Abstract
Recovery of consciousness has been associated with connectivity in the frontal cortex and parietal regions modulated by the thalamus. To examine this model and to relate alterations to deficits in cognitive functioning and conscious processing, we investigated topological network properties in patients with chronic disorders of consciousness recovered from coma.
Resting state fMRI data of 34 patients with unresponsive wakefulness syndrome and 25 in minimally conscious state were compared to 28 healthy controls. We investigated global and local network characteristics. Additionally, behavioral measures were correlated with the local metrics of 28 regions within the fronto-parietal network and the thalamus.
In chronic disorders of consciousness, modularity at the global level was reduced suggesting a disturbance in the optimal balance between segregation and integration. Moreover, network properties were altered in several regions which are associated with conscious processing (particularly, in medial parietal, and frontal regions, as well as in the thalamus). Between minimally conscious and unconscious patients the local efficiency of medial parietal regions differed. Alterations in the thalamus were particularly evident in non-conscious patients. Most of the regions affected in patients with impaired consciousness belong to the so-called ‘rich club’ of highly interconnected central nodes. Disturbances in their topological characteristics have severe impact on information integration and are reflected in deficits in cognitive functioning probably leading to a total breakdown of consciousness.
Abbreviations: DOC, disorders of consciousness; ACC, anterior cingulate cortex; PCC, posterior cingulate cortex; MCS, minimally conscious state; VS/UWS, vegetative state/unresponsive wakefulness syndrome
Keywords: Consciousness, Vegetative state, Network, Graph theory, Connectivity, Small world
Highlights
-
•
We investigated network properties in patients with a disorder of consciousness.
-
•
Patients showed reduced global modularity.
-
•
Alterations in regions of the rich club were related to impaired consciousness.
-
•
These alterations have severe impact on information integration and segregation.
-
•
Disturbances in overall integration may lead to breakdown of consciousness.
1. Introduction
In the last decade, research on patients with severe brain injury and chronic disorders of consciousness (DOC) has provided important insights into the complex patterns of neuronal activity associated with consciousness (e.g., Boly et al., 2004; Crone et al., 2013; Fernandez-Espejo et al., 2011, 2012; Laureys et al., 2002; Owen et al., 2006).
Patients with DOC, that is, patients with unresponsive wakefulness syndrome, formerly vegetative state (VS/UWS), and patients in the minimally conscious state (MCS), have recovered from coma after severe brain injury and are characterized by a present sleep-wake-cycle but loss of or only minimal awareness of themselves and their environment.
Recently, Laureys and Schiff (Laureys and Schiff, 2012) proposed a model of recovery of consciousness focusing on the connectivity between and within frontal and parietal regions influenced by specific circuit modulations of the thalamus. Evidence for this model comes from two different sets of findings: studies showing disrupted functional connectivity in a widespread fronto-parietal network in patients with impaired consciousness (Boly et al., 2009; Cauda et al., 2009; Soddu et al., 2012; Vanhaudenhuyse et al., 2010) and studies emphasizing an important role of the thalamus (Alkire et al., 2000; Guldenmund et al., 2013; Laureys and Schiff, 2012; Laureys et al., 2000; Lull et al., 2010; Nakayama et al., 2006; Xie et al., 2011; Zhou et al., 2011). In a next step, the brain network of patients with DOC should be investigated as a whole with focus on the thalamo-cortical network to identify specific changes in its characteristics associated with impaired consciousness.
For such detailed analyses of complex brain networks, a new scientific approach using graph theoretical methods has recently been introduced. In complex systems, functionally associated clusters show a high density of local connections with few long-range connections between segregated areas (Achard et al., 2006; Salvador et al., 2005). This constellation ensures a high efficiency in information processing at a relatively low cost of connection length. These so-called small-world properties can be quantified with graph theoretical methods. In graph theory, complex networks are defined as a set of nodes connected by edges and described by network metrics.
The network topology of the brain tends to show alterations in a number of disorders like Alzheimer's disease (Supekar et al., 2008), schizophrenia (Alexander-Bloch et al., 2012), and major depressive disorder (Zhang et al., 2011) and such alterations were also found to be related to cognitive deficits (Stam et al., 2007; Zhang et al., 2011).
Severe brain injury has also a high impact on network organization. Particularly, if main nodes with high centrality (so-called hubs) are affected, the stability and organization of a network is disturbed with serious consequences for its integrity and functioning (Chen et al., 2008; He et al., 2007; Honey and Sporns, 2008). A recent study demonstrates that shortly after severe brain injury, central hubs throughout the network show a fundamental reorganization (Achard et al., 2012). However, Achard et al. could not relate alterations in the topology directly to consciousness since they investigated patients in deep coma. Hence, the differences they found are related to the severity of brain injury, and even though it has an important prognostic value, it cannot be associated with the level or the content of consciousness. To acquire knowledge about the relationship between alterations of brain networks and consciousness, we investigated network properties during resting state in chronic patients with different degrees of impaired consciousness.
To our knowledge, this is the first study applying graph theoretical methods in patients with DOC to investigate global and local network topology. We are also the first to compare deficits in cognitive functioning and conscious processing to impairments of the fronto-parietal and thalamo-cortical network organization using fMRI resting state data from a very large group of 59 patients with DOC.
2. Materials and methods
2.1. Subjects
The study was approved by the Ethical Committee of Salzburg (Ethics Commission Salzburg/Ethikkommission Land Salzburg; number 415-E/952). Resting state data of patients with DOC were compared to a group of 28 age-matched healthy subjects. Participants were investigated at 2 different sites (at the Department of Neurology, Christian Doppler Klinik, Paracelsus Medical University, Salzburg, and at the CHU Sart Tilman University Hospital, Liège) and with 3 different scanners (see Section 2.2. Data acquisition). Since patients with DOC are not easily investigated, experience in diagnosis is required, and suitable patients are rare, it is necessary to cooperate with other diagnostic teams to get a suitable sample size. This is a common practice (e.g., Monti et al., 2010). To make sure that the different sites do not influence the effects of group differences, site was included as a covariate (see Section 2.6. Statistical Analysis). After excluding 5 VS/UWS and one MCS patient due to large movement and artifacts (see Data preprocessing), the patient group consisted of 34 VS/UWS (mean age = 52; age range = 26–87; 13 female) and 25 MCS (mean age = 48; age range = 28–74; 10 female) patients. All patients participating in this study were examined with the Coma Recovery Scale-Revised (CRS-R) (Giacino et al., 2004) and showed preserved auditory functioning, largely preserved brainstem reflexes, and a fairly preserved sleep-wake-cycle based on neurological examination. None of the patients were artificially ventilated or sedated at time of scanning. Additional information of the patients is listed in Inline Supplementary Table S1. The control group consisted of 28 volunteers with no history of neurological or psychiatric disease (mean age 51 years; age range 28–75 years; 15 female). Control subjects were recruited at the Paris Lodron University of Salzburg and at the University of Liège. There were no differences between healthy controls, MCS patients, and VS/UWS patients in age (F = 0.265, p = 0.768). Written informed consent was obtained from all healthy subjects and from the guardianship of all patients according to the Declaration of Helsinki.
Inline Supplementary Table S1.
Table S1.
Patients' information.
| Subjects | Age | Etiology | Time since onset (in days) |
Auditory function | Visual function | Motor function | Verbal/Oromotor function | Communication | Arousal |
|---|---|---|---|---|---|---|---|---|---|
| MCS | |||||||||
| MCS01 | 51 | Intracerebral Hemorrhage | 102 | Localization to sound | Visual pursuit | Abnormal posturing | Oral reflexive movement | None | Without stimulation |
| MCS02 | 37 | Respiratory failure | 67 | Auditory startle | Fixation | Flexion withdrawal | Oral reflexive movement | None | Without stimulation |
| MCS03 | 47 | PICA infarct | 49 | Auditory startle | Fixation | Abnormal posturing | Oral reflexive movement | Intentional | Without stimulation |
| MCS04 | 47 | Traumatic brain injury | 52 | Localization to sound | Fixation | Abnormal posturing | Oral reflexive movement | None | Without stimulation |
| MCS05 | 55 | Subarachnoidal hemorrhage | 74 | Consistent movement to command | Object recognition | Flexion withdrawal | Oral reflexive movement | Functional: Accurate | Attention |
| MCS06 | 28 | Limbic encephalopathy | 148 | None | Visual pursuit | Flexion withdrawal | Oral reflexive movement | None | Without stimulation |
| MCS07 | 63 | Subarachnoidal hemorrhage | 62 | Auditory startle | Object localization: Reaching | Flexion withdrawal | None | None | Without stimulation |
| MCS08 | 62 | Subarachnoidal hemorrhage | 74 | Localization to sound | Visual pursuit | Flexion withdrawal | Oral reflexive movement | None | Attention |
| MCS09 | 46 | Multiple cerebral infarct | 41 | Auditory startle | Visual startle | Automatic motor response | Oral reflexive movement | None | With stimulation |
| MCS10 | 65 | Cardiopulmonary resuscitation | 85 | Localization to sound | Fixation | Object manipulation | Oral reflexive movement | None | Attention |
| MCS11 | 31 | Traumatic brain injury | 66 | None | None | Localization to noxious stimuli | Oral reflexive movement | None | With stimulation |
| MCS12 | 52 | Subarachnoidal hemorrhage | 146 | Localization to sound | Visual startle | Localization to noxious stimuli | Oral reflexive movement | None | Without stimulation |
| MCS13 | 71 | Subarachnoidal hemorrhage | 362 | None | Visual pursuit | Localization to noxious stimuli | Vocalization/oral movement | Intentional | With stimulation |
