Abstract
Purpose
To maintain the patient's quality of life, surgeons strive to preserve the sphincter during rectal cancer surgery. This study evaluated the oncologic safety of a sphincter-saving resection with a distal resection margin (DRM) <1 cm without radiotherapy in T3, mid- or low-rectal cancer.
Methods
This retrospective study enrolled 327 patients who underwent a sphincter-saving resection for proven T3 rectal cancer located <10 cm from the anal verge and without radiotherapy between January 1995 and December 2011. The oncologic outcomes included the 5-year cancer-specific survival, the local recurrence, and the systemic recurrence rates.
Results
In groups A (DRM ≤1 cm) and B (DRM >1 cm), the 5-year cancer-specific survival rates were 81.57% and 80.03% (P = 0.8543), the 5-year local recurrence rates were 6.69% and 9.52% (P = 0.3981), and the 5-year systemic recurrence rates were 19.46% and 23.11% (P = 0.5750), respectively.
Conclusion
This study showed that the close DRM itself should not be a contraindication for a sphincter-saving resection for T3 mid- or low-rectal cancer without radiotherapy. However, a prospective randomized controlled trial including the effect of adjuvant therapy will be needed.
Keywords: Rectal neoplasms, Distal resection margin, Recurrence
INTRODUCTION
The most important factor in the treatment of rectal cancer is the control of recurrence. If the quality of life after rectal cancer surgery is to be optimized and control of bowel movements maintained, preserving the sphincter of the anus is desirable. Traditionally, the success or failure of a sphincter-saving resection depends on the distance between the tumor and the anal verge (AV). Microinvasion at the lower part of the tumor may be possible, so an abdominoperineal resection is recommended for all rectal cancers within 5 cm from the anus [1-4]. However, Karanjia et al. [5] and Heald et al. [6] reported a lower rate of local recurrence even though the distal resection margin (DRM) was less than 1 cm. Also, several studies have indicated that a shorter DRM did not increase the recurrence rate [7-9]. Furthermore, because the effectiveness of preoperative neoadjuvant therapy in rectal cancer has been demonstrated, a shorter DRM should not be a great barrier anymore. Thus, the 2010 National Comprehensive Cancer Network (NCCN) guidelines recommended this therapy for locally-advanced rectal cancer stage T3 with positive nodes [10]. Recent studies reported good oncological outcomes of a small DRM if accompanied by chemoradiotherapy [11, 12]. However, the accessibility to hospitals, as well as patient refusal, performance status, and economic status, all make the use of radiotherapy difficult. Therefore, a small DRM may be an important prognostic factor in advanced rectal cancer in case of surgery without radiotherapy. For that reason, this study aimed to evaluate whether a small DRM should be a contraindication for a sphincter-saving resection for T3 mid- or low-rectal cancer when the surgery would not be accompanied radiotherapy.
METHODS
Patients
Between January 1995 and December 2011, 1,755 patients with primary rectal cancer underwent a surgical resection at the Department of Surgery, Inje University Busan Paik Hospital, Inje University College of Medicine. All patients who underwent a surgical resection for rectal cancer were registered in a prospectively collected colorectal cancer database. From this database, we enrolled 327 patients with histologically proven T3 rectal cancer with the tumor located <10 cm from the AV, who underwent a curative sphincter-saving resection, did not receive radiotherapy before or after surgery, and had a negative DRM. Patients with distant metastasis, palliative resection, or no information about the preoperative serum carcinoembryonic antigen (CEA) level or distance from the AV were excluded.
Rectal cancer was classified into three groups based on the distance from the AV: low (<5 cm), mid (5≤ to <10 cm), and upper (10≤ to <15 cm). This study included the mid- and low-rectal cancer groups. The survival rate was analyzed using data from the Korean National Cancer Registry. Anastomotic leakage was defined as definite disruption of the anastomosis with high fever, abdominal pain, fecal material drainage, and leukocytosis that required surgical intervention, such as a diverting enterostomy. Circumferential resection margin involvement was defined as a distance of <1 mm between the outer margin and the mesorectal fascia.
Adjuvant chemotherapy
Adjuvant chemotherapy was not administered routinely. Postoperative chemotherapy was given in cases in which local surgical control was in doubt, such as tumor invasion of multiple lymph nodes and lymphovascular invasion. Chemotherapy comprised 425 mg/m2 5-fluorouracil for 5 days and 20 mg/m2 leucovorin for 5 days intravenously, monthly for six cycles.
Follow-up
In our hospital, if the DRM was less than 1 cm, we recommended a re-resection or adjuvant chemoradiotherapy. However, no adjuvant chemoradiotherapy was administered to patients who refused chemoradiotherapy, were 75 years of age or older, or showed a poor performance status.
Patients were followed every 3 months for the first 2 years after surgery and then every 6 months for 3 years, for a total of 5 years. The history, physical examination, and serum CEA level were evaluated at each follow-up visit. A chest X-ray and abdominopelvic computed tomography were performed at 6-month intervals, and colonoscopy was performed annually. Recurrence was identified using imaging studies and colonoscopy and was confirmed by colonoscopic or percutaneous biopsy. When histological confirmation was impossible, radiologically-identified tumor growth within the previous surgical field was considered to indicate recurrence.
Measuring the DRM
The distal margin was grossly measured as the closest distance between the distal edge of the tumor and the distal mucosal end of the resected specimen. It was measured usually after pinning and formalin fixation of the specimen. For a stapled anastomosis, the donut rings were not included in the measurement.
