Abstract
Background/Aims
The association between clinical symptoms, gastric emptying, quality of life and sleep disorders in distinct functional dyspepsia (FD) patients has not been studied yet in detail.
Methods
We enrolled 79 FD patients (postprandial distress syndrome [PDS], n = 65; epigastric pain syndrome [EPS], n = 47; EPS-PDS overlap, n = 33) and 44 healthy volunteers. Gastric motility was evaluated. We used Rome III criteria to evaluate clinical symptoms and State-Trait Anxiety Inventory (STAI) scores to determine anxiety status. Sleep disorder was evaluated using the Pittsburgh Sleep Quality Index scores.
Results
There were no significant differences in age, sex and Helicobacter pylori positivity between FD subtypes and healthy volunteers. The scores of Glasgow dyspepsia severity scores (GDSS), SF-8 and Pittsburgh Sleep Quality Index (PSQI) in distinct subtypes of FD patients were significantly different from those in healthy volunteers. However, there were not significant differences in these scores, Tmax and T1/2 among 3 subtypes of FD patients. PSQI score was significantly (P = 0.027, P = 0.002 and P = 0.039, respectively) associated with GDSS among EPS, PDS and EPS-PDS overlap patients. In addition, 8-item short form health survey (SF-8; Physical Component Score and Mental Component Score) was significantly associated with global PSQI score in PDS and EPS-PDS overlap patients. In contrast, SF-8 (Mental Component Score) only was significantly linked to global PSQI score in EPS patients.
Conclusions
Prevalences for sleep disorders, gastric motility and quality of life in 3 subtypes of FD patients were similar levels. In PDS and EPS-PDS overlap patients, SF-8 was significantly associated with global PSQI score.
Keywords: Dyspepsia, Functional gastrointestinal disorders, Sleep disorders
Introduction
Functional dyspepsia (FD), irritable bowel syndrome (IBS) and gastroesophageal reflux disease (GERD) are highly prevalent and almost endemic gastrointestinal (GI) disorders in the general population. FD has been subclassified into 2 disease categories under the Rome III classification criteria: epigastric pain syndrome (EPS) and postprandial distress syndrome (PDS).1 Impairment of gastric motility such as gastric emptying is strongly associated with the pathophysiology of FD, one of the most common gastrointestinal disorders.2 In a previous study, we reported that maximum time (Tmax) value as a marker of gastric emptying in PDS patients was significantly greater compared to healthy volunteers.3 We also reported that nizatidine significantly improved clinical symptoms by affecting the Tmax value in FD patients with impaired gastric emptying compared to those without impairment of gastric emptying.4 In addition, acotiamide has been reported to improve significantly clinical symptoms in PDS patients compared to placebo treatment.5 As for the treatment of FD patients, it might be useful to consider subtypes of FD and classification in view of impairment of gastric emptying. Therefore, we have considered subtypes of FD patients as well as evaluating gastric motility to manage the treatment of FD patients.
Sleep disorder is a common medical problem, and has been associated with several diseases, including pulmonary disease, GERD and fibromyalgia.6 Sleep disorder, in turn, causes significant morbidity, as evidenced by the increased need for general medical and mental health treatment for emotional problems.7 However, a number of studies have found an association between sleep disorders and functional GI disorders.8-11 Sleep disturbance reported by patients with GERD was substantially improved by the use of proton pump inhibitor (PPI) therapy or anti-reflux surgery.12,13 Patients whose treatment protocol included histamine H2 receptor antagonist medication at bedtime in addition to PPI therapy showed overall improvement in GI symptoms and GERD-associated sleep disturbance.14 In contrast, few studies have focused on the relationship between sleep disorders and functional dyspepsia.15,16 We also reported that there was a significant relationship between subjective sleep quality and both Tmax and T1/2 values in FD patients by the 13C-acetate breath test.4 In this study, we investigated (1) the prevalence and relationship for sleep disorders and status of health-related quality of life (HRQOL) in FD patients according by subtypes and (2) the degree of impairment for gastric emptying in subtypes of FD patients.
Materials and Methods
Subjects
Seventy-nine consecutive patients presenting typical symptoms of PDS (n = 65), EPS (n = 47) and EPS-PDS overlap (n = 33) were enrolled after upper gastrointestinal endoscopy and abdominal ultrasonography. Patients presented with various types of abdominal symptoms including nausea and upper abdominal discomfort, in addition to the four typical upper abdominal symptoms defined by the Rome III criteria17; bothersome postprandial fullness, early satiation, epigastric pain and epigastric burning. Dyspeptic symptoms were defined as pain or discomfort in the upper abdomen for the past 3 months, with symptom onset at least 6 months prior to medical check-up. Patients completed a self-administrated questionnaire for the diagnosis of FD according to the Rome III criteria. Forty-four healthy volunteers with no clinical history of gastroduodenal disease including symptoms of FD, were recruited from our medical staffs and students at Nippon Medical School. Exclusion criteria included severe heart disease, renal or pulmonary failure, liver cirrhosis, severe systemic illness and history of malignant disease. Patients with previous gastroduodenal surgery, duodenal ulcer scars, diabetes mellitus, and recent use of non-steroidal anti-inflammatory drugs, PPIs or anticoagulants at endoscopy were also excluded. Helicobacter pylori infection was determined by both the 13C-acetate breath test and by histological identification. Written informed consent was obtained from all subjects prior to upper gastrointestinal endoscopy and abdominal ultrasonography for evaluation of dyspeptic symptoms. The study protocol was approved by the Ethics Review Committee of Nippon Medical School Hospital.
