Abstract
Objective
To examine whether the clinical severity of cervical dystonia (CD) significantly correlates with 18F-fluorodeoxyglucose positron emission tomography/computed tomography (18F-FDG PET/CT) findings as well as to determine the threshold of the clinical severity of CD for positive 18F-FDG PET/CT study findings.
Methods
Forty-seven subjects with torticollis as one of the symptoms of CD were included. The clinical severity of CD was evaluated with the Toronto Western Spasmodic Torticollis Rating Scale (TWSTRS) at the time of 18F-FDG PET/CT. The correlation between the clinical severity of CD and the highest SUVmax was examined. The threshold of the clinical severity of CD necessary for positive 18F-FDG PET/CT findings was determined using receiver operating characteristics curve analysis.
Results
Thirty-three of the 47 subjects (70.21%) showed positive 18F-FDG PET/CT findings. The ipsilateral splenius capitis/cervicis, oblique capitis inferior, and longus colli/capitis were the rotators most frequently involved. The highest SUVmax of 18F-FDG PET/CT was significant correlated with the TWSTRS. Subjects with a total TWSTRS exceeding 39 showed positive 18F-FDG PET/CT findings, with those having a total TWSTRS ≤22 showing negative 18F-FDG PET/CT results. The cutoff value of the total TWSTRS for positive 18F-FDG PET/CT findings was set at 27.5 with 90.9% sensitivity and 64.3% specificity.
Conclusion
A significant correlation was evident between the clinical severity of CD and 18F-FDG PET/CT findings, providing a threshold of the clinical severity of CD for acquisition of positive 18F-FDG PET/CT findings.
Keywords: Cervical dystonia, Fluorodeoxyglucose F18, Positron-emission tomography
INTRODUCTION
Cervical dystonia (CD) is a chronic neurological disorder characterized by involuntary contractions of the cervical musculature that lead to abnormal movements and postures of the head and neck [1,2]. CD is the most common form of focal dystonia with a prevalence of 0.006% in Europe [3] and 0.280% in the United States [2]. While CD causes variable postures of the head and neck, torticollis is the most common symptom [4].
Botulinum neurotoxin (BoNT) injection, physical therapy, deep brain stimulation, and oral medications including anticholinergics and benzodiazepines have been used for the management of CD. Among these treatments, BoNT injection has been the first-line therapy for CD for nearly 25 years [5]. Symptomatic relief has been reported with BoNT injection in more than 85% of those affected [6-10]. However, not all subjects show satisfactory improvement of abnormal posture of the head and neck after BoNT administration. The most important determinants in achieving a favorable response to BoNT treatment are proper identification of the involved muscles and the appropriate dosage of BoNT [10].
Inspection and palpation of dystonic muscles and electromyography have been used to identify the dystonic muscles in CD [11]. Sung et al. [12] reported the usefulness of 18F-fluorodeoxyglucose positron emission tomography/computed tomography (18F-FDG PET/CT) for identifying dystonic muscles in subjects with CD. Lee et al. [13] compared the outcome of BoNT injection between a 18F-FDG PET/CT-assisted clinically targeted group and a clinically targeted group and reported that the 18F-FDG PET/CT-assisted clinically targeted group was superior to the clinically targeted group in terms of the reduction rate of the Toronto Western Spasmodic Torticollis Rating Scale (TWSTRS) and the BoNT-A reinjection-free living period. 18F-FDG PET/CT has subsequently become a useful tool in the identification of dystonic muscles of CD, which results in better outcome with BoNT injection.
However, not all subjects with CD show positive 18F-FDG PET/CT findings [13]. Our clinical experiences with 18F-FDG PET/CT indicate that positive findings increase with the clinical severity of CD [13]. However, it has not yet been established whether there is any correlation between the clinical severity of CD and 18F-FDG PET/CT findings. If there is a significant correlation, the threshold of the clinical severity of CD for acquiring positive 18F-FDG PET/CT study findings would be able to be determined. This would help prevent clinicians from conducting negative 18F-FDG PET/CT studies.
The objectives of this study were to examine whether there is a significant correlation between the clinical severity of CD and 18F-FDG PET/CT findings and, if so, to determine the threshold of the clinical severity of CD for the acquisition of positive 18F-FDG PET/CT study findings.
MATERIALS AND METHODS
This was a retrospective study conducted at a torticollis clinic in a single tertiary medical center from November 2009 to May 2012. This research was approved by the Institutional Review Board of Ajou Medical Center.
