Abstract
Background and Purpose
Cerebral ischemia has been shown to result in peripheral inflammatory responses followed by long-lasting immunosuppression. Our recent study demonstrated that intravenous delivery of Tregs markedly protected against transient cerebral ischemia by suppressing neutrophil-derived matrix metallopeptidase-9 production in the periphery. The impact of Tregs on systemic inflammatory responses and immune status, however, have not been fully characterized.
Methods
Cerebral ischemia was induced by middle cerebral artery occlusion (MCAO) for 60 minutes in mice or 120 minutes in rats. Tregs were isolated from donor animals by CD4 and CD25 double selection and transferred intravenously to ischemic recipients at 2 hours after MCAO. Animals were sacrificed on different days after reperfusion. The effects of Tregs on systemic inflammation and immune status were evaluated using flow cytometry, ELISAs, and immunohistochemistry.
Results
Systemic administration of purified Tregs raises functional Tregs in the blood and peripheral organs, including spleen and lymph nodes. These exogenous Tregs remain in the blood and peripheral organs for at least 12 days. Functionally, Treg adoptive transfer markedly inhibits MCAO-induced elevation of inflammatory cytokines (IL-6 and TNF-α) in the blood. Furthermore, Treg treatment corrects long-term lymphopenia and improves cellular immune functions after ischemic brain injury. As a result, Treg-treated animals exhibit decreased bacterial loads in the blood during the recovery from cerebral ischemic attack.
Conclusions
Treg treatment did not exacerbate post-stroke immunosuppression. On the contrary, Treg-treated animals displayed improved immune status after focal cerebral ischemia.
Keywords: stroke, regulatory T cell, inflammation, immunosuppression
Introduction
Cerebral ischemia induces prompt and robust local inflammation in the brain, which is characterized by the activation of glial cells and infiltration of leukocytes1. Mounting evidence has demonstrated that overactivation of these inflammatory cells releases a large number of cytotoxic molecules and exacerbates brain injury. Interestingly, the immune responses elicited by focal cerebral ischemia are not restricted to the brain but extend into the periphery. Activation of cytokines, chemokines, and chemokine receptors have been observed in peripheral blood after cerebral ischemia and are associated with rapid neurological deterioration and poor functional outcomes in stroke patients2–4. In response to such widespread and pervasive inflammation, multiple anti-inflammatory mechanisms are launched5. However, these anti-inflammatory mechanisms negatively affect the function and composition of the systemic innate and adaptive immune systems5. As a consequence, there is post-stroke immunosuppression6, predisposing stroke victims to infections and associated complications. Although it is well accepted that inflammation amplifies brain damage and represents a promising target for stroke management, the application of anti-inflammatory agents in stroke patients must be carefully titrated to avoid catastrophic immunosuppression.
Regulatory T cells (Tregs) are a specialized population of T cells that play essential roles in suppressing inflammatory responses and maintaining immune homeostasis7. As a result of their potent immunomodulatory properties, much of the stroke literature has focused its recent attention on Tregs. Clinical and animal studies demonstrate that the number of circulating Tregs increases after stroke and remains elevated for several weeks4, 8. Some of these Tregs exit the peripheral circulation, infiltrate into the ischemic brain, and continue to accumulate there for over one month9, 10. Increasing numbers of studies suggest that endogenous Tregs influence the development of ischemic brain injury9, 11. In our recent study, we reported that intravenous delivery of isolated CD4+CD25+ Tregs after transient ischemia markedly inhibits cerebral inflammation, reduces brain infarct size, and improves long-term neurological functions12. Notably, we demonstrated that Tregs exert neuroprotection from a peripheral location by suppressing neutrophil-derived matrix metallopeptidase-9 and reducing subsequent proteolytic damage of the blood brain barrier (BBB)12. These findings reveal that Tregs do not necessarily need to enter the brain in order to modulate CNS damage. However, whether and how transferred Tregs impact systemic inflammatory responses and immune status are not well understood.
Using two rodent models of focal transient ischemia, we show in this study that intravenous injection of Tregs 2 hours after ischemia significantly reduces peripheral inflammation. We further demonstrate that Treg treatment did not exacerbate post-stroke immunosuppression. Remarkably, Treg-treated animals showed robust improvements in immune status after focal cerebral ischemia.
Methods
Rodent models of transient focal cerebral ischemia
All animal experiments were approved by the University of Pittsburgh Institutional Animal Care and Use Committee and performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and with STAIR criteria. Male 10- to 12-week-old C57/BL6 mice (Jackson Laboratory, Bar Harbor, Maine USA) were anesthetized with 1.5% isoflurane in a 30% O2/68.5% N2O mixture under spontaneous breathing conditions. Focal cerebral ischemia was produced by intraluminal occlusion of the left middle cerebral artery (MCA) for 60 minutes as described previously.13 This results in moderate brain damage 3d later with an approximately 55 ± 8 mm3 infarct size. Rectal temperature was controlled at 37.0°C±0.5°C during surgery and MCA occlusion (MCAO) using a temperature-regulated heating pad. Regional cerebral blood flow was measured in all stroke animals using laser Doppler flowmetry. Animals that did not show a regional cerebral blood flow reduction to <25% of pre-ischemia baseline levels during MCAO were excluded from further experimentation. Sham operated animals underwent the same anesthesia and surgical procedures but were not subjected to MCAO. All animals were randomly assigned to different treatment groups. Finally, all assessments were performed by investigators who were blinded to experimental group assignments.
