Abstract
Background
Despite the extensive information available in the literature, cell surface marker signature of human Amniotic Epithelial Cells (hAECs) remains controversial. The aim of the present study was to characterize immunophenotypic features, proliferative capacity and immunogenicity of hAECs. We also tested whether expression of some cell surface markers is influenced by the type of trypsin used for tissue digestion.
Methods
Single cell suspensions of amniotic membranes from four human placentas were isolated by enzymatic digestion and expression of CD9, CD10, CD29, CD34, CD38, CD44, CD45, CD73, CD105, CD133, HLA-I, HLA-DR, HLA-G, SSEA-4, STRO-1 and OCT-4 was then evaluated by flow cytometry. The differential impact of four trypsin types on the yield and expression of CD105 and HLA-I was also determined. The proliferative capacity of cultured hAECs was assessed and compared in the presence and absence of Epidermal Growth Factor (EGF). To test their immunogenicity, hAECs were injected into Balb/c mice and the reactivity of hyperimmunized sera was examined by immunofluorescence staining.
Results
Nearly all purified cells expressed mesenchymal markers, CD9, CD10, CD29, and CD73 and the embryonic marker, SSEA-4. A large proportion of the cells also expressed STRO-1 and OCT-4. The purified cells also expressed HLA-G and HLA-I. A very small proportion of hAECs expressed CD34, CD38, CD44, CD133 and HLA-DR. The type of trypsin used for enzymatic digestion affected both the percentage and expression of HLA-I and CD105. hAECs revealed substantial proliferative capacity only when cultured in the medium supplemented with EGF. These cells were shown to be capable of inducing high amounts of anti-donor antibodies.
Conclusion
Here we provided evidence that hAECs are immunogenic cells with high level of HLA-I expression. Furthermore, this work highlighted the impact of isolation procedure on the immunophenotype of hAEC.
Keywords: Cell proliferation, Epithelial cells, Immunophenotyping, Placenta, Stem cell, Trypsin
Introduction
Human placenta is composed of three layers: amnion, chorion, and decidua. Amnion is derived from the embryo and suggested to contain cells with pluripontential capacity (1, 2). Amniotic epithelial and stromal cells are the major cell types of the amnion layer. hAECs, in particular, consist of flat, cuboidal and columnar cells in direct contact with amniotic fluid (3).
hAECs can self renew and differentiate into the three embryonic germ layers. Furthermore, hAECs have a high proliferation capacity, and are positive for such markers as SSEA-4, a marker specific to pluripotent stem cells (2, 4, 5). Due to their differentiation potential, hAECs have been reported to express organspecific molecules when transplanted into organ/ tissue of interest (6, 7). Under certain circumstances, hAECs could differentiate into neural cells synthesizing and releasing such neurotransmitters as acetylcholine, norepinephrine, and dopamine (8, 9).
Amniotic epithelial cells are easily accessible which makes them advantageous over other stem cell types. Amnion is normally discarded at birth; therefore, hAECs could be prepared through a rather simple and low-cost procedure which does not raise serious ethical issues. In contrast, to obtain human adult and embryonic stem cells one needs to go through laborious experimental, religious, and ethical processes. All the aforementioned advantages suggest hAECs as a useful stem cell source to be used in future clinical treatments (10). In addition, hAECs show low immunogenic and substantial immunomodulatory properties introducing them as promising tools to be used in regenerative medicine (11). These cells were shown to neither express Human Leukocyte Antigen (HLA)-A, B, and C nor induce anti-HLA humoral immune responses (11).
Despite the extensive information available in the literature, cell surface marker signature of hAECs remains controversial so far. Particularly, expression of HLA-I, HLA-G, CD105, CD34 has been the matter of debate (12–15). Also among the markers with general consensus on expression status in hAECs, great variability exists in the percentage of positive or negative populations. Accordingly, we sought to determine the immunophenotype of hAECs and investigate whether expression of certain markers is affected by the type of trypsin used for tissue digestion. Moreover, we explored the proliferative capacity and immunogenicity of these cells.
Materials and Methods
Reagents and antibodies
Fluorescein isothiocyanate (FITC) conjugated monoclonal antibodies (mAb) against cytokeratin, CD9, CD34, CD38, HLA-DR, HLA-G, and phycoerythrin (PE)-conjugated mAbs against CD29, CD44, CD133, SSEA-4, CD10, CD73, and HLA-I were all purchased from BD biosciences (USA). PE-conjugated anti-CD105 and unconjugated anti-STRO-1 mAb were from R&D System (USA). FITCconjugated sheep anti-mouse Ig was obtained from Avicenna Research Institute (Iran). Polyclonal rabbit anti-human OCT-4, and FITCconjugated goat anti-rabbit antibodies were purchased from Abcam (USA), and Sigma (USA), respectively. 2, 3-Bis-(2-methoxy- 4- nitro-5-sulfophenyl)-2H-tetrazolium-5 carboxanilide (XTT) and Phenazine Methosulfate (PMS) were obtained from Sigma (USA). All the isotype-matched control antibodies were from BD Biosciences (USA). Trypsin was purchased from Gibco (cat no. 27250018 and 15090046), Sigma (cat no. T4799), and Fluka (cat no. 93613) companies (Germany). DNase I was purchased from Roche (Germany).