Subjects
Subjects with CD with torticollis as one of the symptoms and who had undergone 18F-FDG PET/CT examination were included. CD was diagnosed as abnormal posture of the head and neck caused by involuntary contractions, along with morning benefit, sensory trick or aggravation of physical/emotional stress [13]. Torticollis was defined as a condition in which the head was turned toward the right or left side on the horizontal plane [14]. Subjects who had one or more of the following criteria were excluded: surgery for CD, limited range of motion of the head and neck, generalized dystonia or other neurological symptoms, CD caused by known gene mutations, such as TOR1 and GCH1, or a copper metabolism disorder, such as Wilson disease. Clinical data were collected through a review of medical records for information on age, gender, duration of CD, clinical severity of CD, and CD-related abnormal posture of the head and neck caused by torticollis, laterocollis, retrocollis, anterocollis or a combination of them. The clinical severity of CD at the time of 18F-FDG PET/CT was measured using TWSTRS. The total score of TWSTRS ranged from 0 to 85 points on three subscales; severity (0-35 points), disability (0-30 points), and pain (0-20 points) [15,16].
Analysis of 18F-FDG PET/CT findings
Subjects fasted for at least 4 hours before 18F-FDG PET/CT, which was done as previously described [17,18]. 18F-FDG PET/CT findings were evaluated in two ways. The first involved visual image interpretation by the second author (a nuclear medicine specialist) as positive or negative according to the presence of increased uptake of 18F-FDG at any muscle among the rotators of the head and neck compared to the adjacent or contralateral muscles. The rotators of the head and neck with increased 18F-FDG uptake were listed for each subject with positive 18F-FDG PET/CT findings. Secondly, 18F-FDG PET/CT findings were quantified using maximum standardized uptake value (SUVmax). SUV is defined as the amount of radioactivity released per 1 g of tissue divided by the amount of radioactivity administered per 1 kg of body weight [17,18]. SUVmax was measured for 10 rotators of the head and neck for each subject by setting the globular-shaped volume of interest (0.96 cm3). Therefore, 10 SUVmax values were measured for each subject, after which the highest SUVmax among the SUVmax values of 10 rotators in each subject was determined. The rotators of the head and neck are the muscles that cause torticollis. Based on functional anatomy of the head and neck, 10 rotators of the head and neck were identified: ipsilateral splenius capitis/cervicis, ipsilateral longus colli/longus capitis, ipsilateral oblique capitis inferior, ipsilateral oblique capitis superior, ipsilateral rectus capitis posterior major, contralateral semispinalis capitis, contralateral sternocleidomastoid, contralateral multifidus, contralateral trapezius and contralateral levator scapulae muscles.
Correlation between CD clinical severity and the highest SUVmax of 18F-FDG PET/CT
The correlation between the clinical severity of CD and the highest SUVmax was examined through correlation and linear regression analyses. The clinical severity of CD and related diminution of activities of daily living were measured using TWSTRS. TWSTRS is composed of severity, disability, and pain subscales and is widely used to assess clinical severity and treatment effect due to its outstanding inter-observer reliability [15,16].
Threshold of CD clinical severity for positive 18F-FDG PET/CT findings
The threshold of the clinical severity of CD necessary to obtain positive 18F-FDG PET/CT results was determined using the receiver operating characteristics (ROC) curve analysis.
Statistical analyses
IBM SPSS Statistics ver. 20.0 (IBM, Armonk, NY, USA) was used for statistical analysis. ROC curve analysis was used to determine the optimal cutoff scores of TWSTRS for prediction of positive findings of 18F-FDG PET/CT Correlation analysis with Pearson γ correlation coefficient and linear regression analysis were done to evaluate the relationship between TWSTRS and the highest SUV-max in 18F-FDG PET/CT. Statistical significance was set at p<0.001.
RESULTS
Subject characteristics
The characteristics of the 47 subjects are presented in Table 1. The 47 subjects comprised 21 men and 26 women with a mean age of 38.23±12.05 years (range, 18-64 years). The mean duration of CD was 28.96±32.63 months (range, 0.25-125 months). The total TWSTRS was 34.26±10.08 (range, 14-60 points). Torticollis was present in the form of torticollis alone (40.4%) or as a combination of other abnormal postures of the head and neck (59.6%). Fifteen subjects (31.9%) were previously treated with BoNT injection. The mean periodic interval between the BoNT injection and 18F-FDG PET/CT was 9.04±10.44 months (range, 2-42 months).
Table 1.