| MCS14 | 61 | Intracerebral Hemorrhage | 75 | None | Visual pursuit | Localization to noxious stimuli | Oral reflexive movement | None | With stimulation |
| MCS15 | 34 | Traumatic brain injury | 3034 | Reproducible movement to command | Visual pursuit | Flexion withdrawal | Vocalization/oral movement | None | Without stimulation |
| MCS16 | 74 | Hemorrhage | 18 | Auditory startle | None | Abnormal posturing | Oral reflex | Intentional | With stimulation |
| MCS17 | 43 | Hemorrhage | 31 | Auditory startle | Visual pursuit | Flexion withdrawal | Oral reflex | None | Without stimulation |
| MCS18 | 52 | Status epilepticus | 20 | Reproducible movement to command | Visual pursuit | Flexion withdrawal | Vocalization/oral movement | Intentional | Without stimulation |
| MCS19 | 47 | Traumatic brain injury | 533 | Reproducible movement to command | Object recognition | Flexion withdrawal | Oral reflex | None | Without stimulation |
| MCS20 | 38 | Atrioventricular conduction | 1854 | Auditory startle | Visual pursuit | Flexion withdrawal | Vocalization/oral movement | None | With stimulation |
| MCS21 | 29 | Traumatic brain injury & anoxia | 64 | Auditory startle | Visual pursuit | Flexion withdrawal | Oral reflex | None | Without stimulation |
| MCS22 | 41 | Anoxia | 9900 | Reproducible movement to command | Visual pursuit | Flexion withdrawal | Vocalization/oral movement | None | Without stimulation |
| MCS23 | 37 | Traumatic brain injury | 342 | Reproducible movement to command | Object recognition | Localisation to noxious stimulation | Oral reflex | None | Without stimulation |
| MCS24 | 60 | Traumatic brain injury | 12 | Reproducible movement to command | Object recognition | Automatic motor reaction | Intelligible verbalization | Intentional | With stimulation |
| MCS25 | 36 | Anoxia | 460 | Reproducible movement to command | Visual pursuit | Flexion withdrawal | Vocalization/oral movement | Intentional | Without stimulation |
| UWS | |||||||||
| UWS01 | 36 | Traumatic brain injury | 347 | Auditory startle | None | None | Oral reflexive movement | None | With stimulation |
| UWS02 | 45 | Traumatic brain injury | 64 | Auditory startle | None | Abnormal posturing | Oral reflexive movement | None | With stimulation |
| UWS03 | 45 | Cardiopulmonary resuscitation | 635 | Auditory startle | None | None | Oral reflexive movement | None | With stimulation |
| UWS04 | 50 | Cardiopulmonary resuscitation | 204 | Auditory startle | None | Flexion withdrawal | Oral reflexive movement | None | Without stimulation |
| UWS05 | 69 | Cardiopulmonary resuscitation | 58 | Auditory startle | None | Flexion withdrawal | Oral reflexive movement | None | Without stimulation |
| UWS06 | 39 | Respiratory failure | 73 | Auditory startle | Visual startle | Abnormal posturing | None | None | With stimulation |
| UWS07 | 47 | Cardiopulmonary resuscitation | 65 | Localization to sound | Visual startle | Flexion withdrawal | Oral reflexive movement | None | Without stimulation |
| UWS08 | 78 | Cardiopulmonary resuscitation | 39 | Auditory startle | None | None | Oral reflexive movement | None | With stimulation |
| UWS09 | 47 | Multiple ischemic infarct | 51 | Auditory startle | None | Flexion withdrawal | Oral reflexive movement | None | With stimulation |
| UWS10 | 63 | Cerebral hypoxia | 16 | Auditory startle | None | Abnormal posturing | Oral reflexive movement | None | With stimulation |
| UWS11 | 51 | Cardiopulmonary resuscitation | 30 | None | None | None | Oral reflexive movement | None | With stimulation |
| UWS12 | 50 | Ischemic brainstem infarct | 165 | Auditory startle | None | Flexion withdrawal | Oral reflexive movement | None | With stimulation |
| UWS13 | 51 | Cardiopulmonary resuscitation | - | Auditory startle | None | Flexion withdrawal | Oral reflexive movement | None | Without stimulation |
| UWS14 | 55 | Cardiopulmonary resuscitation | 121 | Auditory startle | None | None | None | None | Without stimulation |
| UWS15 | 61 | Traumatic brain injury | 116 | None | None | None | Oral reflexive movement | None | Without stimulation |
| UWS16 | 32 | Basilaris thrombosis | 922 | Auditory startle | None | Flexion withdrawal | Oral reflexive movement | None | With stimulation |
| UWS17 | 47 | Cardiopulmonary resuscitation | 3627 | Auditory startle | None | Abnormal posturing | Vocalization/oral movement | None | Without stimulation |
| UWS18 | 66 | Traumatic brain injury & subarachnoidal hemorrhage | 82 | None | Visual startle | Flexion withdrawal | None | None | Without stimulation |
| UWS19 | 36 | Cardiopulmonary resuscitation | 66 | Auditory startle | None | Abnormal posturing | Oral reflexive movement | None | Without stimulation |
| UWS20 | 26 | Traumatic brain injury | 128 | Auditory startle | None | Flexion withdrawal | Oral reflexive movement | None | Without stimulation |
| UWS21 | 55 | Cardiopulmonary resuscitation | 82 | None | Visual startle | None | Oral reflexive movement | None | With stimulation |
| UWS22 | 54 | Cardiopulmonary resuscitation | 70 | Auditory startle | Visual startle | None | None | None | With stimulation |
| UWS23 | 35 | Status epilepticus | 49 | None | None | Flexion withdrawal | Oral reflexive movement | None | Without stimulation |
| UWS24 | 74 | Cardiac arrest | 92 | Auditory startle | None | Abnormal posturing | oral reflex | None | With stimulation |
| UWS25 | 44 | Ventricular fibrillation | 8 | None | None | Abnormal posturing | None | None | With stimulation |
| UWS26 | 63 | Ischemia | 30 | Auditory startle | Visual startle | Flexion withdrawal | Vocalization | None | Without stimulation |
| UWS27 | 67 | Hemorrhage | 43 | Auditory startle | None | Flexion withdrawal | Oral reflex | None | With stimulation |
| UWS28 | 87 | Hemorrhage | 7 | None | None | Flexion withdrawal | Oral reflex | None | With stimulation |
| UWS29 | 63 | Anoxia | 1210 | Auditory startle | None | Abnormal posturing | Oral reflex | None | With stimulation |
| UWS30 | 44 | ARCA | 27 | Auditory startle | None | Abnormal posturing | None | None | Without stimulation |
| UWS31 | 41 | Anoxia | 1572 | Auditory startle | None | Abnormal posturing | Oral reflex | None | Without stimulation |
| UWS32 | 69 | Anoxia | 50 | Auditory startle | None | Flexion withdrawal | Oral reflex | None | With stimulation |
| UWS33 | 49 | ARCA & Hypoxia | 129 | Auditory startle | None | None | Oral reflex | None | Without stimulation |
| UWS34 | 36 | Anoxia | 2031 | Auditory startle | None | Abnormal posturing | Vocalization | None | Without stimulation |
MCS = minimally conscious state patients; UWS = unresponsive wakefulness syndrome patients.
The study was approved by the Ethical Committee of Salzburg (Ethics Commission Salzburg/Ethikkommission Land Salzburg; number 415-E/952). Resting state data of patients with DOC were compared to a group of 28 age-matched healthy subjects. Participants were investigated at 2 different sites (at the Department of Neurology, Christian Doppler Klinik, Paracelsus Medical University, Salzburg, and at the CHU Sart Tilman University Hospital, Liège) and with 3 different scanners (see Section 2.2. Data acquisition). Since patients with DOC are not easily investigated, experience in diagnosis is required and suitable patients are rare, it is necessary to cooperate with other diagnostic teams to get a suitable sample size. This is a common practice (e.g., Monti et al., 2010). To make sure that the different sites do not influence the effects of group differences, site was included as a covariate (see Section 2.6. Statistical analysis). After excluding 5 VS/UWS and one MCS patient due to large movement and artifacts (see Section 2.3. Data preprocessing), the patient group consisted of 34 VS/UWS (mean age = 52; age range = 26–87; 13 female) and 25 MCS (mean age = 48; age range = 28–74; 10 female) patients. All patients participating in this study were examined with the Coma Recovery Scale-Revised (CRS-R) (Giacino et al., 2004) and showed preserved auditory functioning, largely preserved brainstem reflexes, and a fairly preserved sleep-wake-cycle based on neurological examination. None of the patients were artificially ventilated or sedated at time of scanning. Additional information of the patients is listed in Inline Supplementary Table S1. The control group consisted of 28 volunteers with no history of neurological or psychiatric disease (mean age 51 years; age range 28–75 years; 15 female). Control subjects were recruited at the Paris Lodron University of Salzburg and at the University of Liège. There were no differences between healthy controls, MCS patients, and VS/UWS patients in age (F = 0.265, p = 0.768). Written informed consent was obtained from all healthy subjects and from the guardianship of all patients according to the Declaration of Helsinki.
Inline Supplementary Table S1 can be found online at http://dx.doi.org/10.1016/j.nicl.2013.12.005.
2.2. Data acquisition
Subjects were instructed to let their thoughts run free and not to think about anything special. Resting state functional MRI data were obtained using three 3 Tesla MR systems (Philips Achieva and 2 Siemens TIM TRIO). Two control subjects, 3 MCS and 11 VS/UWS patients were scanned with the Philips scanner at the Christian Doppler Klinik, Salzburg (116 T2*-weighted images were obtained with a gradient EPI in axial plane; 25 slices with 4.5 mm thickness; interslice gap = 0.5 mm; matrix size = 64 × 64; FoV = 210 mm2; TR = 2200 ms; TE = 45 ms; flip angle = 90°). Thirteen control subjects, 11 MCS and 12 VS/UWS patients were scanned with the Siemens scanner at the Christian Doppler Klinik, Salzburg (250 T2*-weighted images were obtained; 36 slices with 3 mm thickness; FoV = 192 mm2; TR = 2250 ms; TE = 30 ms; flip angle = 70°). Thirteen control subjects, 11 MCS and 11 VS/UWS patients were scanned with the Siemens scanner at the CHU Sart Tilman University Hospital, Liège (300 T2*-weighted images were obtained; 32 slices with 3 mm thickness; FoV = 192 mm2; TR = 2000 ms; TE = 30 ms; flip angle = 78°). In addition, high-resolution, T1-weighted MPRAGE sequences for anatomic information were acquired for each participant.
2.3. Data preprocessing
Functional data were preprocessed and analyzed using Statistical Parametric Mapping (version SPM8; Wellcome Department of Cognitive Neurology, London, UK; http://www.fil.ion.ucl.ac.uk/spm/). The first 6 functional scans were considered as dummy scans and were discarded. Preprocessing steps included the following procedures: segmentation of the T1-weighted image to compute the gray matter images; realignment to compensate for motion; unwarping (adjustment for movement-related artifacts); coregistration of the mean EPI to the participant's own anatomical scan; normalization to standard stereotaxic anatomical Montreal Neurological Institute (MNI) space; data were spatially smoothed using a Gaussian Kernel of 8 mm full width at half maximum (FWHM). Voxel size was resampled to 3 × 3 × 3 mm2.