Statistical analysis
All data were presented as mean ± standard deviation for continuous variables and as counts and percentages for discrete variables. The clinical variables were compared between the distal margin groups (DRM ≤1 cm 1 vs. DRM >1 cm) by using the t-test or Wilcoxon rank-sum test and by using the chi-square test or Fisher exact test, as appropriate. Survival time was analyzed using the Kaplan-Meier method with a log-rank test. A Cox proportional model with stepwise selection was used to identify risk factors for cancer-specific survival (CSS) and recurrence (local and systemic). P-values less than 0.05 were considered to indicate statistical significance. The statistical analysis was performed using SAS ver. 9.3 (SAS Institute Inc., Cary, NC, USA).
RESULTS
Clinical and pathological characteristics
Of the 372 patients, 129 (34.68%) had a DRM ≤1 cm (group A) and 198 (65.32%) had a DRM >1 cm (group B). The median ages of groups A and B were 62.81 and 62.42 years, respectively. Midrectal cancer was present in 118 patients (91.47%) in group A and 189 (95.45%) in group B. In both groups, moderately-differentiated adenocarcinomas were the most common cancers. On histopathological examination, lymphovascular invasion was seen in 54 (41.86%) and 85 patients (42.93%) in groups A and B, respectively, circumferential resection margin involvement was seen in 4 (3.10%) and 8 patients (4.04%), and lymph nodes were positive in 61 (47.29%) and 101 patients (51.01%) (Table 1). Group A was divided fiducially at 5 mm: 63 (48.8%) had a DRM ≤5 mm (group C) and 66 (51.2%) had 5 mm < DRM ≤1 cm (group D) (Table 2).
Table 1.
Clinical characteristics of all the patients
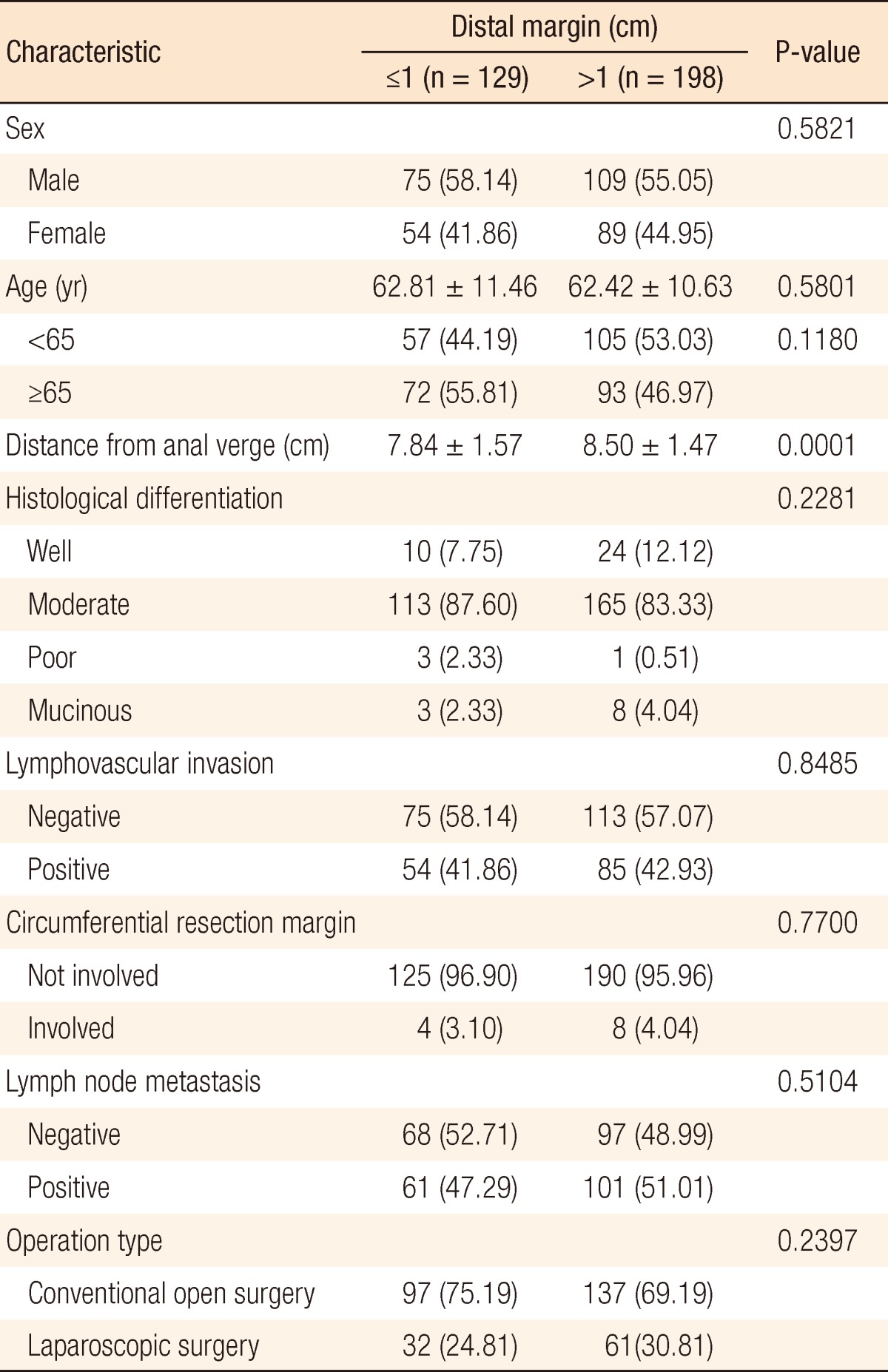
Values are presented as number (%) or mean ± standard deviation.
Table 2.
