Abstract
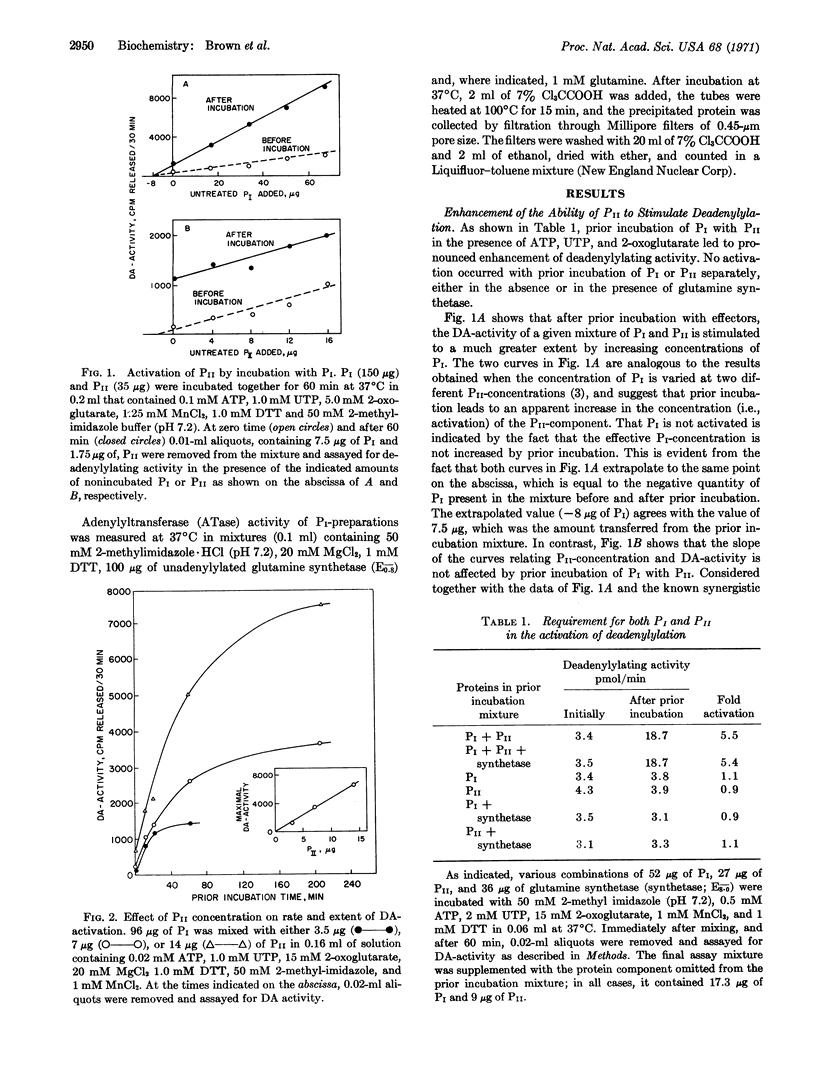
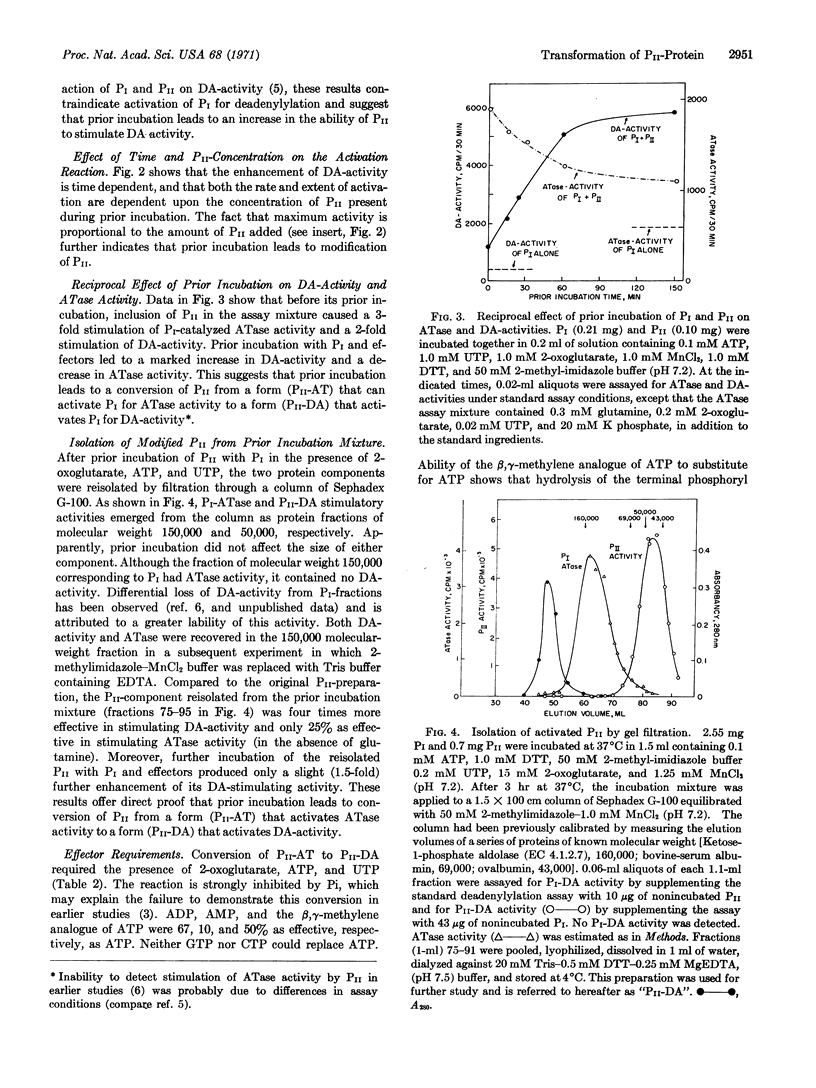
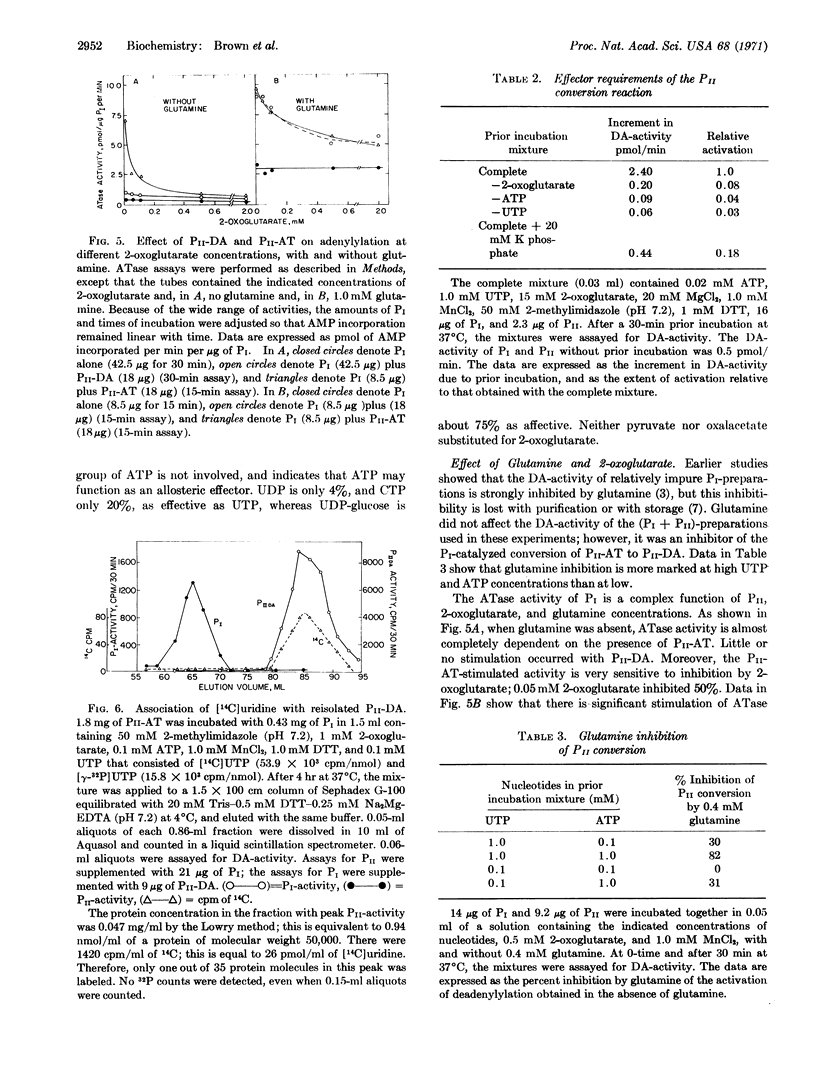
Earlier studies showed that two protein components, PI and PII, are concerned with the adenylylation and deadenylylation of Escherichia coli glutamine synthetase (EC 6.3.1.2). PI by itself catalyzes both adenylylation and deadenylylation, but its activity is modulated by the PII-protein and by glutamine, 2-oxoglutarate, ATP, and UTP, The PII-protein exists in two forms: one form, PII-AT, stimulates PI-catalyzed adenylylation activity in the absence of glutamine and makes this activity very sensitive to inhibition by 2-oxoglutarate; it does not affect deadenylylation activity. The other form, PII-DA, stimulates adenylylation only if glutamine is present, and also stimulates the deadenylylation activity of PI, which is then dependent upon the presence of ATP and 2-oxoglutarate. Conversion of PII-AT to PII-DA requires the presence of UTP, ATP, and 2-oxoglutarate; it is catalyzed by an enzyme present in PI preparations. UTP may be directly involved in this conversion since PII-DA fractions reisolated by filtration through Sephadex G-100 contain small