Abstract
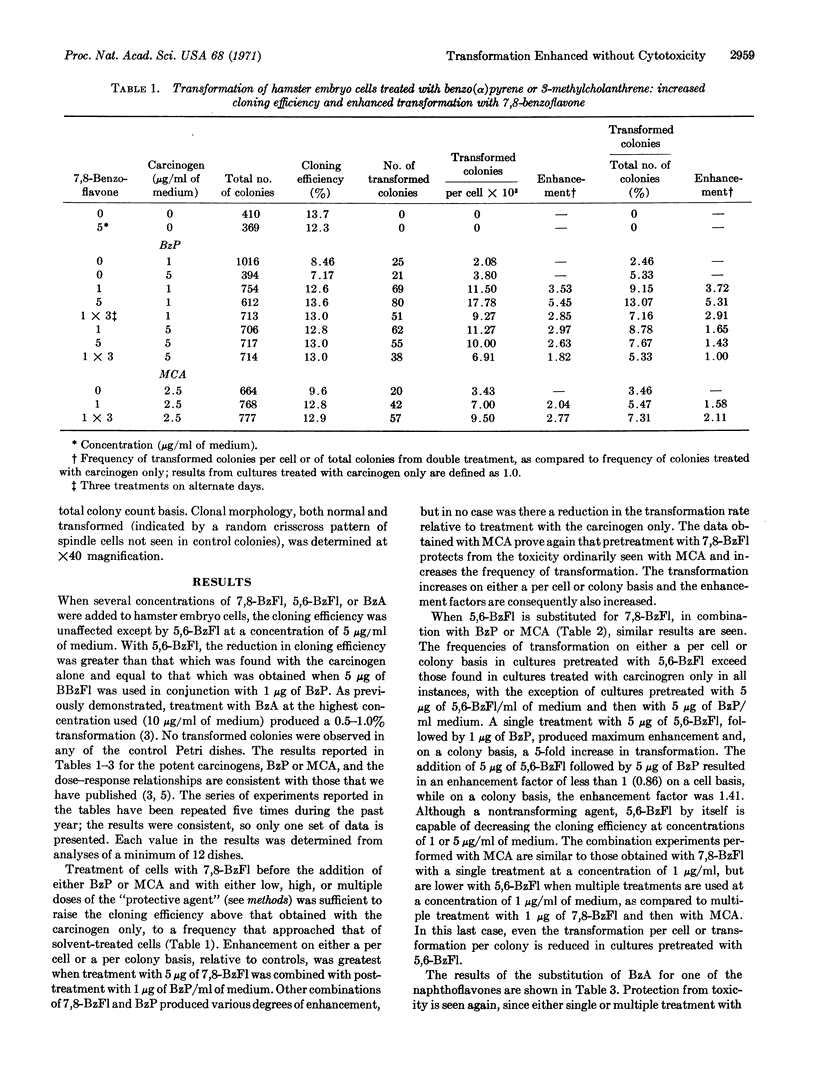
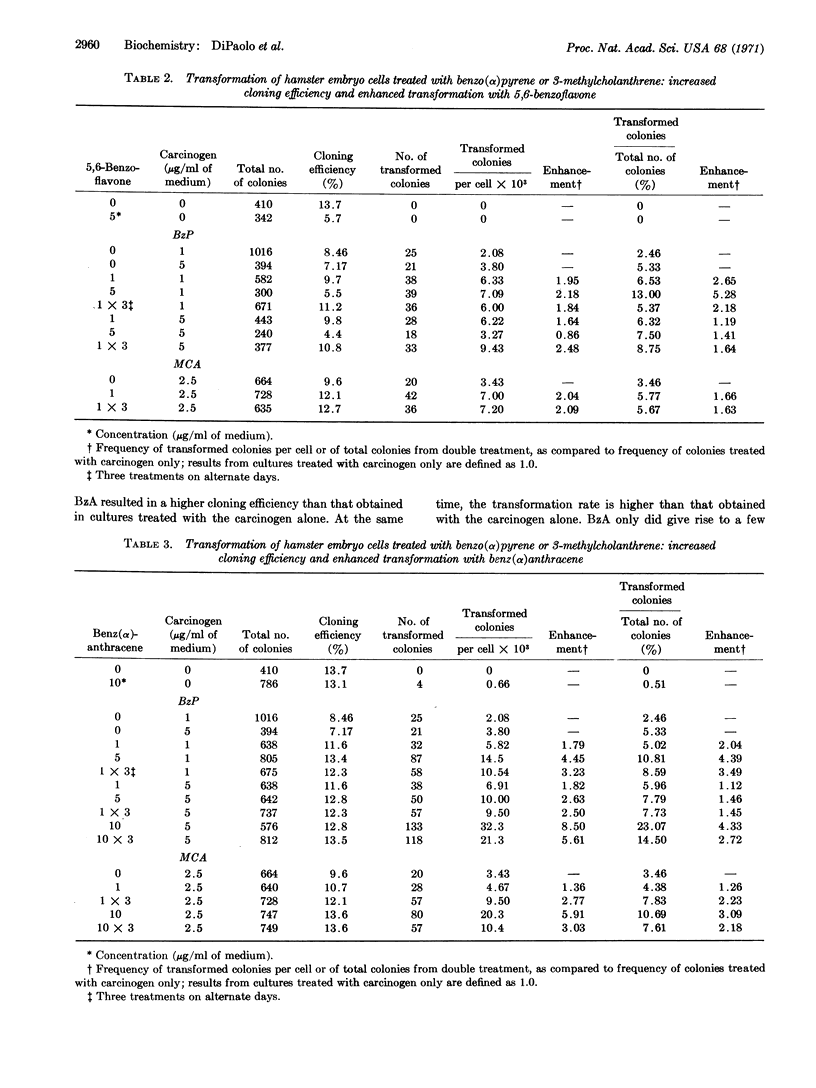
Treatment with either a flavone or benz(α)anthracene before the addition of a potent carcinogen, benzo(α)pyrene or 3-methylcholanthrene, enhanced the transformation of Syrian hamster cells that are seeded in order to form colonies. 7,8-benzoflavone and benz(α)anthracene prevented the cytotoxicity by the carcinogens, while 5,6-benzoflavone did not. The results clearly indicate that it is possible to dissociate the transforming from the toxic metabolic properties of benzo(α)pyrene and 3-methylcholanthrene.