Clinical Symptoms
Clinical symptoms of FD were evaluated according to the Rome III criteria.17 Abdominal symptoms were assessed with a previously validated questionnaire.18-20 We assessed abdominal symptoms using the modified Glasgow dyspepsia severity score (GDSS),18-21 which is based on frequency (never, score 0; on only 1 or 2 days, score 1; on approximately 1 day per week, score 3; on approximately 50% of days, score 4; on most days, score 5), duration (minimum score, 0; maximal score 5), and intensity of symptoms (minimum score, 0; maximal score, 3). The degree of anxiety was evaluated by the State-trait Anxiety Inventory (STAI-state/-trait) scores.22
Pittsburgh Sleep Quality Index
A Japanese version of Pittsburgh Sleep Quality Index (PSQI)23 was used to measure the patient's recent history of sleep quality and the sleep duration during the month immediately preceding the study. The PSQI consisted of 17 items which generated 7 components, including subjective sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbance, use of sleep medication and daytime dysfunction. Each component score ranged from 0 to 3. The sum of these 7 component scores provides a global PSQI score, which ranges from 0 to 21. Higher scores indicate poorer sleep.23,24 A cut-off score > 5.5 has a sensitivity of 80.0-85.7% for various patient groups, and a specificity of 86.6% for control subjects in the Japanese version of the PSQI.24
Health-related Quality of Life
The 8-item short form health survey (SF-8) was used as a measurement scale for HRQOL, using eight questions scored according to the "Manual of the SF-8 Japanese version."25,26 Scores for the 8 domains ("general health," "physical functioning," "role-physical," "bodily pain," "vitality," "social functioning," "mental health" and "role-emotional") and 2 summaries (physical component summary [PCS] and mental component summary [MCS]) were derived. A score < 50 indicates impaired QOL, and an increasingly low score is considered to indicate greater damage to QOL.
Measurement of Gastric Motility
Sodium acetate (water-soluble; 13C-acetate) for emptying of liquids was used as tracer (Cambridge Isotope Laboratories, Cambridge, MA, USA). The liquid test meal consisted of 100 mg of 13C-acetate dissolved in 200 mL of a liquid meal (Racol, 1 mL/1 kcal; Otsuka Pharmacia Company, Tokyo, Japan). Breath samples were collected 0, 10 seconds, 5, 10, 15, 20, 30, 40, 50, 60, 75 and 90 minutes after ingestion of the test meal at 10:00 a.m. Patients were instructed not to drink, eat or smoke during the test. Probes were analyzed by non-dispersive infrared spectroscopy (IRIS, Wagner Analyzentechnik, Bremen, Germany). The subject's own production of 300 mmol CO2 per m2 body surface area and per hour was set as default. We used an Integrated Software Solutions program to calculate the half gastric emptying time (T1/2) and the lag phase (Tmax, minutes) as the point of maximum gastric emptying according to Hellmig et al.27 The T1/2 represents the time at which 50% of the initial gastric content was emptied.27,28 Tmax value greater than 60 minutes, representing the mean Tmax in healthy volunteers plus SD, was defined to represent relative disturbances in gastric emptying according to the diagnostic criteria of the Japan Society of Smooth Muscle Research, and our own study.3,29
Statistical Methods
For statistical evaluation of group data, Student's t test for paired data and ANOVA for multiple comparisons were followed by Scheffe's F test. Mann-Whitney U test was used for analysis of categorical data. The data analysis were performed using a standard software package (SPSS version 13.0; SPSS Inc., Chicago, IL, USA). A P-value of < 0.05 was statistically significant.
Results
Characteristics of EPS, PDS and EPS-PDS Overlap Patients and Healthy Volunteers
We have compared age, sex, H. pylori positivity, STAI-trait/-state score and Tmax value among EPS, PDS and EPS-PDS overlap patients and healthy volunteers. There were no significant differences in age, sex and H. pylori positivity among EPS, PDS and EPS-PDS overlap patients and healthy volunteers. Although there were significant (P < 0.05) differences in STAI-trait/-state, Tmax and T1/2 values between 3 subtypes of FD patients and healthy volunteers, there were no significant differences in STAI-trait/-state, Tmax and T1/2 values among EPS, PDS and EPS-PDS overlap patients (Table 1).
Table 1.
Characteristics of Subtypes of Functional Dyspepsia Patients and Healthy Volunteers
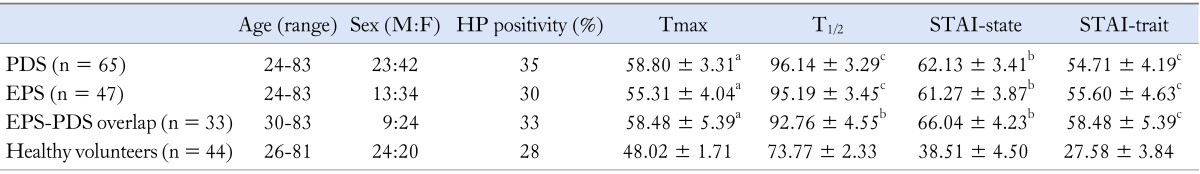
avs. healthy volunteers, P < 0.05; bvs. healthy volunteers, P < 0.01; cvs. healthy volunteers, P < 0.001.
HP, Helicobacter pylori; Tmax, the lag phase as the point of maximum gastric emptying; T1/2, half gastric emptying time; STAI, state-trait anxiety inventory; PDS, postprandial distress syndrome; EPS, epigastric pain syndrome.
Values are mean ± SD.
Comparison of GDSS, SF-8 and Global PSQI Scores Among EPS, PDS and EPS-PDS Overlap Patients and Healthy Volunteers
The scores of GDSS, SF-8 and global PSQI scores in EPS, PDS and EPS-PDS overlap patients were significantly different from those in healthy volunteers (Table 2). Especially, SF-8 (PCS) in EPS, PDS and EPS-PDS overlap patients were significantly lower (P < 0.001, P < 0.001 and P < 0.001) compared to that of healthy volunteers. The ratio of sleep disorders (global PSQI score > 5.5) in EPS, PDS and EPS-PDS overlap patients and healthy volunteers was 36.2%, 35.4%, 33.0% and 19.0%, respectively. However, there was no significant difference in GDSS, SF-8 and global PSQI scores among EPS, PDS and EPS-PDS overlap patients.
Table 2.