Clinical characteristics of the subgroup with distal resection margin ≤1 cm
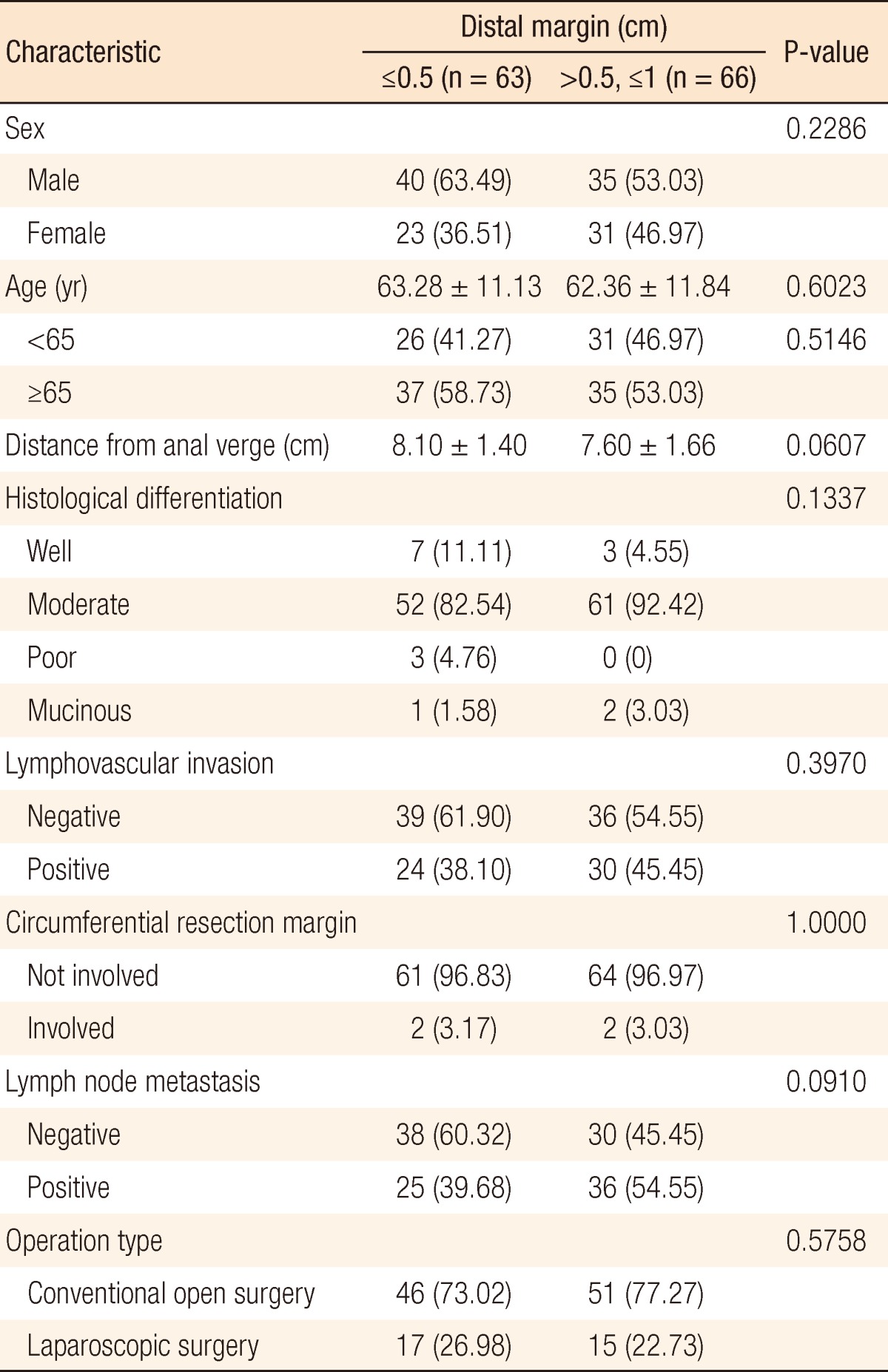
Values are presented as number (%) or mean ± standard deviation.
Analysis of local and systemic recurrence
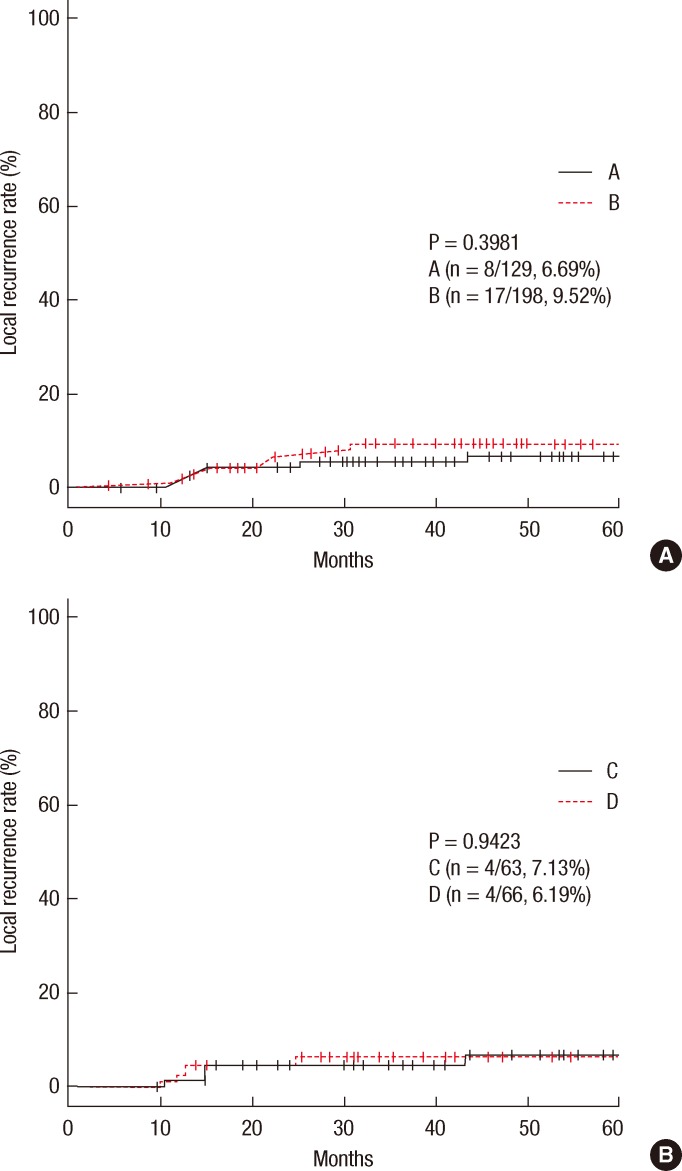
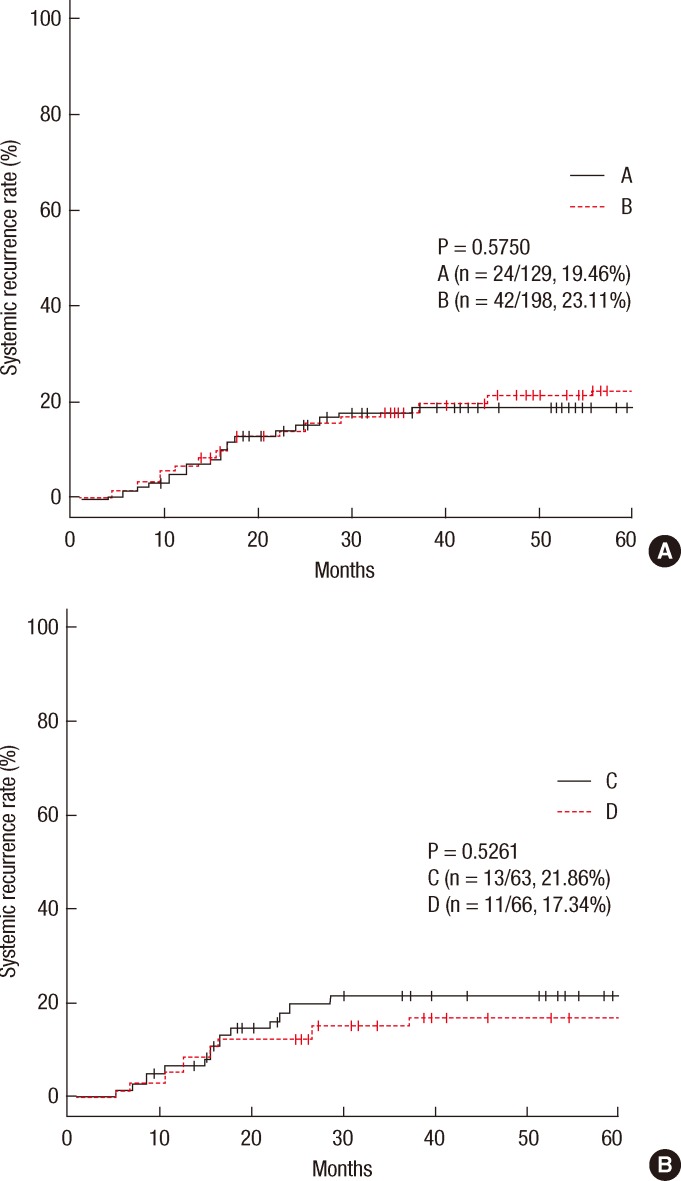
In group A, local recurrence occurred in 8 patients (6.69%): at the anastomosis in 5 cases, in the pelvic cavity in 2 cases, and in the presacral area in 1 case. In group B, local recurrence occurred in 17 patients: at the anastomosis in 8 cases, in the pelvic cavity in 7 cases; and in the presacral area in 2 cases (Table 3). The 5-year local recurrence rates according to DRM were 6.69% in group A and 9.52% in group B (P = 0.3981) (Fig. 1A). In the univariate analysis, the risk factors for local recurrence were age (P = 0.0235), positive lymphovascular invasion (P = 0.0082), and lymph node metastasis (P = 0.0181) (Table 4). In the multivariate analysis, the risk factors for local recurrence were age (P = 0.0269) and positive lymphovascular invasion (P = 0.0483) (Table 5). In group A, systemic recurrence occurred in 24 patients (19.46%), including 13 cases of liver metastasis and 9 cases of lung metastasis. In group B, systemic recurrence occurred in 42 patients (23.11%), including 15 cases of liver metastasis, 11 cases of lung metastasis, 3 cases of bone metastasis, and 2 cases of brain metastasis (Table 3). The 5-year systemic recurrence rate according to DRM was 19.46% in group A and 23.11% in group B (P = 0.5750) (Fig. 2A). In the univariate and the multivariate analyses, the risk factors for systemic recurrence were positive lymphovascular invasion and lymph node metastasis (Tables 4 and 5). The analysis between the groups C and D presented no significant difference in either the local recurrence rate (7.13% vs 6.19%, P = 0.9423) or the systemic recurrence rate (21.86% vs. 17.34%; P = 0.5261) (Figs. 1B and 2B).
Table 3.