Placental unit and sample selection
Human placentas were obtained from uncomplicated term pregnancies delivered by elective cesarean from 4 healthy women aged 22 to 32 years. The study was approved by the ethical committee of Avicenna Research Institute and Shahid Beheshti University of Medical Sciences and all participants signed a written consent form before enrolment in this study. All women were tested for blood borne viral infections during pregnancy and checked to make sure they did not have the evidence of congenital malformation, genital aberration or severe diseases. All manipulations were car ried out under sterile conditions.
Hematoxylin and eosin staining
A small fragment of placenta unit containing amniotic membrane was fixed in 10% formalin solution and embedded in paraffin. Sections (5 μm thick) were cut from the paraffin blocks and stained with Hematoxylin and Eosin (H&E) according to standard methods.
Isolation of hAECs
Placentas were delivered immediately in the cold chain to the laboratory and placed in a biological safety cabinet with fetal side up in a sterile stainless steel dish. Amnion was mechanically peeled free from underlying chorion (Figure 1). Remaining residual chorion was carefully removed by the slow slipping of a scalpel blade over the membrane. Isolated amniotic membrane was transferred to a sterile beaker and washed several times with cold RPMI-1640 (Sigma, USA) to remove blood and cellular debris. Next, bloodfree amnion was divided in 3 to 4 pieces. Each piece was then transferred into a cell culture flask (75 cm 2) containing 20 ml 0.05% trypsin-EDTA and incubated at 37°C in a shaking water bath for 20 min. In some settings, 20 μg/ml DNase was added to the digestion medium.
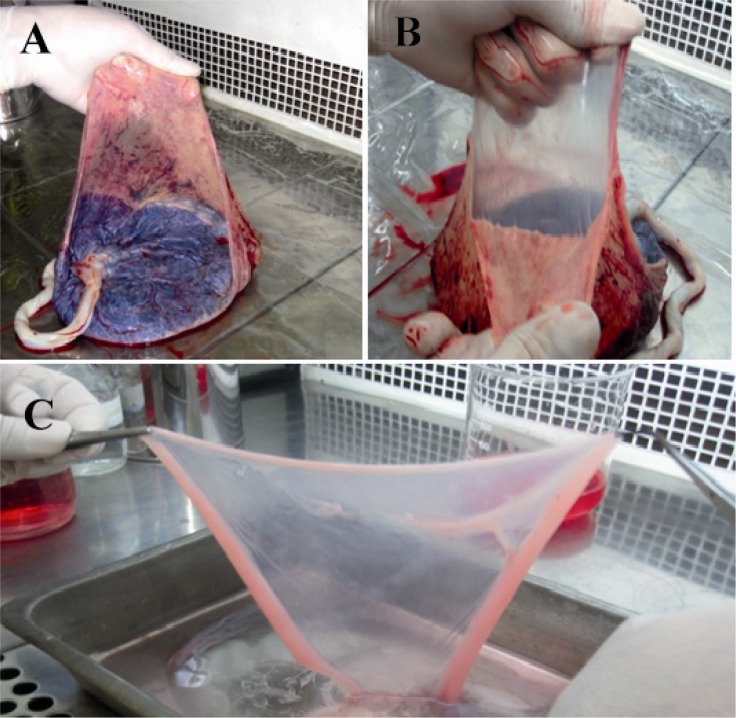
Figure 1.
Separation of amniotic membrane. Placenta was placed under a biological safety cabinet with fetal side up. A) Membranes were extended with left hand; B) Amniotic membrane was mechanically peeled free from underlying chorion; C) Amnion was washed several times with ice-cold isotonic buffer or culture medium to remove blood and cellular debris
Thereafter, the digest was passed through a stainless steel mesh (300 μm) to separate the dispersed amniotic epithelial cells from the undigested tissue. Cells were then suspended in 30 ml complete medium [(RPMI + 10% heat inactivated Fetal Bovine Serum (FBS)] and pelleted by centrifugation at 300×g for 10 min. The supernatant resulting from the first trypsinization was discarded to eliminate red blood cells. The remaining tissue was subjected to second, third, and fourth trypsinization steps each for 20 min. The cells obtained in each enzymatic digestion step were kept on ice and the dispersed epithelial cells obtained from all steps were pooled together, poured over a cell strainer (100 μm) (BD Biosciences, USA), and analyzed by flow cytometry. Trypan blue dye exclusion test was used to determine cell viability.
Furthermore, to assess the impact of trypsin type on the expression of cell surface markers, each tissue was digested by four types of trypsins and the expression of CD105 and HLA-I was measured as above.
Proliferation assay
To study their proliferation capacity, hAECs were cultured in different seed numbers ranging from 5000 to 160000 in 96-well cell culture plates containing 150 μl 10% heat-inactivated FBS-supplemented phenol red-free RPMI. In some wells, Epidermal Growth Factor (EGF) (Invitrogen, Germany) at the final concentration of 10 ng/ml was added. Cells were incubated at 37°C in a humidified incubator (5% CO2) and cell proliferation was evaluated using XTT assay immediately after initiation of culture and every 24 hr up to 72 hr. XTT assay was performed according to the protocol we published recently (16). Briefly, an XTT stock solution (1 mg/ml) was prepared in pre-warmed phenol red-RPMI-1640. PMS (Phenazin Methosulfate) stock solution (5 mM in PBS) was mixed with XTT solution to achieve 0.025 mM PMS-XTT. Fifty μl of this mixture was added to each well and cells were incubated for 2 hr in a 37°C incubator with 5% CO2. Next, the plate was shaken and absorbance of wells was measured at 450 nm using a spectrophotometer (BioTek, USA).
