Abstract
Objective
To investigate the effect of botulinum toxin type A (BTA) injection into the salivary gland and to evaluate the changes of drooling in varied postures in tetraplegic patients with brain injury.
Methods
Eight tetraplegic patients with brain injury were enrolled. BTA was injected into each parotid and submandibular gland of both sides under ultrasonographic guidance. Drooling was measured by a questionnaire-based scoring system for drooling severity and frequency, and the sialorrhea was measured by a modified Schirmer test for the patients before the injection, 3 weeks and 3 months after the injection. Drooling was evaluated in each posture, such as supine, sitting, and tilt table standing, and during involuntary mastication, before and after the injection.
Results
The severity and frequency of drooling and the modified Schirmer test improved significantly at 3 weeks and 3 months after the injection (p<0.05). Drooling was more severe and frequent in tilt table standing than in the sitting position and in sitting versus supine position (p<0.05). The severity of drooling was significantly increased in the patients with involuntary mastication (p<0.05).
Conclusion
Salivary gland injection of BTA in patients with tetraplegia resulting from brain injury who had drooling and sialorrhea could improve the symptoms for 3 months without complications. The severity and frequency of drooling were dependent on posture and involuntary mastication. Proper posture and involuntary mastication of the patients should be taken into account in planning drooling treatment.
Keywords: Botulinum toxins, Drooling, Posture, Salivary glands, Ultrasonography
INTRODUCTION
Drooling is a condition that commonly occurs in patients with neurological diseases, such as cerebral palsy, motor neuron disease or Parkinson disease [1-3]. The reasons for drooling include inadequate oral and facial motor control or interruption of swallowing reflexes rather than excess saliva production. Excessive drooling leads to functional, social, psychological, and physical problems, such as dehydration, unpleasant odor, and aspiration [4-6]. Drooling also occurs frequently in tetraplegic patients with severe brain injury and persistent drooling may cause nausea, vomiting, and coughing due to continuous sucking of saliva, aspiration into the airway and, if severe, recurrent aspiration pneumonia as well as hygiene problems that may significantly decrease quality of life. A variety of surgical and non-surgical treatments have been used to diminish drooling; those with few, uncertain or unsatisfactory results lead to complications [7].
The present pilot study evaluated the efficacy of botulinum toxin type A (BTA) injection into the parotid and submandibular glands under ultrasonic guidance in patients with severe drooling and aspiration symptoms after brain injury. Posture-related changes in drooling were also evaluated with the aim of improving the interventions adopted for patients at different levels of activity.
MATERIALS AND METHODS
Among patients with tetraplegia who suffered brain injury at least 3 months previously, eight patients (six males and two females; age range, 30-63 years; mean±standard deviation, 46.6±13.2 years) were enrolled; these patients had previously been withdrawn from treatment with oral anticholinergic agents because of complications that included dry mouth, blurred vision, urinary retention, and constipation. The diagnoses were hypoxic brain injury in three, pontine hemorrhage in four, and cerebral infarction in one (Table 1). All patients had undergone percutaneous endoscopic gastrostomy due to severe dysphagia and showed symptoms of frequent aspiration of saliva and excessive drooling. An appropriate informed verbal consent was obtained from the caregivers of all patients as per the recommendations of the Pusan National University Hospital Research Ethics Review Board with Institutional Review Board approval.
Table 1.
Characteristics of subjects (n=8)

Values are presented as mean±standard deviation or number.
