Abstract
Germline mutations of the MEN1 gene cause multiple endocrine neoplasia type 1 (MEN1), an autosomal dominant disorder characterized by tumors of the parathyroids, the pancreas, and the anterior pituitary. Paraganglioma (PGL) is a rare endocrine tumor, which can be sporadic or genetically determined. To date, PGL has never been reported as a feature of MEN1.
We report here a patient presenting three features of MEN1 syndrome (hyperparathyroidism, pancreatic neuroendocrine tumor, and adrenocortical adenoma) associated with PGL. Genetic analysis of MEN1 gene revealed a new missense mutation in exon 5 (AGG→AAG), causing the substitution of arginine by lysine at codon 275. Screening for other genetic disorders (SDHx, TMEM127, MAX, CDKN1B) causing PGL was negative. Immunohistochemical analyses showed normal levels of succinate dehydrogenase (SDH)A and SDHB in the PGL. The proband's sister, bearing the mutation, had primary hyperparathyroidism. It was the first typical MEN1 syndrome reported with an extra-adrenal PGL.
Keywords: multiple endocrine neoplasia type 1, MEN1, germline mutation, paraganglioma
Introduction
Multiple endocrine neoplasia type 1 (MEN1) is an autosomal dominant disease, with penetrance reaching 100% with age. It is characterized by hyperplastic and neoplastic lesions arising principally from the parathyroid, anterior pituitary, and endocrine pancreas. On locus 11q13, the causative MEN1 gene consists of 10 exons and encodes a 610-amino-acid protein called menin.1 Menin interacts with a wide number of proteins involved in transcriptional regulation, genome stability, cell division, and proliferation.2 Germline mutations of the MEN1 gene along with the loss of heterozygosity (LOH) result in menin structural changes and subsequent loss of tumor-suppressing function.3 As for many other tumor suppressor genes, there is no genotype–phenotype correlation in MEN1. More than 20 different other endocrine and non-endocrine tumors may occur in the course of the disease.
Pheochromocytoma (PHEO) and paraganglioma (PGL) are rare neuroendocrine tumors arising in the adrenal medulla or the sympathetic or parasympathetic ganglia. Heredity accounts for about 35% of such tumors. The occurrence of PHEO and PGL is well characterized within Von Hippel-Lindau disease, multiple endocrine neoplasia type 2 (MEN2), and neurofibromatosis type 1. Recently, mutations in the genes encoding subunits of the succinate dehydrogenase (SDH), that is, SDHA, SDHB, SDHC, and SDHD genes, have been shown to determine the hereditary PGL-PHEO syndromes. These mutations concern 85% of familial aggregations of PHEO and PGL. Several other genes have been demonstrated to be predisposing for PHEO/PGL (ie TMEM127, MAX, SDHAF2, and HIF2A).4, 5 Also, the Carney-Stratakis syndrome associates PGL and gastrointestinal stromal tumors and is linked with SDHs gene mutations.6 Another new syndrome, which associates pituitary adenoma and PHEO or PGL, has been speculated by Xekouki et al7 and could be linked with germline mutations of SDHs genes.
We report here a patient bearing a typical MEN1 syndrome with an extra-adrenal PGL. The new germline mutation p.Arg275Lys of the MEN1 gene was identified in the proband and in the affected sister, bearing a hyperparathyroidism.