For the rat model of transient focal cerebral ischemia, transient (120 min) cerebral focal ischemia was induced in Sprague Dawley rats as previously described.14 This results in moderate brain damage 3d later with an approximately 150 ± 10 mm3 infarct size. Blood was collected 3 days after reperfusion onset.
Isolation and adoptive transfer of CD4+CD25+ regulatory T cells and splenocytes
Single-cell suspensions were prepared from inguinal and axillary lymph nodes and spleens of C57/BL6 mice (8-week-old). CD4+CD25+ Treg populations were enriched by negative selection and positive selection with the regulatory T cell isolation kit (Miltenyi Biotec) according to the manufacturer’s instructions. For the rat study, CD4+CD25+ Treg were prepared from Sprague Dawley rats using the regulatory T cell isolation kit (R&D system) according to the manufacturer’s instructions. The CD4+CD25+ Tregs isolated in this manner were more than 95% enriched, with 82% of the isolated CD25+ cells expressing the Treg immunophenotypic marker Foxp3. Recipients received a tail vein injection of 2×106 freshly enriched Tregs or freshly isolated splenocytes in 0.2 mL DPBS at 2 hours after reperfusion. For Treg labeling and tracking, Tregs was incubated with 0.5 µM cell tracker orange CMTMR (Invitrogen) at 37 °C for 30 min before iv injection.
Cell preparation for flow cytometry
Spleen, lymph nodes, bone marrow, blood, lung, and brain were collected after MCAO and single cell suspensions were prepared for flow cytometric analysis. Briefly, lung and brain were first flushed with PBS and chopped into fine particles in 4 mL of complete RPMI 1640 medium supplemented with 10% fetal calf serum. Tissues were then incubated in 10 mL of digestion buffer (2% FBS, 1 mg/mL collagenase II, 0.5 mg/mL of DNase I in RPMI 1640 medium) for 1 hour in a 37°C water bath. The suspension was passed through a 70 μm cell strainer, resuspended in 40 mL of complete RPMI 1640, and pelleted at 2000g for 10 min at 4 °C. Cells were fractionated on a 30%–60% percoll gradient (GE Health) at 1000g for 25 min. The mononuclear cells in the interface were washed prior to staining. Bone marrow was prepared from femur and tibia bones. Peripheral blood was obtained from mice by cardiac puncture, and the red blood cells were lysed by ACK lysis buffer (Sigma-Aldrich). Lymphocytes were isolated from spleens and lymph nodes by mechanical homogenization followed by lysis of red blood cells using ACK lysis buffer. Isolated cells were resuspended at 1×106/mL and stained with CD4 and CD45.1.
Flow cytometry
Cells were stained with anti-mouse CD3, CD4, CD8, B220, NK1.1, Gr-1, CD45.1 and the appropriate isotype controls following the manufacturer’s instructions (eBioscience). For Foxp3 intracellular staining, cells were surface-stained with PB-conjugated anti-CD4 and PE-conjugated anti-CD25, and then permeabilized with the intracellular staining Kit (eBioscience) according to the manufacturer's protocol. Cells were then stained with APC-conjugated anti-Foxp3. Flow cytometric analysis was performed using a FACS flow cytometer (BD Biosciences).
Analysis of ex vivo cytokine production
Whole blood was diluted 1:5 in RPMI 1640 and incubated at 37°C. For analysis of TNF-α synthesis, samples were stimulated with 100 ng/mL lipopolysaccharide (LPS; Sigma-Aldrich) for 4 hours. For analysis of IFN-γ and IL-4 production, blood samples were stimulated with 100 µg/mL Concanavalin A (Con A; Sigma-Aldrich) for 24 hours. For analysis of IL-10 production, blood samples were stimulated with 50 ng/mL phorbol 12-myristate 13-acetate (PMA) and 500 ng/mL ionomycin (Sigma-Aldrich) for 5 hours. For analysis of TNF-α, IFN-γ, IL-4 and IL-10 production in spleen cells, 106 cells/mL were stimulated as above. Concentrations of cytokines in culture supernatants were determined using commercially available kits (manufacturer names).
Cytokine enzyme-linked immunosorbent assay
Blood plasma and cell culture media were collected as described above. Protein concentrations were measured with commercial ELISA quantification kits for TNF-α, IL-10, IL-6, IL-4 and IFN-γ (R&D Systems) according to the manufacturer’s instructions.
Bacteriological Analysis
Sacrificed mice were washed with 70% ethanol. Blood was collected by cardiac puncture. The lungs were removed after thoracotomy and homogenized in sterile PBS. 100 µl tissue homogenate or blood was serially diluted, plated onto sheep blood agar plates (Hardy Diagnosis), and incubated at 37 °C for 24 hours. Bacterial colonies were counted by an observer blinded to experimental group assignment.
