Abstract
The identification of women with a high probability of being carriers of pathogenic BRCA mutation is not straightforward and a major improvement would be the availability of markers of mutations that could be directly evaluated in individuals asking for genetic testing. The FMR1 gene testing was recently proposed as a candidate prescreening tool because an association between BRCA pathogenic mutations and FMR1 genotypes with ‘low alleles' (CGG repeat number <26) was observed. To confirm this hypothesis, we evaluated the distribution of FMR1 alleles and genotypes between BRCA mutation carriers and non-carriers in a cohort of 147 Italian women, free of cancer or affected by breast and/or ovarian cancer, who were tested for the presence of BRCA mutation in a clinical setting. The distribution of FMR1 CGG repeat numbers in the two groups was similar (lower allele median/mean were 30/27.4 and 30/27.9, respectively; Mann–Whitney test P=0.997) and no difference in the FMR1 genotype distribution was present (χ2=0.503, d.f.=2, P=0.78). This result is in contrast with literature data and suggests that FMR1 genetic testing is not a candidate BRCA prescreening tool.
Keywords: FMR1, BRCA, HBOC, mutation screening
Introduction
The decision to offer testing for BRCA mutations is challenging in many women in whom an inherited predisposition to breast/ovarian cancer is suspected, because of the associated costs, but also for the difficult interpretation of test results when genetic variants of uncertain significance are detected. Although several tools have been developed to help in this decision, their performance is unsatisfactory.1
A major improvement would be the availability of markers of pathogenic BRCA mutations that could be directly evaluated in individuals candidate to (or asking for) genetic testing.
A low ovarian response rate was observed in BRCA1 mutation-positive women with breast cancer undergoing in vitro fertilization, suggesting an association between BRCA1 mutation and occult primary ovarian insufficiency.2 Impairment of functional ovarian reserve is associated with another genetic trait, the polymorphic CGG repeat of the Fragile X Mental Retardation 1 (FMR1) gene.3 Female FMR1 premutation carriers (55–200 CGG repeats) are at increased risk of primary ovarian insufficiency (FXPOI). However, an increased risk of ovarian insufficiency was also reported in women with CGG repeat numbers still within the conventional normal range (<55 CGG repeats) but lower (<26 CGG repeats) or higher (35–55 CGG repeats) of the range of CGG repeats associated with normal folliculogenesis (26–34 CGG repeats).4, 5, 6
Recently, Weghofer et al7 have investigated whether BRCA mutations and FMR1 ovarian genotypes are interdependent: in Austrian women carrying a BRCA mutation they observed almost exclusively the presence of genotypes containing at least one FMR1 allele with a CGG repeat number below 26 (so called ‘low FMR1 genotypes').7 To explain this unexpected finding, they speculated that BRCA mutations could be embryo-lethal unless rescued by genotypes containing low FMR1 alleles and that the risk of prematurely diminished ovarian reserve reported as being associated to BRCA1 mutations could be FMR1 mediated.
If their findings are confirmed, FMR1 testing could be considered as a potential prescreening tool since BRCA mutations carriers could be expected, almost entirely, amongst the 25% of women of the general population carrying low FMR1 alleles.8
The present study was aimed at comparing the distribution of FMR1 genotypes between BRCA mutation carriers and non-carriers in a cohort of Italian women, free of cancer or affected by breast and/or ovarian cancer, who were tested for the presence of BRCA mutation in a clinical setting because of their personal and/or familial cancer history.
Materials and methods
The study was approved by the local Ethical Committee.
Eligible to the study were unrelated Italian women, with or without breast cancer, who were enrolled at our center from January 2006 to April 2012 in a prospective study on familial breast cancer risk (Familial Breast Cancer Risk and Mutagen Sensitivity, AIRC Study), and had been tested for BRCA mutations for suspected Hereditary Breast Ovarian Cancer (HBOC) syndrome. To enrich the study sample of BRCA mutation carriers, unrelated women identified as BRCA mutation carriers at any time since our cancer genetics laboratory offered this type of testing (year 2004) were also selected. Eligible cases should have given their consent to the use of genetic information and DNA for research purposes at the time of genetic testing.