Comparison of GDSS, SF-8 and PSQI Scores Among Subtypes of Functional Dyspepsia Patients and Healthy Volunteers
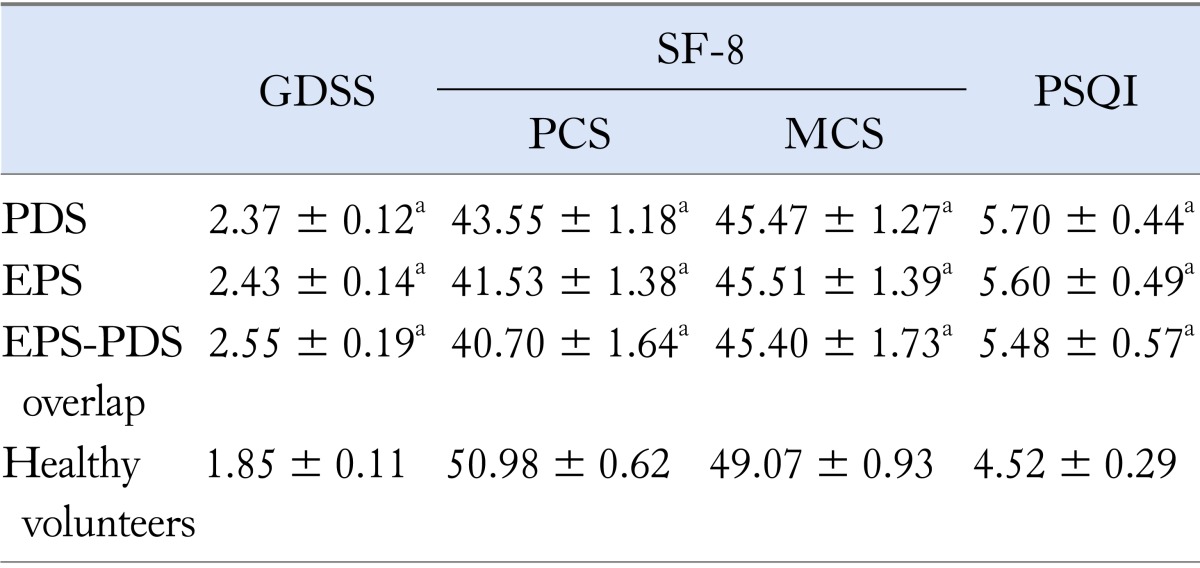
avs. healthy volunteers, P < 0.05.
GDSS, Glasgow dyspepsia severity score; SF-8, 8-item short form health survey; PCS, physical component summary; MCS, mental component summary; PSQI, Pittsburgh Sleep Quality Index; PDS, postprandial distress syndrome; EPS, epigastric pain syndrome.
Values are mean ± SD.
Comparison of Each PSQI Score Among EPS, PDS and EPS-PDS Overlap Patients and Healthy Volunteers
Since we previously reported that global PSQI score of FD subjects was significantly higher compared to healthy volunteers, we have compared each PSQI score in distinct subtypes of FD subjects compared to those in healthy volunteers. Sleep latency, sleep duration, habitual sleep efficiency, sleep disturbance and use of sleep medication in 3 subtypes of FD subjects were significantly higher compared to those in healthy volunteers (Table 3). Especially, sleep duration in EPS, PDS and EPS-PDS overlap syndrome was significantly (P < 0.001, P < 0.01 and P < 0.05) lower compared to that of healthy volunteers. However, there was no significant difference in each PSQI score among three subtypes of FD subjects (Table 3).
Table 3.
Comparison of PSQI Scores Among Subtypes of Functional Dyspepsia Patients and Healthy Volunteers
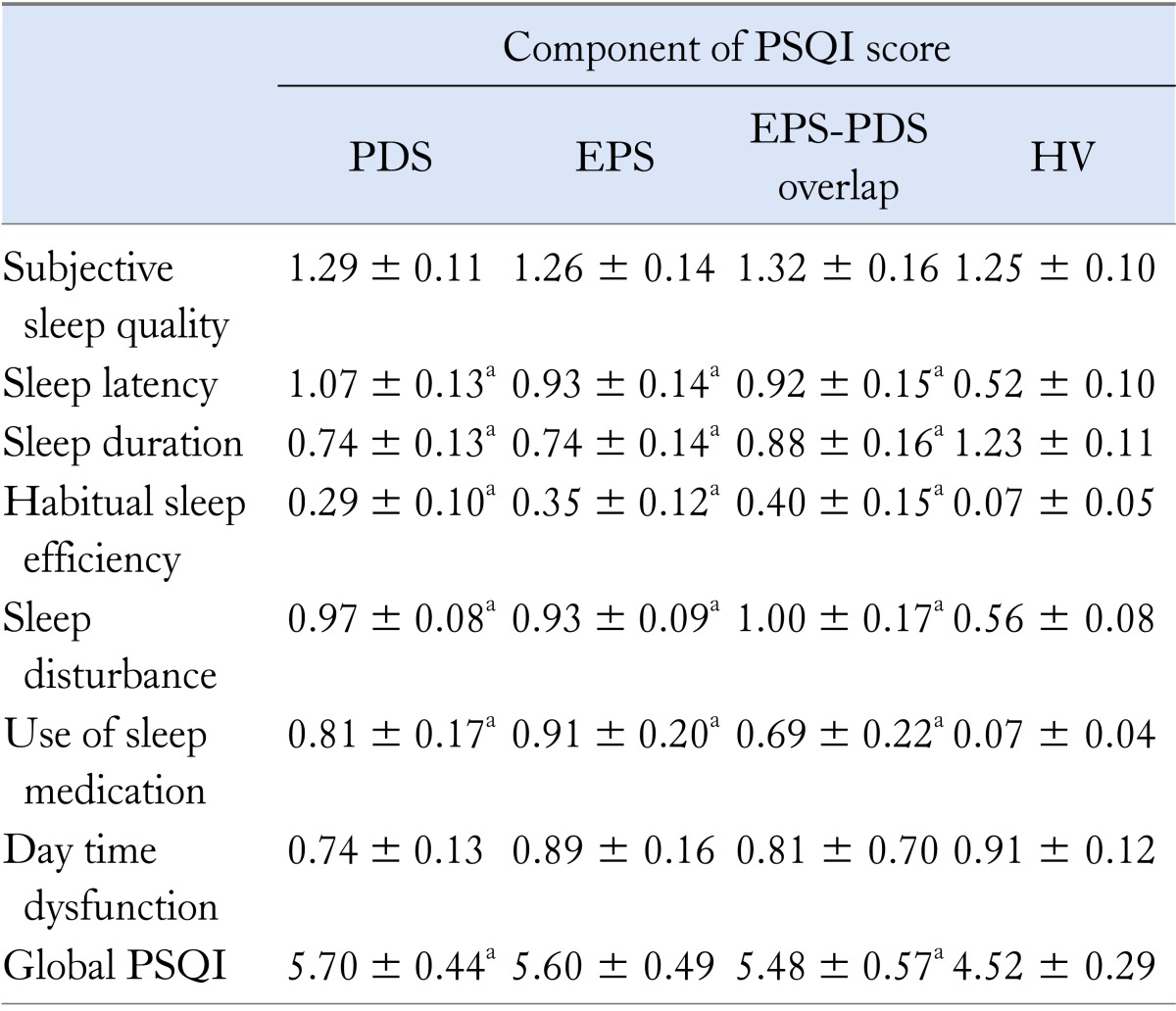
avs. healthy volunteers, P < 0.05.