Patients and methods
Patients
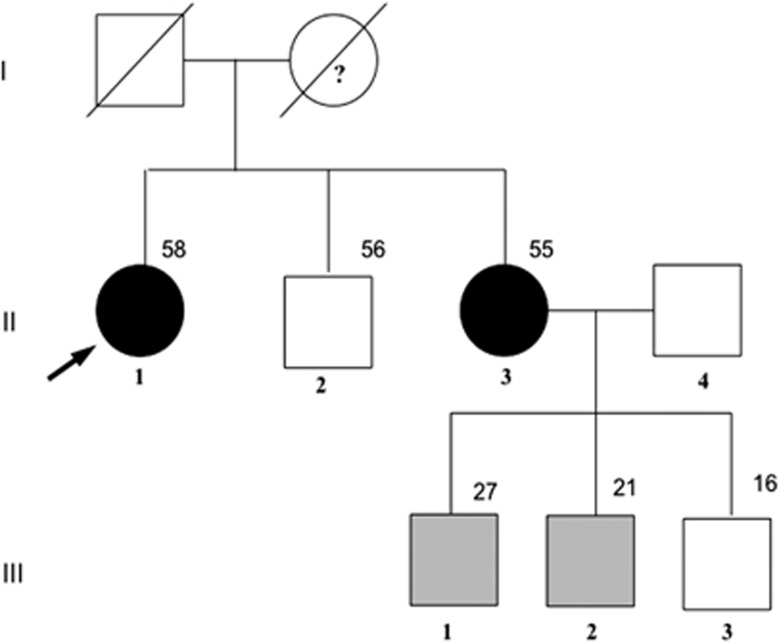
The proband was a 58-year-old woman (Figure 1, II-1) referred to us for a ‘dropped head syndrome' owing to electromyography-confirmed muscle fatigability. She was diagnosed with a jugulotympanic PGL at the age of 50 and the tumor was surgically removed. She had a mild mental retardation. On clinical examination, centripetal obesity (body mass index=33 kg/m2), moon face, lower limb edema, and sweating were observed. She had a high blood pressure (190/100 mm Hg) despite two antihypertensive drugs. Routine laboratory findings were normal except increased level of serum calcium (Ca, 2.86 mmol/l). There was no polycythemia. Further analyses revealed primary hyperparathyroidism (PTH, 147 pg/ml) and ACTH-independent Cushing syndrome. Serum chromogranin A, gastrin, insulin, somatostatin, glucagon, 5-hydroxyindoleacetic acid, calcitonin, androgens, and adrenocortical precursors were normal. Computed tomography disclosed a 35-mm tumor of the right adrenocortical gland and a second tumor located in the tail of the pancreas. Images were consistent with endoscopic ultrasound findings and somatostatin receptor imaging with Indium 111 (OctreoScan, Mallinckrodt Medical, Petten, The Netherlands). Parathyroid glands, right adrenocortical gland and the distal portion of the pancreas were surgically removed. Histological analyses revealed a diffuse hyperplasia of the four parathyroid glands, a benign adenoma of the adrenal cortex, and a well-differentiated pancreatic endocrine tumor associated with disseminated microadenomas. On microscopic examination, the pancreatic adenoma cells were arranged in cords and pseudoacini, with regular nuclei, rare mitoses, and rich endocrine vascularization. Neighboring Langerhans islets were hyperplastic, and multiple microadenomas with the same morphology as the adenoma were observed. Immunohistochemical analysis revealed that the tumor was positive for synaptophysin, chromogranin, heterogeneous for glucagon, and negative for N-Cam. Ki-67-positive fraction was estimated to be <1% of tumoral cells. Two months after surgery, the patient completely recovered from the ‘dropped head syndrome' and biological markers returned to normal. Her sister (Figure 1, II-3) was 55 years old, and had a history of surged salivary gland tumor without available histological data. She had no complaint. However, laboratory and imaging tests revealed isolated PTH and enlargement of parathyroid glands. She underwent surgery and recovered. At the time of submitting this article, the latest news from her revealed multiple neuroendocrine tumors within the pancreas (OctreoScan). The proband's mother had a history of complete thyroidectomy but the reason could not be established. Individuals II-2 and III-1, 2, and 3 were asymptomatic and standard laboratory tests were normal.
Figure 1.
Pedigree of the kindred. The arrow indicates the proband. Squares represent male family members, circles female family members, and slashes deceased family members; dark symbols denote symptomatic carriers of the MEN1 gene mutation, gray asymptomatic carriers, and blank non-carriers. Note: members I-1 and I-2 were not tested; the question mark represents a clinical presumption for the presence of the mutation.
Sequencing analysis of genomic DNA
To examine the MEN1 gene alterations in this family, blood samples were drawn from the affected (Figure 1, II-1 and 3) and asymptomatic members (Figure 1, II-2 and III-1, 2, and 3) after written informed consent had been obtained. Genomic DNA from leukocytes was extracted and the coding exons and exon-intron boundaries of the MEN1 gene (NM_003977.2) were PCR-amplified and screened by direct sequencing.8 Moreover, sequencing of the SDHA, SDHAF2, SDHB, SDHC, and SDHD genes (NM_004168.2, NM-017841.1, NM_003000.2, NM_003001.3, NM_003002.2), TMEM127 (NM_017849.1), MAX (NM_002382.3) and CDKN1B gene (NM_004064) were performed on the proband's DNA. The potential effect of the missense variant on menin protein was evaluated in silico using a battery of tools: Polyphen2 (http//:www.genetics.bwh.harvard.edu), UMD predictor,9 and Alamut 2.2.0 software (Interactive Biosoftware, Rouen, France).