Measurement of infarct volume
For 2,3,5-triphenyltetrazolium chloride (TTC) staining, brains were removed and sliced into 7 coronal sections, 1 mm thick each. Sections were immersed in prewarmed 2% TTC (Sigma-Aldrich) in saline for 10 min and then fixed in 4% paraformaldehyde. Infarct volume was determined using NIH Image J by an observer blinded to experimental group assignment. Infarct volume with correction for brain edema was calculated as the volume of the contralateral hemisphere minus the non-infarcted volume of the ipsilateral hemisphere.
Two-dimensional laser speckle imaging techniques
Cortical blood flow (CBF) was monitored using the laser speckle technique. Briefly, a CCD camera (PeriCam PSI System; Perimed) was positioned above the head, and a laser diode (785 nm) illuminated the intact skull surface to allow penetration of the laser in a diffuse manner through the brain. Speckle contrast— defined as the ratio of the SD of pixel intensity to the mean pixel intensity—was used to measure CBF as it is derived from the speckle visibility relative to the velocity of the light-scattering particles (blood). This was then converted to correlation time values, which are inversely and linearly proportional to the mean blood velocity. Laser speckle perfusion images were obtained 10 min before MCAO and continuing throughout the ischemic period until 5 min into the reperfusion. CBF was measured again in the same animals at 2 hours after reperfusion. CBF changes were recorded over time and expressed as % of pre-MCAO baseline.
Statistical Analysis
Results are presented as mean ± SEM. The difference in means between 2 groups was assessed by the two-tailed Student's t test. Differences in means between multiple groups were analyzed using one- or two-way ANOVA with time or treatment as the independent factors. When the ANOVA showed significant differences, pairwise comparisons between means were performed by post hoc Bonferroni/Dunn tests. In all analyses, p < 0.05 was considered statistically significant.
Results
Treg treatment elevates the number of functional Tregs in the blood and spleen after MCAO
In order to study the peripheral effect of Treg transfer, we first confirmed that adoptive transfer of the therapeutic dose of Tregs (2×106/mouse) at 2 hours after MCAO elevated the number of functional Tregs in the periphery. Flow cytometric analysis demonstrated increases in Treg populations in the blood, spleen, and lymph node of Treg-treated animals compared to splenocyte-injected controls at 1 day after MCAO (Figure 1A and 1B). Similarly, Foxp3 immunostaining revealed increases in Treg populations in the spleens of Treg-treated animals at 1 day after MCAO (Figure 1C). We then examined PMA (50 ng/mL) and ionomycin (500 ng/mL)-induced IL-10 production in whole blood cultures and splenocyte cultures that were obtained from Treg-treated or splenocyte-treated MCAO animals. ELISAs on the culture media revealed enhanced capacity for IL-10 production in cultures isolated from Treg-treated animals (Figure 1D). These data suggest that Treg transfer significantly elevates the number of functional Tregs, and that these viable Tregs produce suppressive cytokines such as IL-10 in the periphery.
Figure 1. Treg treatment elevates the number of functional Tregs in the blood and spleen after MCAO.
(A) Flow cytometric comparison of CD25+Foxp3+ Tregs among the CD4+ T cell populations in blood, spleens, and lymph nodes at 1 day after MCAO and cell transfer. (B) Flow cytometric quantification of CD4+CD25+Foxp3+ Treg population in the blood (n=4/group), spleen (n=6–8/group), and lymph nodes (n=4/group) of Treg-treated animals compared to splenocyte-treated or sham-operated animals at 1 day after MCAO. (C) Immunohistochemical staining of Foxp3 in the spleen shows an increased number of Tregs in Treg-treated animals at 1 day after stroke. Images are representative of four animals per group. (D) IL-10 production capacity in ex vivo blood and spleen cultures. Blood and spleen cells isolated from Treg- or splenocyte-treated MCAO animals were cultured for 5 hours with PMA (50 ng/ml) and ionomycin (500 ng/ml). IL-10 in the medium was quantified by ELISA. n=3 in each group. Data are mean ± SE. *P<0.05, **P<0.01.