Patterns of recurrence
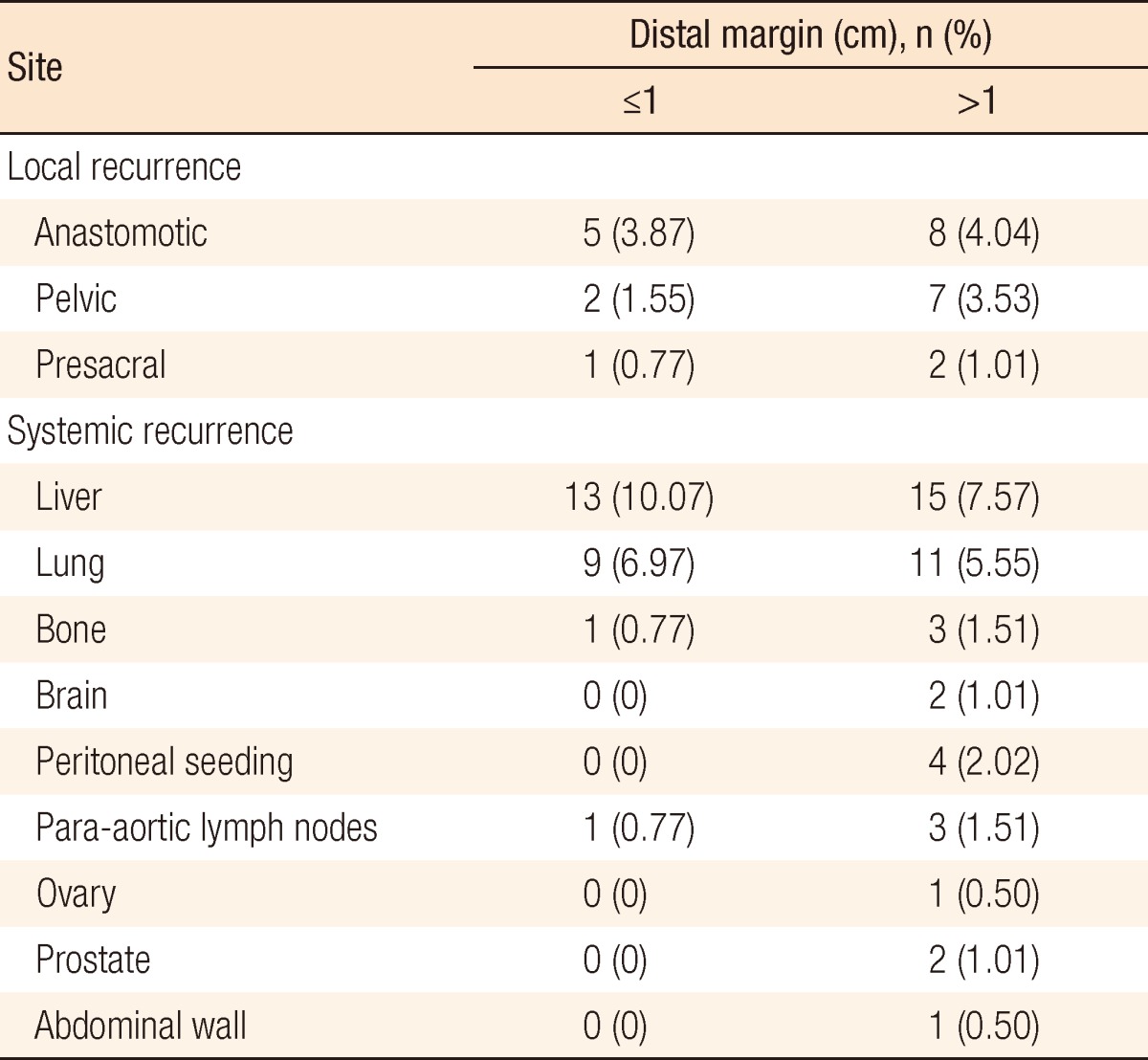
Fig. 1.
(A) Local recurrence rates in group A vs B. (B) Local recurrence rates in group C vs D.
Table 4.
Univariate analysis of risk factors for all the patients
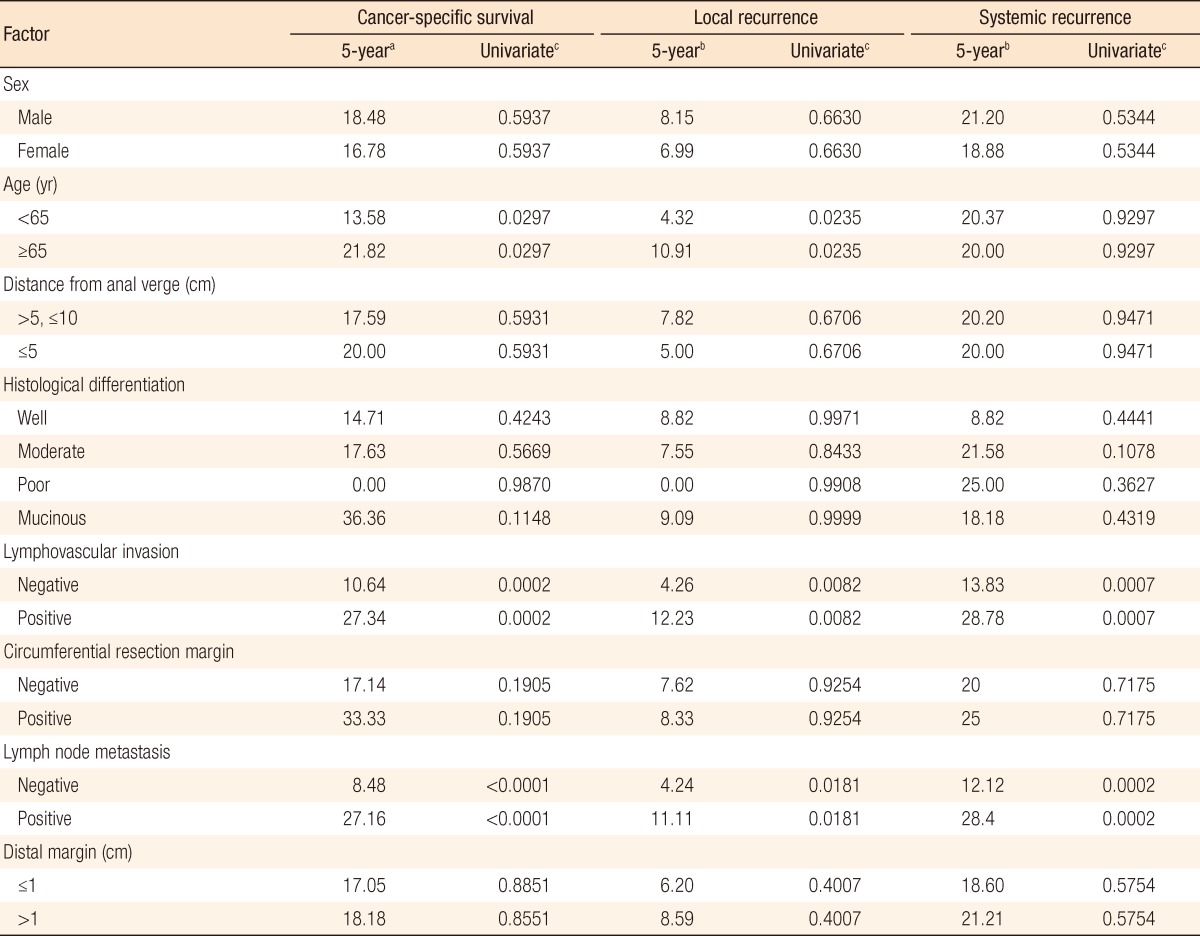
a5-year death rate. b5-year recurrence rate. cUnivariate analysis (P-value).