Keywords: cloning efficiency, carcinogen, protective agents, frequency of transformation
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berwald Y., Sachs L. In vitro transformation of normal cells to tumor cells by carcinogenic hydrocarbons. J Natl Cancer Inst. 1965 Oct;35(4):641–661. [PubMed] [Google Scholar]
- Chen T. T., Heidelberger C. Quantitative studies on the malignant transformation of mouse prostate cells by carcinogenic hydrocarbons in vitro. Int J Cancer. 1969 Mar 15;4(2):166–178. doi: 10.1002/ijc.2910040207. [DOI] [PubMed] [Google Scholar]
- Connell D. I., Riechers L. A., DiPaolo J. A. Radioautographic analysis of 7,12-dimethyl-benz(a)anthracene-3H incorporation and cell survival of Syrian hamster embryo cells during exposure to nucleic acid inhibitors. J Natl Cancer Inst. 1971 Jan;46(1):183–193. [PubMed] [Google Scholar]
- DiPaolo J. A., Donovan P. J., Nelson R. L. In vitro transformation of hamster cells by polycyclic hydrocarbons: factors influencing the number of cells transformed. Nat New Biol. 1971 Apr 21;230(16):240–242. doi: 10.1038/newbio230240a0. [DOI] [PubMed] [Google Scholar]
- DiPaolo J. A., Donovan P., Nelson R. Quantitative studies of in vitro transformation by chemical carcinogens. J Natl Cancer Inst. 1969 May;42(5):867–874. [PubMed] [Google Scholar]
- Diamond L., Gelboin H. V. Alpha-naphthoflavone: an inhibitor of hydrocarbon cytotoxicity and microsomal hydroxylase. Science. 1969 Nov 21;166(3908):1023–1025. doi: 10.1126/science.166.3908.1023. [DOI] [PubMed] [Google Scholar]
- Dipaolo J. A., Donovan P. J. Properties of Syrian hamster cells transformed in the presence of carcinogenic hydrocarbons. Exp Cell Res. 1967 Nov;48(2):361–377. doi: 10.1016/0014-4827(67)90361-8. [DOI] [PubMed] [Google Scholar]
- Gelboin H. V. A microsome-dependent binding of benzo[a]pyrene to DNA. Cancer Res. 1969 Jun;29(6):1272–1276. [PubMed] [Google Scholar]
- Gelboin H. V., Wiebel F., Diamond L. Dimethylbenzanthracene tumorigenesis and aryl hydrocarbon hydroxylase in mouse skin: inhibition by 7,8-benzoflavone. Science. 1970 Oct 9;170(3954):169–171. doi: 10.1126/science.170.3954.169. [DOI] [PubMed] [Google Scholar]
- Grover P. L., Sims P. Enzyme-catalysed reactions of polycyclic hydrocarbons with deoxyribonucleic acid and protein in vitro. Biochem J. 1968 Nov;110(1):159–160. doi: 10.1042/bj1100159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grover P. L., Sims P., Huberman E., Marquardt H., Kuroki T., Heidelberger C. In vitro transformation of rodent cells by K-region derivatives of polycyclic hydrocarbons. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1098–1101. doi: 10.1073/pnas.68.6.1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HILL W. T., STANGER D. W., PIZZO A., RIEGEL B., SHUBIK P., WARTMAN W. B. Inhibition of 9,10-dimethyl-1,2-benzanthracene skin carcinogenesis in mice by polycyclic hydrocarbons. Cancer Res. 1951 Nov;11(11):892–897. [PubMed] [Google Scholar]
- Huberman E., Sachs L. Cell susceptibility to transformation and cytotoxicity by the carcinogenic hydrocarbon benzo[a]pyrene. Proc Natl Acad Sci U S A. 1966 Oct;56(4):1123–1129. doi: 10.1073/pnas.56.4.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nebert D. W., Gelboin H. V. Substrate-inducible microsomal aryl hydroxylase in mammalian cell culture. II. Cellular responses during enzyme induction. J Biol Chem. 1968 Dec 10;243(23):6250–6261. [PubMed] [Google Scholar]
- Selkirk J. K., Huberman E., Heidelberger C. An epoxide is an intermediate in the microsomal metabolism of the chemical carcinogen, dibenz(a,h)anthracene. Biochem Biophys Res Commun. 1971 Jun 4;43(5):1010–1016. doi: 10.1016/0006-291x(71)90562-6. [DOI] [PubMed] [Google Scholar]
- Wattenberg L. W., Leong J. L. Inhibition of the carcinogenic action of benzo(a)pyrene by flavones. Cancer Res. 1970 Jul;30(7):1922–1925. [PubMed] [Google Scholar]
- Wheatley D. N. Enhancement and inhibition of the induction by 7,12-dimethylbenz(a)anthracene of mammary tumours in female Sprague-Dawley rats. Br J Cancer. 1968 Dec;22(4):787–797. doi: 10.1038/bjc.1968.93. [DOI] [PMC free article] [PubMed] [Google Scholar]