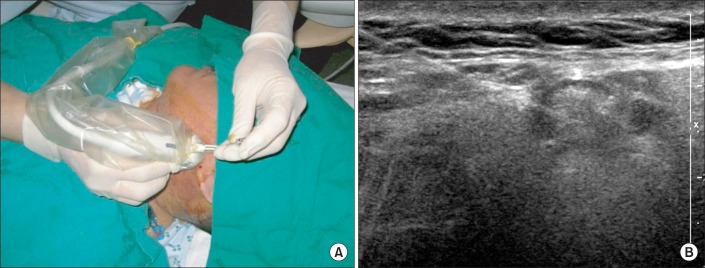
For each patient, 100 units of Botox (Allergan, Irvine, CA, USA) was diluted with 8 mL of normal saline so that 0.6 mL was equated to approximately 8 units and 1 mL to 12 units. Using a 25-gauge spinal needle and under ultrasonic guidance, a total of 24 units was injected into each parotid gland at three sites and 24 units into each submandibular gland at two sites, totaling 96 units per patient (Fig. 1). For the clinical scale, the questionnaire-based scoring system for drooling severity and frequency was used (Table 2) [3]. The severity and frequency of drooling was measured for the patients in each posture (supine, sitting, and tilt table standing) using the clinical scale before the injection, 3 weeks and 3 months after the injection. We also checked the presence or absence of involuntary mastication with all patients before the injection.
Fig. 1.
Representative ultrasound data. (A) Ultrasound-guided injection into the salivary glands (left parotid gland) and (B) ultrasound image of the injected parotid gland.
Table 2.
Questionnaire-based scoring system for drooling severity and frequency

We objectively assessed the effect of BTA injection in sialorrhea, regardless of involuntary mastication. The Schirmer test, which uses paper strips inserted into the eye for several minutes to measure the production of tears, is used for the evaluation of dry eye syndrome [8]. Modifying this method, salivation was evaluated by placing the Schirmer test strip (EagleVision, Memphis, TN, USA) near the submandibular gland of the patient and measuring the length of saliva permeation after 5 minutes because it was not possible to measure the weight of secreted saliva using dental rolls due to patient characteristics that included involuntary mastication and excretion saliva from dental rolls (Fig. 2). The test was performed on all patients in the supine position before the injection, 3 weeks and 3 months after the injection. We also surveyed the caregivers about the degree of satisfaction of BTA injection using 5 scales (very satisfied, satisfied, neither, dissatisfied or very dissatisfied) and existence of complications.
Fig. 2.
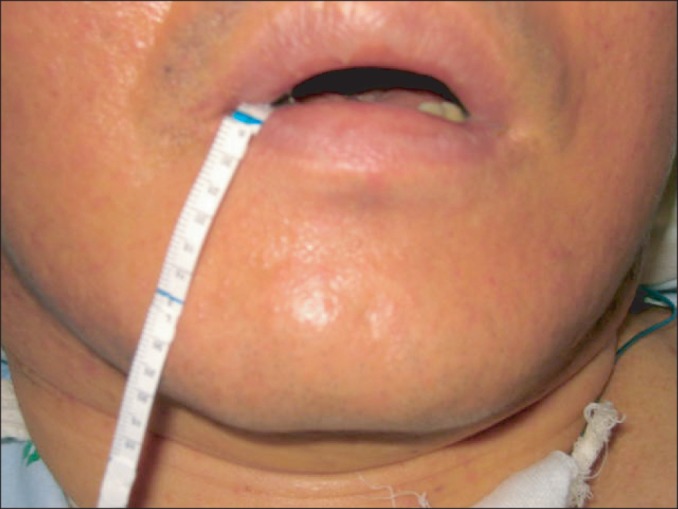
Performance of the modified Schirmer test. For objective measurement of drooling, the Schirmer test strip was placed near the submandibular gland of the patient and salivation was measured as the length of saliva permeation after 5 minutes.
The primary outcome measures of this study were the therapeutic effect of BTA injection in drooling and sialorrhea in tetraplegia with brain injury. The second outcome measures were drooling severity and frequency according to postural change and the presence or absence of involuntary mastication.
SPSS ver. 18.0 for Windows (SPSS Inc., Chicago, IL, USA) was used for statistical analysis with the significance level determined at p<0.05. A Friedman test was used to compare the mean scores for each position and severity and frequency before and after the injection. The test was also used to evaluate the results of the modified Schirmer test and the presence of involuntary mastication. When a statistically significant difference was noted in the Friedman test, Wilcoxon signed rank test was used to compare paired matches of each states as post-hoc comparison. The Mann-Whitney U test was used in the statistical analysis between with and without involuntary mastication groups.