Adoptively transferred Tregs remain in the peripheral blood and organs for at least 1 week after injection
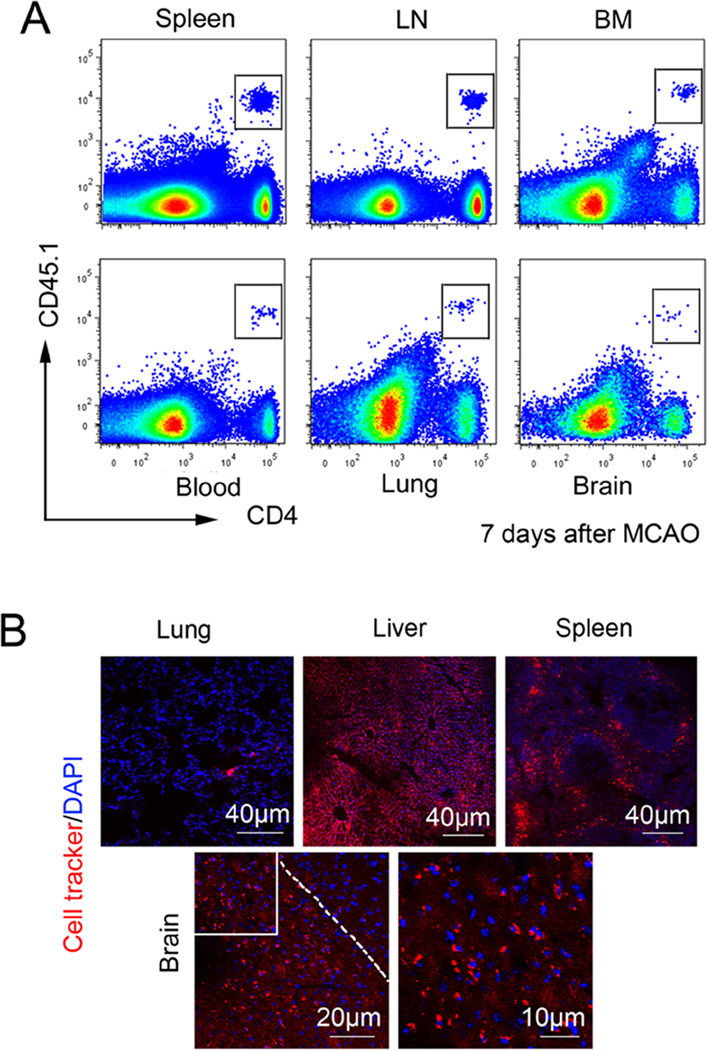
In an attempt to track the adoptively transferred Tregs in recipient mice over the long term, we used congenic mouse strains bearing allelic variants of CD45. Tregs were isolated from pooled spleens and lymph nodes of CD45.1 mice and then intravenously injected into CD45.2 recipient mice 2 hours after MCAO. Using flow cytometry, we detected transplanted CD45.1+CD4+ Tregs in the spleen, lymph nodes, bone marrow, lung, and blood 1 week after MCAO (Figure 2A). A small number of Tregs had also infiltrated into the brain. In another series of experiments, we injected cell tracker-labeled Tregs into recipient mice 2 hours after MCAO. Similarly, cell tracker-labeled Tregs were found in multiple peripheral organs as well as in the ischemic area of the brain at 1 week after MCAO (Figure 2B). Taken together, these data demonstrate that majority of transferred Tregs remain in the peripheral blood and organs for at least one week after injection, and may thus influence the systemic immune status of the recipients within this timeframe.
Figure 2. Adoptive transferred Tregs remain in peripheral blood and organs for at least 7 days after injection.
(A) Adoptively transferred CD45.1+CD4+ Tregs are present in the spleen, bone 17 marrow (BM), lymph node (LN), blood, lung, and brain at 7 days after MCAO. Plots are representative of four animals. (B) Cell tracker-labeled Tregs were observed in the lung, liver, spleen, and brain 7 days after MCAO. Images are representative of four animals.
Tregs attenuate post-ischemic peripheral inflammation
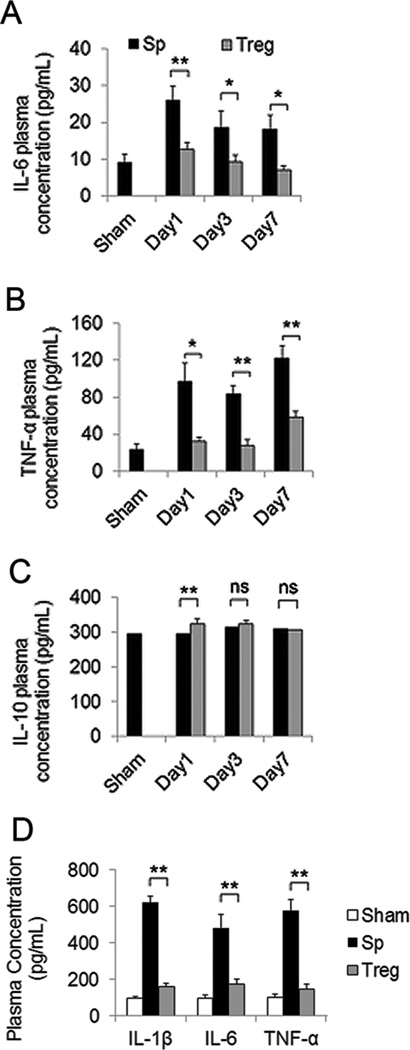
It is known that ischemic stroke induces dynamic and widespread inflammatory responses that involve not only the central but also the peripheral immune system2, 3. Indeed, the concentrations of plasma pro-inflammatory cytokines IL-6 and TNF-α were increased as early as 1 day after MCAO and remained elevated until at least 7 days post-injury. Adoptive therapy with Tregs at 2 hours after MCAO ameliorated these ischemia-induced inflammatory responses at all time points measured (Figure 3A–3B). Plasma concentrations of anti-inflammatory cytokine IL-10 exhibited a slight and transient increase in the Treg-treated group at 1 day after stroke (Figure 3C). We also tested the peripheral effects of Treg transfer in a rat model of stroke. Consistent with results in mice, rats with Treg post-treatment exhibited significantly lower levels of IL-6, TNF-α and IL-1β than splenocyte-treated animals 3 days after ischemia (Figure 3D). Collectively, these data reveal that adoptive transfer of Tregs attenuates post-ischemic inflammation in peripheral blood.
Figure 3. Tregs attenuate post-ischemic inflammation in the periphery.