To address the ongoing debate concerning motion effects on resting state fMRI data, only subjects with movement parameters smaller than 3 mm translation and 3° rotation were included in this study. Additionally, movement parameters were compared between the 3 groups using One-way ANOVA with group as a factor. There were no significant differences between groups (F < 1.85, p > 0.163). Furthermore, we included the mean change in BOLD signal as a regressor in all group analyses (see Section 2.6. Statistical analyses for details).
Preprocessed functional data were further processed with the functional connectivity toolbox v12 (Whitfield-Gabrieli and Nieto-Castanon, 2012). Sources of noise and movement-related covariates were removed (using a white matter and CSF template file created from 37 healthy subjects, as well as realignment parameter of each participant) with the CompCor method (Behzadi et al., 2007) implemented in the conn toolbox. These processing steps substantially reduce noise from non-neural sources and increase the sensitivity and reliability of functional connectivity analyses (Whitfield-Gabrieli and Nieto-Castanon, 2012). The residual BOLD time-series were band-pass filtered in a low-frequency range of 0.01 to 0.1 Hz.
2.4. Network construction
For each individual dataset, the time-series of all voxels in 110 regions (55 regions for each hemisphere) according to the Harvard–Oxford atlas was averaged and extracted. To construct correlation matrices from the extracted fMRI time-series data, the correlation coefficient for each pair of the 110 regions was computed resulting in a 110 × 110 correlation matrix for each participant using Pearson correlation. In contrast to partial correlation, Pearson correlation is gaining higher values of reproducibility (Telesford et al., 2013).
A network consists of 2 basic elements, that is, nodes and edges. In this functional brain connectivity network, nodes are represented by each of the 110 brain regions defined by the Harvard–Oxford atlas and edges are represented by the association of the BOLD signals between different regions of the brain described by the correlation matrices.
2.5. Network analysis
2.5.1. Threshold selection
For analysis of graph metrics, we applied 2 different strategies for selecting a threshold. First, we used the same correlation thresholds for all correlation matrices. This approach is useful to investigate the absolute network efficiency in different groups. Because there is still no consensus on which correlation threshold is the best to choose, we applied a wide range of different thresholds 0.1 ≤ R ≤ 0.6 with an increment of 0.05. We selected this range because values beyond 0.6 tend to divide a network into disconnected subgroups of nodes (Watts and Strogatz, 1998).
Second, we applied an individual defined threshold to each correlation matrix to ensure that graphs in both groups have the same number of edges. This was done by calculating the ratio of the number of actual connections divided by the maximum number of all possible connections. The resulting value is known as the connection density (δ) or cost of the network. The threshold was selected over a whole range of density values (0.10 ≤ δ ≤ 0.6) with an increment of 0.05. This approach allows the investigation of the relative network efficiency and makes it possible to compare groups with each other. If a correlation threshold is not normalized, it is likely that the number of edges is smaller in the patient groups, which, consequentially, would influence the comparison between groups.
Range selection was based on following criteria to address the problem of disconnectedness and networks without small-world features: (1) the average degree of all nodes in a network was larger than 2 × log(N) with N = 110 nodes in the network; (2) the small-worldness (σ) at each threshold was larger than 1.1 for all subjects (Fig. 1). This procedure certifies that all thresholded networks have small-world properties and only very few false connections (Zhang et al., 2011).
Fig. 1.
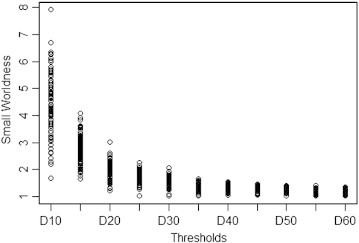
Small worldness for all participants in the density range 0.1 to 0.6.
Small worldness (σ) at each threshold was larger than 1.1 for all subjects to ensure that networks at all thresholds show small-world properties.
2.5.2. Network metrics
For each threshold, we calculated global and local network metrics using the brain connectivity toolbox (Rubinov and Sporns, 2010). Global metrics were chosen as followed: characteristic path length (L); global efficiency (Eglob); modularity (M). Additionally, the following local metrics were investigated: degree (k); clustering coefficient (C); local efficiency (Eloc);
Metrics were compared with the corresponding values (e.g., Crand; Lrand) obtained and averaged from 50 random networks with the same number of nodes and degree distribution to evaluate the network for small-world properties (Maslov and Sneppen, 2002). Note that only 50 random networks were computed due to computational reasons.
The characteristic path length describes the number of edges between one node and any other node in a network and quantifies the effectiveness of information transfer throughout the whole network. Note that here we used weighted matrices since binarization does not provide any information on important differences between weak and strong connections. In the case of the characteristic path length, this may falsify the topological characterization of long-distance shortcuts since the strength, that is, the functional distance of connectivity is not taken into account. Global efficiency is another measure of effectiveness of information transfer (it is inversely related to the characteristic path length but has some advantages when computing disconnected graphs). The modularity is the strength of division of a network in clusters. Networks with high modularity have dense connections between nodes within clusters but sparse connections between nodes of different clusters. A high modularity minimizes the cost of a network. The degree describes the number of edges one node is connected with and gives information on how functionally connected a node is. The clustering coefficient is a measure of degree to which nodes in a graph are forming a cluster. The local efficiency is computed on node neighborhoods and is related to the clustering coefficient reflecting the efficiency of parallel information transfer, robustness, and fault tolerance of a network.
Small-world networks are characterized by a high normalized clustering coefficient averaged over the whole brain (γ), that is, (C/Crand) > 1, and low normalized characteristic path length (λ), that is, (L/Lrand) ~ 1. The ratio of the normalized and averaged clustering coefficient (γ) to the normalized characteristic path length (λ) is the so-called small-worldness (σ) which has also been assessed.
For each metric of the δ-thresholded data, we calculated the area under the curve (AUC) to compare the metrics over the whole range of thresholds between groups. This approach ensures a topological characterization of brain networks independent of the single threshold and has been successfully applied in previous studies (Achard and Bullmore, 2007; Wang et al., 2009; Zhang et al., 2011).
2.6. Statistical analysis
Values of all metric parameters were computed with Matlab (MATLAB 7.6.0, The MathWorks Inc., Natick, MA, 2008). To search for significant group differences between controls and patients in the AUC values of each metric, we performed ANCOVA with the factor group at 3 levels using permutation tests with the package lmPerm implemented in R (The R Project for Statistical Computing, URL http://www.R-project.org/.). Additionally, the correlation values of all 110 anatomical regions resulting in 5995 pairs were compared between groups to search for significant differences in connectivity. Estimations of statistical significance were based on 100,000 permutations. Iterations were terminated when the estimated standard deviation of the estimated proportion p was less than p ∗ 0.1. Iterations continued until all sources and coefficients met this criterion or until 100,000 permutations were reached (Anscombe, 1953). This procedure reduces the uncertainty near p = 0.05 to +/− 0.1% and addresses the problem of multiple comparisons (i.e., number of regions and/or number of measures).
Because participants were scanned with 3 different MR scanners and at 2 different sites, we included scanner as a covariate. Moreover, the root mean squared change in BOLD signal from volume to volume (DVARS) was assessed (Power et al., 2011) using FSL (Jenkinson et al., 2002) and implemented in the group analysis as a covariate to account for possible motion effects.
For all group analyses, additional post-hoc testing was performed with Tukey honest significant differences based on the adjusted p-values of the permutation testing. This test is essentially a t-test except that it corrects for experiment-wise error rate when the factor has more than 2 levels.
The significance level of all adjusted p-values was set to p < 1/N, that is, N = number of regions investigated. Note that because of this additional correction, we expect less than one false-positive per comparison.
To examine the relationship between cognitive functioning and network efficiency, we correlated the score of the Coma Recovery Scale-Revised (CRS-R) for each patient with the AUC values of degree, clustering coefficient, and local efficiency of 28 regions within the fronto-parietal network and the thalamus using Matlab (MATLAB 7.6.0, The MathWorks Inc., Natick, MA, 2008). Results of the correlation analysis were corrected for multiple comparisons using a false discovery rate (FDR).
3. Results
3.1. Analyses of absolute small world metrics
Networks were investigated for absolute small world properties at different correlation thresholds (0.1 ≤ R ≤ 0.6). The functional brain networks showed a higher clustering coefficient compared to random networks (γ > 1) and a linear increase with the increase of threshold in all 3 groups. The characteristic path length, on the contrary, was very similar compared to random networks (λ ~ 1) and showed similar values for the whole threshold range. In line with the higher γ values, small-worldness is present with σ > 1 and increases with correlation threshold. Please note that small world metrics at higher threshold levels are difficult to interpret because the higher the threshold value the lower the number of edges of the nodes which questions the meaningfulness of connectivity.
As expected, healthy subjects as well as both DOC groups demonstrated an efficient and economic small-world topology with a high density of connections between nearest neighbors and a low average path length. This is in line with previous studies of functional brain networks demonstrating that complex systems have small world properties (Achard and Bullmore, 2007; Bullmore and Bassett, 2011; Bullmore and Sporns, 2009; Salvador et al., 2005).
3.2. Alterations in global metrics
To compare metrics of the three groups (controls, MCS, and VS/UWS), a relative threshold was applied to control for the number of edges in a graph. Significance level was corrected for multiple comparisons.
In the global network topology, groups differed significantly in modularity (p < 0.001). The posthoc tests showed that the MCS group (p < 0.001) as well as the VS/UWS group (p = 0.001) showed a significant reduced modularity compared to the control group (Fig. 2).
Fig. 2.
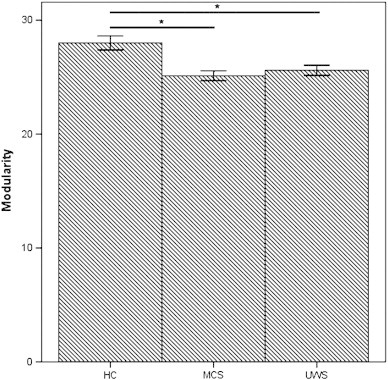
Global group differences in network metrics.
A. Significant reduced modularity for MCS and VS/UWS compared to controls; B. Significant reduced averaged clustering coefficient for MCS and VS/UWS compared to controls; HC = healthy control group; MCS = minimally conscious state; and UWS = unresponsive wakefulness syndrome.
The other global metrics (characteristic path length and global efficiency) did not differ at a corrected threshold level (p ≥ 0.354).
3.3. Alterations in regional metrics
At the nodal level, there are several regions of the fronto-parietal network that differ in their topological characteristics between groups (see Fig. 3). All presented results of the permutation tests are significant at p < 1/N to additionally correct for false positives.
Fig. 3.