RESULTS
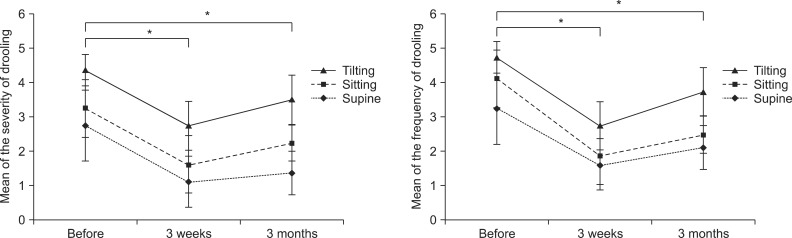
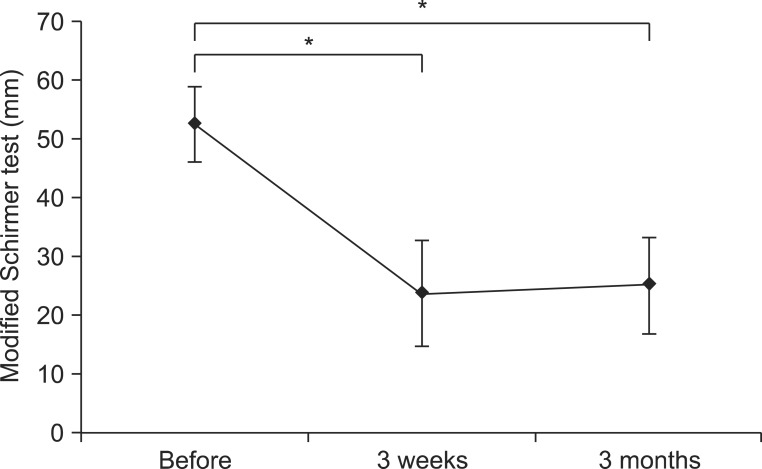
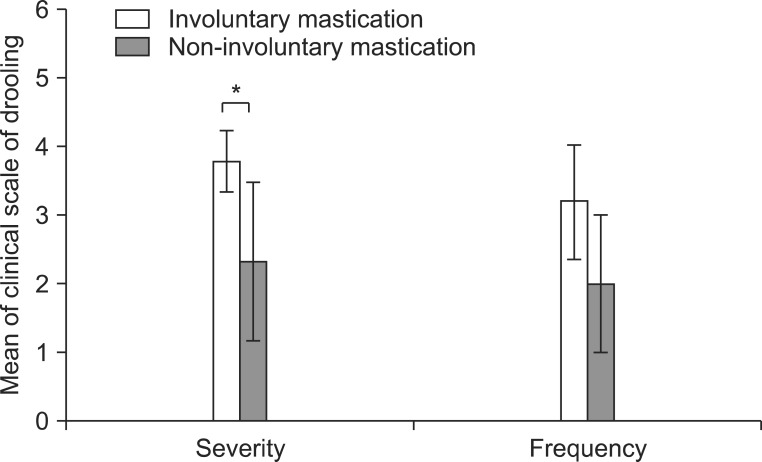
The severity and frequency of drooling measured by the clinical scale decreased significantly 3 weeks and 3 months after the injection (p<0.05) (Fig. 3). Though severity and frequency tended to increase again at 3 months after the injection compared with 3 weeks, improvement of drooling at 3 months after injection was maintained significantly compared with before the injection (p<0.05). After injection of BTA, the modified Schirmer test in the supine position, which was performed to objectively examine the severity of sialorrhea, also revealed significantly decreased salivation 3 weeks and 3 months after injection (p<0.05) (Table 3, Fig. 4). The results of evaluation according to the posture revealed that the severity and frequency of drooling were highest in the tilting position and greater in the sitting position than in the supine position at all time points following injection (p<0.05) (Fig. 3). The severity of drooling before the injection was significantly increased in the patients with involuntary mastication (p<0.05) (Fig. 5), though the frequency of drooling was not. The satisfaction survey results of the caregivers were very satisfied for five patients and satisfied for two patients, with one caregiver not responding.