PSQI, Pittsburgh Sleep Quality Index; PDS, postprandial distress syndrome;
EPS, epigastric pain syndrome; HV, healthy volunteers.
Summary values are mean ± SD.
Relationship Between GDSS Score and PSQI Score in 3 Subtypes of Functional Dyspepsia Subjects
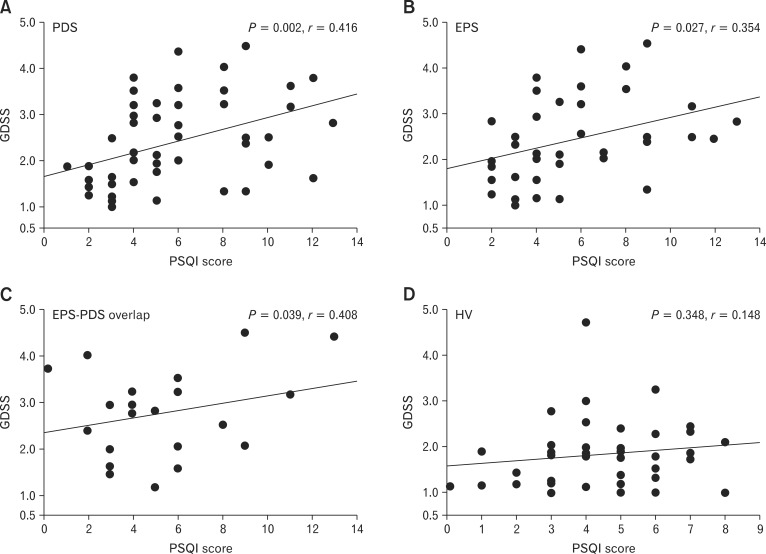
Although we could not get any significant difference in GDSS, SF-8 and global PSQI scores among EPS, PDS and EPS-PDS overlap patients, we have previously reported that there was a significant relationship between clinical symptoms and global PSQI in FD patients. Therefore, we investigated whether there were any differences in GDSS, SF-8 and global PSQI scores in FD subtypes. Total GDSS score in PDS patients was significantly (P = 0.002, r = 0.416) associated with global PSQI score (Fig. 1A). In EPS patients, total GDSS score was significantly (P = 0.027, r = 0.354) associated with global PSQI score (Fig. 1B). In EPS-PDS overlap patients, total GDSS score was also significantly (P = 0.039, r = 0.408) associated with global PSQI score (Fig. 1C). In contrast, in healthy volunteers, there was no significant relationship (P = 0.348, r = 0.148) between total GDSS score and global PSQI score (Fig. 1D).
Figure 1.
Relationship between total Glasgow dyspepsia severity score (GDSS) and global Pittsburgh Sleep Quality Index (PSQI) score among subtypes of functional dyspepsia patients. (A) There was a significant (P = 0.002, r = 0.416) relationship between total GDSS and global PSQI score in postprandial distress syndrome (PDS) patients. (B) In epigastric pain syndrome (EPS) patients, there was a significant (P = 0.027, r = 0.354) relationship between total GDSS and global PSQI score. (C) In EPS-PDS overlap patients, there was a significant (P = 0.039, r = 0.408) relationship between total GDSS and global PSQI score. (D) In healthy volunteers, there was no significant (P = 0.348, r = 0.148) relationship between total GDSS and global PSQI score.
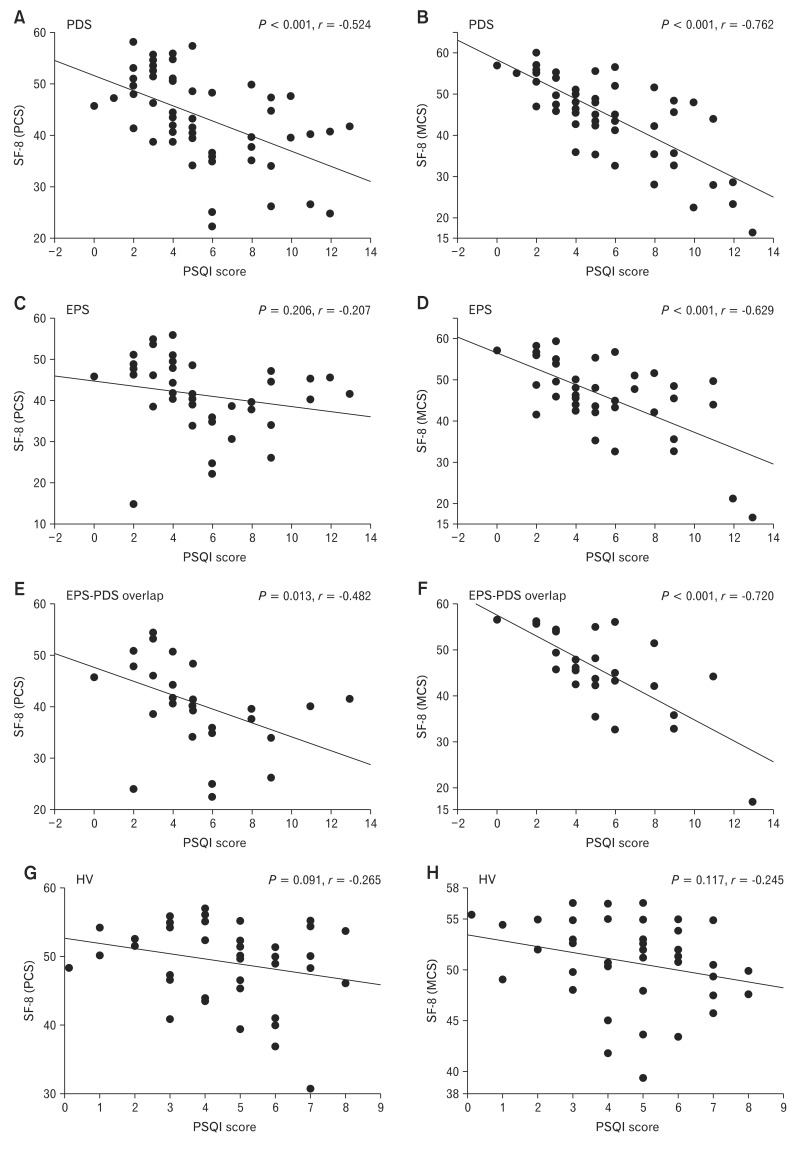
Relationship Between Total SF-8 and Global PSQI Score Among EPS, PDS and EPS-PDS Overlap Patients
In our data, quality of life in both subtypes of FD patients was significantly impaired, and we have investigated whether there was any significant correlations between SF-8 score and global PSQI score. In PDS patients, there were significant (PCS and MCS; P < 0.001, r = -0.524 and P < 0.001, r = -0.762, respectively) correlations between SF-8 score and global PSQI score (Fig. 2A and 2B). In EPS-PDS overlap patients, there were significant (PCS and MCS; P < 0.013, r = -0.482 and P < 0.001, r = -0.720, respectively) correlations between SF-8 score and global PSQI score (Fig. 2E and 2F). In contrast, in EPS patients, there was no significant (P = 0.206) relationship between SF-8 (PCS) score and global PSQI score (Fig. 2C). SF-8 (MCS) in EPS patients was significantly (P < 0.001, r = -0.629) associated with global PSQI score (Fig. 2D). In healthy volunteers, there were no significant correlations (PCS and MCS; P = 0.091, r = -0.265 and P = 0.117, r = -0.245, respectively) between SF-8 and global PSQI score (Fig. 2G and 2H).
