Abstract
Background: The aim of the study is to detect apoptosis in granular cell ameloblastoma by annexin v affinity assay, a novel technique. Materials & Methods: Excitional biopsy of two patients with granular cell ameloblastoma were fixed in buffered formalin and later embedded in paraffin wax. Blocks were sliced into 3m thick sections for routine histological and subsequent immunohistochemical examinations. For electron microscopic examination tissues were fixed in 2.5% glutaraldehyde.electron microscopic examination was carried out to find the nature of granularity in granular cell ameloblastoma which was finally confirmed by annexin v technique. Results: Annexin v is a sensitive marker to detect early apoptosis. Fluorescence in granular cell clusters showed that apoptotic cell death is higher in granular cells. Both early and late events of apoptosis were identified in annexin v staining and electron microscopic study respectively. Conclusion: Our study confirms that increased apoptotic cell death and subsequent phagocytosis is responsible for granular appearance of cells in granular cell ameloblastoma compared with that of conventional ameloblastoma. How to cite this article: Balaji N, Devy AS, Sumathi MK, Vidyalakshmi S, Kumar GS, D’Silva S. Annexin V – Affinity Assay – Apoptosis Detection System in Granular Cell Ameloblastoma. J Int Oral Health 2013; 5(6):25-30 .
Key words: : Affinity assay, annexinv, apoptosis, phagocytosis
Introduction
Ameloblastoma is an epithelial odontogenic tumour of the jaw and it exhibits diverse microscopic patterns which occur either singly or in combination with others. Granular ameloblastoma is an unusual variant of ameloblastoma which is characterized by nests of large, eosinophilic granular cells. These granular cells have been the subject of debate and about which there is controversy regarding its origin and nature. Previous studies have showed increase apoptotic cells in granular cell ameloblastoma both immunohistoche -mically and ultrastructually. Present study is an attempt to detect apoptosis in granular cell ameloblastoma by Annexin V Affinity Assay – A noval technique.
Ameloblastoma is most frequently encountered tumour arising from odontogenic epithelium and exhibits considerable histological variations. 1 , 2 Granular cell variant of ameloblastoma shows granular transmission of neoplastic cells. 1 - 4 Previous studies which includes ultrastructural, 5 - 7 histochemical 7 , 8 and immunohistochemical 9 - 11 methods have revealed that cytoplasmic granularity is caused by lysosomal overload, though the mechanism involved is poorly understood.
Apoptosis is a programmed, physiological mode of cell death that plays an vital role in tissue homeostasis as well as in oncogenesis. 12 , 13 Apoptotic cells and Apoptotic related factors have been investigated in 2 germs and ameloblastoma suggesting that apoptotic reactions have important roles in tooth development and tumour growth. 14 - 19
Numerous studies shown increased apoptotic cells and decreased expression of several apoptosis related factors such as Bcl – 2 family proteins and p53 protein cells in ameloblastoma. 14 , 19 This study emphasis on a Noval technique Annexin V affinity assay system which detects early stages of apoptotic cell death in granular cell ameloblastoma.
Materials and Methods
Tissue Preparation:
Excitional Biopsy specimens from 2 patients with granular cell ameloblastoma were received and the clinical characteristics of the patients are shown in Table 1. Tumour tissues were fixed in buffered formalin and later embedded in paraffin wax. Tissues blocks were sliced into 3m thick sections for routine histological and subsequent immunohistochemical examinations. Tissue sections were stained with H & E. For electron microscopic examinations, tissues were fixed in 2.5% glutaraldehyde and post fixed in 1%
Table 1
| Case | Age | Sex | Location | Treatment |
| 1 | 45 | F | Molar-ramus | Extirpation |
| 2 | 37 | F | Molar - Premolar | Extirpation |
osmium tetraoxide, dehydrated in a graded series of ethanol & embedded in epoxy resin. Semithin sections were stained with 0.1% tolidineblue for light microscopy. Sections to be examined were selected and ultrathin sections were double stained with uranyl acetate and lead citrate.