Characteristics of the subjects (n=47)
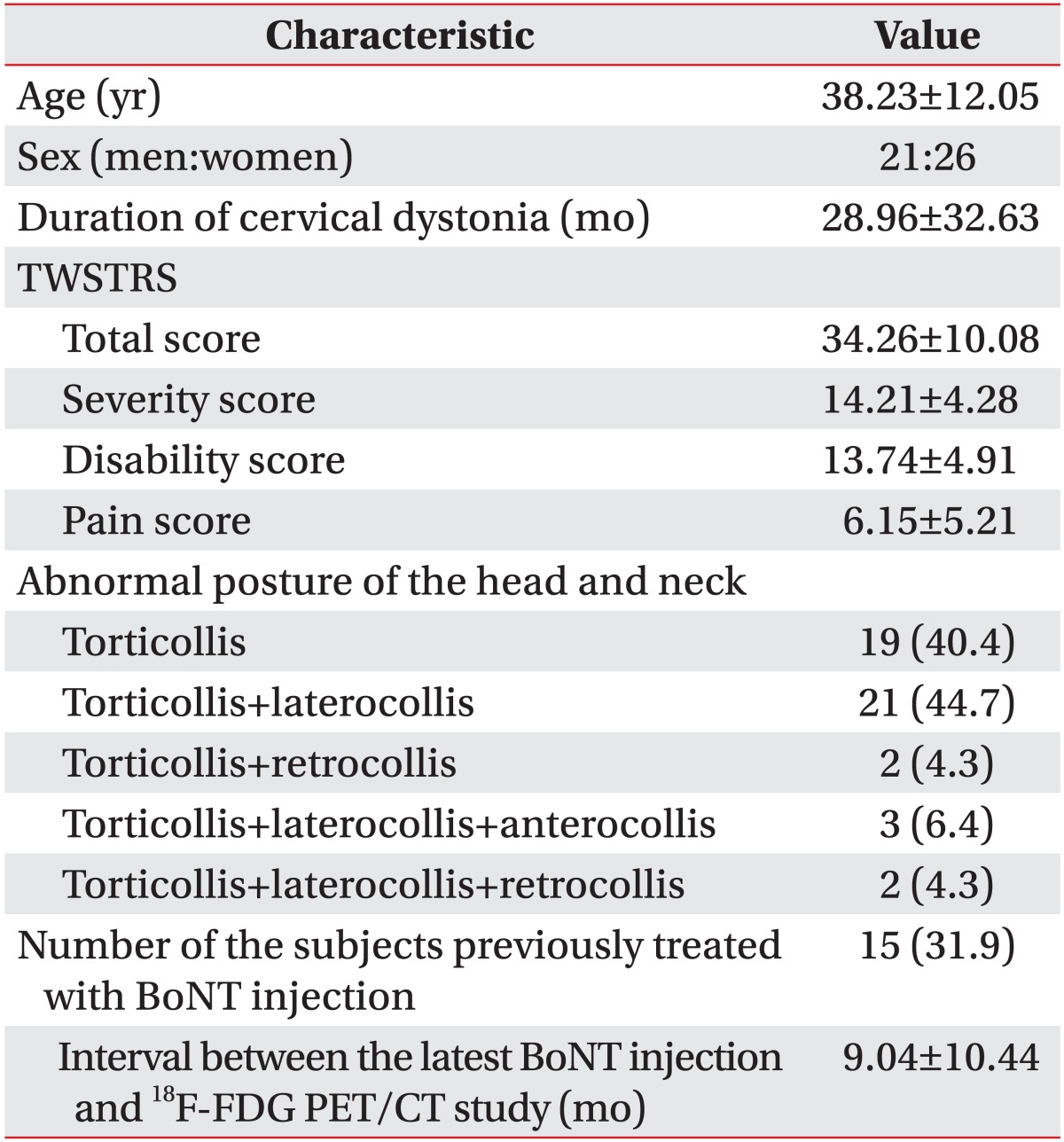
Values are presented as mean±standard deviation or number (%).
TWSTRS, Toronto Western Spasmodic Torticollis Rating Scale; BoNT, botulinum neurotoxin; 18F-FDG PET/CT, 18F-fluorodeoxyglucose positron emission tomography/computed tomography.