Results of BRCA genetic testing were derived from clinical records. Until May 2010, BRCA1/BRCA2 mutation screening was performed by denaturation high-performance liquid chromatography (dHPLC) followed by the direct DNA sequence analysis of fragments with altered dHPLC profiles. Subsequently, DNA direct sequencing was utilized as mutation screening method. Large deletions and duplications were excluded by multiple ligation probe amplification (MLPA) analysis using a commercial kit (MRC-Holland, Amsterdam, The Netherlands). BRCA variant classification followed international rules.9 In particular, variants were classified as pathogenic if (i) they introduced a premature stop codon (frameshift, nonsense), (ii) they affected the highly conserved splice site positions AG/GT or (iii) they were missense variants whose clinical significance had already been demonstrated.
BRCA1 and BRCA2 pathogenic variants are listed in Supplementary Table 1 and they have been submitted to the Breast Cancer Information Core database (http://research.nhgri.nih.gov/bic/).
The CGG repeat number of FMR1 alleles was determined by capillary gel electrophoresis of fluorescent-labeled DNA fragments amplified using primers F (labeled with FAM fluorochrome) and C described by Fu et al.10 The reactions were performed with the GC Rich PCR System (Roche Diagnostics, Basel, Switzerland); the fragments were separated on an automatic sequencer 3130xl Genetic Analyzer (Life Technologies, Foster City, CA, USA) using a 36-cm capillary, the POP7 polymer, and the Genescan ROX-500 (Life Technologies) as internal standard markers. Data were elaborated using Genescan v. 3.2.
For the purpose of comparison with Weghofer et al,7 FMR1 genotypes were defined according to the recently reported ovarian genotypes and sub-genotypes classification by Gleicher et al.6 Based on a normal range of 26–34 repeats (median 30), FMR1 alleles containing less than 26 CGG repeats were defined as ‘low alleles' and FMR1 alleles containing more than 34 CGG repeats as ‘high alleles'. Genotypes were classified as norm (normal) if both alleles were in the normal range (CGGn=26–34), het (heterozygous) if one allele was outside the normal range and hom (homozygous) if both alleles were outside normal range. Het individuals were subdivided into sub-genotypes depending whether abnormal alleles were above (high) or below (low) normal range.
The distribution of the CGG repeat number in women with and without BRCA mutation was compared by means of the Mann–Whitney test. The χ2-test was used to compare the frequency of genotypes in the two groups.
Results
In total, 147 unrelated Italian women were included in the study: 128 women consecutively enrolled in the prospective familial breast cancer AIRC study and additional 19 women identified as BRCA mutation carriers from year 2004.
Thirty-seven women (25.2%) were free of cancer, while the remaining women had a history of breast cancer (n=101, 68.7%), ovarian cancer (n=3, 2%) or both breast and ovarian cancers (n=6, 4.1%).
In the AIRC group, a pathogenic BRCA variant was found in 24 of 128 cases (18.75%). In the remaining 104 cases, the reported methods of BRCA screening varied with time (dHPLC in 58 cases, 55.8%, and direct DNA sequencing in 46 cases, 44.2%) and large deletions and duplications were excluded by MLPA analysis in 64 cases (61.5%).
Overall, 43 of 147 cases (29.25%) carried a mutation in a BRCA gene (23 in BRCA1 and 20 in BRCA2): 24 women from the AIRC study and 19 known mutation carriers.