Fig. 3.
The severity and frequency scales of drooling before botulinum toxin A injection and at 3 weeks and 3 months after the injection in different postures. Both scales of drooling decreased significantly at 3 weeks and 3 months after the injection (*p<0.05).
Table 3.
Modified Schirmer test in the supine position before and after botulinum toxin type A injection
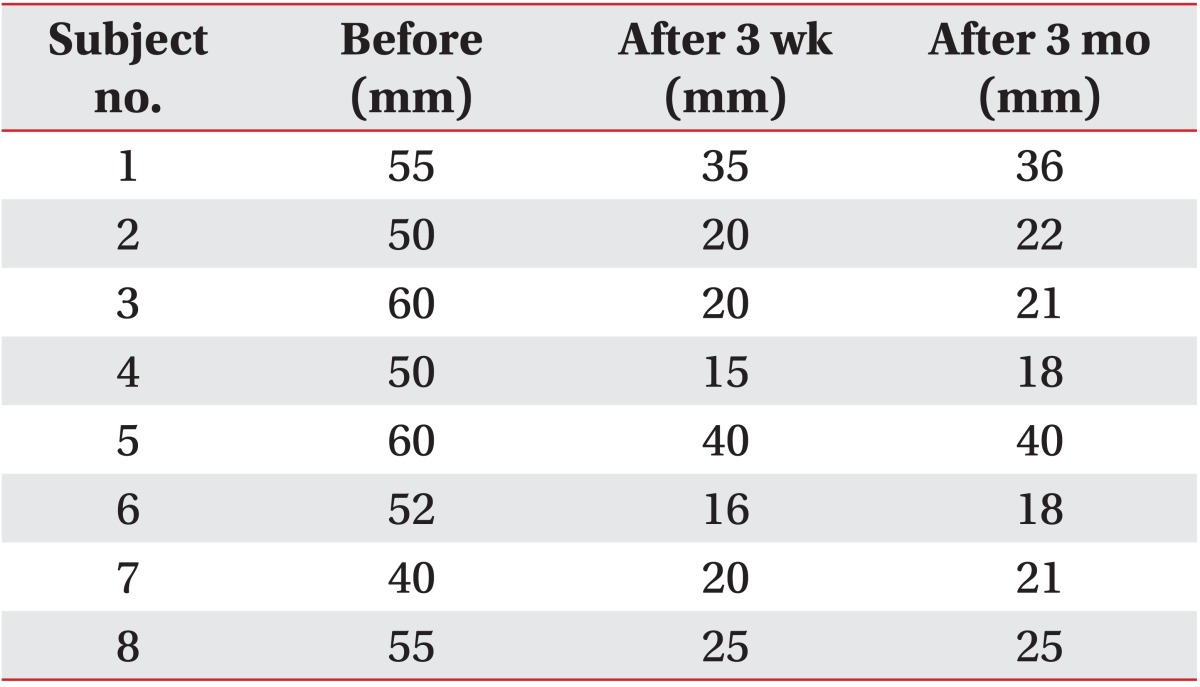
Fig. 4.
Modified Schirmer test results in the supine position before botulinum toxin A injection and 3 weeks and 3 months after injection. Excessive salivation improved significantly 3 weeks and 3 months (*p<0.05) after the injection.
Fig. 5.
The severity and frequency scales of drooling with or without involuntary mastication. The severity of drooling increased significantly in the patients with involuntary mastication (*p<0.05).