(A–C) Blood plasma was collected at different times after MCAO in a mouse model. Plasma concentrations of IL-6 (A), TNF-α (B), and IL-10 (C) were measured by ELISA at 1, 3, and 7 days after MCAO in Treg-treated and splenocyte-treated mice (n=8–10/group). (D) Blood plasma was collected at 3 days after MCAO in a rat model. Plasma concentrations of IL-6, IL-1β and TNF-α were measured by ELISA. Data are mean ± SE. *P<0.05, **P<0.01.
Tregs preserve lymphocyte populations after cerebral ischemia
A long-lasting reduction in the number of lymphocytes has been reported in stroke patients and is thought to underlie spontaneous infections after stroke6, 15. We therefore performed differential blood cell counting by flow cytometry to evaluate the effect of Treg transfer on stroke-induced lymphopenia. The number of CD3+CD4+ T helper lymphocytes and CD3+CD8+ cytotoxic T lymphocytes in the blood was significantly reduced at 12 days after MCAO, but this effect was reversed by Treg treatment (Figure 4A). Treg treatment also attenuated loss of blood B220+ B lymphocytes following MCAO. No difference was observed in the number of blood NK1.1+ NK cells between Treg-treated and splenocyte-treated groups. A similar trend was observed in spleen T lymphocyte populations (Figure 4B). These results suggest that Treg treatment corrects the long-term lymphopenia that is observed after ischemic brain injury.
Figure 4. Tregs preserve lymphocyte populations after cerebral ischemia.
At 12 days after stroke, (A) blood cells were isolated and stained for CD3+CD4+ T helper cells, CD3+CD8+ T cytotoxic cells, B220+ B cells, and NK1.1+ NK cells. Flow cytometric analysis revealed that the decrease in CD4, CD8, and B cells after stroke in the splenocyte-treated mice was reversed by Treg treatment. n=4–6/group. (B) Treg treatment did not exacerbate the loss of T cells in the spleen after stroke. The numbers of CD3+CD4+, CD3+CD8+ T cells were significantly decreased in the spleen after stroke in the splenocyte-treated mice. Treg treatment did not increase T cell loss. n=4–6/group. Data are mean ± SE. *P<0.05, **P<0.01.
Treg treatment improves cellular immune functions after MCAO
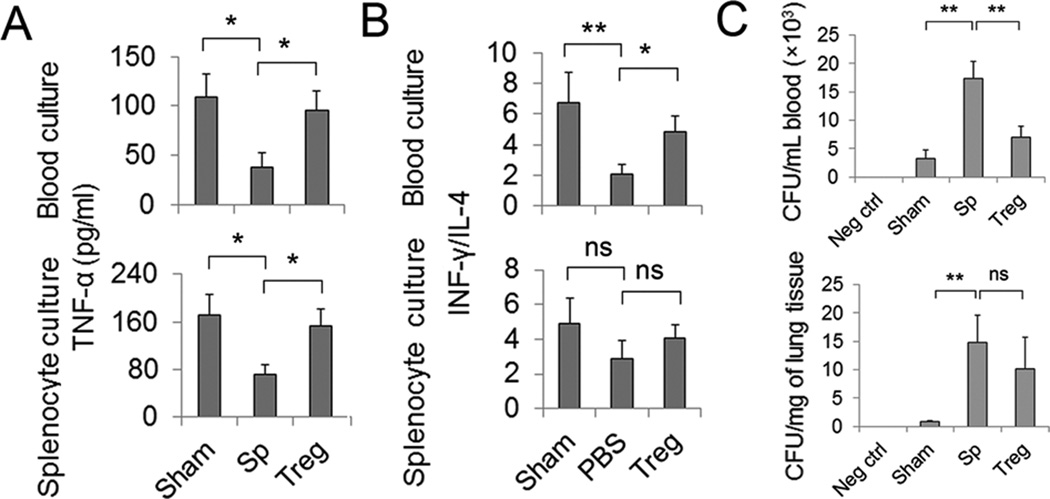
Another negative post-stroke alteration in the immune system is the impairment of cell-mediated immunity, which is characterized by impaired monocyte function and a shift from T helper cell (Th)1 to Th2 cytokine production6. We therefore performed ex vivo stimulation tests in blood cultures and splenocyte cultures to examine LPS-induced TNF-α production and ConA-induced IFN-γ production as indicators of monocyte and Th cell functions, respectively. As shown in Figure 5A, blood and splenocyte cultures collected from Treg-treated mice at 3 days after MCAO showed enhanced capacity for TNF-α production following LPS stimulation compared to the blood and splenocytes collected from splenocyte-treated animals. The production of IFN-γ (Th1 cytokine) decreased whereas the production of IL-4 (Th2 cytokine) increased after Con-A treatment, resulting in a significantly reduced ratio of IFN-γ/IL-4 in blood cultures collected from MCAO mice. Treg treatment reinstated the blood IFN-γ/IL-4 balance upon Con-A stimulation (Figure 5B). These results suggest that Treg treatment improves cellular immune functions after MCAO.
Figure 5. Treg treatment improves cellular immune functions and improves antimicrobial host defense after MCAO.