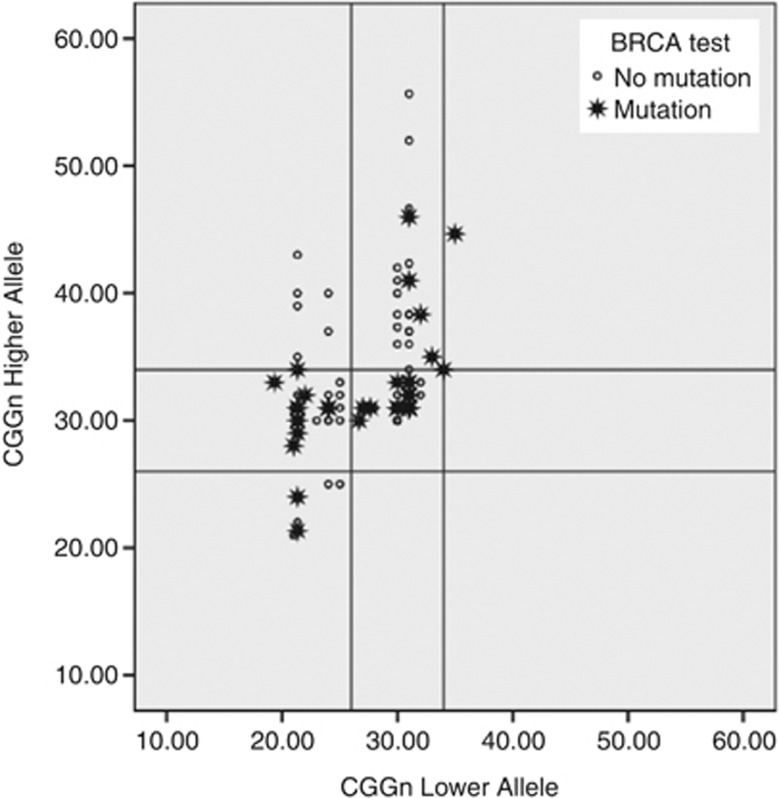
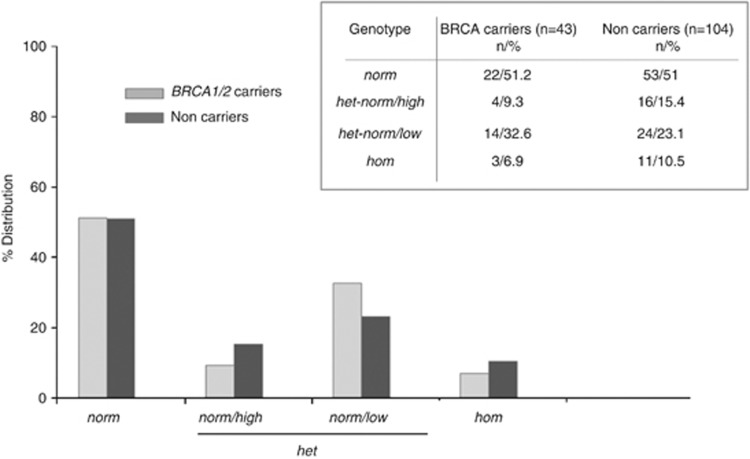
The distribution of FMR1 CGG repeat numbers in women with and without BRCA mutations is shown in Figure 1 and was similar in the two groups of women: the median/mean(SE) CGG repeat numbers in the lower allele were 30/27.4(0.71) and 30/27.9(0.37) in BRCA carriers and non-carriers, respectively (Mann–Whitney test P=0.997). No difference in the FMR1 genotype distribution is present among the two groups (Figure 2): among BRCA mutation carriers, 22 (51.2%) were norm, 18 (41.9%) were het and 3 (6.9%) were hom, while in non-carriers 53 (51%) were norm, 40 (38.5%) were het and 11 (10.5%) were hom (χ2=0.503, d.f.=2, P=0 0.78).
Figure 1.
Distribution of both FMR1 alleles in women with BRCA mutations (black stars) and in women with BRCA test inconclusive (white dots) in form of scatter plot. The norm genotype (CGGn=26–34) is defined by horizontal and vertical parallel lines.
Figure 2.
FMR1 genotypes and sub-genotypes in women with (gray bars) and without (black bars) BRCA mutations.
Similarly, no difference in the distribution of FMR1 genotypes was observed when women with or without cancer were compared (data not shown).
Discussion
In the present study we evaluated the association between FMR1 CGG repeat genotypes and BRCA mutations in 147 unrelated Italian women who have undergone BRCA genetic testing in a clinical setting because of their personal and/or familial history of breast/ovarian cancer. No difference in the FMR1 CGG repeat numbers or in the distribution of FMR1 genotypes and sub-genotypes was observed between BRCA-positive and BRCA-negative groups (Figures 1 and 2).
This result is in contrast with the finding reported in Austrian BRCA mutation carriers by Weghofer et al.7 In their study, these authors showed a significantly different distribution of FMR1 genotypes in BRCA women compared with a control group of women (patients with infertility problems). In particular, the FMR1 alleles distribution in the control population was comparable to the one reported in the general population (and in the present study), while almost all (93 of 99) Austrian BRCA mutation carriers presented with at least one low FMR1 allele (<26 CGG repeats).