Immunohistochemistry
Immunohistochemistry was performed on the PGL using a standard procedure. Briefly, slides were deparaffinized, rehydrated and antigen retrieval was performed by boiling the slides in Tris-EDTA buffer pH 9 for 45 min, followed by incubation in 3% H2O2 for 20 min. Incubation with anti-SDHB (HPA002868, Sigma-Aldrich, Saint-Quentin Fallavier, France, 1:250) and anti-SDHA (Abcam, ab14715, Paris, France 1:1000) was performed at room temperature for 1 h, and was followed by incubation with biotinylated secondary antibody (Vector Laboratories, ABCYS, Les Ulis, France 1:400) and avidin–biotin–peroxidase complex (VectastainABC Elite; Vector Laboratories, ABCYS, Les Ulis, France). Revelation was achieved using Histogreen (ABCYS) as a chromogen.
Results and discussion
This patient presented an undoubted MEN1 syndrome according to the Stockholm and Gubbio criteria,10 including hyperparathyroidism, a pancreatic endocrine tumor, and a benign adrenocortical adenoma. Moreover, the hyperplasia of the four parathyroid glands found in the proband and her sister were highly suggestive of the MEN1 history. In this context, the presence of a PGL was extremely unexpected.
Sequencing of the MEN1 gene of the proband revealed a new heterozygous missense mutation with transition from AGG to AAG at codon 275 of exon 5, causing an amino-acid change from arginine to lysine, p.Arg275Lys. The same substitution was identified in the affected proband's sister (Figure 1, II-3) and 2/3 of her asymptomatic sons (Figure 1, III-1 and 2). Patients II-2 and III-3 did not carry the mutation. This variant was never described, including in the paper from Lemos et al11 reporting 1336 mutations of MEN1. It is localized on a highly conserved amino acid in the menin domain interacting with Smad3, NM23H1, NMHCII-A, FANCD2, HDAC1, and is not reported in the ESV database (Exome Variant Server from Exome Sequencing Project) and the dbSNP database (NCBI's Variant database). Although the in silico analysis only showed a moderate likelihood of a deleterious effect (Polyphen, 0.2 (range 0–1), UMD predictor, 59 (range 0–100)), considering the clinical MEN1 history of this family, we classified this variant as probably damaging.
The younger sons (III-1 and 2), 27 and 21 years old, respectively, were diagnosed as asymptomatic carriers. Indeed, the penetrance of MEN1 for 20- and 25-year-old mutation carriers is estimated around 52% and 78%, respectively.12 Genetic testing permitted to enable a strict follow-up for these two patients and to avoid periodic screening of non-carriers.