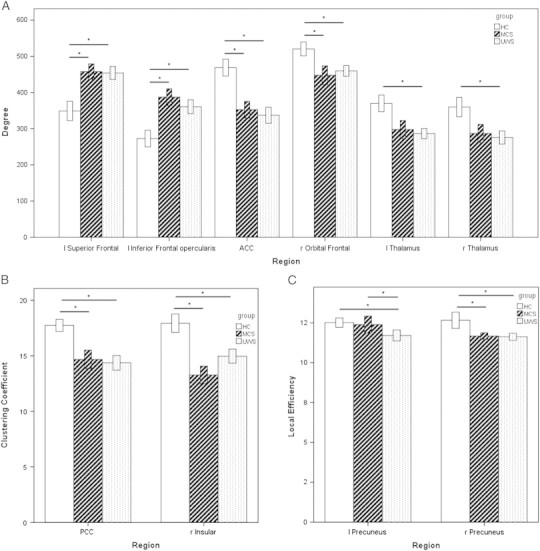
Regional group differences in network metrics of the fronto-parietal network and the thalamus.
A. Significant differences in the degree of functional connectivity for MCS and VS/UWS compared to controls; B. Significant reduced clustering coefficient for MCS and VS/UWS compared to controls; C. Significant reduced local efficiency for MCS and VS/UWS compared to MCS and for VS/UWS compared to MCS; HC = healthy control group; MCS = minimally conscious state; and UWS = unresponsive wakefulness syndrome.
The degree showed differences in several frontal regions (Fig. 3A) with a higher degree for patients in left frontal regions including the left superior frontal gyrus (controls vs. MCS: p = 0.009; controls vs. VS/UWS: p = 0.009), and the pars opercularis of the left inferior frontal gyrus (controls vs. MCS: p < 0.001; controls vs. VS/UWS: p = 0.009), as well as a lower degree in medial frontal regions such as the anterior cingulate cortex (ACC) (controls vs. MCS: p = 0.009; controls vs. VS/UWS: p = 0.009), and the frontal orbital cortex (controls vs. MCS: p = 0.009; controls vs. VS/UWS: p = 0.009). Furthermore, the degree was decreased for VS/UWS in the left (p = 0.009) and right thalamus (p = 0.009).
The clustering coefficient was reduced in patients in the posterior cingulate cortex (PCC) (controls vs. MCS: p = 0.004; controls vs. VS/UWS: p = 0.008), as well as in the right insular cortex (controls vs. MCS: p < 0.001; controls vs. VS/UWS: p = 0.009) (Fig. 3B).
The local efficiency differed in the precuneus between all 3 groups (controls vs. MCS: p = 0.004; controls vs. VS/UWS: p = 0.004; MCS vs. VS/UWS: p = 0.009) (Fig. 3C).
For additional information on differences of network metrics in other regions see Table 1.
Table 1.
Significant differences in regional network metrics between groups.
| HC vs. MCS | HC vs. VS/UWS | MCS vs. VS/UWS | |
|---|---|---|---|
| Degree | |||
| Anterior cingulate cortex | * | * | |
| Left superior frontal gyrus | * | * | |
| Left inferior frontal gyrus, pars opercularis | * | * | |
| Left supramarginal gyrus, anterior division | * | ||
| Right orbital frontal cortex | * | * | |
| Right temporal pole | * | * | |
| Right superior temporal gyrus, anterior division | * | ||
| Right superior temporal gyrus, posterior division | * | ||
| Right fusiform temporal gyrus, anterior division | * | ||
| Right planum polare | * | ||
| Left thalamus | * | ||
| Right thalamus | * | ||
| Clustering coefficient | |||
| Posterior cingulate cortex | * | * | |
| Right insular cortex | * | * | |
| Right parahippocampal gyrus, posterior division | * | * | |
| Left superior temporal gyrus, anterior division | * | ||
| Right superior temporal gyrus, anterior division | * | ||
| Left inferior temporal gyrus, temporooccipital part | * | ||
| Left central operculum cortex | * | * | |
| Right central operculum cortex | * | * | |
| Left parietal operculum cortex | * | * | |
| Left planum polare | * | ||
| Right planum polare | * | ||
| Right Heschl's gyrus | * | * | |
| Right planum temporale | * | * | |
| Local efficiency | |||
| left precuneus | * | * | |
| Right precuneus | * | * | |
| Left angular gyrus | * | ||
| Left intracalcarine cortex | * | ||
| Left planum polare | * | ||
| Right postcentral | * | ||
| Right parahippocampal gyrus, posterior division | * | * | |
| Left putamen | * | ||
*Significant differences for the area under the curve (AUC), p < .05 corrected for multiple comparisons; HC = healthy control group; MCS = minimally conscious state; and VS/UWS = unresponsive wakefulness syndrome.
3.4. Alterations in connectivity
Of the 5995 pairs of anatomical regions, functional connectivity differed in 455 pairs significantly between healthy controls and MCS, in 510 pairs between control subjects and VS/UWS, and in 31 pairs between MCS and VS/UWS.
In the fronto-parietal network, we found numerous impaired connections in patients (see Inline Supplementary Table S2). Significant reduced connectivity for both patient groups compared to healthy controls could be identified specifically for medial frontal regions with medial parietal regions and with the left and right thalamus, as well as with the left and right temporal parietal junction (TPJ) and with the right frontal gyrus. Connectivity in patients between medial frontal and left frontal regions was greater compared to healthy subjects (except for the frontal pole). Reduced connectivity was also identified within medial frontal regions, whereas connectivity was enhanced in patients between the frontal pole and the orbital frontal cortex. Medial parietal regions showed also reduced connectivity for both patient groups within and with the left and right thalamus, as well as with the left and right TPJ, the right frontal cortex, and within medial parietal regions. Connectivity between medial parietal regions and the left frontal gyrus was greater for both patient groups. Reduced connectivity was also significant for both hemispheres of the frontal cortex with the left and right thalamus, between both hemispheres of the frontal cortex, between both hemispheres of the TPJ, and between the lateral frontal cortex with the lateral TPJ. Within the right frontal cortex, reduced connectivity was only significant for the VS/UWS group. Connectivity between the left and right thalamus was only significantly reduced for the MCS group.
Inline Supplementary Table S2.
Table S2.
Differences in functional connectivity between groups within the fronto-parietal network and the thalamus.
| ROI | ROI | HC vs. MCS | HC vs. VS/UWS | MCS vs. VS/UWS |
|---|---|---|---|---|
| Medial frontal to medial parietal | ||||
| Right Frontal Pole | Right Posterior Cingulate Cortex | HC > VS/UWS | ||
| Left Medial Frontal Cortex | Left Posterior Cingulate Cortex | HC > MCS | HC > VS/UWS | |
| Left Medial Frontal Cortex | Right Posterior Cingulate Cortex | HC > MCS | HC > VS/UWS | |
| Right Medial Frontal Cortex | Left Posterior Cingulate Cortex | HC > MCS | HC > VS/UWS | |
| Right Medial Frontal Cortex | Right Posterior Cingulate Cortex | HC > VS/UWS | ||
| Left Medial Frontal Cortex | Left Precuneus | HC > MCS | ||
| Left Medial Frontal Cortex | Right Precuneus | HC > MCS | ||
| Right Medial Frontal Cortex | Left Precuneus | HC > MCS | HC > VS/UWS | |
| Left Anterior Cingulate Cortex | Left Posterior Cingulate Cortex | HC > MCS | HC > VS/UWS | |
| Left Anterior Cingulate Cortex | Right Posterior Cingulate Cortex | HC > MCS | HC > VS/UWS | |
| Right Anterior Cingulate Cortex | Left Posterior Cingulate Cortex | HC > MCS | HC > VS/UWS | |
| Right Anterior Cingulate Cortex | Right Posterior Cingulate Cortex | HC > MCS | HC > VS/UWS | |
| Left Anterior Cingulate Cortex | Left Precuneus | HC > MCS | ||
| Left Anterior Cingulate Cortex | Right Precuneus | HC > MCS | ||
| Right Anterior Cingulate Cortex | Right Precuneus | HC > MCS | ||
| Left Supplementary Motor Areal | Left Posterior Cingulate Cortex | HC > VS/UWS | ||
| Left Supplementary Motor Areal | Left Precuneus | HC > VS/UWS | ||
| Right Supplementary Motor Areal | Left Precuneus | HC > MCS | HC > VS/UWS | |
| Right Supplementary Motor Areal | Right Precuneus | HC > MCS | ||
| Within medial frontal | ||||
| Left Frontal Pole | Right Orbital Frontal Cortex | HC < MCS | ||
| Right Frontal Pole | Right Orbital Frontal Cortex | HC < MCS | HC < VS/UWS | |
| Right Frontal Pole | Right Anterior Cingulate Cortex | HC > VS/UWS | ||
| Left Medial Frontal | Left Supplementary Motor Areal | HC > MCS | ||
| Right Medial Frontal | Left Supplementary Motor Areal | HC > MCS | ||
| Right Medial Frontal | Right Supplementary Motor Areal | HC > MCS | ||
| Left Orbital Frontal Cortex | Right Orbital Frontal Cortex | HC > MCS | ||
| Right Orbital Frontal Cortex | Left Anterior Cingulate Cortex | HC > MCS | HC > VS/UWS | |
| Right Orbital Frontal Cortex | Right Anterior Cingulate Cortex | HC > MCS | HC > VS/UWS | |
| Medial frontal to left frontal | ||||
| Left Frontal Pole | Left Superior Frontal Gyrus | HC > VS/UWS | MCS > VS/UWS | |
| Left Frontal Pole | Left Middle Frontal Gyrus | HC > VS/UWS | ||
| Right Medial Frontal Cortex | Left Frontal Operculum | HC < VS/UWS | ||
| Left Orbital Frontal Cortex | Right Inferior Frontal Gyrus, pars triangularis | HC > MCS | HC > VS/UWS | |
| Left Anterior Cingulate Cortex | Left Inferior Frontal Gyrus, pars opercularis | HC < MCS | ||
| Right Anterior Cingulate Cortex | Left Inferior Frontal Gyrus, pars opercularis | HC < MCS | ||
| Right Supplementary Motor Areal | Left Middle Frontal Gyrus | HC < MCS | ||
| Medial frontal to right frontal | ||||
| Right Frontal Pole | Right Superior Frontal Gyrus | HC > VS/UWS | MCS > VS/UWS | |
| Right Frontal Pole | Right Middle Frontal Gyrus | HC > MCS | HC > VS/UWS | |
| Right Orbital Frontal Cortex | Right Middle Frontal Gyrus | HC > VS/UWS | ||
| Right Orbital Frontal Cortex | Right Inferior Frontal Gyrus, pars triangularis | HC > MCS | ||
| Left Anterior Cingulate Cortex | Right Inferior Frontal Gyrus, pars triangularis | HC > MCS | HC > VS/UWS | |
| Left Anterior Cingulate Cortex | Right Frontal Operculum | HC > MCS | HC > VS/UWS | |
| Left Supplementary Motor Areal | Right Frontal Operculum | HC > MCS | HC > VS/UWS | |
| Right Supplementary Motor Areal | Right Frontal Operculum | HC > MCS | HC > VS/UWS | |
| Medial frontal to temporo-parietal junction | ||||