(A–B) Blood and spleen cells were prepared from sham, Treg-treated, and splenocyte-treated MCAO mice at 3 day after sham operation or MCAO. Cells were cultured under different stimulation conditions for cytokine induction. (A) TNF-α production in ex vivo blood and spleen cell cultures in response to 4 hour LPS stimulation (100ng/ml). (B) IFN-γ/ IL-4 production in ex vivo blood or spleen cell cultures in response to 24 hour ConA stimulation (100 µg/ml). (C) Treg treatment improves antimicrobial host defense in blood. Blood and lung homogenates were collected 3 days after MCAO or sham operation and cultured on blood agar plates. Cultures from splenocyte-treated MCAO mice demonstrated significant bacterial loads after 24 hours in culture. Blood cultures from Treg-treated animals showed reduced bacterial loads whereas cultures of lung homogenates showed no difference between splenocyte- and Treg-treated animals. N=6/group. Data are mean ± SE. *P<0.05, **P<0.01.
Treg treatment reduces the risk of spontaneous infection after MCAO
We further tested whether Treg treatment affects antimicrobial host defense. Blood and lung homogenates collected 3 days after MCAO demonstrated significant bacterial loads after 24 hours in culture. Cultures of lung homogenates showed no difference between splenocyte- and Treg-treated animals, whereas blood cultures from Treg-treated animals showed decreased bacterial loads than splenocyte-treated animals (Figure 5C). Collectively, our results indicate that Treg treatment does not exacerbate post-stroke immunosuppression; on the contrary, it improves immune status following MCAO.
Treg treatment reduced ischemic brain injury without impairing reperfusion after MCAO
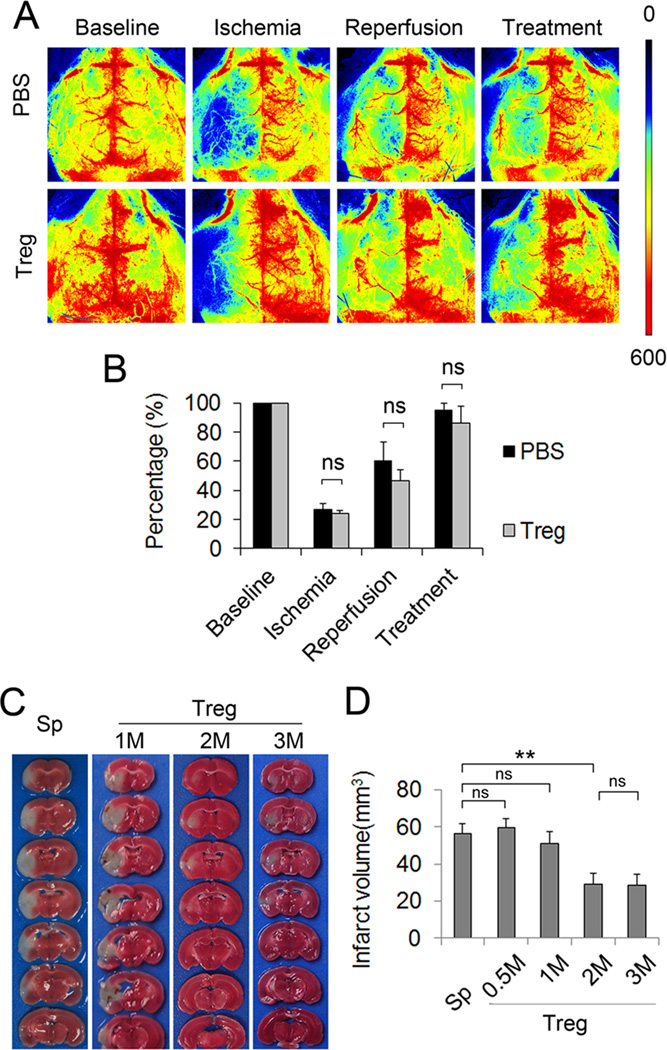
Our data showed that Treg treatment dose-dependently reduced the size of infarct after 60 min MCAO in mice (Figure 6C–6D). A recent study suggests that Treg transfer causes microvascular thrombi, thereby impairing reperfusion to the ischemic brain11. To address this concern, we monitored cerebral blood flow at different time points before, during and after reperfusion and Treg (2×106/mouse) injection using two dimensional laser speckle imaging technique. In contrast to laser Doppler flowmetry, which measures blood flow at a single spot, laser speckle can generate whole-brain images of blood flow. Therefore, laser speckle permits a robust comparison between nonischemic and ischemic hemispheres and can better demonstrate the changes in blood flow in the entire ischemic territory. As shown in Figure 6A–6B, Treg-treated animals subjected to transient MCAO demonstrated similar extents of reperfusion as PBS-treated animals.
Figure 6. Treg treatment reduces ischemic brain injury without impairing reperfusion after MCAO.
Tregs were injected intravenously 2 hours after reperfusion. Regional cerebral blood flow (CBF) was monitored using 2-D laser speckle imaging techniques. (A) Representative images of CBF before MCAO, during MCAO, and at 5 min and 24 hours after reperfusion (22 hours after treatment with 2×106 Tregs/mouse). (B) Quantification of CBF. Results were expressed as percent change from baseline (pre-MCAO). Treg-treated mice showed similar CBF compared to PBS-treated mice. n=3–4 per group. (C–D) Effect of various doses of Treg treatment 3 days after MCAO. M: 1×106 cells. Data are mean ± SE. *P<0.05, ** P< 0.01.