Flow cytometry analysis of molecular markers
Immunophenotyping of hAECs was performed by flow cytometry according to our recently published protocol (16, 17). To this end, after isolation, hAEC cells were washed in cold PBS-2% heat-inactivated FBS (stain buffer) and incubated for 30 min in stain buffer containing manufacturer-recommended concentrations of either FITC- or PE-labeled antibodies. All the staining steps were carried out at 4°C unless otherwise mentioned. In all tests, the isotype-matched antibodies were used as negative controls. The expression of OCT-4 was determined through intracellular flow cytometry staining. To do so, cells were washed twice in stain buffer and fixed with 4% formalin for 15 min at room temperature. Thereafter, cells were incubated in PBS-0/1% saponin (permeabilization buffer) containing anti-human OCT-4 (1:1000) for 30 min. Cells were then washed twice in permeabilization buffer. Next, FITC-conjugated goat anti-rabbit Ig (diluted 1:150 in permeabilization buffer) was added and cells were incubated for another 30 min. Afterwards, the unbound Abs were washed away and the cells were kept on ice until analysis. For cell surface STRO-1 staining, cells were incubated sequentially with unconjugated mouse anti-human STRO-1 and FITC-conjugated sheep anti-mouse antibodies each for 30 min. Thereafter, cells were washed twice with stain buffer and analyzed using flow cytometry (Partec, Germany).
Assessment of hAEC immunogenicity
hAECs were isolated as above and cultured for 24 hr in complete culture medium. Thereafter, cells were harvested, washed three times in Phosphate Buffered Saline (PBS), suspended in 50 μl of the same buffer and injected (1×106 cells) subcutaneously into the dorsal flank of four 8-12 week Balb/c mice (Pasture institute; Iran). Immunization was repeated two more times with a one week interval. As the control group, mice were injected with PBS. One week after completion of the immunization schedule mice were bled by retroorbital sinus sampling after induction of anesthesia by a mixture of xylazine and ketamine and sera were collected. Immunoreactivity of the hyperimmune sera was then tested by immunoflourescent staining on the cytospined hAECs according to the protocol we published recently (18).
In brief, cells were fixed with -20°C acetone 100% for 2 min followed by three 5 min washing steps with PBS + 0.5% Bovine Serum Albumin (BSA). The cells were incubated for 90 min at room temperature with 1:200 dilution of hyperimmunized sera. Slides were washed as above and incubation was continued for 45 min in the presence of FITCconjugated sheep anti-mouse Ig. Afterwards, nuclei were counterstained with DAPI (Invitrogen, USA) and signals were visualized under an epifluorescent microscope (BX51 Olympus, Japan). In negative control slides, primary antibody was substituted by equivalent dilutions of preimmune mice sera.
Statistical analysis
hAECs isolated from four placentas were analyzed in this study. XTT assay was performed in quadruplicate wells. Where appropriate, data were expressed as mean±SD. Comparative analysis of cell proliferation in the presence and absence of EGF was performed by Wilcoxon signed-rank test and pvalues less than 0.05 were considered statistically significant.
Results
H&E staining of placenta sections
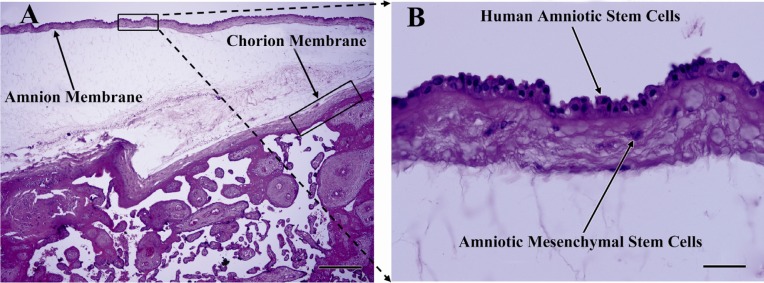
To provide an overview of anatomical location of the amniotic membrane from which hAECs were isolated, H&E staining of paraffin- embedded placenta sections was performed. As depicted in Figure 2, amniotic membrane is composed of a single layer of flattened cuboidal cells resting on a basal lamina. Beneath this layer, a stromal layer composed of amniotic mesenchymal cells is seen. Figure 2 also shows the intermediate spongy layer between amniotic and chorionic membranes.
Figure 2.
H&E staining of placenta section. A) Amnion and chorion membranes and intermediate spongy layer are easily distinguishable; B) As seen in this close view of the amnion layer, amniotic membrane composed of single layer of flattened cuboidal cells resting on a basement membrane. Beneath this layer, stromal layer composed of amniotic mesenchymal cells is seen; Upright light microscope, A) scale bar; 500 µm, B) scale bar; 50 µm
Isolation of hAECs
Amniotic epithelial cells were isolated from the amnion layers obtained from healthy women. On average, 80-130×106 hAECs with viability of more than 98% were isolated from each placenta unit. Upon Giemsa staining, cells showed relatively dense nuclei, abundant cytoplasms and high cytoplasm/nuclear ratios (Figure 3). The purity, as evaluated by cytokeratin expression, was demonstrated to be more than 98% (Figure 4). Most of the cells were isolated during the first and second run of trypsin digestion and extending trypsinization steps to more than four steps, not only did not alter the final yield, but also decreased cell viability by about 10%. Trypsins from different suppliers resulted in different cell yields (data not shown). Inclusion of DNase in the digestion medium substantially reduced the gelatinization of digested tissues which hindered proper passage of the cells through cell strainer. Microscopic examinations showed fresh hAECs as large, refractile round cells with great capacity to adhere to the plastic surfaces. The cells exhibited adherence to the plastic surface within the first hours of culture initiation. The cells were gradually flattened by extension of culture period and became more flattened with fibroblastic-like morphology after 48 hr (Figure 5).