Figure 2.
Relationship between 8-item short form health survey (SF-8) and global Pittsburgh Sleep Quality Index (PSQI) score among subtypes of functional dyspepsia patients. (A) There was a significant (P < 0.001, r = -0.524) relationship between SF-8 (physical component summary [PCS]) and global PSQI score in postprandial distress syndrome (PDS) patients. (B) In PDS patients, there was a significant (P < 0.001, r = -0.762) relationship between SF-8 (mental component summary [MCS]) and global PSQI score. (C) There was no significant (P = 0.206, r = -0.207) relationship between SF-8 (PCS) and global PSQI score in epigastric pain syndrome (EPS) patients. (D) There was a significant (P < 0.001, r = -0.629) relationship between SF-8 (MCS) and global PSQI score in EPS patients. (E) There was a significant (P = 0.013, r = -0.482) relationship between SF-8 (PCS) and global PSQI score in EPS-PDS overlap patients. (F) In EPS-PDS overlap patients, there was a significant (P < 0.001, r = -0.720) relationship between SF-8 (MCS) and global PSQI score. (G) There was no significant (P = 0.091, r = -0.265) relationship between SF-8 (PCS) and global PSQI score in healthy volunteers. (H) There was no significant (P = 0.117, r = -0.245) relationship between SF-8 (MCS) and global PSQI score in healthy volunteers.
Multiple Logistic Regression Analysis of Subtypes of Functional Dyspepsia Patients
Because diagnosis of subtypes of FD patients seemed to be useful for effective treatment, we compared subtypes of FD patients with the following parameters: GDSS score, PSQI score, SF-8 score, Tmax value and STAI score. A multiple logistic regression analysis revealed that GDSS, PSQI, SF-8 (MCS and PCS) and STAI-trait/-state score were not significantly (P = 0.211, P = 0.877, P = 0.547, P = 0.269, P = 0.868 and P = 0.889) associated with classification of distinct subtypes of FD patients (Table 4). In addition, Tmax value as a useful marker for gastric emptying was not significantly (P = 0.182) associated with classification of subtypes of FD patients (Table 4).
Table 4.
Multiple Logistic Regression Analysis of Factors Associated With Subtypes of Functional Dyspepsia Patients
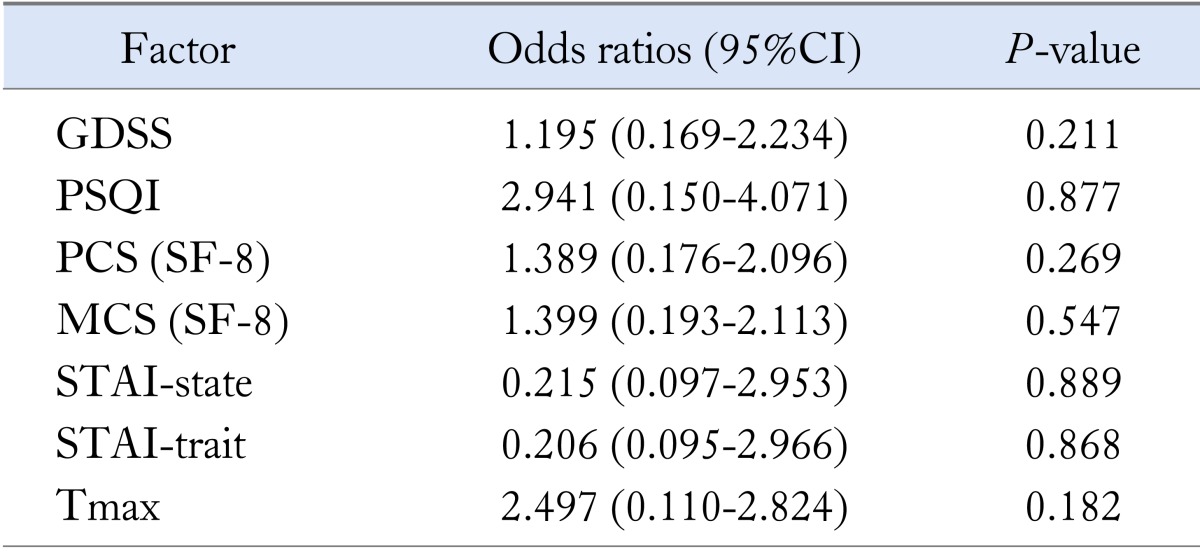
GDSS, Glasgow dyspepsia severity scores; PSQI, Pittsburgh Sleep Quality Index; PCS, physical component summary; MCS, mental component summary; STAI, State-Trait Anxiety Inventory; Tmax, the lag phase as the point of maximum gastric emptying.