| Left Frontal Pole | Left Angular Gyrus | HC > VS/UWS | ||
| Right Frontal Pole | Right Angular Gyrus | HC > MCS | HC > VS/UWS | |
| Left Anterior Cingulate Cortex | Left Angular Gyrus | HC > VS/UWS | ||
| Left Anterior Cingulate Cortex | Right Angular Gyrus | HC > MCS | HC > VS/UWS | |
| Right Anterior Cingulate Cortex | Right Angular Gyrus | HC > MCS | HC > VS/UWS | |
| Medial frontal to thalamus | ||||
| Left frontal Pole | Right Thalamus | HC > VS/UWS | ||
| Right frontal Pole | Right Thalamus | HC > VS/UWS | ||
| Left Medial Frontal Cortex | Right Thalamus | HC > MCS | HC > VS/UWS | |
| Right Medial Frontal Cortex | Left Thalamus | HC > MCS | HC > VS/UWS | |
| Right Medial Frontal Cortex | Right Thalamus | HC > MCS | ||
| Left Orbital Frontal Cortex | Left Thalamus | HC > MCS | ||
| Right Orbital Frontal Cortex | Left Thalamus | HC > MCS | HC > VS/UWS | |
| Right Orbital Frontal Cortex | Right Thalamus | HC > VS/UWS | ||
| Medial frontal to insular | ||||
| Left Anterior Cingulate Cortex | Right Insular Cortex | HC > MCS | ||
| Right Anterior Cingulate Cortex | Right Insular Cortex | HC > MCS | ||
| Left Supplementary Motor Areal | Left Insular Cortex | HC > MCS | HC > VS/UWS | |
| Left Supplementary Motor Areal | Right Insular Cortex | HC > MCS | HC > VS/UWS | |
| Right Supplementary Motor Areal | Left Insular Cortex | HC > MCS | HC > VS/UWS | |
| Right Supplementary Motor Areal | Right Insular Cortex | HC > MCS | HC > VS/UWS | |
| Within medial posterior | ||||
| Left Posterior Cingulate Cortex | Left Precuneus | HC > VS/UWS | ||
| Left Posterior Cingulate Cortex | Right Precuneus | HC > MCS | ||
| Right Posterior Cingulate Cortex | Left Precuneus | HC > MCS | HC > VS/UWS | |
| Right Posterior Cingulate Cortex | Right Precuneus | HC > MCS | HC > VS/UWS | |
| Left Posterior Cingulate Cortex | Right Posterior Cingulate Cortex | HC > MCS | HC > VS/UWS | |
| Left Precuneus | Right Precuneus | HC > MCS | ||
| Medial posterior to left frontal | ||||
| Right Posterior Cingulate Cortex | Left Inferior Frontal Gyrus, pars triangularis | HC < MCS | ||
| Right Posterior Cingulate Cortex | Left Inferior Frontal Gyrus, pars opercularis | HC < MCS | ||
| Left Precuneus | Left Inferior Frontal Gyrus, pars triangularis | HC < MCS | ||
| Right Precuneus | Left Inferior Frontal Gyrus, pars triangularis | HC < MCS | HC < VS/UWS | |
| Left Precuneus | Left Inferior Frontal Gyrus, pars opercularis | HC < MCS | ||
| Right Precuneus | Left Inferior Frontal Gyrus, pars opercularis | HC < MCS | HC < VS/UWS | |
| Medial posterior to temporo-parietal junction | ||||
| Left Posterior Cingulate Cortex | Left Angular Gyrus | HC > VS/UWS | ||
| Left Posterior Cingulate Cortex | Right Angular Gyrus | HC > MCS | ||
| Right Posterior Cingulate Cortex | Right Angular Gyrus | HC > VS/UWS | ||
| Medial posterior to thalamus | ||||
| Left Posterior Cingulate Cortex | Right Thalamus | HC > MCS | HC > VS/UWS | |
| Right Posterior Cingulate Cortex | Left Thalamus | HC > VS/UWS | ||
| Left Precuneus | Left Thalamus | HC > MCS | HC > VS/UWS | |
| Left Precuneus | Right Thalamus | HC > MCS | HC > VS/UWS | |
| Right Precuneus | Left Thalamus | HC > VS/UWS | ||
| Right Precuneus | Right Thalamus | HC > MCS | HC > VS/UWS | |
| Between lateral frontal | ||||
| Left Inferior Frontal Gyrus, pars triangularis | Right Inferior Frontal Gyrus, pars triangularis | HC > MCS | HC > VS/UWS | |
| Left Inferior Frontal Gyrus, pars triangularis | Right Inferior Frontal Gyrus, pars opercularis | HC > VS/UWS | ||
| Left Inferior Frontal Gyrus, pars opercularis | Right Inferior Frontal Gyrus, pars triangularis | HC > MCS | HC > VS/UWS | |
| Left Inferior Frontal Gyrus, pars opercularis | Right Inferior Frontal Gyrus, pars opercularis | HC > MCS | ||
| Left frontal to temporo-parietal junction | ||||
| Left Superior Frontal Gyrus | Left Angular Gyrus | HC > VS/UWS | ||
| Left Middle Frontal Gyrus | Left Angular Gyrus | HC > VS/UWS | ||
| Within right frontal | ||||
| Right Superior Frontal Gyrus | Right Inferior Frontal Gyrus, pars triangularis | HC > VS/UWS | ||
| Right frontal to temporo-parietal junction | ||||
| Right Superior Frontal Gyrus | Right Angular Gyrus | HC > VS/UWS | ||
| Right Middle Frontal Gyrus | Right Angular Gyrus | HC > MCS | HC > VS/UWS | |
| Right frontal to insular | ||||
| Right Middle Frontal Gyrus | Left Insular Cortex | HC > VS/UWS | ||
| Right Frontal Operculum | Left Insular Cortex | HC > MCS | HC > VS/UWS | |
| Right Frontal Operculum | Right Insular Cortex | HC > VS/UWS | ||
| Left temporo-parietal junction to right temporo-parietal junction | ||||
| Left Angular Gyrus | Right Angular Gyrus | HC > MCS | HC > VS/UWS | |
| Within thalamus | ||||
| Left Thalamus | Right Thalamus | HC > MCS | ||
| Within insular | ||||
| Left Insular Cortex | Right Insular Cortex | HC > MCS | HC > VS/UWS | |
p < .05 corrected for multiple comparisons; HC = healthy control group; MCS = minimally conscious state; UWS = unresponsive wakefulness syndrome.
In the fronto-parietal network, we found numerous impaired connections in patients (see Inline Supplementary Table S2). Significant reduced connectivity for both patient groups compared to healthy controls could be identified specifically for medial frontal regions with medial parietal regions and with the left and right thalamus, as well as with the left and right temporal parietal junction (TPJ) and with the right frontal gyrus. Connectivity in patients between medial frontal and left frontal regions was greater compared to healthy subjects (except for the frontal pole). Reduced connectivity was also identified within medial frontal regions, whereas connectivity was enhanced in patients between the frontal pole and the orbital frontal cortex. Medial parietal regions showed also reduced connectivity for both patient groups within and with the left and right thalamus, as well as with the left and right TPJ, the right frontal cortex, and within medial parietal regions. Connectivity between medial parietal regions and the left frontal gyrus was greater for both patient groups. Reduced connectivity was also significant for both hemispheres of the frontal cortex with the left and right thalamus, between both hemispheres of the frontal cortex, between both hemispheres of the TPJ, and between the lateral frontal cortex with the lateral TPJ. Within the right frontal cortex, reduced connectivity was only significant for the VS/UWS group. Connectivity between the left and right thalamus was only significantly reduced for the MCS group.
VS/UWS compared to MCS showed significant reduced connectivity between medial frontal regions and lateral frontal cortex.
Inline Supplementary Table S2 can be found online at http://dx.doi.org/10.1016/j.nicl.2013.12.005.
3.5. Relation between network topology and cognitive functioning
The CRS-R score correlated significantly with the AUC value of the clustering coefficient in the right middle frontal gyrus (r = 0.40, p = 0.006) and in the frontal pole (r = 0.29, p = 0.048). p-Values are corrected for FDR.
4. Discussion
To our knowledge, this study is the first to investigate brain network properties in patients with DOC using graph-theoretical approaches. Our findings are threefold: (i) we identified alterations between healthy controls and patients at the global level; (ii) regions in the fronto-parietal network and the thalamus differed in their network properties between healthy subjects and patients while only the precuneus showed significant differences between patient groups in its local efficiency of information transfer; and (iii) behavioral responsiveness in patients was related to the clustering coefficient in frontal regions.
At the global brain level, both patient groups showed lower modularity than the control group. Patients exhibited a network less well decomposed into non-overlapping sets of nodes in a way that should minimize the number of between-set edges and maximize the number of within-set edges. This finding suggests a disturbance in the optimal balance between segregation and integration in patients with DOC. Such a balance is essential for the high level of functioning of human brain networks (Honey and Sporns, 2008). Interferences between segregation and integration may lead to deficits and fluctuations in cognitive functioning which can be observed in patients with DOC.
This is contrary to a previous investigation of network properties in 17 comatose patients which found no differences in global metrics compared to healthy subjects, neither in modularity nor in any other global metric (Achard et al., 2012), even though findings on a finer-grade level indicate that the organization of the comatose brain is indeed radically affected. However, they examined patients in deep coma within days of brain injury. In contrast, the patients of this study all evolved from coma to different states of consciousness with severe impairments extensively examined with standardized tests differentiating at low levels of consciousness and scanned after weeks following injury. Moreover, their preprocessing and network construction differed from ours in several ways which makes the results perhaps difficult to compare. For instance, a much smaller interval was used for filtering which has been shown to reduce the reliability of findings (Braun et al., 2012), and the time-series was extracted from 417 regions instead of 110.
