Abstract
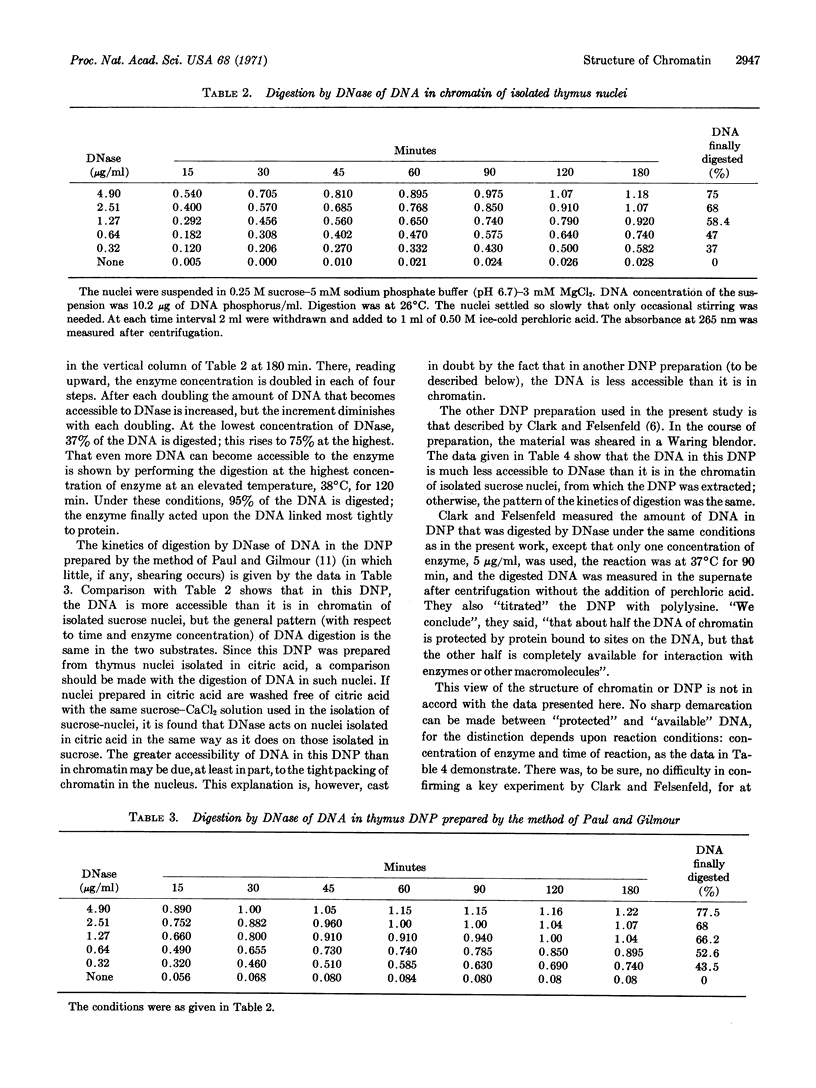
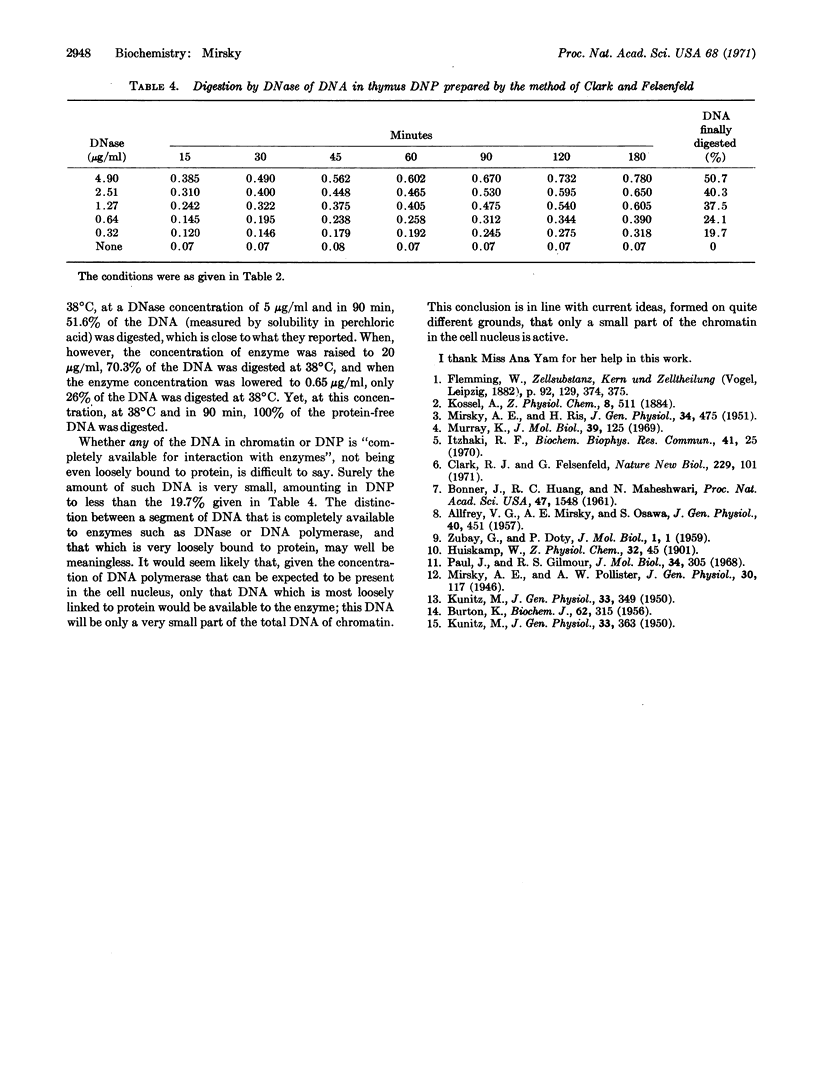
The DNA in chromatin of isolated thymus nuclei, and in two different preparations of deoxyribonucleoprotein extracted from chromatin, has been digested with DNase. By variation of the time of reaction and the concentration of enzyme, and by comparison of the action of the enzyme on (protein-free) DNA with its action on DNA complexed with protein in chromatin and deoxyribonucleoprotein, it was found that most, if not all, of the DNA is linked to protein. The linkage is loose, as shown by the fact that all the DNA is accessible to the enzyme; however, the wide variations in accessibility in a given complex show that the looseness of the DNA-protein link varies considerably. Accessibility of DNA in one type of deoxyribonucleoprotein preparation is greater than it is in chromatin, while in the other type it is less than in chromatin.
Keywords: DNA, DNase, chromatin, deoxyribonucleoprotein, cell nucleus
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALLFREY V. G., MIRSKY A. E., OSAWA S. Protein synthesis in isolated cell nuclei. J Gen Physiol. 1957 Jan 20;40(3):451–490. doi: 10.1085/jgp.40.3.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BONNER J., HUANG R. C., MAHESHWARI N. The physical state of newly synthesized RNA. Proc Natl Acad Sci U S A. 1961 Oct 15;47:1548–1554. doi: 10.1073/pnas.47.10.1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark R. J., Felsenfeld G. Structure of chromatin. Nat New Biol. 1971 Jan 27;229(4):101–106. doi: 10.1038/newbio229101a0. [DOI] [PubMed] [Google Scholar]