Figure 3.
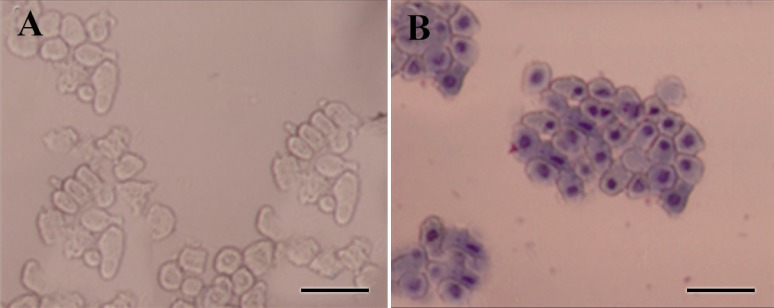
Morphological assessment of freshly isolated hAECs. A) Freshly isolated hAECs was investigated morphologically under invert microscope; B) or after Gimsa staining. These cells appeared as flat cuboidal cells with abundant cytoplasm and high cytoplasm/nuclear ratio. Scale bar; 50 µm
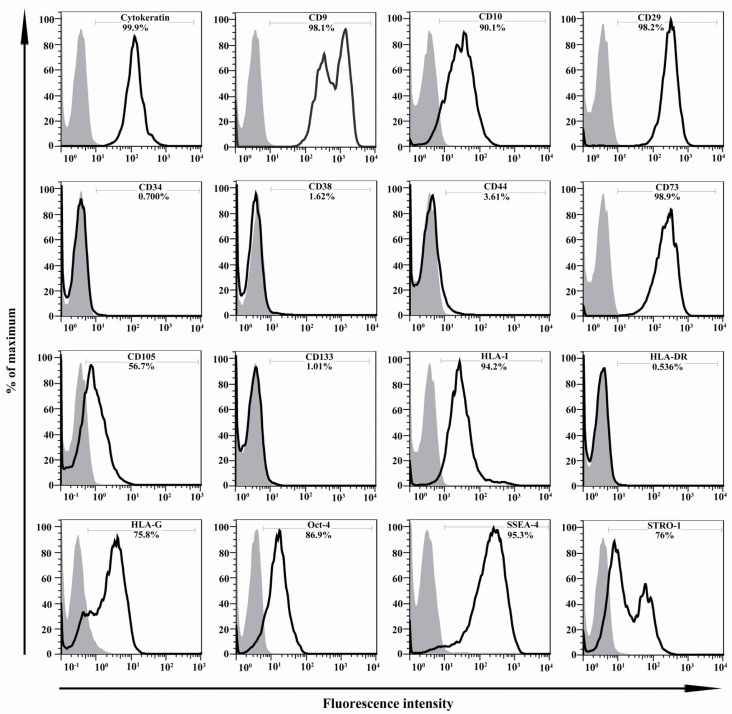
Figure 4.
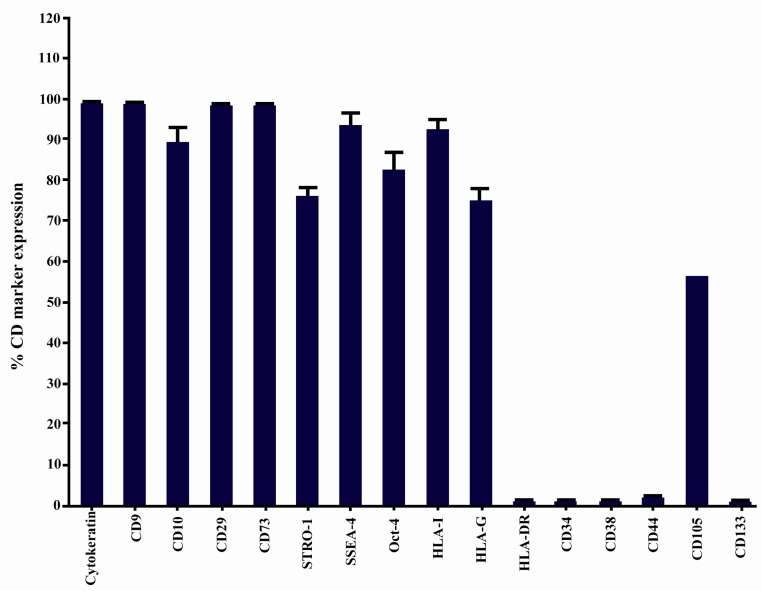
Immunophenotyping of hAECs. The representative results of four independent experiments have been shown. In each graph, open and filled histograms represent test and isotype-matched control Abs, respectively
Figure 5.