However, there were no differences between the MCS group and the VS/UWS group in any of the global metrics. Thus, it is not clear if the alterations in modularity are related to consciousness per se. This could be due to the fact that fundamental lesions of the brain are present in both patient groups and may have led to a comparable reduction of connection density and reorganization throughout the brain. As Achard et al. (2012) have already speculated, the topological organization required for consciousness especially at such a low level might be too complex to be sufficient reflected by global properties.
At the nodal level, the network properties of regions within the fronto-parietal network were altered in both patient groups compared to healthy controls. Patients demonstrated a reduced embeddedness of the PCC and reduced local efficiency of the precuneus compared to controls. Alterations in frontal regions revealed a more complex pattern: In left frontal regions, patients with DOC showed increased interaction with other regions. In medial frontal regions like the ACC and the orbitofrontal cortex, the interaction was decreased. The resulting pattern of alterations exposes deficits in the centrality of medial frontal regions with a shift of increased functional connectivity towards left frontal regions especially for subjects with minimal conscious responses.
Most of the affected regions are part of the so-called ‘rich club’ defined as a set of highly central nodes that are more densely interconnected than other nodes such as medial parietal regions, the superior frontal gyrus, the ACC, the orbitofrontal cortex, the insular, and the thalamus (van den Heuvel and Sporns, 2011). Nodes belonging to the rich club act as a strongly interlinked entity and are suggested to play a central role in overall brain communication with connections tending to span long distances enabling highly efficient integration. Thus, impairments in the functional connectivity of these regions reflect dysfunctions in overall information integration and have impact on many different cognitive functions. It may be interesting to note that there is some overlap between regions of the rich club and the default mode network (Greicius et al., 2003; Raichle and Snyder, 2007) which is altered in impaired consciousness and patients with traumatic brain injury (for a review see Boly et al., 2008; Bonnelle et al., 2011; Cauda et al., 2009; Crone et al., 2011; Fernandez-Espejo et al., 2010; Horovitz et al., 2009; Sharp et al., 2011; Soddu et al., 2011). van den Heuvel and Sporns (2011) hypothesize that the rich club serves as a functional link between different resting state networks including the default mode network.
In the thalamus, deficits in the degree of interaction were significantly present in VS/UWS which are, by definition, not consciously aware. In MCS patients, who show at least minimal signs of conscious behavior, the topology did not differ from healthy controls significantly. Contrary to what was expected though, the network properties of the thalamus showed no significant differences between patients in MCS and with VS/UWS. Thus, it is not clear if deficits are related to impaired consciousness per se or to abnormalities caused by severe brain lesions.
The thalamus plays a key role in theories of neural correlation of consciousness (Edelman, 2003; Tononi and Edelman, 1998; Ward, 2011). It is highly reciprocal connected with cortical areas, especially with frontal regions, and is also a region belonging to the rich club. This makes it a perfect candidate for integrating information computed by cortical areas, and therefore, being an important part of the network involved in generating conscious awareness. One hypothesis is that consciousness is generated by a dynamic pattern of information binding within spatial distributed but highly connected neural networks which has its origin in the thalamo-cortical circuits emphasizing the critical role of thalamic connectivity (Edelman, 2003). Previous studies investigating impaired consciousness provide evidence for a crucial involvement of the thalamus (Adams et al., 2000; Alkire et al., 2000; Fernandez-Espejo et al., 2011; Schiff and Fins, 2007; Zhou et al., 2011). However, most of the studies did not compare unconscious patients with minimal conscious patients directly. Merely in respect to the structural organization, a difference between MCS and VS/UWS in thalamic regions has been demonstrated (Fernandez-Espejo et al., 2011).
Furthermore, previous studies investigating functional connectivity have been conducted in relative small samples of patients. In contrast, this work has examined the data of 59 patients with DOC.
One of the most interesting findings of this study is that only the precuneus differed significantly between VS/UWS and MCS patients in its efficiency of local information transfer. This is in line with a previous investigation of functional connectivity in DOC indicating that only medial parietal regions are particularly sensitive to differences in functional connectivity between MCS and VS/UWS (Vanhaudenhuyse et al., 2010). The efficient embeddedness of this area seems to hold a key position in impaired consciousness and is sensitive to different levels of consciousness in severe brain injury. This finding supports the postulation of Laureys and Schiff (2012) that medial parietal regions are closely associated with recovery of consciousness. Nevertheless, alterations of medial parietal regions do not seem to be specific for impaired consciousness since they have been identified in a vast amount of different studies (for an overview see Cavanna and Trimble, 2006).
Another important finding is that behavioral responsiveness (CRS-R scores) was associated with network properties only in frontal regions. The grade of behavioral responsiveness of a patient was related to measures of segregation in medial frontal regions and the right middle frontal gyrus. In other words, the less responsive and conscious a patient is, the less embedded medial and right frontal regions.
The results concerning functional connectivity complement the picture, showing reduction in connectivity between almost all regions of the fronto-parietal network in DOC patients. Within frontal regions, VS/UWS demonstrated reduced connectivity compared to MCS.
Note that there were also significant differences between groups in some regions which are not part of the fronto-parietal network (see Table 1), particularly in temporal regions comprising the planum temporale, Heschl's gyrus, and planum polare, as well as in the parahippocampal gyrus and parts of the operculum cortex. Differences between both patient groups were also evident in the local efficiency of the left planum polare involved in auditory processing and the right parahippocampal gyrus involved in scene recognition.
Interestingly, only measures of segregation differentiated between minimal conscious and unconscious patients, while the influence of central hubs did not differ between patients and was affected only in frontal regions and the thalamus. One interpretation of this pattern of findings may be that the integration function especially of frontal and thalamic regions is not related to lower levels of consciousness but rather necessary for intact conscious awareness. In contrast, segregation of main regions within the fronto-parietal network seems to play a primary role in recovery of consciousness. However, this interpretation remains a matter of speculation and further investigations are needed specifically controlling for severe brain injury.
Recently, the confounding effects of small movement artifacts concerning functional connectivity measures have come into focus (Power et al., 2011, 2012; Satterthwaite et al., 2012; Van Dijk et al., 2012). We did not use scrubbing to remove motion artifacts since this reduces length and at least 4–5 min are needed for the correlation values to stabilize throughout intrinsic connectivity networks (Van Dijk et al., 2010). Moreover, a shorter duration, that is, fewer time points reduce reliability of findings (Braun et al., 2012). Instead, we excluded the whole dataset of every subject showing movement above a defined threshold. We also applied standard procedures for movement correction and ensured that movement parameters did not differ between groups. Finally, the root mean squared change in BOLD signal from volume to volume (DVARS) was calculated (as described in Power et al., 2011) and included in all group analyses as a regressor. Note that we did not include the global signal in confound regression because of the effects on resting state connectivity (Murphy et al., 2009; Telesford et al., 2013; Weissenbacher et al., 2009).
In patients with severe head injury, processing of fMRI images is a critical issue. Numerous studies demonstrated that spatial normalization methods for group analyses have several shortcomings when performed in patients with focal or wide-spread brain lesions (e.g., Andersen et al., 2010; Ashburner and Friston, 2005; Crinion et al., 2007). The same is true when using parcellation templates for dividing the brain into distinct regions. Especially in this cohort, in which etiology and effects of brain lesions are quite heterogeneous, image processing is very difficult and prone to distorted outcome. We have carefully examined each processing step by visual inspection to avoid falsifications (see Supplementary Fig. 1 for examples on segmentation in patients). Yet, these concerns should be kept in mind when considering group differences.
Supplementary Fig. 1.
Examples of segmentation in patients with disorder of consciousness.
Examples of segmentation into gray (red) and white matter (blue) in patients in minimal consciousness state (MCS) and with unresponsive wakefulness syndrome (UWS).
In patients with severe head injury, processing of fMRI images is a critical issue. Numerous studies demonstrated that spatial normalization methods for group analyses have several shortcomings when performed in patients with focal or wide-spread brain lesions (e.g., Andersen et al., 2010; Ashburner and Friston, 2005; Crinion et al., 2007). The same is true when using parcellation templates for dividing the brain into distinct regions. Especially in this cohort, in which etiology and effects of brain lesions are quite heterogeneous, image processing is very difficult and prone to distorted outcome. We have carefully examined each processing step by visual inspection to avoid falsifications (see Supplementary Fig. 1 for examples on segmentation in patients). Yet, these concerns should be kept in mind when considering group differences.
A methodological limitation when investigating small world properties using graph theoretical approaches is the application of thresholds. There are several possibilities for selecting thresholds, each with their own advantages and disadvantages.
For example, the problem with global thresholding is that it can lead to disconnected graphs. Comparing subjects and groups is problematic if the network of one subject at a given threshold is connected and the network of another is disconnected. We addressed the problem of disconnectedness by certifying that all thresholded networks have small-world properties and only few unconnected nodes. Further, we not only investigated the clustering coefficient and characteristic path length but also local and global efficiency which offers methodological advantages in terms of disconnectedness (Achard and Bullmore, 2007). While the local efficiency includes disconnected nodes with a value of zero, the clustering coefficient removes them from the analysis.
Another problematic issue is the wide range of threshold selection. Until now there has been no consensus on which threshold is the best to apply. Thus, each study uses different ranges of thresholds and results depend on the threshold range selected (Braun et al., 2012).
Furthermore, several preprocessing steps have essential impact on network construction and metrics, for example, range of band-pass filtering, length of time-series, global regression, or type of randomization (Braun et al., 2012; Telesford et al., 2013; Zalesky et al., 2012).
This is a problem that has not yet been solved to a satisfying extent and which should be subject of future studies using graph theoretical approaches for network analyses. In this study, we have implemented approaches that have been successfully applied in previous studies investigating brain network topology (e.g., Achard and Bullmore, 2007; Alexander-Bloch et al., 2010; Zhang et al., 2011).
4.1. Conclusion
Taken together, the present investigation in 59 patients with DOC after recovering from coma demonstrated decreased global modularity and alterations in network properties and connectivity of several regions within fronto-parietal and cortico-thalamic circuits. This pattern of findings suggests alterations in the influence of frontal and thalamic regions and in the segregation function of regions within the fronto-parietal network in patients with DOC.
A direct link to the level of consciousness, that is, differences between minimal conscious and unconscious patients or a correlation with behavioral responsiveness, was only identified for measures of segregation in the precuneus and in medial and right frontal regions. Moreover, the influence of the thalamus seemed to be significantly reduced only in patients without conscious responses.