No notable complications including significant dry mouth or infection were observed after BTA injection, except for one case with mild difficulty in mouth opening. A significant decrease of drooling could be observed until 3 months after the injection, even though oromotor or swallowing functions did not improve, which could be explained by a decreased effect of salivation.
DISCUSSION
Drooling is a problem frequently faced by tetraplegic patients with severe brain injury. Drooling in patients with neurologic disease is caused by inadequate oromotor control secondary to pharyngeal sensory deficit or central interruption of normal swallowing reflexes rather than hypersecretion [9]. Since patients with severe brain injury, in particular, often have accompanying severe dysphagia, their drooling problem should be perceived differently from the drooling caused by other pathological causes. In patients with dysphagia from severe brain injury, tracheal aspiration of food can be prevented by performing a cannulation, such as percutaneous endoscopic gastrostomy, but the problem with saliva aspiration still remains. Most patients with severe brain injury have an accompanying by severe cognitive disorder, which mutes subjective complaints of aspiration symptom by the patients. This makes early treatment difficult. Excessive drooling in patients with severe brain injury is a hygienic problem and can also increase the risks of the occurrence of bronchitis or pneumonia due to infected saliva and aspiration in oral unhygienic condition, which may lead to death. Ending rehabilitation therapy due to risks of dehydration, bad breath and skin deterioration due to continuous drooling also are the additional potential problems.
This study evaluated the efficacy of BTA injection on the severity and frequency of drooling in tetraplegic patients with severe brain injury including stroke and hypoxic brain injury for different postures, with the goal of providing background information for efficient rehabilitation by considering appropriate dosage of BTA injection depending on the range of activity. In addition, this study attempted to use the modified Schirmer test to quantify drooling and to clarify how involuntary mastication affects drooling. To our knowledge, this is the first report of the effect of BTA injection for drooling on salivary gland according to postural change, evaluated including objective and numerical scale.
Saliva is secreted by six major salivary glands consisting of a pair each of parotid, submandibular and sublingual glands. In the resting state, 70% of saliva is secreted by the submandibular and sublingual glands. When stimulated during mastication, salivary flow increases up to five times, with the parotids providing the preponderance of the saliva [10,11]. Salivary secretion depends mainly on the autonomic nervous system in which the salivary glands function under a complex parasympathetic and sympathetic neural control. The parasympathetic nerves supply the main drive for glandular secretion, while sympathetic system modulates the composition of the saliva. Nerve terminals within the parasympathetic postganglionic system secrete acetylcholine and blockade of these receptor sites inhibits nervous stimulation to the salivary glands [12,13]. Various approaches, often used in combination, have been tried to treat drooling. Some noninvasive methods include behavior modification using biofeedback techniques, oral appliances or use of anticholinergic agents, which can be used to decrease secretion by blocking the parasympathetic innervations of the salivary glands. More invasive options in the treatment of drooling are radiotherapy and surgical methods like transtympanic neurectomy, bilateral submandibular gland excision, and parotid duct ligation [7,11,14]. However, it is difficult to apply these methods to the treatment of patients who have serious brain injury. The use of anticholinergic agents can have many side effects that include confusion, memory problems, urinary retention, and paralytic ileus [15]. Therefore, anticholinergics are not appropriate for tetraplegic patients who already have cognitive impairment, and bladder and bowel dysfunction after brain injury. Moreover, malignancies induced by radiation therapy and post-operative complications, such as increased incidence of dental caries, salivary gland calculi, and excessive dryness of oral mucosa, can occur calling for careful consideration of the clinical use of invasive methods [16,17]. The injection of BTA into the salivary glands has emerged as a safe, minimally invasive and clinically effective treatment for drooling in recent years.