Table 5.
Multivariate analysis of risk factors for all the patients
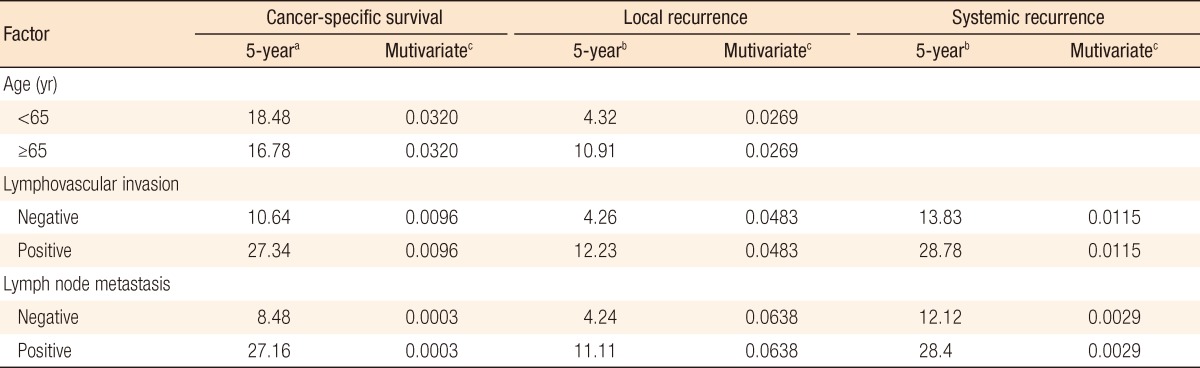
a5-year death rate. b5-year recurrence rate. cUnivariate analysis (P-value).
Fig. 2.
(A) Systemic recurrence rates in group A vs B. (B) Systemic recurrence rates in group C vs D.
Analysis of survival rates
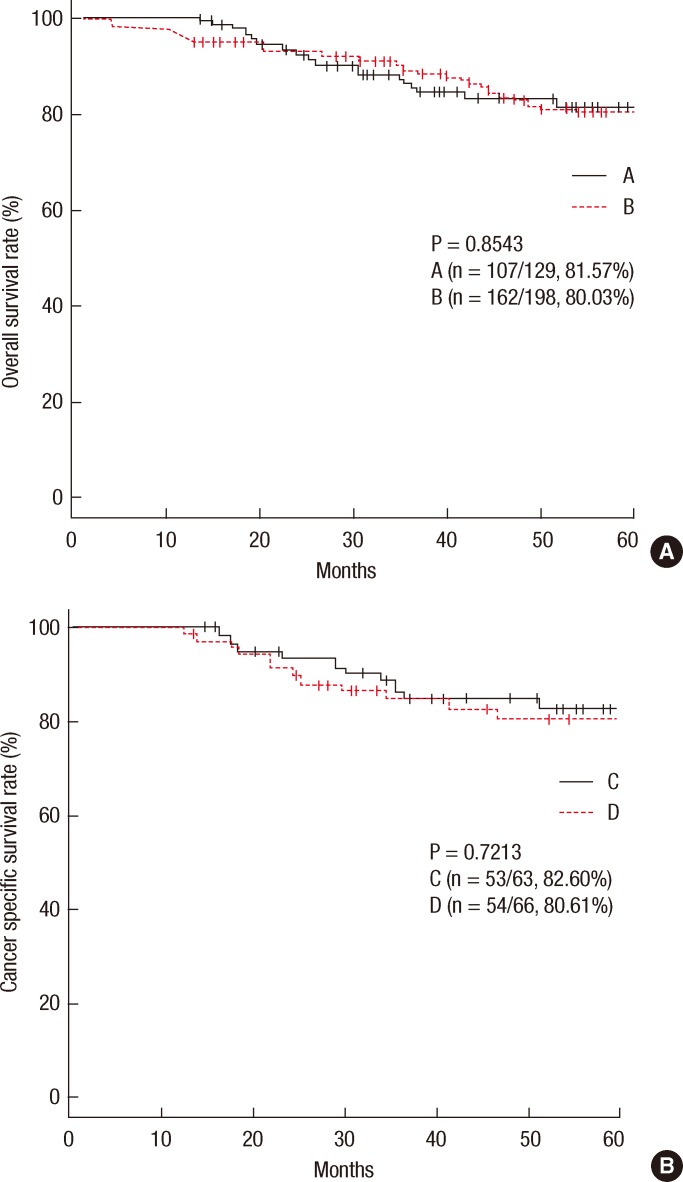
The 5-year CSS rates were 81.57% in group A and 80.03% in group B (P = 0.8543) (Fig. 3A). The significant risk factors in the multivariate analysis were age (P = 0.032), lymphovascular invasion (P = 0.0096), and lymph node metastasis (P < 0.0003) (Table 5). The 5-year CSS rates were 82.60% in group C and 80.61% in group D (P = 0.7213) (Fig. 3B).