Subjects showing increased 18F-FDG uptake on each of the 10 rotators of the head and neck in descending order
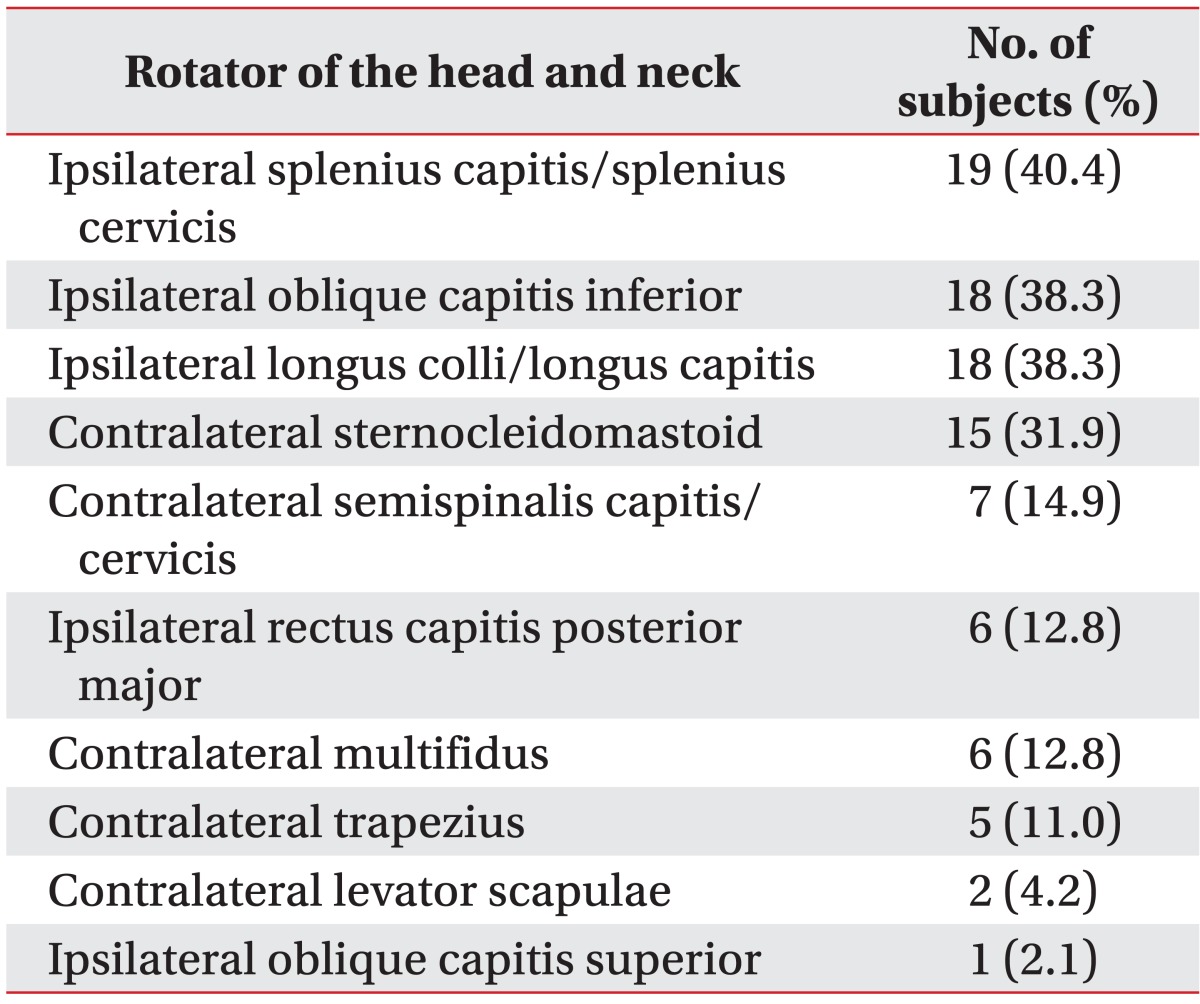
Thirty-three subjects out of 47 (70.21%) showed positive 18F-FDG PET/CT study findings. Table 2 lists the 10 rotators of the head and neck in descending order in terms of the number of the 33 subjects who showed increased 18F-FDG uptake. Increased 18F-FDG uptake was evident in 2.88 rotators on average. The most frequently involved top three rotators were the ipsilateral splenius capitis/cervicis, oblique capitis inferior and longus colli/capitis, indicating frequent involvement of the deep-seated neck muscles in CD.
Table 2.
Number of subjects showing increased 18F-fluorodeoxyglucose uptake on each of 10 rotators of the head and neck
Correlation between CD clinical severity and highest SUVmax of 18F-FDG PET/CT
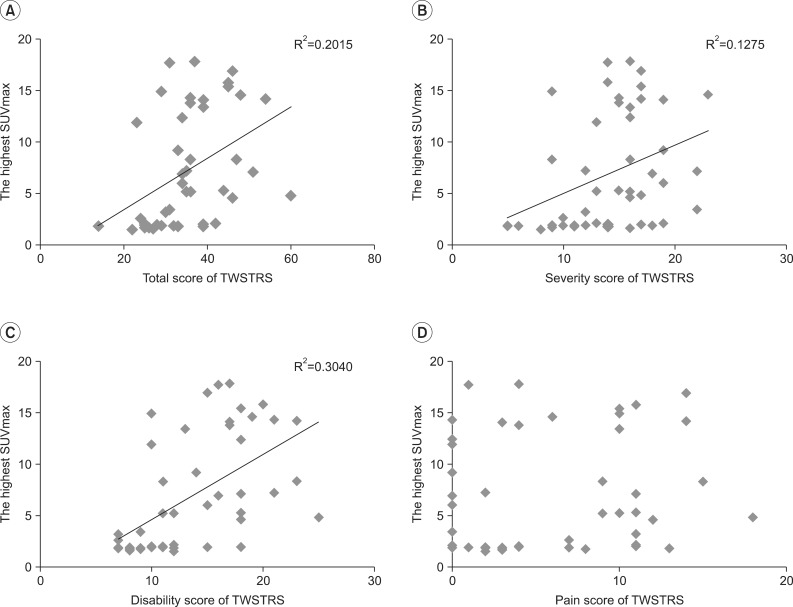
While the mean highest SUVmax of 14 negative instances of 18F-FDG PET/CT was 1.79±0.13 (range, 1.5-1.9), the mean highest SUVmax of 33 positive 18F-FDG PET/CT studies was 9.17±5.34 (range, 2-17.8). The highest SUVmax of 18F-FDG PET/CT was significantly positively correlated with the total TWSTRS (Pearson γ correlation coefficient=0.449, p=0.01), severity subscale score (Pearson γ correlation coefficient=0.357, p=0.01), and disability subscale score (Pearson γ correlation coefficient=0.551, p=0.01). The linear regression analysis with R2 is shown in Fig. 1. There was no significant correlation between the pain subscale score and the highest SUVmax (p=0.582).