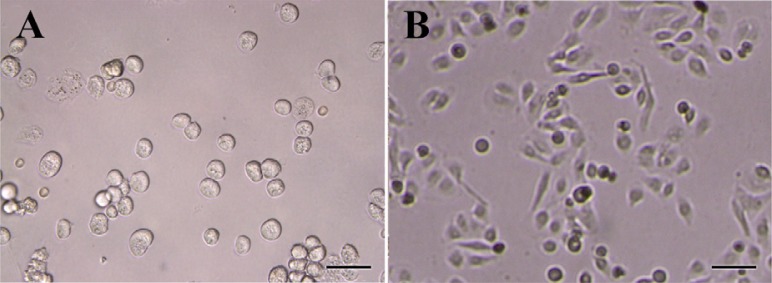
Morphology of isolated hAECs during the culture period. A) Isolated hAECs were cultured and their morphology was monitored immediately after culture; B) and after 48 hr. Scale bar; 50 µm
Proliferation assay
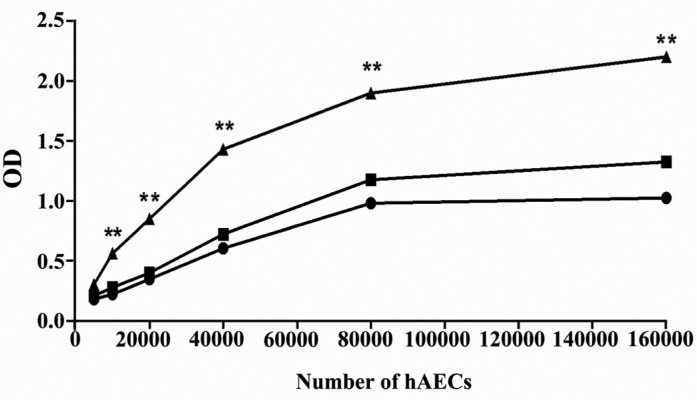
XTT assay was carried out to explore the proliferative ability of hAECs. To assess the dependency of hAEC proliferation on EGF treatment, this growth factor was added to some wells. Cell proliferation was determined with different cell numbers during a 72 hr of culture period. According to the results, hAECs cultured for 72 hr in the presence of EGF revealed substantial proliferative capacity which increased with cell concentration. More importantly, deprivation of EGF significantly halted the proliferation of hAECs (p < 0.01) (Figure 6).
Figure 6.
Proliferative capacity of hAECs. The proliferation of hAECs, cultured for 72 hr in the presence (filled triangle) or absence (filled rectangle) of EGF, was determined using XTT. Moreover, cell proliferation was investigated immediately after seeding (0 hr) (filled circle) and every 24 hr until 72 hr. For simplicity, only the results of proliferation at 0 hr and after 72 hr are provided in the figure. The comparison was made between proliferation of hAECs cultured in the presence and absence of EGF for 72 hr (**: p < 0.01)
Immunophenotyping of hAECs
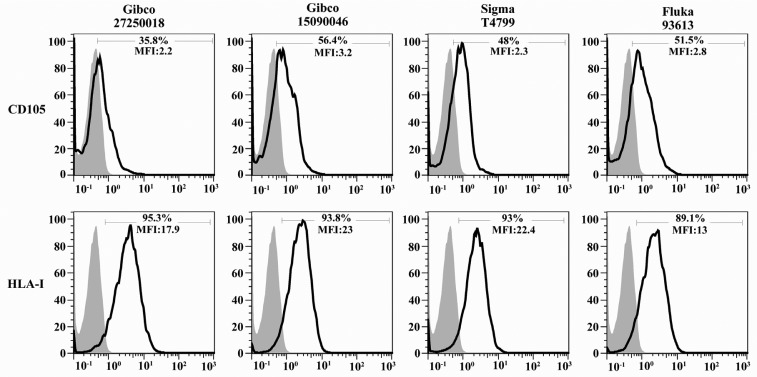
Immunophenotype of isolated hAECs was determined using flow cytometry. The average results of four independent experiments and representative results for each examined marker are shown in Figures 4 and 7, respectively. hAECs strongly expressed cytokeratin, a marker associated with cells from the epithelial origin. Flow cytometry data revealed that hAECs were a heterogeneous population consisting of immunophenotipically mixed cells. Nearly all purified cells expressed mesenchymal markers, CD9, CD10, CD29, and CD73. A large proportion of cells also expressed STRO-1 and CD105. Interestingly, embryonic stem cell markers, OCT-4 and SSEA-4 were expressed by the great majority of isolated cells. Unlike their anatomically adjacent trophoblast cells, hAECs expressed HLA-I on the surface. Non-classical HLA-I, HLA-G, which is mainly expressed by placental trophoblasts was also expressed by hAECs. Isolated cells failed to express the hematopoietic stem cell marker, CD34. No expression of CD38, CD44, CD133, and HLA-DR was found on the surface of the cells (Figure 4). More importantly, the percentage of cells positive for CD105 and also its expression level (mean fluorescence intensity; MFI) were considerably affected by the type of trypsin. In the case of HLA-I, although the percentage of positive cells was not considerably affected by the type of trypsin, different trypsins affected differentially the number of HLA-I molecules on the cell surface, as judged indirectly by MFI (Figure 8).
Figure 7.
Stem cell marker expression by hAECs. Data are shown as mean±SD obtained from four independent experiments
Figure 8.
Effect of trypsin type on percentage and expression of CD105 and HLA-I. Amniotic membranes were digested by four commercially available trypsins and the percentage and expression level (mean fluorescence intensity; MFI) of CD105 and HLA-I was determined using flow cytometry. The open and filled histograms represent test and isotype control, respectively
Immunogenicity of hAECs
Immunofluorescent staining was used to assess the immunogenicity of hAECs in Balb/c mice. According to the results, hAECs were positively stained with the sera obtained from hyperimmunized mice, while they showed no immunoreactivity with the sera of control group mice (Figure 9).