Fig. 3.
(A) Cancer-specific survival rates in group A vs B. (B) Cancer-specific survival rates in group C vs D.
DISCUSSION
Local recurrence is common in rectal cancer after a radical resection, unlike in colon cancer. It is also rare for repeat resection to succeed after local recurrence [13]. Therefore, securing the DRM because of the risk of local recurrence is crucial. In addition, the DRM is an important factor in deciding whether to perform anal sphincter-saving resection surgery, which optimizes the quality of life of the patient. The distance between the tumor and the AV is important when deciding on anal-sphincter-saving surgery due to infiltration in the lower wall of the rectum [1]. Until the 1980s, it was recommended that a DRM of at least 5 cm be secured and that an abdominoperineal resection be performed for low-rectal cancer. In a histopathological study, Scott et al. [14] found that it was uncommon for cancer cells to spread distally more than 2-3 cm after a total mesorectal excision for rectal cancer. The NCCN guidelines suggested a 4- to 5-cm DRM for upper-rectal cancer and 1- to 2-cm for low-rectal cancer [15]. More recent studies reported that a DRM <1 cm did not influence the survival rate after an anal-sphincter-saving resection for rectal cancer [11, 12, 16-19]. Kwak et al. [11] reported no significant difference in the local recurrence rates (9.7% vs 6.0%, P = 0.122) and the 5-year survival rates (80.3% vs 76.8%, P = 0.340) in 376 patients with a standard distal margin of 5 mm who underwent both a sphincter-saving resection and radiotherapy for rectal cancer. In this study, patients undergoing a sphincter-saving resection without radiotherapy presented with similar results. Conversely, other studies reported that a small DRM increased the local and the systemic recurrence rates [7-9]. Nash et al. [7] reported that the 5-year mucosal recurrence (5% vs 2%, P < 0.001) was higher in the group with a DRM <8 mm than it was in the group with a DRM >8 mm. Most studies of rectal surgery have not proposed an unquestioned DRM, and most included patients who received preoperative and postoperative chemoradiotherapy. However, due to patients' circumstances, routine chemoradiotherapy might not be performed. Therefore, clearly, the DRM is a significant factor in locally-advanced rectal cancer. Our study examined pT3 patients with a standard DRM of 1 cm without radiotherapy for 5 years. For groups A and B, respectively, the 5-year local recurrence rates were 6.69% vs 9.52% (P = 0.3981), the 5-year systemic recurrence rates were 19.46% vs 23.11% (P = 0.5750), and the 5-year CSS rates were 81.57% vs 80.03% (P = 0.8543); none of these differences were significant. Lim et al. [18] studied patients who underwent a sphincter-saving resection for rectal cancer without neoadjuvant therapy. Their results were similar; no significant differences among their three groups were identified; the overall recurrence rates for DRM <1, 1-2, and >2 cm were 23.6%, 39.5%, and 32.6%, respectively (P = 0.078).
The length of the DRM differs according to the time and the method of measurement. The DRM has been reported to shrink to 40% of the in vivo DRM 10-20 minutes after resection and to 17% of the in vivo DRM after fixation [20]. Our study was based on pathology reports in which the DRM was measured after fixation; we also did not consider the width of the stapled "doughnut ring." Thus, this study was limited because a longer DRM than that in vivo might have been measured for the above-mentioned reasons.
Age and lymphovascular invasion were identified as risk factors for local recurrence, and lymphovascular invasion and lymph node metastasis were identified as risk factors for systemic recurrence. The risk factors associated with the 5-year CSS rate were age, lymphovascular invasion, and lymph node metastasis. These results are similar to those reported for a total mesorectal excision for rectal cancer.