Fig. 1.
(A-C) Correlation between TWSTRS and the highest SUVmax of 18F-fluorodeoxyglucose positron emission tomography/computed tomography. The SUVmax showed significant correlation with (A) the total TWSTRS, (B) severity subscale scores, and (C) disability subscale scores of TWSTRS (p<0.001). (D) There was no correlation between the scores of pain subscale and the SUVmax (p=0.582). The highest value among SUVmaxes of 10 rotators in each subject was defined as the highest SUVmax. SUVmax, maximum standardized uptake value; TWSTRS, Toronto Western Spasmodic Torticollis Rating Scale.
Threshold of CD clinical severity for positive 18F-FDG PET/CT
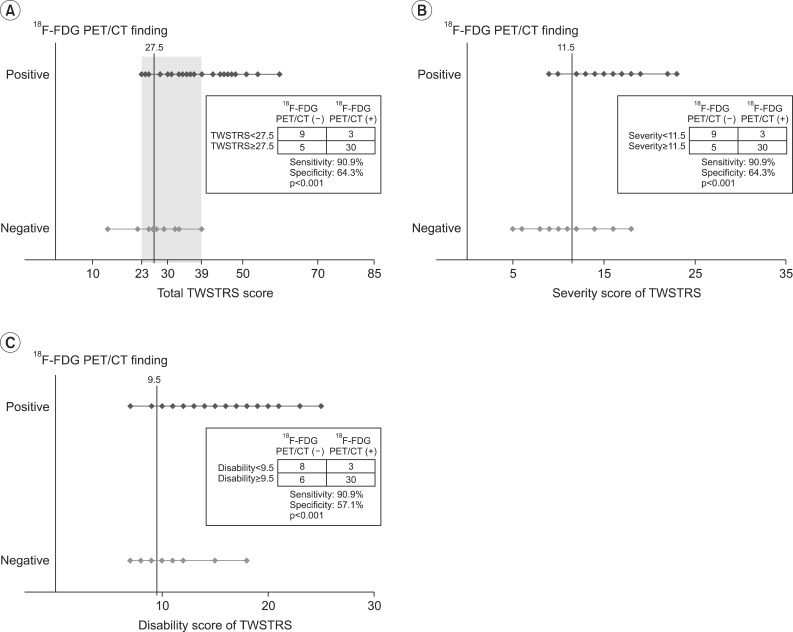
The 33 subjects with positive 18F-FDG PET/CT findings had total TWSTRS exceeding 39, and subjects with total TWSTRS ≤22 showed negative 18F-FDG PET/CT findings (Fig. 2). ROC curve analysis was done to reveal the total TWSTRS score with reasonable sensitivity and specificity. The cutoff value of the total TWSTRS for positive 18F-FDG PET/CT study was set as 27.5 with 90.9% sensitivity and 64.3% specificity (confidence interval [CI]=75.2%-97.3%, area under the ROC curve [AUC]=0.863). The cutoff value of severity score, which shows positive 18F-FDG PET/CT, was 11.5 with 90.9% sensitivity and 64.3% specificity (CI=70.3%-96.8%, AUC=0.835). The cutoff value of disability score for positive TWSTRS was 9.5 with sensitivity of 90.9% and 57.1% of specificity (CI=68.1%-94.5%, AUC=0.813) (Fig. 2).
Fig. 2.