The idea of the therapeutic use of botulinum toxin to the neuroglandular junction was first suggested by Justinus Kerner in 1822. He mentioned the severe dryness of mouth of the patients with botulism and suggested that the toxin could be applied to treat hypersalivation [18,19]. The possibility of such use was confirmed by animal studies beginning in the late 1990s. Subsequent clinical trials involving patients with cerebral palsy, Parkinson disease, and amyotrophic lateral sclerosis reported successful results. Jost [20] used parotid gland injections of BTA in Parkinson disease patients with severe drooling. Although they did not describe detailed measurement method, they reported that three patients had improvements that were satisfactory to the patients and their guardians. In 1997, Bushara [21] hypothesized that the injection of botulinum toxin into parotid glands in amyotrophic lateral sclerosis with severe sialorrhea was therapeutic. For accurate and safe injection, Ellies et al. [22] used parotid and submandibular gland injections of 55 and 66 units of BTA, respectively, under ultrasonic guidance and reported a positive effect within one week that continued up to 8 weeks.
The injection of botulinum toxin into salivary glands is applied to patients with varied conditions because of its advantages of minimal complications, ease of treatment and satisfactory clinical results. Presently, significantly positive effects were observed in eight brain injury patients treated with BTA injections. No notable complications were evident, except for one case with mild difficulty in mouth opening. The use of ultrasonic guidance allowed accurate evaluation of the structures of head and neck, which enables precise positioning of the needle and minimizing the irritation of surrounding tissues and vascular structure [23]. Considering the fact that the effect of botulinum toxin as seen from the autonomic dysfunction in botulism, namely skeletal muscle paralysis in botulism and botulinum toxin-induced anhidrosis, may last up to 8 months, while the duration of the action of BTA at the neuromuscular junction appears to be approximately 3 months, it may be predicted that its effect will last longer in neuroglandular junctions than in neuromuscular junctions [21]. This is also why botulinum treatment became popular as a new treatment for patients who are facing various problems due to chronic hypersalivation. Another advantage of botulinum injection is that appropriate amount of salivation is maintained after the injection, minimizing the complaint of severe dry mouth. This is because sufficient basal secretion of saliva from minor salivary glands distributed in the oral mucosa and sublingual glands is maintained and sympathetic nerve-controlled salivation is preserved [22].
In this study, the questionnaire-based scoring system for drooling severity and frequency developed by Thomas-Stonell and Greenberg [3] as well as the modified Schirmer test used for the evaluation of eye dryness syndrome were used for the clinical evaluation of drooling. In other studies, the weight of saliva using dental rolls is usually used for the measurement of salivation [3]. However, use of this method in this study was not possible because most of the subjects had cognitive disorders so serious that their pathological chewing reflex destroyed the dental roll during gagging or attempted swallowing of the cotton, making this approach impossible. Instead, we used the Schirmer test strip (EagleVision) for the evaluation of salivation by placing it near the submandibular gland of the patients and measuring the length of saliva permeation after 5 minutes.
It was possible to examine the changes in the drooling in tetraplegia patients with serious brain injury in different postures. The drooling in different positions for rehabilitation, such as table standing, wheelchair ambulation, and sitting, also affects the lives of patients. Therefore, we evaluated the severity and frequency of drooling with patients kept standing using a tilt table, sitting, and supine. The severity and frequency of drooling after the injection of BTA decreased in all postures without change in the order of the amount of salivation for different postures. In other words, drooling was more severe in tilt table standing than in sitting or in supine position. Salivation worsened when the patient's head was positioned parallel to the direction of gravity due to decreased oral movement. In rehabilitation medicine, tetraplegic patients with severe brain injury have varied levels of activities of daily living. Patients tend to remain in bed, but many engage in tilt table standing to reduce stiffness, prevent osteoporosis and improve bowel movement and control of the autonomic nervous system. The present findings point the way to further studies assessing doses and sites of BTA injections for patients with sialorrhea according to target level of daily living activity.
Salivation increases in patients with severe brain injury, probably because the primitive reflex that triggers mastication increases with more severe brain injury. Increased chewing movement then increases salivation from the parotid gland, which normally secrets saliva during mastication, exacerbating the drooling problem. Drooling is worse in cerebral palsy with moderate or severe mental retardation [13]. Therefore, administration of BTA injection to the submandibular glands and parotid glands with appropriate dose for the degree of chewing reflex should help to control the drooling in patients with severe brain injury.