- Itzhaki R. F. Structure of deoxyribonucleoprotein as revealed by its binding to polylysine. Biochem Biophys Res Commun. 1970 Oct 9;41(1):25–32. doi: 10.1016/0006-291x(70)90463-8. [DOI] [PubMed] [Google Scholar]
- KUNITZ M. Crystalline desoxyribonuclease; digestion of thymus nucleic acid; the kinetics of the reaction. J Gen Physiol. 1950 Mar;33(4):363–377. doi: 10.1085/jgp.33.4.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KUNITZ M. Crystalline desoxyribonuclease; isolation and general properties; spectrophotometric method for the measurement of desoxyribonuclease activity. J Gen Physiol. 1950 Mar;33(4):349–362. doi: 10.1085/jgp.33.4.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MIRSKY A. E., RIS H. The composition and structure of isolated chromosomes. J Gen Physiol. 1951 May;34(5):475–492. doi: 10.1085/jgp.34.5.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray K. Stepwise removal of histones from native deoxyribonucleoprotein by titration with acid at low temperature and some properties of the resulting partial nucleoproteins. J Mol Biol. 1969 Jan 14;39(1):125–144. doi: 10.1016/0022-2836(69)90338-6. [DOI] [PubMed] [Google Scholar]
- Paul J., Gilmour R. S. Organ-specific restriction of transcription in mammalian chromatin. J Mol Biol. 1968 Jul 14;34(2):305–316. doi: 10.1016/0022-2836(68)90255-6. [DOI] [PubMed] [Google Scholar]