Optimal cutoff value of TWSTRS for 18F-FDG PET/CT positive finding selected by receiver operating characteristics curve analysis. (A) The cutoff value of total score of TWSTRS was 27.5. The lowest total score of TWSTRS for the positive 18F-FDG PET/CT findings was 23. All subjects with a total TWSTRS of more than 39 showed positive 18F-FDG PET/CT findings. (B) The cutoff value of severity score of TWSTRS was 11.5. (C) The cutoff value of disability score of TWSTRS was 9.5. TWSTRS, Toronto Western Spasmodic Torticollis Rating Scale; 18F-FDG PET/CT, 18F-fluorodeoxyglucose positron emission tomography/computed tomography.
DISCUSSION
To the best of our knowledge, this is the first report to demonstrate a significant correlation between the clinical severity of CD and 18F-FDG PET/CT findings.
The present study aimed to examine the clinical usefulness of 18F-FDG PET/CT by assessing whether the degree of 18F-FDG uptake is related with the extent of clinical symptoms by examining the level of clinical severity that can result in positive findings under the 18F-FDG PET/CT test in CD with torticollis symptoms. 18F-FDG PET/CT is useful since it can determine the metabolism of the deep and superficial neck muscles simultaneously and non-invasively by overcoming the shortcomings of physical examination and electromyography mapping [12,19]. 18F-FDG PET/CT is also useful in the selection of muscles in CD, as evident with the assessment of the effects of BoNT treatment [13]. However, no research has assessed the correlation between the clinical severity expressed by means of TWSTRS score and SUVmax as a means of illustrating the extent of increase in 18F-FDG uptake in 18F-FDG PET/CT.
To study the relationship between clinical severity and 18F-FDG PET/CT, we presently conducted a correlation analysis between the TWSTRS score and the highest SUVmax. The highest SUVmax significantly correlated with TWSTRS. Although significant correlations with total TWSTRS score, severity subscale score, and disability subscale score were evident, there was no correlation with the pain subscale score. Pain is a relatively common symptom of CD, with a reported rate of 68% [20]. In the present study, 37 subjects (78.72%) had pain but 24 subjects (51.06%) displayed a low level of pain, including 10 patients who were pain-free. This may reflect the difficulty of achieving an objective level of severity symptoms only by means of pain, the most subjective subscale of TWSTRS. Due to differences in pain perception, and because pain tolerance is associated with other factors including ethnicity, genetics and sex, pain is a very subjective and complex entity that varies among individuals.
Based on this correlation, we were able to provide the threshold of the clinical severity of CD for acquisition of positive 18F-FDG PET/CT study findings. The cutoff value of TWSTRS for 18F-FDG PET/CT to display positive findings was set at a score of 27.5 with 90.3% sensitivity and 64.3% specificity. We set the sensitivity as the major value and, as such, selected a point at which the specificity is at its peak when the sensitivity is high at 90.3%. Those with a TWSTRS score <22 did not show increased 18F-FDG uptake due to relatively low glucose metabolism, probably caused by insufficient contractility of the dystonic muscles compared to adjacent muscles. Thus, we suggest that it would be better for clinicians to use conventional methods, such as physical examination and electromyography to identify dystonic muscles instead of 18F-FDG PET/CT study for patients with TWSTRS score <27.5. The use of the suggested cutoff value in determining whether to perform 18F-FDG PET/CT for patients with CD with symptoms of torticollis may have value, given that the examination is expensive. Use of the cutoff aids in determining whether 18F-FDG PET/CT will be useful in discriminating dystonic muscles in patients with moderate severity. There are no decision guidelines concerning the implementation of 18F-FDG PET/CT. This study is the first to present a cutoff value for clinical severity that can be used in predicting the presence of 18F-FDG PET/CT positive findings.
Among the head and neck rotator muscles, the deep-seated neck muscles including the oblique capitis inferior, longus colli and rectus capitis posterior major are frequently involved. The superficial neck muscle is the dystonic muscle in the previously reported majority of CD patients. Only a few prior studies used 18F-FDG PET/CT to examine the involvement of deep neck muscles, such as the longus colli/capitis, oblique capitis inferior and rectus capitis posterior major, as the dystonic muscles [12,13,19]. These studies did not administer BoNT injections into these deep neck muscles. Presently, the longus colli, oblique capitis inferior and rectus capitis posterior major displayed a high frequency of 18F-FDG uptake increase in 18F-FDG PET/CT examination of patients with CD with symptoms of torticollis. Thus, the muscles should be considered as targets for BoNT injection.