Figure 9.
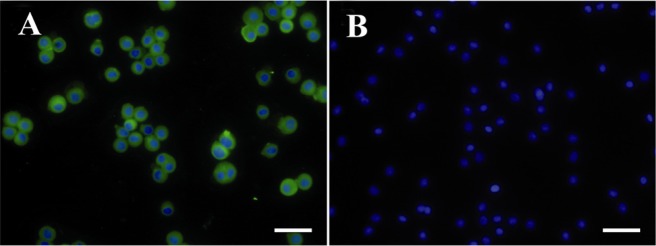
Assessment of hAEC immununogenicity. hAEC were subcutaneously injected into Balb/c mice three times with a one week interval. A) Immunoreactivity of mice sera with hAEC was tested by immunofluorescent staining; B) Nuclei were counterstained with DAPI. In negative reagent control slides, sera from mice injected with PBS was used as primary antibody. Scale bar; 50 μm
Discussion
In this study, we described the isolation of human amniotic epithelial cells and investigated the morphological, proliferative, and immunophenotypic properties of this interesting cell type.
Like placental trophoblast cells, this cell population is unique in that they exist only during nine month period of pregnancy. Of utmost importance, these cells are capable of differentiating into all three germ cell layers (13). Indeed, placenta is largely available as a discarded tissue following normal or cesarean delivery. Moreover, as we showed here, large numbers of stem cells could be achieved without any concern about ethical and religious issues usually being encountered with embryonic stem cells. The advantageous features mentioned above make amniotic membrane an easy to access and attractive source for regenerative medicine.
Here, we isolated hAECs with high purity and viability. We observed that discarding the cells obtained from the first step of tissue digestion, although reduces isolation yield, would increase cell viability by about 6-10% and decrease red blood cell contamination. It is of note that, depending on gestational age, the extent of blood contamination and the type of trypsin, the cell yield at the first trypsinization step varied from 20 to 70%.
It is needless to say that in cases where the majority of hAECs are isolated at the first digestion step, discarding the cells existing in the supernatant would result in a significant loss of final yield. Contamination with blood is the most important factor influencing the isolation yield due to the presence of serum, which is known to inactivate trypsin (19). In this context, washing of amniotic membrane and discarding the first step product of digestion could remove blood contamination which is considered to be the most important factor inhibiting the activity of trypsin. On the other hand, increasing the digestion period, although increases cell yield, will reduce cell viability and also cell purity due to contamination with underlying mesenchymal cells.
We found that more than 98% of purified cells express cytokeratin, a marker of intermediate filaments that is almost exclusively expressed in epithelia. The absence of HLA- DR on hAEC surface, as we showed here, and lack of tumorigenecity and immunogenicity (20) are the interesting features for stem cell-based therapeutic approaches. In spite of this, we showed that a considerable proportion of this cell type expresses HLA-I which could potentially induce immune system-based rejection mechanisms.
There is a great controversy on HLA-I status in freshly isolated hAECs. Although according to some reports these cells express low levels of HLA-I immediately after isolation (8, 21, 22) there are some reports indicating that majority of these cell types express considerable amounts of HLA-I (15, 16). Equally important, in the studies reporting low levels of HLA-I on the isolated hACEs, the level of HLA-I expression has been found to be a function of culture period and after the first few passages, these cells expressed significant level of this cell surface antigen (22, 23). We suggest that this discrepancy may arise from the type and specific activity of trypsin usually used for cell dissociation. In line with this assumption it has recently been shown that trypsin could alter cell proteome through enzymatically cleaving of some cell surface markers (24). We also showed that the utilization of different trypsin types to digest tissues would result in different percentages and expression levels for CD105 and different expression levels in the case of HLA-I.
Whatever the HLA-I status on hAECs is, exposure to IFNγ promotes cell surface expression of HLA-I on these cells (12), a condition that might happen in vivo soon after transplantation. So lack of immunogenicity seems to stem in part from immunomodulatory factors produced by these cells. In line with the assumption, it has been reported that hAECs have potent immunomodulatory properties. hAECs inhibit peripheral blood mononuclear cell proliferative responses to mitogen, alloantigen, and recall antigen (14). Viable hAECs have been shown to have beneficial effects on secretion of anti-inflammatory factors. Culture supernatant from hAECs has profound suppressive effect on neovascularization and inhibits recruitment of Major Histocompatibility Complex (MHC) class II + antigen- presenting cells, macrophages and neutrophils and induces apoptosis of T and B lymphocytes (25, 26). Moreover, expression of HLA-G in these cells, as we showed here, could restrain harmful NK cell-mediated cytotoxicity.
We observed that hAECs exhibit substantial proliferative capacity only when they are treated with EGF. In the absence of this growth factor, their proliferation was considerably diminished to the background level. It was reported that selection of a suitable growth medium is a critical step influencing growth rate of hAECs (15).
The substantial proliferation of hAECs could be attributed to the expression of OCT- 4, an embryonic stem cell marker essential for the establishment and maintenance of undifferentiated pluripotent stem cells (27). OCT-4 is downregulated during embryonic stem cell differentiation. It has been shown that loss of OCT-4 at the blastocyst stage results in the differentiation of inner cell mass to trophectodermal cells (27). Our results revealed expression of OCT-4 in a high percentage of freshly isolated hAEC, supporting the pluripotency capacity of these cells.