The discordant results of the two studies are difficult to explain, even though differences in the study design do exist. In our study, only unrelated women were included while it is not clear if this is the case also in the study by Weghofer et al,7 particularly for the BRCA-positive group (64 different BRCA mutations are reported for 99 index cases). Another difference between the two studies is the source of the control group, as in our study the control population is represented by a prospective cohort of women who performed BRCA genetic testing for clinical purposes while the control population of the Weghofer et al7 study was not tested for BRCA mutation. However, this fact does not explain the difference in results of the two studies, which was due entirely to the difference in the two groups of BRCA carriers.
Alternatively, this difference might not be due to study bias but to a true difference between Italian and Austrian BRCA mutation carriers. This hypothesis, though unlikely, cannot be ruled out because at present little is known about the nature and distribution among different populations of BRCA-associated functional interacting factors.
A limitation of our study is the sample size. However, the frequency of normal genotypes in BRCA carriers (22/43, 51%) is similar to that observed in non-carriers, in sharp contrast with the almost complete absence of all constitutional genotypes except for sub-genotypes with low FMR1 alleles detected in the Austrian BRCA mutation carriers, and cannot be attributed to chance alone.
Certainly, these contradictory data require further investigation in other cohorts of women undergone BRCA genetic testing, including series of cases with different ethnic background.
In conclusion, our findings suggest that, so far, FMR1 testing cannot be considered as a prescreening tool to identify individuals with a higher probability of being carriers of pathogenic BRCA mutations.
Acknowledgments
This work was supported by the Italian Association for Research on Cancer (AIRC) (Project IG 5706 2008). We thank Dr Francesca Faravelli for helpful discussions on the ethical implications of the study.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on European Journal of Human Genetics website (http://www.nature.com/ejhg)
Supplementary Material
References
- Amir E, Freedman OC, Seruga B, Evans DG. Assessing women at high risk of breast cancer: a review of risk assessment models. J Natl Cancer Inst. 2010;102:680–691. doi: 10.1093/jnci/djq088. [DOI] [PubMed] [Google Scholar]
- Oktay K, Kim JY, Barad D, Babayev SN. Association of BRCA1 mutations with occult primary ovarian insufficiency: a possible explanation for the link between infertility and breast/ovarian cancer risks. J Clin Oncol. 2010;28:240–244. doi: 10.1200/JCO.2009.24.2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willemsen R, Levenga J, Oostra BA. CGG repeat in the FMR1 gene: size matters. Clin Genet. 2011;80:214–225. doi: 10.1111/j.1399-0004.2011.01723.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretherick KL, Fluker MR, Robinson WP. FMR1 repeat sizes in the gray zone and high end of the normal range are associated with premature ovarian failure. Hum Genet. 2005;117:376–382. doi: 10.1007/s00439-005-1326-8. [DOI] [PubMed] [Google Scholar]
- Gleicher N, Weghofer A, Oktay K, Barad D. Relevance of triple CGG repeats in the FMR1 gene to ovarian reserve. Reprod Biomed Online. 2009;19:385–390. doi: 10.1016/s1472-6483(10)60173-3. [DOI] [PubMed] [Google Scholar]
- Gleicher N, Weghofer A, Barad DH. Ovarian reserve determinations suggest new function of FMR1 (fragile X gene) in regulating ovarian ageing. Reprod Biomed Online. 2010;20:768–775. doi: 10.1016/j.rbmo.2010.02.020. [DOI] [PubMed] [Google Scholar]
- Weghofer A, Tea MK, Barad DH, et al. BRCA1/2 mutations appear embryo-lethal unless rescued by low (CGG n<26) FMR1 sub-genotypes: explanation for the ‘BRCA paradox'. PLoS One. 2012;7:e44753. doi: 10.1371/journal.pone.0044753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peprah E. Fragile X syndrome: the FMR1 CGG repeat distribution among world populations. Ann Hum Genet. 2012;76:178–191. doi: 10.1111/j.1469-1809.2011.00694.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plon SE, Eccles DM, Easton D, et al. Sequence variant classification and reporting: recommendation for improving the interpretation of cancer susceptibility genetic test results. Hum Mutat. 2008;29:1282–1291. doi: 10.1002/humu.20880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu YH, Kuhl DP, Pizzuti A, et al. Variation of the CGG repeat at the fragile X site results in genetic instability: resolution of the Sherman paradox. Cell. 1991;67:1047–1058. doi: 10.1016/0092-8674(91)90283-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.