Presently, we determined the cutoff value that can be used to decide whether to implement 18F-FDG PET/CT in accordance with the severity of clinical symptoms. This advantageous, since 18F-FDG PET/CT involves exposure to radiation and is expensive to do, and so should not be performed indiscriminately. The present results indicate that 18F-FDG PET/CT usefully determines dystonic muscles in CD with higher than moderate severity. In addition, it was possible to present guidelines to prioritizing the muscles for BoNT injection for those not subjected to 18F-FDG PET/CT, by computing the frequencies of the muscles for which the 18F-FDG uptake was increased under 18F-FDG PET/CT. This will provide assistance in selecting the appropriate muscle for BoNT injection by illustrating that not only the widely known superficial neck muscles, but also the deep neck muscles, such as the longus colli/capitis, oblique capitis inferior and rectus capitis posterior major, are frequently involved in CD. Lastly, the results could also be helpful in determining the dosage of BoNT injection in accordance with the extent of 18F-FDG uptake at the time of injection, since a high correlation between clinical severity and SUVmax was confirmed.
This study had several limitations. First, the results cannot be applied if the symptoms of patients including laterocollis, anterocollis, and retrocollis manifest singly or in combination, since the study was conducted only on subjects with CD with symptoms of torticollis. Second, the maximum TWSTRS score was 60, which indicates that there is a limitation in generalizing the results of this study to CD patients whose TWSTRS score exceeds 60. Third, the subjects were not divided into a BoNT injection group and a non-BoNT injection group. Among 15 subjects previously treated with BoNT injection, 8 proceeded 18F-FDG PET/CT <6 months after BoNT injection and 2 underwent 18F-FDG PET/CT <3 months later (Table 1). The effect of BoNT lasts for 12 to 24 weeks [21,22]. So to investigate whether previous BoNT injection affected the existence of 18F-FDG uptake in 18F-FDG PET/CT, binomial logistic regression analysis was performed. In our analysis, previous BoNT injection did not significantly affect our study results. Fourth, since we did not compare baseline TWSTRS with the TWSTRS score following BoNT injection treatment after 18F-FDG PET/CT, it was not possible to determine the resultant effect of 18F-FDG PET/CT on the treatment of CD with BoNT.
Future studies should be broadened to include patients with a diverse range of symptoms including laterocollis, retrocollis, and anterocollis, singly or in combination, and patients with severe condition who have a TWSTRS score >60. Moreover, the scope of the analysis of the muscles should include not only the ones that function as rotators but also those that cause a wide range of head and neck movements including flexion, lateral flexion, and extension. In addition, research on the extent of improvement of clinical symptoms prior to and following BoNT injection would enable the determination of the usefulness of 18F-FDG PET/CT in the selection of target muscles and the treatment effect.
In conclusion, a significant correlation was identified between the clinical severity of CD and 18F-FDG PET/CT findings. Based on this correlation, we were able to provide a threshold of the clinical severity of CD for acquisition of positive 18F-FDG PET/CT study findings. 18F-FDG PET/CT enabled an in-depth understanding of the frequent involvement of the deep-seated neck muscles in CD. These findings are suggestive of the usefulness of 18F-FDG PET/CT for the identification of dystonic muscles of moderately severe CD. Further studies are required to verify these findings.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Jankovic J, Tolosa E. Parkinson's disease and movement disorders. 5th ed. Philadelphia: Lippincott Williams & Wilkins; 2007. [Google Scholar]
- 2.Jankovic J, Tsui J, Bergeron C. Prevalence of cervical dystonia and spasmodic torticollis in the United States general population. Parkinsonism Relat Disord. 2007;13:411–416. doi: 10.1016/j.parkreldis.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 3.Epidemiological Study of Dystonia in Europe (ESDE) Collaborative Group. A prevalence study of primary dystonia in eight European countries. J Neurol. 2000;247:787–792. doi: 10.1007/s004150070094. [DOI] [PubMed] [Google Scholar]
- 4.Camargo CH, Teive HA, Becker N, Baran MH, Scola RH, Werneck LC. Cervical dystonia: clinical and therapeutic features in 85 patients. Arq Neuropsiquiatr. 2008;66:15–21. doi: 10.1590/s0004-282x2008000100005. [DOI] [PubMed] [Google Scholar]