Discussion
The major findings of this study are: (1) The scores of STAI-state/-trait and global PSQI in 3 subtypes of FD patients were significantly greater compared to those in healthy volunteers. (2) The scores of SF-8 in 3 subtypes of FD patients were significantly lower compared to that in healthy volunteers. (3) Sleep disorders were similarly observed in distinct subtypes of FD. (4) In PDS and EPS-PDS overlap patients, both SF-8 (PCS) and SF-8 (MCS) were significantly associated with global PSQI score. In contrast, only SF-8 (MCS) in EPS patients was significantly linked to global PSQI score.
The pathophysiology of FD is related to symptoms characterized by multifunctional disorders of the upper GI tract, such as disorders of GI motility, abnormal acid secretion, visceral hyper-sensitivity, H. pylori infection and potent psychological factors. Distinct differences may exist in pathophysiology among the three subgroups, PDS, EPS and EPS-PDS overlap patients. Although following a therapeutic approach for FD in line with pathophysiology of each subgroup is appropriate, it is very difficult to investigate the factors of each FD patient at clinical investigations, including primary care hospitals. Therefore, in this study, we tried to clarify whether there were any differences such as sleep disorders, quality of life and gastric motility among 3 subgroups. Sleep disorders are common medical problems, and have been associated with several diseases, including GERD and pulmonary disease.6 In addition, an epidemiological survey on insomnia demonstrated that almost 20% of the general Japanese population suffer from sleep disorders.24,30,31 Higher prevalence of sleep disorders in FD patients has been reported by other studies conducted in Japan and in the USA.16,32 Miwa32 have also reported that the distribution of subjects who thought that they got enough sleep was significantly lower for the FD-IBS overlap subjects than that for control subjects. In our study, prevalence of sleep disorder in EPS (36.2%), PDS (35.4%) and EPS-PDS overlap patients (33%) was significantly higher than that in healthy volunteers (19%). We have first reported the ratio of sleep disorders in distinct subtypes in functional dyspepsia. Sleep disorders distribution in three FD subtypes was significantly higher compared to that in healthy volunteers.
Moreover, it is possible that both sleep disorders and functional GI disturbances may be the result of some other underlying problem, such as depression, anxiety or other psychological conditions. Studies have shown that the prevalence of psychological disorders is significantly higher in patients with FD than in the general population.33-36 Lee et al33 have reported a significant correlation between clinical depression and FD. We have also reported that Self-Rating Questionnaire for Depression scores in FD patients are relatively higher than that in healthy volunteers.4 Some studies have suggested that the presence of anxiety can modulate the gut function and produce gastrointestinal disorders.37-41 In EPS patients, there was a significant correlation between mental component score and global PSQI score. In contrast, sleep disorders in PDS and EPS-PDS overlap patients were significantly associated with psychological factors as well as physical factors. These results suggest that psychological factors significantly affect sleep disorders compared to physiological factors in EPS patients. In addition, in our study, STAI scores in subtypes of FD patients were significantly higher compared to that in healthy volunteers. We have first demonstrated STAI scores in subtypes of FD patients. Further studies will be needed to clarify the reason why psychological factors strongly affected on sleep disorders, especially in EPS patients.
Tack et al1 have reported that we could better select a treatment for FD patients based on the classification of subtypes of FD.1 They recommended anti-acid therapy for EPS patients and prokinetics for PDS patients were recommended.1 Therefore, treatment for FD patients based on subtypes of FD or impairment of gastric emptying might be very important for the effective treatment of FD patients.4,42,43 However, we could not find significant differences in gastric motility, sleep disorders, anxiety and quality of life among EPS, PDS and EPS-PDS overlap patients. Although it is very much important for the effective treatment of FD patients to classify FD, precise diagnosis for the subtypes of FD is sometimes difficult because it depends on subjective complaints. Further studies will be needed to determine the certain tools for classifying the distinct subtypes of FD.
Taken together, prevalences for sleep disorders, gastric motility and quality of life in subtypes of FD patients were similar level. However, there is the limitation of sample size and evaluation of sleep disorders in this study.
Footnotes
Financial support: This work was supported in part by grants from the Ministry of Education, Culture, and Science and from the Ministry of Health, Japan (Grant No. 24590928).
Conflicts of interest: None.
Author contributions: Hiroshi Yamawaki: data collection, data processing and data analysis. Seiji Futagami: study designing, data collection and writing manuscript. Mayumi Shimpuku: data collection. Hitomi Sato: data collection. Taiga Wakabayashi: data collection. Yuuta Maruki: data collection. Yasuhiro Kodaka: data collection. Hiroyuki Nagoya: data collection. Tomotaka Shindo: data collection. Tetsuro Kawagoe: data collection. Choitsu Sakamoto: writing manuscript.