In conclusion, our study showed that was no differences in the 5-year recurrence rate and the 5-year CSS rate between rectal cancer patients with DRM ≤1 cm and those with DRM >1 cm who underwent a sphincter-saving resection for T3 mid- or low-rectal cancer without radiotherapy. Therefore, a small DRM itself should not be a contraindication for a sphincter-saving resection for T3 mid- or low-rectal cancer without radiotherapy. However, a prospective randomized controlled trial including the effect of adjuvant therapy will be needed.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Goligher JC, Dukes CE, Bussey HJ. Local recurrences after sphincter saving excisions for carcinoma of the rectum and rectosigmoid. Br J Surg. 1951;39:199–211. doi: 10.1002/bjs.18003915504. [DOI] [PubMed] [Google Scholar]
- 2.Williams NS, Dixon MF, Johnston D. Reappraisal of the 5 centimetre rule of distal excision for carcinoma of the rectum: a study of distal intramural spread and of patients' survival. Br J Surg. 1983;70:150–154. doi: 10.1002/bjs.1800700305. [DOI] [PubMed] [Google Scholar]
- 3.Pollett WG, Nicholls RJ. The relationship between the extent of distal clearance and survival and local recurrence rates after curative anterior resection for carcinoma of the rectum. Ann Surg. 1983;198:159–163. doi: 10.1097/00000658-198308000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nicholls RJ, Hall C. Treatment of non-disseminated cancer of the lower rectum. Br J Surg. 1996;83:15–18. doi: 10.1002/bjs.1800830105. [DOI] [PubMed] [Google Scholar]
- 5.Karanjia ND, Schache DJ, North WR, Heald RJ. 'Close shave' in anterior resection. Br J Surg. 1990;77:510–512. doi: 10.1002/bjs.1800770512. [DOI] [PubMed] [Google Scholar]
- 6.Heald RJ, Smedh RK, Kald A, Sexton R, Moran BJ. Abdominoperineal excision of the rectum: an endangered operation. Norman Nigro Lectureship. Dis Colon Rectum. 1997;40:747–751. doi: 10.1007/BF02055425. [DOI] [PubMed] [Google Scholar]
- 7.Nash GM, Weiss A, Dasgupta R, Gonen M, Guillem JG, Wong WD. Close distal margin and rectal cancer recurrence after sphincter-preserving rectal resection. Dis Colon Rectum. 2010;53:1365–1373. doi: 10.1007/DCR.0b013e3181f052d4. [DOI] [PubMed] [Google Scholar]
- 8.Bokey EL, Ojerskog B, Chapuis PH, Dent OF, Newland RC, Sinclair G. Local recurrence after curative excision of the rectum for cancer without adjuvant therapy: role of total anatomical dissection. Br J Surg. 1999;86:1164–1170. doi: 10.1046/j.1365-2168.1999.01216.x. [DOI] [PubMed] [Google Scholar]
- 9.Vernava AM, 3rd, Moran M, Rothenberger DA, Wong WD. A prospective evaluation of distal margins in carcinoma of the rectum. Surg Gynecol Obstet. 1992;175:333–336. [PubMed] [Google Scholar]
- 10.National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology (NCCN Guidelines): Colon/recta cancer. ver. 1. 2010 [Internet] Fort Wathington: NCCN; c2012. [cited 2013 Feb 7]. Available from: http://www.nccn.org/professionals/physician_gls/f_guidelines.asp. [Google Scholar]
- 11.Kwak JY, Kim CW, Lim SB, Yu CS, Kim TW, Kim JH, et al. Oncologically safe distal resection margins in rectal cancer patients treated with chemoradiotherapy. J Gastrointest Surg. 2012;16:1947–1954. doi: 10.1007/s11605-012-1988-1. [DOI] [PubMed] [Google Scholar]
- 12.Moore HG, Riedel E, Minsky BD, Saltz L, Paty P, Wong D, et al. Adequacy of 1-cm distal margin after restorative rectal cancer resection with sharp mesorectal excision and preoperative combined-modality therapy. Ann Surg Oncol. 2003;10:80–85. doi: 10.1245/aso.2003.04.010. [DOI] [PubMed] [Google Scholar]
- 13.Stipa S, Nicolanti V, Botti C, Cosimelli M, Mannella E, Stipa F, et al. Local recurrence after curative resection for colorectal cancer: frequency, risk factors and treatment. J Surg Oncol Suppl. 1991;2:155–160. doi: 10.1002/jso.2930480532. [DOI] [PubMed] [Google Scholar]
- 14.Scott N, Jackson P, al-Jaberi T, Dixon MF, Quirke P, Finan PJ. Total mesorectal excision and local recurrence: a study of tumour spread in the mesorectum distal to rectal cancer. Br J Surg. 1995;82:1031–1033. doi: 10.1002/bjs.1800820808. [DOI] [PubMed] [Google Scholar]
- 15.Nelson H, Petrelli N, Carlin A, Couture J, Fleshman J, Guillem J, et al. Guidelines 2000 for colon and rectal cancer surgery. J Natl Cancer Inst. 2001;93:583–596. doi: 10.1093/jnci/93.8.583. [DOI] [PubMed] [Google Scholar]
- 16.Rutkowski A, Bujko K, Nowacki MP, Chmielik E, Nasierowska-Guttmejer A, Wojnar A, et al. Distal bowel surgical margin shorter than 1 cm after preoperative radiation for rectal cancer: is it safe? Ann Surg Oncol. 2008;15:3124–3131. doi: 10.1245/s10434-008-0125-6. [DOI] [PubMed] [Google Scholar]
- 17.Moore HG, Riedel E, Minsky BD, Saltz L, Paty P, Wong D, et al. Adequacy of 1-cm distal margin after restorative rectal cancer resection with sharp mesorectal excision and preoperative combined-modality therapy. Ann Surg Oncol. 2003;10:80–85. doi: 10.1245/aso.2003.04.010. [DOI] [PubMed] [Google Scholar]
- 18.Lim JW, Chew MH, Lim KH, Tang CL. Close distal margins do not increase rectal cancer recurrence after sphincter-saving surgery without neoadjuvant therapy. Int J Colorectal Dis. 2012;27:1285–1294. doi: 10.1007/s00384-012-1467-x. [DOI] [PubMed] [Google Scholar]
- 19.Bujko K, Rutkowski A, Chang GJ, Michalski W, Chmielik E, Kusnierz J. Is the 1-cm rule of distal bowel resection margin in rectal cancer based on clinical evidence? A systematic review. Ann Surg Oncol. 2012;19:801–808. doi: 10.1245/s10434-011-2035-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goldstein NS, Soman A, Sacksner J. Disparate surgical margin lengths of colorectal resection specimens between in vivo and in vitro measurements: the effects of surgical resection and formalin fixation on organ shrinkage. Am J Clin Pathol. 1999;111:349–351. doi: 10.1093/ajcp/111.3.349. [DOI] [PubMed] [Google Scholar]