Parotid and submandibular gland injections of BTA in tetraplegic patients with brain injury improve drooling symptoms. Also, BTA injection to salivary glands, under ultrasonic guidance, is relatively safe without significant complication and produced improvement that satisfied caregivers including decreased aspiration symptoms, such as repetitive cough, halitosis or sudden hypoxia. Our study was limited by the small sample size. However, it forms the foundation for further research investigating appropriate doses for the levels of activity of daily living, the presence of involuntary mastication and the duration of the effect of botulinum toxin with maximum results and minimal complications.
In conclusion, BTA injection to parotid and submandibular salivary glands under ultrasonographic guidance in tetraplegic patients with severe brain injury with drooling and aspiration symptoms improves the symptoms for 3 months. The severity and frequency of drooling was dependent on the posture and the presence of involuntary mastication. Drooling increased when in the standing position than when sitting, and in the sitting position than when supine. Involuntary mastication aggravated drooling. For tetraplegic patients with severe brain injury whose symptoms are not improved by the administration of oral anticholinergic agents or for whom drug treatment is ruled out due to complications, the injection of BTA is an effective and safe method of control the drooling. Also, proper posture and involuntary mastication of the patients should be taken into account in planning the treatment of drooling.
ACKNOWLEDGMENTS
This work was supported by a clinical research grant from Pusan National University Hospital in 2013.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Lim M, Mace A, Nouraei SA, Sandhu G. Botulinum toxin in the management of sialorrhoea: a systematic review. Clin Otolaryngol. 2006;31:267–272. doi: 10.1111/j.1749-4486.2006.01263.x. [DOI] [PubMed] [Google Scholar]
- 2.Bhatia KP, Munchau A, Brown P. Botulinum toxin is a useful treatment in excessive drooling in saliva. J Neurol Neurosurg Psychiatry. 1999;67:697. doi: 10.1136/jnnp.67.5.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thomas-Stonell N, Greenberg J. Three treatment approaches and clinical factors in the reduction of drooling. Dysphagia. 1988;3:73–78. doi: 10.1007/BF02412423. [DOI] [PubMed] [Google Scholar]
- 4.van der Burg JJ, Jongerius PH, van Limbeek J, van Hulst K, Rotteveel JJ. Social interaction and self-esteem of children with cerebral palsy after treatment for severe drooling. Eur J Pediatr. 2006;165:37–41. doi: 10.1007/s00431-005-1759-z. [DOI] [PubMed] [Google Scholar]
- 5.Suskind DL, Tilton A. Clinical study of botulinum-A toxin in the treatment of sialorrhea in children with cerebral palsy. Laryngoscope. 2002;112:73–81. doi: 10.1097/00005537-200201000-00014. [DOI] [PubMed] [Google Scholar]
- 6.Raval TH, Elliott CA. Botulinum toxin injection to the salivary glands for the treatment of sialorrhea with chronic aspiration. Ann Otol Rhinol Laryngol. 2008;117:118–122. doi: 10.1177/000348940811700209. [DOI] [PubMed] [Google Scholar]
- 7.Nunn JH. Drooling: review of the literature and proposals for management. J Oral Rehabil. 2000;27:735–743. doi: 10.1046/j.1365-2842.2000.00575.x. [DOI] [PubMed] [Google Scholar]
- 8.Cho P, Yap M. Schirmer test. I. A review. Optom Vis Sci. 1993;70:152–156. doi: 10.1097/00006324-199302000-00011. [DOI] [PubMed] [Google Scholar]