References
- 1.Tack J, Talley NJ, Camilleri M, et al. Functional gastroduodenal disorders. Gastroenterology. 2006;130:1466–1479. doi: 10.1053/j.gastro.2005.11.059. [DOI] [PubMed] [Google Scholar]
- 2.Quigley EM. Gastric emptying in functional gastrointestinal disorders. Aliment Pharmacol Ther. 2004;20(suppl 7):56–60. doi: 10.1111/j.1365-2036.2004.02186.x. [DOI] [PubMed] [Google Scholar]
- 3.Shindo T, Futagami S, Hiratsuka T, et al. Comparison of gastric emptying and plasma ghrelin levels in patients with functional dyspepsia and non-erosive reflux disease. Digestion. 2009;79:65–72. doi: 10.1159/000205740. [DOI] [PubMed] [Google Scholar]
- 4.Futagami S, Yamawaki H, Izumi N, et al. Impact of sleep disorders in Japanese patients with functional dyspepsia (FD): nizatidine improves clinical symptoms, gastric emptying and sleep disorders in FD patients. J Gastroenterol Hepatol. 2013;28:1314–1320. doi: 10.1111/jgh.12236. [DOI] [PubMed] [Google Scholar]
- 5.Matsueda K, Hongo M, Tack J, Saito Y, Kato H. A placebo-controlled trial of acotiamide for meal-related symptoms of functional dyspepsia. Gut. 2012;61:821–828. doi: 10.1136/gutjnl-2011-301454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Parish JM. Sleep-related problems in common medical conditions. Chest. 2009;135:563–572. doi: 10.1378/chest.08-0934. [DOI] [PubMed] [Google Scholar]
- 7.Weissman MM, Greenwald S, Niño-Murcia G, Dement WC. The morbidity of insomnia uncomplicated by psychiatric disorders. Gen Hosp Psychiatry. 1997;19:245–250. doi: 10.1016/s0163-8343(97)00056-x. [DOI] [PubMed] [Google Scholar]
- 8.Fass R, Fullerton S, Tung S, Mayer EA. Sleep disturbances in clinic patients with functional bowel disorders. Am J Gastroenterol. 2000;95:1195–2000. doi: 10.1111/j.1572-0241.2000.02009.x. [DOI] [PubMed] [Google Scholar]
- 9.Jarrett M, Heitkemper M, Cain KC, Burr RL, Hertig V. Sleep disturbance influences gastrointestinal symptoms in women with irritable bowel syndrome. Dig Dis Sci. 2000;45:952–959. doi: 10.1023/a:1005581226265. [DOI] [PubMed] [Google Scholar]
- 10.Goldsmith G, Levin JS. Effect of sleep quality on symptoms of irritable bowel syndrome. Dig Dis Sci. 1993;38:1809–1814. doi: 10.1007/BF01296103. [DOI] [PubMed] [Google Scholar]
- 11.David D, Mertz H, Fefer L, et al. Sleep and duodenal motor activity in patients with severe non-ulcer dyspepsia. Gut. 1994;35:916–925. doi: 10.1136/gut.35.7.916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vakil N, van Zanten SV, Kahrilas P, Dent J, Jones R Global Consensus Group. The Montreal definition and classification of gastroesophageal reflux disease: a global evidence-based consensus. Am J Gastroenterol. 2006;101:1900–1920. doi: 10.1111/j.1572-0241.2006.00630.x. [DOI] [PubMed] [Google Scholar]
- 13.Orr WC, Goodrich S, Robert J. The effect of acid suppression on sleep patterns and sleep-related gastro-oesophageal reflux. Aliment Pharmacol Ther. 2005;21:103–108. doi: 10.1111/j.1365-2036.2005.02310.x. [DOI] [PubMed] [Google Scholar]
- 14.Rackoff A, Aqrawal A, Hila A, Mainie I, Tutuian R, Castell DO. Histamine-2 receptor antagonists at night improve gastroesophageal reflux disease symptoms for patients on proton pump inhibitor therapy. Dis Esophagus. 2005;18:370–373. doi: 10.1111/j.1442-2050.2005.00518.x. [DOI] [PubMed] [Google Scholar]
- 15.Vege SS, Locke GR, 3rd, Weaver AL, Farmer SA, Melton LJ, 3rd, Talley NJ. Functional gastrointestinal disorders among people with sleep disturbances: a population-based study. Mayo Clin Proc. 2004;79:1501–1506. doi: 10.4065/79.12.1501. [DOI] [PubMed] [Google Scholar]
- 16.Lacy BE, Everhart K, Crowell MD. Functional dyspepsia is associated with sleep disorders. Clin Gastroenterol Hepatol. 2011;9:410–414. doi: 10.1016/j.cgh.2011.02.010. [DOI] [PubMed] [Google Scholar]
- 17.Drossman DA. The functional gastrointestinal disorders and the Rome III process. Gastroenterology. 2006;130:1377–1390. doi: 10.1053/j.gastro.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 18.Futagami S, Shimpuku M, Song JM, et al. Nizatidine improves clinical symptoms and gastric emptying in patients with functional dyspepsia accompanied by impaired gastric emptying. Digestion. 2012;86:114–121. doi: 10.1159/000339111. [DOI] [PubMed] [Google Scholar]
- 19.McColl K, Murray L, El-Omar E, et al. Symptomatic benefit from eradicating Helicobacter pylori infection in patients with nonulcer dyspepsia. N Engl J Med. 1998;339:1869–1874. doi: 10.1056/NEJM199812243392601. [DOI] [PubMed] [Google Scholar]
- 20.Portincasa P, Altomare DF, Moschetta A, et al. The effect of acute oral erythromycin on gallbladder motility and on upper gastrointestinal symptoms in gastrectomized patients with and without gallstones: a randomized, placebo-controlled ultrasonographic study. Am J Gastroenterol. 2000;95:3444–3451. doi: 10.1111/j.1572-0241.2000.03282.x. [DOI] [PubMed] [Google Scholar]
- 21.Shimpuku M, Futagami S, Kawagoe T, et al. G-protein β3 subunit 825CC genotype is associated with postprandial distress syndrome with impaired gastric emptying and with the feeling of hunger in Japanese. Neurogastroenterol Motil. 2011;23:1073–1080. doi: 10.1111/j.1365-2982.2011.01781.x. [DOI] [PubMed] [Google Scholar]
- 22.Spielberger CD, Goursuch RL, Lushene RE. Manual for the State Trait Anxiety Inventory. Mountain View: Counsulting Psychologist Press; 1983. [Google Scholar]
