:3272–3279. doi: 10.1161/CIRCULATIONAHA.104.532259. [DOI] [PubMed] [Google Scholar]
- 37.Ikonomidis I, Tzortzis S, Lekakis J, et al. Lowering interleukin-1 activity with anakinra improves myocardial deformation in rheumatoid arthritis. Heart. 2009;95:1502–1507. doi: 10.1136/hrt.2009.168971. [DOI] [PubMed] [Google Scholar]
- 38.Goebel B, Gjesdal O, Kottke D, et al. Detection of irregular patterns of myocardial contraction in patients with hypertensive heart disease: a two-dimensional ultrasound speckle tracking study. J Hypertens. 2011;29:2255–2264. doi: 10.1097/HJH.0b013e32834bdd09. [DOI] [PubMed] [Google Scholar]
- 39.Kosmala W, Plaksej R, Strotmann JM, et al. Progression of left ventricular functional abnormalities in hypertensive patients with heart failure: an ultrasonic two-dimensional speckle tracking study. J Am Soc Echocardiogr. 2008;21:1309–1317. doi: 10.1016/j.echo.2008.10.006. [DOI] [PubMed] [Google Scholar]
- 40.Mor-Avi V, Lang RM, Badano LP, et al. Current and evolving echocardiographic techniques for the quantitative evaluation of cardiac mechanics: ASE/EAE consensus statement on methodology and indications endorsed by the Japanese Society of Echocardiography. J Am Soc Echocardiogr. 2011;24:277–313. doi: 10.1016/j.echo.2011.01.015. [DOI] [PubMed] [Google Scholar]
- 41.Park SJ, Miyazaki C, Bruce CJ, et al. Left ventricular torsion by two-dimensional speckle tracking echocardiography in patients with diastolic dysfunction and normal ejection fraction. J Am Soc Echocardiogr. 2008;21:1129–1137. doi: 10.1016/j.echo.2008.04.002. [DOI] [PubMed] [Google Scholar]