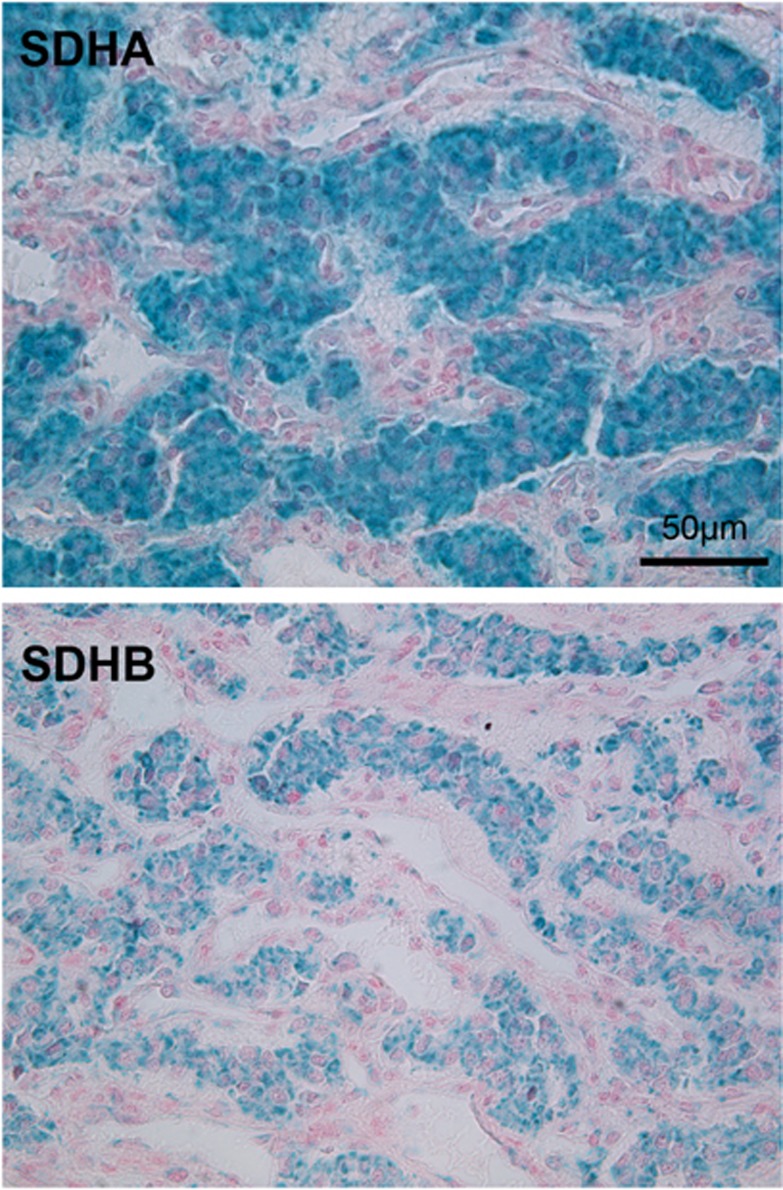
The occurrence of an extra-adrenal PGL has never been reported during the course of genetically confirmed MEN1, although it was described in association with pituitary tumors.4, 7 Conversely, MEN1 gene mutation has never been identified while investigating familial or sporadic PGL etiology. In the literature, <10 cases (<1%) of PHEO have been reported in the course of MEN1.4 However, the LOH on chromosome 11q might be critical for the development of PGL or PHEO.13 As PGL is more likely to occur in SDHs gene-related syndromes, we investigated SDHA, SDHB, SDHC, SDHD, and SDHAF2 gene sequences and found no mutation. We evaluated the expression of SDHA and SDHB in the PGL tissue. Both proteins were present at normal levels (Figure 2), thus confirming the absence of any SDHx gene mutation in this tumor.14
Figure 2.
SDHA and SDHB immunohistochemistry in the patient's PGL. Normal SDHA and SDHB mitochondrial staining was observed in tumor cells. Scale bar=50 μm.
Testing for TMEM127 and MAX mutations were also negative. HIF2A analysis on PGL tissue was not performed because of preservation conditions and lack of polycythemia. Finally, we excluded the recently reported MEN4 syndrome, which shares features of MEN1 and MEN2, by sequencing the CDKN1B gene that was not mutated.15
In conclusion, we report here the first association of an extra-adrenal PGL with an authentic MEN1 syndrome revealing a new missense mutation of MEN1 gene, p.Arg275Lys. Unfortunately, the quality of PGL tissue did not allow searching a LOH 11q13 to confirm that this MEN1 mutation is really involved in the PGL of the patient. Other cases of MEN1 syndrome with PGL are required to know whether PGL might be considered or not as a ‘MEN1-associated tumor'.16
Acknowledgments
This work was supported by the Association pour le Développement des Recherches Biologiques et Médicales au Centre Hospitalier Régional de Marseille (ADEREM), Oncogenetic Network of the French Ministry of Health, CNRS.
The authors declare no conflict of interest.