Most regions altered in DOC belong to the so-called rich club of highly interconnected central nodes. Alteration in their functional connectivity may reflect serious impact on the overall information integration of the brain. This altered interaction has far-reaching consequences associated with impairment of cognitive functioning possibly culminating in a total breakdown of conscious awareness.
Nevertheless, more research is required to further disentangle the mechanisms of interaction between distinct regions of the brain to understand impairments of consciousness in patients with severe brain injury. Particular difficulties related to the population investigated and to the method applied which are discussed in detail in this work should be addressed in future studies.
The following is the supplementary data related to this article.
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.nicl.2013.12.005.
Acknowledgements
This work was supported by the Jubiläumsfonds of the National Bank of Austria [grant number 14201]; and the Scientific Funds of the Paracelsus Medical University [grant number E-10/12/062-KRO].
We want to thank Fabio Richlan and Benjamin Gagl for their review of the manuscript, and Joe Miller for proof reading.
The authors declare no competing financial interests.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-No Derivative Works License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
References
- Achard S., Bullmore E. Efficiency and cost of economical brain functional networks. PLoS Comput. Biol. 2007;3:e17. doi: 10.1371/journal.pcbi.0030017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achard S., Salvador R., Whitcher B., Suckling J., Bullmore E. A resilient, low-frequency, small-world human brain functional network with highly connected association cortical hubs. J. Neurosci. 2006;26:63–72. doi: 10.1523/JNEUROSCI.3874-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achard S., Delon-Martin C., Vertes P.E., Renard F., Schenck M., Schneider F., Heinrich C., Kremer S., Bullmore E.T. Hubs of brain functional networks are radically reorganized in comatose patients. Proc. Natl. Acad. Sci. U. S. A. 2012;109(50):20608–20613. doi: 10.1073/pnas.1208933109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams J.H., Graham D.I., Jennett B. The neuropathology of the vegetative state after an acute brain insult. Brain. 2000;123(Pt 7):1327–1338. doi: 10.1093/brain/123.7.1327. [DOI] [PubMed] [Google Scholar]
- Alexander-Bloch A.F., Gogtay N., Meunier D., Birn R., Clasen L., Lalonde F., Lenroot R., Giedd J., Bullmore E.T. Disrupted modularity and local connectivity of brain functional networks in childhood-onset schizophrenia. Front. Syst. Neurosci. 2010;4:147. doi: 10.3389/fnsys.2010.00147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander-Bloch A., Lambiotte R., Roberts B., Giedd J., Gogtay N., Bullmore E. The discovery of population differences in network community structure: new methods and applications to brain functional networks in schizophrenia. Neuroimage. 2012;59:3889–3900. doi: 10.1016/j.neuroimage.2011.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkire M.T., Haier R.J., Fallon J.H. Toward a unified theory of narcosis: brain imaging evidence for a thalamocortical switch as the neurophysiologic basis of anesthetic-induced unconsciousness. Conscious. Cogn. 2000;9:370–386. doi: 10.1006/ccog.1999.0423. [DOI] [PubMed] [Google Scholar]
- Andersen S.M., Rapcsak S.Z., Beeson P.M. Cost function masking during normalization of brains with focal lesions: still a necessity? Neuroimage. 2010;53:78–84. doi: 10.1016/j.neuroimage.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anscombe F.J. Sequential estimation. J. R. Stat. Soc. B. 1953;15:1–29. [Google Scholar]
- Ashburner J., Friston K.J. Unified segmentation. Neuroimage. 2005;26:839–851. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Behzadi Y., Restom K., Liau J., Liu T.T. A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. Neuroimage. 2007;37:90–101. doi: 10.1016/j.neuroimage.2007.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boly M., Faymonville M.E., Peigneux P., Lambermont B., Damas P., Del F.G., Degueldre C., Franck G., Luxen A., Lamy M., Moonen G., Maquet P., Laureys S. Auditory processing in severely brain injured patients: differences between the minimally conscious state and the persistent vegetative state. Arch. Neurol. 2004;61:233–238. doi: 10.1001/archneur.61.2.233. [DOI] [PubMed] [Google Scholar]
- Boly M., Phillips C., Tshibanda L., Vanhaudenhuyse A., Schabus M., Dang-Vu T.T., Moonen G., Hustinx R., Maquet P., Laureys S. Intrinsic brain activity in altered states of consciousness: how conscious is the default mode of brain function? Ann. N. Y. Acad. Sci. 2008;1129:119–129. doi: 10.1196/annals.1417.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boly M., Tshibanda L., Vanhaudenhuyse A., Noirhomme Q., Schnakers C., Ledoux D., Boveroux P., Garweg C., Lambermont B., Phillips C., Luxen A., Moonen G., Bassetti C., Maquet P., Laureys S. Functional connectivity in the default network during resting state is preserved in a vegetative but not in a brain dead patient. Hum. Brain Mapp. 2009;30:2393–2400. doi: 10.1002/hbm.20672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnelle V., Leech R., Kinnunen K.M., Ham T.E., Beckmann C.F., De Boissezon X., Greenwood R.J., Sharp D.J. Default mode network connectivity predicts sustained attention deficits after traumatic brain injury. J. Neurosci. 2011;31:13442–13451. doi: 10.1523/JNEUROSCI.1163-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun U., Plichta M.M., Esslinger C., Sauer C., Haddad L., Grimm O., Mier D., Mohnke S., Heinz A., Erk S., Walter H., Seiferth N., Kirsch P., Meyer-Lindenberg A. Test–retest reliability of resting-state connectivity network characteristics using fMRI and graph theoretical measures. Neuroimage. 2012;59:1404–1412. doi: 10.1016/j.neuroimage.2011.08.044. [DOI] [PubMed] [Google Scholar]
- Bullmore E.T., Bassett D.S. Brain graphs: graphical models of the human brain connectome. Annu. Rev. Clin. Psychol. 2011;7:113–140. doi: 10.1146/annurev-clinpsy-040510-143934. [DOI] [PubMed] [Google Scholar]
- Bullmore E., Sporns O. Complex brain networks: graph theoretical analysis of structural and functional systems. Nat. Rev. Neurosci. 2009;10:186–198. doi: 10.1038/nrn2575. [DOI] [PubMed] [Google Scholar]
- Cauda F., Micon B.M., Sacco K., Duca S., D'Agata F., Geminiani G., Canavero S. Disrupted intrinsic functional connectivity in the vegetative state. J. Neurol. Neurosurg. Psychiatry. 2009;80:429–431. doi: 10.1136/jnnp.2007.142349. [DOI] [PubMed] [Google Scholar]
- Cavanna A.E., Trimble M.R. The precuneus: a review of its functional anatomy and behavioural correlates. Brain. 2006;129:564–583. doi: 10.1093/brain/awl004. [DOI] [PubMed] [Google Scholar]
- Chen Z.J., He Y., Rosa-Neto P., Germann J., Evans A.C. Revealing modular architecture of human brain structural networks by using cortical thickness from MRI. Cereb. Cortex. 2008;18:2374–2381. doi: 10.1093/cercor/bhn003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crinion J., Ashburner J., Leff A., Brett M., Price C., Friston K. Spatial normalization of lesioned brains: performance evaluation and impact on fMRI analyses. Neuroimage. 2007;37:866–875. doi: 10.1016/j.neuroimage.2007.04.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crone J.S., Ladurner G., Holler Y., Golaszewski S., Trinka E., Kronbichler M. Deactivation of the default mode network as a marker of impaired consciousness: an fMRI study. PLoS One. 2011;6:e26373. doi: 10.1371/journal.pone.0026373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crone J.S., Holler Y., Bergmann J., Golaszewski S., Trinka E., Kronbichler M. Self-related processing and deactivation of cortical midline regions in disorders of consciousness. Front. Hum. Neurosci. 2013;7:504. doi: 10.3389/fnhum.2013.00504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelman G.M. Naturalizing consciousness: a theoretical framework. Proc. Natl. Acad. Sci. U. S. A. 2003;100:5520–5524. doi: 10.1073/pnas.0931349100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Espejo D., Junque C., Cruse D., Bernabeu M., Roig-Rovira T., Fabregas N., Rivas E., Mercader J.M. Combination of diffusion tensor and functional magnetic resonance imaging during recovery from the vegetative state. BMC Neurol. 2010;10:77. doi: 10.1186/1471-2377-10-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Espejo D., Bekinschtein T., Monti M.M., Pickard J.D., Junque C., Coleman M.R., Owen A.M. Diffusion weighted imaging distinguishes the vegetative state from the minimally conscious state. Neuroimage. 2011;54:103–112. doi: 10.1016/j.neuroimage.2010.08.035. [DOI] [PubMed] [Google Scholar]
- Fernandez-Espejo D., Soddu A., Cruse D., Palacios E.M., Junque C., Vanhaudenhuyse A., Rivas E., Newcombe V., Menon D.K., Pickard J.D., Laureys S., Owen A.M. A role for the default mode network in the bases of disorders of consciousness. Ann. Neurol. 2012;72:335–343. doi: 10.1002/ana.23635. [DOI] [PubMed] [Google Scholar]
- Giacino J.T., Kalmar K., Whyte J. The JFK Coma Recovery Scale-Revised: measurement characteristics and diagnostic utility. Arch. Phys. Med. Rehabil. 2004;85:2020–2029. doi: 10.1016/j.apmr.2004.02.033. [DOI] [PubMed] [Google Scholar]
- Greicius M.D., Krasnow B., Reiss A.L., Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc. Natl. Acad. Sci. U. S. A. 2003;100:253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guldenmund P., Demertzi A., Boveroux P., Boly M., Vanhaudenhuyse A., Bruno M.A., Gosseries O., Noirhomme Q., Brichant J.F., Bonhomme V., Laureys S., Soddu A. Thalamus, brainstem and salience network connectivity changes during propofol-induced sedation and unconsciousness. Brain Connect. 2013;3:273–285. doi: 10.1089/brain.2012.0117. [DOI] [PubMed] [Google Scholar]