According to flow cytometric evaluations, hAECs were positive for CD9, CD10, CD29, CD73, SSEA-4, STRO-1 and CD105; however, they failed to express CD34, CD38, CD44, and CD133. As with other stem cells (17, 28–30), amniotic membrane contained stem cells with different cell surface markers suggesting heterogenicity of hAECs. Whereas chorion is derived from the trophoblast layer, the amnion is derived from the epiblast as early as 8 days after fertilization. Thus, amnion may retain the pluripotent properties of early epiblast cells (2, 10).
The overall pattern of molecular marker expression in hAECs, especially OCT-4 and SSEA-4, is in line with their embryonic origin. It is important to note that cell surface marker expression in hAECs is greatly influenced by cell isolation method, culture condi tion and the number of culture passages. For instance, freshly isolated hAECs lose CD10 and CD73 expression and gain CD34 at passage 5 (15). These factors may account for the great variability of hAECs markers which is mentioned in the literature.
Conclusion
Altogether, surface markers and such features as proliferation capacity of hAECs are among the characteristics that are strongly dependent upon isolation and cell culture conditions. Thus such variables should be taken into consideration when stem cell properties of hAECs are to be interpreted.
Acknowledgement
The authors wish to thank Ms. Vahedian for her technical assistance. This study was a part of Meraj Tabatabaei M.Sc. thesis and supported by grants from Avicenna Research Institute and Shahid Beheshti University of Medical Sciences.
References
- 1.Diwan S, Stevens LC. Development of teratomas from the ectoderm of mouse egg cylinders. J Natl Cancer Inst. 1976;57:937–942. doi: 10.1093/jnci/57.4.937. [DOI] [PubMed] [Google Scholar]
- 2.Akle CA, Adinolfi M, Welsh KI, Leibowitz S, Mc Coll I. Immunogenicity of human amniotic epithelial cells after transplantation into volunteers. Lancet. 1981;2(8254):1003–1005. doi: 10.1016/s0140-6736(81)91212-5. [DOI] [PubMed] [Google Scholar]
- 3.Toshio M, Thomas L, Hongbo C, Donna BS. Stem cell characteristics of amniotic epithelial cells. Stem Cells. 2005;23:1549–1559. doi: 10.1634/stemcells.2004-0357. [DOI] [PubMed] [Google Scholar]
- 4.Sakuragawa N, Kakinuma K, Kikuchi A, Okano H, Uchida S, Kamo I, et al. Human amnion mesenchyme cells express phenotypes of neuroglial progenitor cells. J Neurosci Res. 2004;78(2):208–214. doi: 10.1002/jnr.20257. [DOI] [PubMed] [Google Scholar]
- 5.Alviano F, Fossati V, Marchionni C, Arpinati M, Bonsi L, Franchina M, et al. Amniotic membrane is a high throughput source for multipotent mesenchymal stem cells with the ability to differentiate into endothelial cells in vitro. BMC Dev Biol. 2007;7:11–20. doi: 10.1186/1471-213X-7-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zheng YB, Gao ZL, Xie C, Zhu HP, Peng L, Chen JH, Chong YT. Characterization and hepatogenic differentiation of mesenchymal stem cells from human amniotic fluid and human bone marrow: a comparative study. Cell Biol Int. 2008;32(11):1439–1448. doi: 10.1016/j.cellbi.2008.08.015. [DOI] [PubMed] [Google Scholar]
- 7.Liu ZS, Xu YF, Feng SW, Li Y, Yao XL, Lu XL, Zhang C. Baculovirus-transduced mouse amniotic fluid-derived stem cells maintain differentiation potential. Ann Hematol. 2009;88(6):565–572. doi: 10.1007/s00277-008-0634-1. [DOI] [PubMed] [Google Scholar]
- 8.Perin L, Giuliani S, Jin D, Sedrakyan S, Carraro G, Habibian R, et al. Renal differentiation of amniotic fluid stem cells. Cell Prolif. 2007;40(6):936–948. doi: 10.1111/j.1365-2184.2007.00478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elwan M, Sakuragawa N. Evidence for synthesis and release of catecholamines by human amniotic epithelial cells. Neuroreport. 1997;8(16):3435–3438. doi: 10.1097/00001756-199711100-00004. [DOI] [PubMed] [Google Scholar]
- 10.Kakishita K, Elwan MA, Nakao N, Itakura T, Sakuragawa N. Human amniotic epithelial cells produce dopamine and survive after implantation into the striatum of a rat model of Parkinson's disease: a potential source of donor for transplantation therapy. Exp Neurol. 2000;165(1):27–34. doi: 10.1006/exnr.2000.7449. [DOI] [PubMed] [Google Scholar]
- 11.Dobreva MP, Pereira PN, Deprest J, Zwijsen A. On the origin of amniotic stem cells: of mice and men. Int J Dev Biol. 2010;54(5):761–777. doi: 10.1387/ijdb.092935md. [DOI] [PubMed] [Google Scholar]
- 12.Hunt JS, Wood GW. Interferon-gamma induces class I HLA and beta 2-microglobulin expression by human amnion cells. J Immunol. 1986;136(2):364–367. [PubMed] [Google Scholar]