References
- Larsson C, Skogseid B, Oberg K, Nakamura Y, Nordenskjöld M. Multiple endocrine neoplasia type 1 gene maps to chromosome 11 and is lost in insulinoma. Nature. 1988;332:85–87. doi: 10.1038/332085a0. [DOI] [PubMed] [Google Scholar]
- Agarwal SK, Kester MB, Debelenko LV, et al. Germline mutations of the MEN1 gene in familial multiple endocrine neoplasia type 1 and related states. Hum Mol Genet. 1997;6:1169–1175. doi: 10.1093/hmg/6.7.1169. [DOI] [PubMed] [Google Scholar]
- Knudson AG, Jr, Strong LC, Anderson DE. Heredity and cancer in man. Prog Med Genet. 1973;9:113–158. [PubMed] [Google Scholar]
- Welander J, Söderkvist P, Gimm O. Genetics and clinical characteristics of hereditary pheochromocytomas and paragangliomas. Endocr Relat Cancer. 2011;18:R253–R276. doi: 10.1530/ERC-11-0170. [DOI] [PubMed] [Google Scholar]
- Gimenez-Roqueplo AP, Dahia PL, Robledo M. An update on the genetics of paraganglioma, pheochromocytoma, and associated hereditary syndromes. Horm Metab Res. 2012;44:328–333. doi: 10.1055/s-0031-1301302. [DOI] [PubMed] [Google Scholar]
- Carney JA, Stratakis CA. Familial paraganglioma and gastric stromal sarcoma: a new syndrome distinct from the Carney triad. Am J Med Genet. 2002;108:132–139. doi: 10.1002/ajmg.10235. [DOI] [PubMed] [Google Scholar]
- Xekouki P, Stratakis CA. Succinate dehydrogenase (SDHx) mutations in pituitary tumors: could this be a new role for mitochondrial complex II and/or Krebs cycle defects. Endocr Relat Cancer. 2012;19:C33–C40. doi: 10.1530/ERC-12-0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu S, Tsukada T, Futami H, et al. Germline mutations of the MEN1 gene in Japanese kindred with multiple endocrine neoplasia type 1. Jpn J Cancer Res. 1997;88:1029–1032. doi: 10.1111/j.1349-7006.1997.tb00325.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frédéric MY, Lalande M, Boileau C, et al. UMD-predictor, a new prediction tool for nucleotide substitution pathogenicity–application to four genes: FBN1, FBN2, TGFBR1, and TGFBR2. Hum Mutat. 2009;30:952–959. doi: 10.1002/humu.20970. [DOI] [PubMed] [Google Scholar]
- Brandi ML, Gagel RF, Angeli A, et al. Guidelines for diagnosis and therapy of MEN type 1 and type 2. J Clin Endocrinol Metab. 2001;86:5658–5671. doi: 10.1210/jcem.86.12.8070. [DOI] [PubMed] [Google Scholar]
- Lemos MC, Thakker RV. Multiple endocrine neoplasia type 1 (MEN1): analysis of 1336 mutations reported in the first decade following identification of the gene. Hum Mutat. 2008;29:22–32. doi: 10.1002/humu.20605. [DOI] [PubMed] [Google Scholar]
- Bassett JH, Forbes SA, Pannett AA, et al. Characterization of mutations in patients with multiple endocrine neoplasia type 1. Am J Hum Genet. 1998;62:232–244. doi: 10.1086/301729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun HY, Cui B, Su DW, et al. LOH on chromosome 11q, but not SDHD and Men1 mutations was frequently detectable in Chinese patients with pheochromocytoma and paraganglioma. Endocrine. 2006;30:307–312. doi: 10.1007/s12020-006-0009-0. [DOI] [PubMed] [Google Scholar]
- Korpershoek E, Favier J, Gaal J, et al. SDHA immunohistochemistry detects SDHA gene mutations in paragangliomas and pheochromocytomas. J Clin Endocrinol Metab. 2011;96:1472–1476. doi: 10.1210/jc.2011-1043. [DOI] [PubMed] [Google Scholar]
- Pellegata NS, Quintanilla-Martinez L, Siggelkow H, et al. Germ-line mutations in p27Kip1 cause a multiple endocrine neoplasia syndrome in rats and humans. Proc Natl Acad Sci USA. 2006;103:15558–15563. doi: 10.1073/pnas.0603877103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakker RV, Newey PJ, Walls GV, et al. Clinical practice guidelines for multiple endocrine neoplasia type 1 (MEN1) J Clin Endocrinol Metab. 2012;97:2990–3011. doi: 10.1210/jc.2012-1230. [DOI] [PubMed] [Google Scholar]