- 23.Doi Y, Minowa M, Okawa M. [Development of the Japanese version of the Pittsburgh Sleep Quality Index] Jpn J Psychiatr Trea. 1998;13:755–763. [Japanese] [Google Scholar]
- 24.Doi Y, Minowa M, Okawa M, Uchiyama M. Prevalence of sleep disturbance and hypnotic medication use in relation to sociodemo-graphic factors in the general Japanese adult population. J Epidemiol. 2000;10:79–86. doi: 10.2188/jea.10.79. [DOI] [PubMed] [Google Scholar]
- 25.Ware JE, Kosinski M, Dewey JE, Gandek B. How to score and interpret single-item health status measures: a manual for users of the SF-8 health survey. Lincolon: Quality Metric Inc; 2001. [Google Scholar]
- 26.Fukuhara S, Suzukamo Y. [Manual of the SF-8 Japanese version] Kyoto: Institute for Health outcomes & Process Evaluation Research; 2004. [In Japanese] [Google Scholar]
- 27.Hellmig S, Von Schöning F, Gadow C, et al. Gastric emptying time of fluids and solids in healthy subjects determined by 13C breath tests: influence of age, sex and body mass index. J Gastroenterol Hepatol. 2006;21:1832–1838. doi: 10.1111/j.1440-1746.2006.04449.x. [DOI] [PubMed] [Google Scholar]
- 28.Ghoos YF, Maes BD, Geypens BJ, et al. Measurement of gastric emptying rate of solids by means of a carbon-labeled octanoic acid breath test. Gastroenterology. 1993;104:1640–1647. doi: 10.1016/0016-5085(93)90640-x. [DOI] [PubMed] [Google Scholar]
- 29.Futagami S, Shindo T, Kawagoe T, et al. Migration of eosinophils and CCR2-/CD68-double positive cells into the duodenal mucosa of patients with postinfectious functional dyspepsia. Am J Gastroenterol. 2010;105:1835–1842. doi: 10.1038/ajg.2010.151. [DOI] [PubMed] [Google Scholar]
- 30.Kim K, Uchiyama M, Okawa M, Liu X, Ogihara R. An epidemiological study of insomnia among the Japanese general population. Sleep. 2000;23:41–47. [PubMed] [Google Scholar]
- 31.Kim K, Uchiyama M, Liu X, et al. Somatic and psychological complaints and their correlates with insomnia in the Japanese general population. Psychosom Med. 2001;63:441–446. doi: 10.1097/00006842-200105000-00013. [DOI] [PubMed] [Google Scholar]
- 32.Miwa H. Life style in persons with functional gastrointestinal disorders--large-scale internet survey of lifestyle in Japan. Neurogastroenterol Motil. 2012;24:464–471. e217. doi: 10.1111/j.1365-2982.2011.01872.x. [DOI] [PubMed] [Google Scholar]
- 33.Lee HJ, Lee SY, Kim JH, et al. Depressive mood and quality of life in functional gastrointestinal disorders: differences between functional dyspepsia, irritable bowel syndrome and overlap syndrome. Gen Hosp Psychiatry. 2010;32:499–502. doi: 10.1016/j.genhosppsych.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 34.Haug TT, Svebak S, Wilhelmsen I, Berstad A, Ursin H. Psychological factors and somatic symptoms in functional dyspepsia. A comparison with duodenal ulcer and healthy controls. J Psychosom Res. 1994;38:281–291. doi: 10.1016/0022-3999(94)90033-7. [DOI] [PubMed] [Google Scholar]
- 35.Wu JC. Psychological co-morbidity in functional gastrointestinal disorders: Epidemiology, mechanisms and management. J Neurogastroenterol Motil. 2012;18:13–18. doi: 10.5056/jnm.2012.18.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miwa H, Ghoshal UC, Gonlachanvit S, et al. Asian consensus report on functional dyspepsia. J Neurogastroenterol Motil. 2012;18:150–168. doi: 10.5056/jnm.2012.18.2.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Elsenbruch S, Thompson JJ, Hamish MJ, Exton MS, Orr WC. Behavioral and physiological sleep characteristics in women with irritable bowel syndrome. Am J Gastroenterol. 2002;97:2306–2314. doi: 10.1111/j.1572-0241.2002.05984.x. [DOI] [PubMed] [Google Scholar]
- 38.Geeraerts B, Vandenberghe J, Van Oudenhove L, et al. Influence of experimentally induced anxiety on gastric sensorimotor function in humans. Gastroenterology. 2005;129:1437–1444. doi: 10.1053/j.gastro.2005.08.020. [DOI] [PubMed] [Google Scholar]
- 39.Lorena SL, Tinois E, Brunetto SQ, Camargo EE, Mesquita MA. Gastric emptying and intragastric distribution of a solid meal in functional dyspepsia: influence of gender and anxiety. J Clin Gastroenterol. 2004;38:230–236. doi: 10.1097/00004836-200403000-00007. [DOI] [PubMed] [Google Scholar]
- 40.Mönnikes H, Tebbe JJ, Hildebrandt M, et al. Role of stress in functional gastrointestinal disorders. Evidence for stress-induced alterations in gastrointestinal motility and sensitivity. Dig Dis. 2001;19:201–211. doi: 10.1159/000050681. [DOI] [PubMed] [Google Scholar]
- 41.De la Roca-Chiapas JM, Solís-Ortiz S, Fajardo-Araujo M, Sosa M, Córdova-Fraga T, Rosa-Zarate A. Stress profile, coping style, anxiety, depression, and gastric emptying as predictors of functional dyspepsia: a case-control study. J Psychosom Res. 2010;68:73–81. doi: 10.1016/j.jpsychores.2009.05.013. [DOI] [PubMed] [Google Scholar]
- 42.Miwa H, Osada T, Nagahara A, et al. Effect of a gastro-protective agent, rebamipide, on symptom improvement in patients with functional dyspepsia: a double-blind placebo-controlled study in Japan. J Gastroenterol Hepatol. 2006;21:1826–1831. doi: 10.1111/j.1440-1746.2006.04446.x. [DOI] [PubMed] [Google Scholar]
- 43.Chen JD, Ke MY, Lin XM, Wang Z, Zhang M. Cisapride provides symptomatic relief in functional dyspepsia associated with gastric myoelectrical abnormality. Aliment Pharmacol Ther. 2000;14:1041–1047. doi: 10.1046/j.1365-2036.2000.00801.x. [DOI] [PubMed] [Google Scholar]