- 13.Parolini O, Alviano F, Bagnara GP, Bilic G, Bühring HJ, Evangelista M, et al. Concise review: isolation and characterization of cells from human term placenta: outcome of the first international Workshop on Placenta Derived Stem Cells. Stem Cells. 2008;26(2):300–311. doi: 10.1634/stemcells.2007-0594. [DOI] [PubMed] [Google Scholar]
- 14.Banas RA, Trumpower C, Bentlejewski C, Marshall V, Sing G, Zeevi A. Immunogenicity and immunomodulatory effects of amnion-derived multipotent progenitor cells. Hum Immunol. 2008;69(6):321–328. doi: 10.1016/j.humimm.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 15.Murphy S, Rosli S, Acharya R, Mathias L, Lim R, Wallace E, Jenkin G. Amnion epithelial cell isolation and characterization for clinical use. Curr Protoc Stem Cell Biol. 2010:1E.6.1–1E.6.25. doi: 10.1002/9780470151808.sc01e06s13. [DOI] [PubMed] [Google Scholar]
- 16.Nikoo S, Ebtekar M, Jeddi-Tehrani M, Shervin A, Bozorgmehr M, Kazemnejad S, Zarnani AH. Effect of menstrual blood-derived stromal stem cells on proliferative capacity of peripheral blood mononuclear cells in allogeneic mixed lymphocyte reaction. J Obstet Gynaecol Res. 2012;38(5):804–809. doi: 10.1111/j.1447-0756.2011.01800.x. [DOI] [PubMed] [Google Scholar]
- 17.Darzi S, Zarnani AH, Jeddi-Tehrani M, Entezami K, Mirzadegan E, Akhondi MM, et al. Osteogenic differentiation of stem cells derived from menstrual blood versus bone marrow in the presence of human platelet releasate. Tissue Eng Part A. 2012;18(15-16):1720–1728. doi: 10.1089/ten.tea.2011.0386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tabatabaei-Panah AS, Jeddi-Tehrani M, Ghods R, Akhondi MM, Mojtabavi N, Mahmoudi AR, et al. Accurate sensitivity of quantum dots for detection of HER2 expression in breast cancer cells and tissues. J Fluoresc. 2013;23(2):293–302. doi: 10.1007/s10895-012-1147-9. [DOI] [PubMed] [Google Scholar]
- 19.Phelan MC. Basic techniques for mammalian cell tissue culture. Curr Protoc Cell Biol. 1998 doi: 10.1002/0471143030.cb0101s36. Chapter 1: Unit 1.1. [DOI] [PubMed] [Google Scholar]
- 20.Dobreva MP, Pereira PN, Deprest J, Zwijsen A. On the origin of amniotic stem cells: of mice and men. Int J Dev Biol. 2010;54(5):761–777. doi: 10.1387/ijdb.092935md. [DOI] [PubMed] [Google Scholar]
- 21.Ilancheran S, Michalska A, Peh G, Wallace EM, Pera M, Manuelpillai U. Stem cells derived from human fetal membranes display multilineage differentiation potential. Biol Reprod. 2007;77(3):577–588. doi: 10.1095/biolreprod.106.055244. [DOI] [PubMed] [Google Scholar]
- 22.Liu YH, Vaghjiani V, Tee JY, To K, Cui P, Oh DY, et al. Amniotic epithelial cells from the human placenta potently suppress a mouse model of multiple sclerosis. PLoS One. 2012;7(4):1–8. doi: 10.1371/journal.pone.0035758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moll R, Franke WW, Schiller DL, Geiger B, Krepler R. The catalog of human cytokeratins: patterns of expression in normal epithelia, tumors and cultured cells. Cell. 1982;31(1):11–24. doi: 10.1016/0092-8674(82)90400-7. [DOI] [PubMed] [Google Scholar]
- 24.Huang HL, Hsing HW, Lai TC, Chen YW, Lee TR, Chan HT, et al. Trypsin-induced proteome alteration during cell subculture in mammalian cells. J Biomed Sci. 2010;17:36. doi: 10.1186/1423-0127-17-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hori J, Wang M, Kamiya K, Takahashi H, Sakuragawa N. Immunological characteristics of amniotic epithelium. Cornea. 2006;25(10 Suppl 1):S53–58. doi: 10.1097/01.ico.0000247214.31757.5c. [DOI] [PubMed] [Google Scholar]
- 26.Li H, Niederkorn JY, Neelam S, Mayhew E, Word RA, McCulley JP, et al. Immunosuppressive factors secreted by human amniotic epithelial cells. Invest Ophthalmol Vis Sci. 2005;46(3):900–907. doi: 10.1167/iovs.04-0495. [DOI] [PubMed] [Google Scholar]
- 27.Pesce M, Scholer HR. Oct-4: gatekeeper in the beginnings of mammalian development. Stem Cells. 2001;19(4):271–278. doi: 10.1634/stemcells.19-4-271. [DOI] [PubMed] [Google Scholar]
- 28.Akiyama K, You YO, Yamaza T, Chen C, Tang L, Jin Y, et al. Characterization of bone marrow derived mesenchymal stem cells in suspension. Stem Cell Res Ther. 2012;3(5):40–47. doi: 10.1186/scrt131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Khanjani S, Khanmohammadi M, Zarnani AH, Talebi S, Edalatkhah H, Eghtesad S, et al. Efficient generation of functional hepatocyte-like cells from menstrual blood-derived stem cells. J Tissue Eng Regen Med. 2013 doi: 10.1002/term.1715. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 30.Rada T, Reis RL, Gomes ME. Distinct stem cells subpopulations isolated from human adipose tissue exhibit different chondrogenic and osteogenic differentiation potential. Stem Cell Rev. 2011;7(1):64–76. doi: 10.1007/s12015-010-9147-0. [DOI] [PubMed] [Google Scholar]