Discussion
Like other biological systems, the immune system is continuously fine-tuned to maintain homeostatic equilibrium. This unremitting process prevents excessive immune activation while at the same time retaining vigilance against foreign invaders or injury. Recent studies have documented that endogenous Tregs or exogenously transferred Tregs dampen cerebral inflammation and reduce brain damage following ischemic stroke9, 12. These findings strongly support a role for Tregs in cerebral immune homeostasis after brain injury. Herein, we extend these findings by showing that Treg transfer after cerebral ischemia also maintains the homeostatic equilibrium of peripheral immune responses, representing an additional mechanism of protection against the damaging sequelae of stroke.
We have previously demonstrated that Tregs quickly distribute into different organs, mainly the lymphoid ones, shortly after injection. Within 1 day after adoptive transfer, we are able to detect exogenous Tregs in bone marrow, spleen, lymph nodes, as well as blood12. In the present study, we confirmed that the number of Tregs increases significantly in the spleen and blood at 1 day after Treg transfer. These exogenous Tregs are capable of exerting functional changes, such as an increase in stimulated IL-10 release in blood and spleen cell cultures. We further showed that exogenous Tregs remain in the peripheral organs for at least one week following injection. Indeed, previous studies have shown that lymphocytes survive and proliferate in lymphoid organs for at least 21 days following transfer into recipient mice16. Adoptively transferred Tregs may therefore modulate peripheral immune responses over an extended timeframe after injury.
The systemic release of several inflammatory markers after stroke is thought to exert a negative impact on the CNS. For example, higher concentrations of plasma pro-inflammatory markers IL-6, TNF-α, and ICAM-1 are associated with rapid neurological deterioration and poor functional outcomes in stroke patients2, 3. Our study shows that Treg treatment mitigates post-ischemic inflammatory cytokines in the periphery and may therefore enhance functional recovery after stroke. However, the potent anti-inflammatory effect of Tregs raised the concern that Treg therapy after stroke might further inhibit the already suppressed immune system and result in undesirable side effects, such as an increased risk of infections. Contrary to this expectation, our results indicate that Treg treatment does not exacerbate post-stroke immunosuppression. Instead, Treg-treated animals maintained greater lymphocyte populations in the blood and spleen 12 days after MCAO. Moreover, the immune cells in Treg-treated animals showed improved cell-mediated immunity, with a balanced Th1/Th2 response. This improvement in immune status in Treg-treated stroke mice might be attributed to a direct modulatory effect of Tregs on the immune system, a secondary effect following reduced brain damage, or both. Concomitantly, Treg treatment reduced the risk of spontaneous infection in the blood following MCAO. Taken as a whole, our data suggest that Treg treatment benefits post-stroke immune status while simultaneously restricting inflammatory overactivation, in line with a critical role for Tregs in immune homeostasis. Relative to other typical anti-inflammatory treatments that only prevent immune overactivation, Treg treatment appears to strike this difficult balance more effectively.
Despite largely positive findings of the protective effects of Tregs in brain, liver, and kidney ischemia/reperfusion models17, a recent study raised several concerns on potentially detrimental effects of Tregs11. The authors suggest that the accumulation of Tregs in the ischemic brain induces microvascular thrombus formation, impairs reperfusion, and further exacerbates brain damage. However, two observations are not consistent with this hypothesis. First, our 2-D laser speckle imaging indicates that Treg-treated mice exhibit similar cerebral blood flow recovery as PBS-treated mice. Second, as illustrated by our flow cytometry and cell-tracer studies, greater numbers of injected Tregs accumulate in the liver than migrate into the brain. The relative abundance of Tregs in liver compared to brain is inconsistent with the observation that Tregs induce microvascular thrombi specifically in the ischemic brain but are beneficial to the ischemic liver18.
In summary, the present study is the first demonstration that Treg adoptive therapy simultaneously dampens peripheral inflammation and corrects immunosuppression after stroke, in addition to its potent central protective effects. Treg treatment may therefore reduce the risk of post-stroke infections and hasten stroke recovery. We conclude that further investigations of Treg therapy in stroke models are highly warranted, both to broaden the list of novel Treg molecular targets for rational drug design as well as to validate Treg neuroprotection in species closer to humans.
Acknowledgments
Funding Sources
XIAOMING HU is supported by a Scientist Development Grant (13SDG14570025) from the American Heart Association. JUN CHEN is supported by National Institutes of Health Grants NS36736, NS43802, and NS45048. JUN CHEN is also supported by Veterans Affairs Pittsburgh Health Care System (Pittsburgh, PA). BAO-LIANG SUN is supported by Chinese Natural Science Foundation grants (81070947, 81271275) and Shandong Natural Science Foundation (ZR2012HZ006).
Footnotes
Disclosure
None.