- 5.Albanese A, Barnes MP, Bhatia KP, Fernandez-Alvarez E, Filippini G, Gasser T, et al. A systematic review on the diagnosis and treatment of primary (idiopathic) dystonia and dystonia plus syndromes: report of an EFNS/MDS-ES Task Force. Eur J Neurol. 2006;13:433–444. doi: 10.1111/j.1468-1331.2006.01537.x. [DOI] [PubMed] [Google Scholar]
- 6.Brans JW, de Boer IP, Aramideh M, Ongerboer de Visser BW, Speelman JD. Botulinum toxin in cervical dystonia: low dosage with electromyographic guidance. J Neurol. 1995;242:529–534. doi: 10.1007/BF00867425. [DOI] [PubMed] [Google Scholar]
- 7.Comella CL, Buchman AS, Tanner CM, Brown-Toms NC, Goetz CG. Botulinum toxin injection for spasmodic torticollis: increased magnitude of benefit with electromyographic assistance. Neurology. 1992;42:878–882. doi: 10.1212/wnl.42.4.878. [DOI] [PubMed] [Google Scholar]
- 8.Anderson TJ, Rivest J, Stell R, Steiger MJ, Cohen H, Thompson PD, et al. Botulinum toxin treatment of spasmodic torticollis. J R Soc Med. 1992;85:524–529. [PMC free article] [PubMed] [Google Scholar]
- 9.Truong D, Duane DD, Jankovic J, Singer C, Seeberger LC, Comella CL, et al. Efficacy and safety of botulinum type A toxin (Dysport) in cervical dystonia: results of the first US randomized, double-blind, placebo-controlled study. Mov Disord. 2005;20:783–791. doi: 10.1002/mds.20403. [DOI] [PubMed] [Google Scholar]
- 10.Jankovic J. Treatment of cervical dystonia with botulinum toxin. Mov Disord. 2004;19(Suppl 8):S109–S115. doi: 10.1002/mds.20024. [DOI] [PubMed] [Google Scholar]
- 11.Brans JW, Aramideh M, Koelman JH, Lindeboom R, Speelman JD, Ongerboer de Visser BW. Electromyography in cervical dystonia: changes after botulinum and trihexyphenidyl. Neurology. 1998;51:815–819. doi: 10.1212/wnl.51.3.815. [DOI] [PubMed] [Google Scholar]
- 12.Sung DH, Choi JY, Kim DH, Kim ES, Son YI, Cho YS, et al. Localization of dystonic muscles with 18F-FDG PET/CT in idiopathic cervical dystonia. J Nucl Med. 2007;48:1790–1795. doi: 10.2967/jnumed.107.044024. [DOI] [PubMed] [Google Scholar]
- 13.Lee HB, An YS, Lee HY, Hwang JH, Lee HJ, Jeong KY, et al. Usefulness of (18)f-fluorodeoxyglucose positron emission tomography/computed tomography in management of cervical dystonia. Ann Rehabil Med. 2012;36:745–755. doi: 10.5535/arm.2012.36.6.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yim SY, Lee IY, Park MC, Kim JH. Differential diagnosis and management of abnormal posture of the head and neck. J Korean Med Assoc. 2009;52:705–718. [Google Scholar]
- 15.Consky ES, Lang AE. Clinical assessments of patients with cervical dystonia. Neurol Dis Ther. 1994;25:211. [Google Scholar]
- 16.Salvia P, Champagne O, Feipel V, Rooze M, de Beyl DZ. Clinical and goniometric evaluation of patients with spasmodic torticollis. Clin Biomech (Bristol, Avon) 2006;21:323–329. doi: 10.1016/j.clinbiomech.2005.11.011. [DOI] [PubMed] [Google Scholar]
- 17.An YS, Sheen SS, Oh YJ, Hwang SC, Yoon JK. Nonionic intravenous contrast agent does not cause clinically significant artifacts to 18F-FDG PET/CT in patients with lung cancer. Ann Nucl Med. 2007;21:585–592. doi: 10.1007/s12149-007-0066-3. [DOI] [PubMed] [Google Scholar]
- 18.An YS, Sun JS, Park KJ, Hwang SC, Park KJ, Sheen SS, et al. Diagnostic performance of (18)F-FDG PET/CT for lymph node staging in patients with operable non-small-cell lung cancer and inflammatory lung disease. Lung. 2008;186:327–336. doi: 10.1007/s00408-008-9109-3. [DOI] [PubMed] [Google Scholar]
- 19.Lee IH, Yoon YC, Sung DH, Kwon JW, Jung JY. Initial experience with imaging-guided intramuscular botulinum toxin injection in patients with idiopathic cervical dystonia. AJR Am J Roentgenol. 2009;192:996–1001. doi: 10.2214/AJR.08.1535. [DOI] [PubMed] [Google Scholar]
- 20.Jankovic J, Leder S, Warner D, Schwartz K. Cervical dystonia: clinical findings and associated movement disorders. Neurology. 1991;41:1088–1091. doi: 10.1212/wnl.41.7.1088. [DOI] [PubMed] [Google Scholar]
- 21.Brashear A, Watts MW, Marchetti A, Magar R, Lau H, Wang L. Duration of effect of botulinum toxin type A in adult patients with cervical dystonia: a retrospective chart review. Clin Ther. 2000;22:1516–1524. doi: 10.1016/s0149-2918(00)83049-0. [DOI] [PubMed] [Google Scholar]
- 22.Brashear A, Ambrosius WT, Eckert GJ, Siemers ER. Comparison of treatment of tardive dystonia and idiopathic cervical dystonia with botulinum toxin type A. Mov Disord. 1998;13:158–161. doi: 10.1002/mds.870130130. [DOI] [PubMed] [Google Scholar]