- 9.Hussein I, Kershaw AE, Tahmassebi JF, Fayle SA. The management of drooling in children and patients with mental and physical disabilities: a literature review. Int J Paediatr Dent. 1998;8:3–11. doi: 10.1046/j.1365-263x.1998.00055.x. [DOI] [PubMed] [Google Scholar]
- 10.Stuchell RN, Mandel ID. Salivary gland dysfunction and swallowing disorders. Otolaryngol Clin North Am. 1988;21:649–661. [PubMed] [Google Scholar]
- 11.Hockstein NG, Samadi DS, Gendron K, Handler SD. Sialorrhea: a management challenge. Am Fam Physician. 2004;69:2628–2634. [PubMed] [Google Scholar]
- 12.Garrett JR. The proper role of nerves in salivary secretion: a review. J Dent Res. 1987;66:387–397. doi: 10.1177/00220345870660020201. [DOI] [PubMed] [Google Scholar]
- 13.Jongerius PH, Rotteveel JJ, van den Hoogen F, Joosten F, van Hulst K, Gabreels FJ. Botulinum toxin A: a new option for treatment of drooling in children with cerebral palsy: presentation of a case series. Eur J Pediatr. 2001;160:509–512. doi: 10.1007/s004310100784. [DOI] [PubMed] [Google Scholar]
- 14.Klem C, Mair EA. Four-duct ligation: a simple and effective treatment for chronic aspiration from sialorrhea. Arch Otolaryngol Head Neck Surg. 1999;125:796–800. doi: 10.1001/archotol.125.7.796. [DOI] [PubMed] [Google Scholar]
- 15.Meningaud JP, Pitak-Arnnop P, Chikhani L, Bertrand JC. Drooling of saliva: a review of the etiology and management options. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101:48–57. doi: 10.1016/j.tripleo.2005.08.018. [DOI] [PubMed] [Google Scholar]
- 16.Borg M, Hirst F. The role of radiation therapy in the management of sialorrhea. Int J Radiat Oncol Biol Phys. 1998;41:1113–1119. doi: 10.1016/s0360-3016(98)00153-9. [DOI] [PubMed] [Google Scholar]
- 17.Stern Y, Feinmesser R, Collins M, Shott SR, Cotton RT. Bilateral submandibular gland excision with parotid duct ligation for treatment of sialorrhea in children: long-term results. Arch Otolaryngol Head Neck Surg. 2002;128:801–803. doi: 10.1001/archotol.128.7.801. [DOI] [PubMed] [Google Scholar]
- 18.Erbguth FJ. Botulinum toxin, a historical note. Lancet. 1998;351:1820. doi: 10.1016/S0140-6736(05)78793-6. [DOI] [PubMed] [Google Scholar]
- 19.Kopera D. Botulinum toxin historical aspects: from food poisoning to pharmaceutical. Int J Dermatol. 2011;50:976–980. doi: 10.1111/j.1365-4632.2010.04821.x. [DOI] [PubMed] [Google Scholar]
- 20.Jost WH. Treatment of drooling in Parkinson's disease with botulinum toxin. Mov Disord. 1999;14:1057. doi: 10.1002/1531-8257(199911)14:6<1057::aid-mds1033>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 21.Bushara KO. Sialorrhea in amyotrophic lateral sclerosis: a hypothesis of a new treatment: botulinum toxin A injections of the parotid glands. Med Hypotheses. 1997;48:337–339. doi: 10.1016/s0306-9877(97)90103-1. [DOI] [PubMed] [Google Scholar]
- 22.Ellies M, Laskawi R, Rohrbach-Volland S, Arglebe C, Beuche W. Botulinum toxin to reduce saliva flow: selected indications for ultrasound-guided toxin application into salivary glands. Laryngoscope. 2002;112:82–86. doi: 10.1097/00005537-200201000-00015. [DOI] [PubMed] [Google Scholar]
- 23.Jongerius PH, Joosten F, Hoogen FJ, Gabreels FJ, Rotteveel JJ. The treatment of drooling by ultrasound-guided intraglandular injections of botulinum toxin type A into the salivary glands. Laryngoscope. 2003;113:107–111. doi: 10.1097/00005537-200301000-00020. [DOI] [PubMed] [Google Scholar]