References
- 1.Iadecola C, Anrather J. The immunology of stroke: From mechanisms to translation. Nature medicine. 17:796–808. doi: 10.1038/nm.2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Castellanos M, Castillo J, Garcia MM, Leira R, Serena J, Chamorro A, et al. Inflammationmediated damage in progressing lacunar infarctions: A potential therapeutic target. Stroke a journal of cerebral circulation. 2002;33:982–987. doi: 10.1161/hs0402.105339. [DOI] [PubMed] [Google Scholar]
- 3.Shenhar-Tsarfaty S, Ben Assayag E, Bova I, Shopin L, Fried M, Berliner S, et al. Interleukin-6 as an early predictor for one-year survival following an ischaemic stroke/transient ischaemic attack. Int J Stroke. 2010;5:16–20. doi: 10.1111/j.1747-4949.2009.00396.x. [DOI] [PubMed] [Google Scholar]
- 4.Offner H, Subramanian S, Parker SM, Afentoulis ME, Vandenbark AA, Hurn PD. Experimental stroke induces massive, rapid activation of the peripheral immune system. J Cereb Blood Flow Metab. 2006;26:654–665. doi: 10.1038/sj.jcbfm.9600217. [DOI] [PubMed] [Google Scholar]
- 5.Meisel C, Schwab JM, Prass K, Meisel A, Dirnagl U. Central nervous system injury-induced immune deficiency syndrome. Nat Rev Neurosci. 2005;6:775–786. doi: 10.1038/nrn1765. [DOI] [PubMed] [Google Scholar]
- 6.Prass K, Meisel C, Hoflich C, Braun J, Halle E, Wolf T, et al. Stroke-induced immunodeficiency promotes spontaneous bacterial infections and is mediated by sympathetic activation reversal by poststroke t helper cell type 1-like immunostimulation. The Journal of experimental medicine. 2003;198:725–736. doi: 10.1084/jem.20021098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vignali DA, Collison LW, Workman CJ. How regulatory t cells work. Nature reviews. 2008;8:523–532. doi: 10.1038/nri2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yan J, Greer JM, Etherington K, Cadigan GP, Cavanagh H, Henderson RD, et al. Immune activation in the peripheral blood of patients with acute ischemic stroke. J Neuroimmunol. 2009;206:112–117. doi: 10.1016/j.jneuroim.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 9.Liesz A, Suri-Payer E, Veltkamp C, Doerr H, Sommer C, Rivest S, et al. Regulatory t cells are key cerebroprotective immunomodulators in acute experimental stroke. Nat Med. 2009;15:192–199. doi: 10.1038/nm.1927. [DOI] [PubMed] [Google Scholar]
- 10.Ishibashi S, Maric D, Mou Y, Ohtani R, Ruetzler C, Hallenbeck JM. Mucosal tolerance to eselectin promotes the survival of newly generated neuroblasts via regulatory t-cell induction after stroke in spontaneously hypertensive rats. J Cereb Blood Flow Metab. 2009;29:606–620. doi: 10.1038/jcbfm.2008.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kleinschnitz C, Kraft P, Dreykluft A, Hagedorn I, Gobel K, Schuhmann MK, et al. Regulatory t cells are strong promoters of acute ischemic stroke in mice by inducing dysfunction of the cerebral microvasculature. Blood. 2013;121:679–691. doi: 10.1182/blood-2012-04-426734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li P, Gan Y, Sun BL, Zhang F, Lu B, Gao Y, et al. Adoptive regulatory t-cell therapy protects against cerebral ischemia. [Accessed May 24, 2013];Annals of neurology. 2012 doi: 10.1002/ana.23815. http://onlinelibrary.wiley.com/doi/10.1002/ana.23815/full. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stetler RA, Cao G, Gao Y, Zhang F, Wang S, Weng Z, et al. Hsp27 protects against ischemic brain injury via attenuation of a novel stress-response cascade upstream of mitochondrial cell death signaling. J Neurosci. 2008;28:13038–13055. doi: 10.1523/JNEUROSCI.4407-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang R, Xue YY, Lu SD, Wang Y, Zhang LM, Huang YL, et al. Bcl-2 enhances neurogenesis and inhibits apoptosis of newborn neurons in adult rat brain following a transient middle cerebral artery occlusion. Neurobiology of disease. 2006;24:345–356. doi: 10.1016/j.nbd.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 15.Klehmet J, Harms H, Richter M, Prass K, Volk HD, Dirnagl U, et al. Stroke-induced immunodepression and post-stroke infections: Lessons from the preventive antibacterial therapy in stroke trial. Neuroscience. 2009;158:1184–1193. doi: 10.1016/j.neuroscience.2008.07.044. [DOI] [PubMed] [Google Scholar]
- 16.Roth R, Mamula MJ. Trafficking of adoptively transferred b lymphocytes in b-lymphocytedeficient mice. J Exp Biol. 1997;200:2057–2062. doi: 10.1242/jeb.200.14.2057. [DOI] [PubMed] [Google Scholar]
- 17.Ferenbach DA, Kluth DC, Hughes J. Regulatory t cells: A brake on ischemic injury or an active promoter of tissue healing? Kidney international. 2009;76:689–691. doi: 10.1038/ki.2009.302. [DOI] [PubMed] [Google Scholar]
- 18.Feng M, Wang Q, Zhang F, Lu L. Ex vivo induced regulatory t cells regulate inflammatory response of kupffer cells by tgf-beta and attenuate liver ischemia reperfusion injury. International immunopharmacology. 2012;12:189–196. doi: 10.1016/j.intimp.2011.11.010. [DOI] [PubMed] [Google Scholar]