- He B.J., Snyder A.Z., Vincent J.L., Epstein A., Shulman G.L., Corbetta M. Breakdown of functional connectivity in frontoparietal networks underlies behavioral deficits in spatial neglect. Neuron. 2007;53:905–918. doi: 10.1016/j.neuron.2007.02.013. [DOI] [PubMed] [Google Scholar]
- Honey C.J., Sporns O. Dynamical consequences of lesions in cortical networks. Hum. Brain Mapp. 2008;29:802–809. doi: 10.1002/hbm.20579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horovitz S.G., Braun A.R., Carr W.S., Picchioni D., Balkin T.J., Fukunaga M., Duyn J.H. Decoupling of the brain's default mode network during deep sleep. Proc. Natl. Acad. Sci. U. S. A. 2009;106:11376–11381. doi: 10.1073/pnas.0901435106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M., Bannister P., Brady M., Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17:825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Laureys S., Schiff N.D. Coma and consciousness: paradigms (re)framed by neuroimaging. Neuroimage. 2012;61:478–491. doi: 10.1016/j.neuroimage.2011.12.041. [DOI] [PubMed] [Google Scholar]
- Laureys S., Faymonville M.E., Luxen A., Lamy M., Franck G., Maquet P. Restoration of thalamocortical connectivity after recovery from persistent vegetative state. Lancet. 2000;355:1790–1791. doi: 10.1016/s0140-6736(00)02271-6. [DOI] [PubMed] [Google Scholar]
- Laureys S., Faymonville M.E., Peigneux P., Damas P., Lambermont B., Del F.G., Degueldre C., Aerts J., Luxen A., Franck G., Lamy M., Moonen G., Maquet P. Cortical processing of noxious somatosensory stimuli in the persistent vegetative state. Neuroimage. 2002;17:732–741. [PubMed] [Google Scholar]
- Lull N., Noe E., Lull J.J., Garcia-Panach J., Chirivella J., Ferri J., Lopez-Aznar D., Sopena P., Robles M. Voxel-based statistical analysis of thalamic glucose metabolism in traumatic brain injury: relationship with consciousness and cognition. Brain Inj. 2010;24:1098–1107. doi: 10.3109/02699052.2010.494592. [DOI] [PubMed] [Google Scholar]
- Maslov S., Sneppen K. Specificity and stability in topology of protein networks. Science. 2002;296:910–913. doi: 10.1126/science.1065103. [DOI] [PubMed] [Google Scholar]
- Monti M.M., Vanhaudenhuyse A., Coleman M.R., Boly M., Pickard J.D., Tshibanda L., Owen A.M., Laureys S. Willful modulation of brain activity in disorders of consciousness. N. Engl. J. Med. 2010;362:579–589. doi: 10.1056/NEJMoa0905370. [DOI] [PubMed] [Google Scholar]
- Murphy K., Birn R.M., Handwerker D.A., Jones T.B., Bandettini P.A. The impact of global signal regression on resting state correlations: are anti-correlated networks introduced? Neuroimage. 2009;44:893–905. doi: 10.1016/j.neuroimage.2008.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama N., Okumura A., Shinoda J., Nakashima T., Iwama T. Relationship between regional cerebral metabolism and consciousness disturbance in traumatic diffuse brain injury without large focal lesions: an FDG-PET study with statistical parametric mapping analysis. J. Neurol. Neurosurg. Psychiatry. 2006;77:856–862. doi: 10.1136/jnnp.2005.080523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen A.M., Coleman M.R., Boly M., Davis M.H., Laureys S., Pickard J.D. Detecting awareness in the vegetative state. Science. 2006;313:1402. doi: 10.1126/science.1130197. [DOI] [PubMed] [Google Scholar]
- Power J.D., Cohen A.L., Nelson S.M., Wig G.S., Barnes K.A., Church J.A., Vogel A.C., Laumann T.O., Miezin F.M., Schlaggar B.L., Petersen S.E. Functional network organization of the human brain. Neuron. 2011;72:665–678. doi: 10.1016/j.neuron.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power J.D., Barnes K.A., Snyder A.Z., Schlaggar B.L., Petersen S.E. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 2012;59:2142–2154. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle M.E., Snyder A.Z. A default mode of brain function: a brief history of an evolving idea. Neuroimage. 2007;37:1083–1090. doi: 10.1016/j.neuroimage.2007.02.041. [DOI] [PubMed] [Google Scholar]
- Rubinov M., Sporns O. Complex network measures of brain connectivity: uses and interpretations. Neuroimage. 2010;52:1059–1069. doi: 10.1016/j.neuroimage.2009.10.003. [DOI] [PubMed] [Google Scholar]
- Salvador R., Suckling J., Coleman M.R., Pickard J.D., Menon D., Bullmore E. Neurophysiological architecture of functional magnetic resonance images of human brain. Cereb. Cortex. 2005;15:1332–1342. doi: 10.1093/cercor/bhi016. [DOI] [PubMed] [Google Scholar]
- Satterthwaite T.D., Wolf D.H., Loughead J., Ruparel K., Elliott M.A., Hakonarson H., Gur R.C., Gur R.E. Impact of in-scanner head motion on multiple measures of functional connectivity: relevance for studies of neurodevelopment in youth. Neuroimage. 2012;60:623–632. doi: 10.1016/j.neuroimage.2011.12.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiff N.D., Fins J.J. Deep brain stimulation and cognition: moving from animal to patient. Curr. Opin. Neurol. 2007;20:638–642. doi: 10.1097/WCO.0b013e3282f1c6e4. [DOI] [PubMed] [Google Scholar]
- Sharp D.J., Beckmann C.F., Greenwood R., Kinnunen K.M., Bonnelle V., De Boissezon X., Powell J.H., Counsell S.J., Patel M.C., Leech R. Default mode network functional and structural connectivity after traumatic brain injury. Brain. 2011;134:2233–2247. doi: 10.1093/brain/awr175. [DOI] [PubMed] [Google Scholar]
- Soddu A., Vanhaudenhuyse A., Demertzi A., Bruno M.A., Tshibanda L., Di H., Melanie B., Papa M., Laureys S., Noirhomme Q. Resting state activity in patients with disorders of consciousness. Funct. Neurol. 2011;26:37–43. [PMC free article] [PubMed] [Google Scholar]
- Soddu A., Vanhaudenhuyse A., Bahri M.A., Bruno M.A., Boly M., Demertzi A., Tshibanda J.F., Phillips C., Stanziano M., Ovadia-Caro S., Nir Y., Maquet P., Papa M., Malach R., Laureys S., Noirhomme Q. Identifying the default-mode component in spatial IC analyses of patients with disorders of consciousness. Hum. Brain Mapp. 2012;33:778–796. doi: 10.1002/hbm.21249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stam C.J., Jones B.F., Nolte G., Breakspear M., Scheltens P. Small-world networks and functional connectivity in Alzheimer's disease. Cereb. Cortex. 2007;17:92–99. doi: 10.1093/cercor/bhj127. [DOI] [PubMed] [Google Scholar]
- Supekar K., Menon V., Rubin D., Musen M., Greicius M.D. Network analysis of intrinsic functional brain connectivity in Alzheimer's disease. PLoS Comput. Biol. 2008;4:e1000100. doi: 10.1371/journal.pcbi.1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telesford Q.K., Burdette J.H., Laurienti P.J. An exploration of graph metric reproducibility in complex brain networks. Front. Neurosci. 2013;7:67. doi: 10.3389/fnins.2013.00067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tononi G., Edelman G.M. Consciousness and complexity. Science. 1998;282:1846–1851. doi: 10.1126/science.282.5395.1846. [DOI] [PubMed] [Google Scholar]
- van den Heuvel M.P., Sporns O. Rich-club organization of the human connectome. J. Neurosci. 2011;31:15775–15786. doi: 10.1523/JNEUROSCI.3539-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dijk K.R., Hedden T., Venkataraman A., Evans K.C., Lazar S.W., Buckner R.L. Intrinsic functional connectivity as a tool for human connectomics: theory, properties, and optimization. J. Neurophysiol. 2010;103:297–321. doi: 10.1152/jn.00783.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dijk K.R., Sabuncu M.R., Buckner R.L. The influence of head motion on intrinsic functional connectivity MRI. Neuroimage. 2012;59:431–438. doi: 10.1016/j.neuroimage.2011.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanhaudenhuyse A., Noirhomme Q., Tshibanda L.J., Bruno M.A., Boveroux P., Schnakers C., Soddu A., Perlbarg V., Ledoux D., Brichant J.F., Moonen G., Maquet P., Greicius M.D., Laureys S., Boly M. Default network connectivity reflects the level of consciousness in non-communicative brain-damaged patients. Brain. 2010;133:161–171. doi: 10.1093/brain/awp313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Wang L., Zang Y., Yang H., Tang H., Gong Q., Chen Z., Zhu C., He Y. Parcellation-dependent small-world brain functional networks: a resting-state fMRI study. Hum. Brain Mapp. 2009;30:1511–1523. doi: 10.1002/hbm.20623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward L.M. The thalamic dynamic core theory of conscious experience. Conscious. Cogn. 2011;20:464–486. doi: 10.1016/j.concog.2011.01.007. [DOI] [PubMed] [Google Scholar]
- Watts D.J., Strogatz S.H. Collective dynamics of ‘small-world’ networks. Nature. 1998;393:440–442. doi: 10.1038/30918. [DOI] [PubMed] [Google Scholar]
- Weissenbacher A., Kasess C., Gerstl F., Lanzenberger R., Moser E., Windischberger C. Correlations and anticorrelations in resting-state functional connectivity MRI: a quantitative comparison of preprocessing strategies. Neuroimage. 2009;47:1408–1416. doi: 10.1016/j.neuroimage.2009.05.005. [DOI] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S., Nieto-Castanon A. Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect. 2012;2:125–141. doi: 10.1089/brain.2012.0073. [DOI] [PubMed] [Google Scholar]
- Xie G., Deschamps A., Backman S.B., Fiset P., Chartrand D., Dagher A., Plourde G. Critical involvement of the thalamus and precuneus during restoration of consciousness with physostigmine in humans during propofol anaesthesia: a positron emission tomography study. Br. J. Anaesth. 2011;106:548–557. doi: 10.1093/bja/aeq415. [DOI] [PubMed] [Google Scholar]
- Zalesky A., Fornito A., Bullmore E. On the use of correlation as a measure of network connectivity. Neuroimage. 2012;60:2096–2106. doi: 10.1016/j.neuroimage.2012.02.001. [DOI] [PubMed] [Google Scholar]
- Zhang J., Wang J., Wu Q., Kuang W., Huang X., He Y., Gong Q. Disrupted brain connectivity networks in drug-naive, first-episode major depressive disorder. Biol. Psychiatry. 2011;70:334–342. doi: 10.1016/j.biopsych.2011.05.018. [DOI] [PubMed] [Google Scholar]
- Zhou J., Liu X., Song W., Yang Y., Zhao Z., Ling F., Hudetz A.G., Li S.J. Specific and nonspecific thalamocortical functional connectivity in normal and vegetative states. Conscious. Cogn. 2011;20:257–268. doi: 10.1016/j.concog.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