quantities of a bound uridine derivative that lacks the γ-phosphoryl group of UTP. The activity of PII-DA, but not of PII-AT, is destroyed by treatment with snake-venom phosphodiesterase. ATP and 2-oxoglutarate apparently function as allosteric effectors for the conversion of PII-AT to PI-DA.
Keywords: E. coli, protein-bound uridine nucleotide, adenylyltransferase, 2-oxoglutarate, cascade regulation
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson W. B., Hennig S. B., Ginsburg A., Stadtman E. R. Association of ATP: glutamine synthetase adenylyltransferase activity with the P1 component of the glutamine synthetase deadenylylation system. Proc Natl Acad Sci U S A. 1970 Nov;67(3):1417–1424. doi: 10.1073/pnas.67.3.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson W. B., Stadtman E. R. Purification and functional roles of the P I and P II components of Escherichia coli glutamine synthetase deadenylylation system. Arch Biochem Biophys. 1971 Apr;143(2):428–443. doi: 10.1016/0003-9861(71)90229-3. [DOI] [PubMed] [Google Scholar]
- Hennig S. B., Anderson W. B., Ginsburg A. Adenosine triphosphate: glutamine synthetase adenylyltransferase of Escherichia coli: two active molecular forms. Proc Natl Acad Sci U S A. 1970 Dec;67(4):1761–1768. doi: 10.1073/pnas.67.4.1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingdon H. S., Shapiro B. M., Stadtman E. R. Regulation of glutamine synthetase. 8. ATP: glutamine synthetase adenylyltransferase, an enzyme that catalyzes alterations in the regulatory properties of glutamine synthetase. Proc Natl Acad Sci U S A. 1967 Oct;58(4):1703–1710. doi: 10.1073/pnas.58.4.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro B. M., Stadtman E. R. The regulation of glutamine synthesis in microorganisms. Annu Rev Microbiol. 1970;24:501–524. doi: 10.1146/annurev.mi.24.100170.002441. [DOI] [PubMed] [Google Scholar]
- Shapiro B. M. The glutamine synthetase deadenylylating enzyme system from Escherichia coli. Resolution into two components, specific nucleotide stimulation, and cofactor requirements. Biochemistry. 1969 Feb;8(2):659–670. doi: 10.1021/bi00830a030. [DOI] [PubMed] [Google Scholar]
- Wulff K., Mecke D., Holzer H. Mechanism of the enzymatic inactivation of glutamine synthetase from E. coli. Biochem Biophys Res Commun. 1967 Sep 7;28(5):740–745. doi: 10.1016/0006-291x(67)90378-6. [DOI] [PubMed] [Google Scholar]