Annexin V Affinity Assay:
This technique is designed to detect apoptosis by targeting for the loss of phospholipid asymmetry of the plasma membrane. Apoptotic cell death is accompanied by a change in plasma membrane structure by surface exposure of phosphatidylserine (PS) while the membrane integrity remains unchallenged. Surface exposed PS can be detected by its affinity for Annexin V, a phospholipid binding protein.
Annexin V was first reported by Bohn and colleagues 20 who isolated the proteins from human placenta and called it placentral protein 4 (PP4) by Reutelingsperger et al 21 who isolated it from umbilical cord by virtue of its anticoagulant activity and called it vascular – anticoagulant - . After cloning and sequencing of the human Annexin V cDNA, the protein got its name because of its homology with family of Annexin proteins. 22 - 24 Using fluoroescently labeled annexin V it could be demonstrated apoptotic cells exposed PS at their outer membrane early after onset of the execution phase of apoptosis. PS appears at the outer leaflet of the plasma membrane, the integrity of which has not been compromised at this stage. PS exposures seem to last from early execution phase of apoptosis until the final stage at which the cell has broken up into apoptotic bodies.
Based on the phenomenon that PS is exposed during apoptosis and on the ability of annexin V to bind to PS with high affinity, Koopman et al 25 were the first to describe a method using extrinsically applied hapten (i.e. FITC or biotin) labeled annexin V to detect apoptosis. Annexin V is not able to bind to normal vital cells since the molecule is not able to penetrate the phospholipid by layer. In the dead cells however the inner leaflet of the membrane is available for binding of extrinsically applied annexin V, since the integrity of plasma membrane is lost.
To discriminate between dead and apoptotic cells a membrane impermiable DNA stain, such as propidium iodide (PI) is added simultaneously to the cell suspension. In this way vital, apoptotic and dead cells are discriminated on basis of a double labeling for annexin V and PI and analysed by fluorescence microscopy. 26
Results
Histological Features:
Specimens from both the granular cell ameloblastomas occurred in follicular pattern that showed odontogenic tumuor in connective tissues stroma [ Figure 1 ]. Follicle is lined by tall columnar cells and central core showing granular cell transformation [ Figure 2 ]. The granular cell clusters showed pyknotic nuclei and bulky cytoplasam filled with eosnophilic granules.
Figure 1: Odontogenic tumuor in connective tissues stroma.
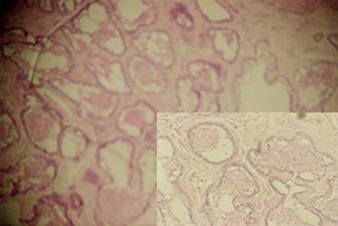
Figure 2: Tall columnar cells.
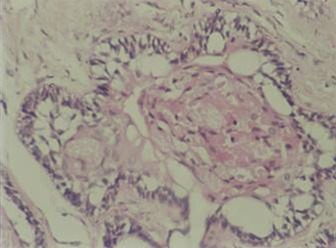
EM & Immunohistochemistry:
On EM examination, granular cell showed lysosomal aggregates [ Figure 3 ]. Lysosomal vacules showed phagocytosed material. In the granular cell clusters, apoptotic cell fragments with condensed chromatin is identified which were consequently phagocytosed by adjacent granular cells and degraded within lysosomes [Figure 4 & 5 ].
Figure 3: EM examination, granular cell showed lysosomal aggregates.
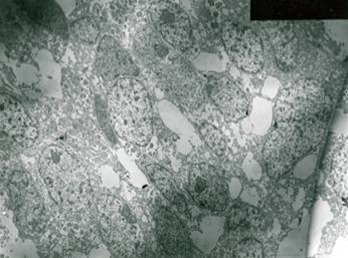
Figure 4: Lysosomal vacules showed phagocytosed material.
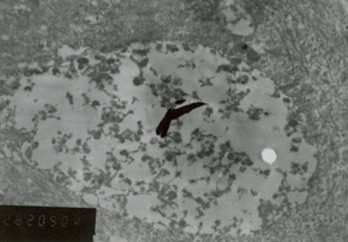
Figure 5: Granular cell clusters, apoptotic cell fragments with condensed chromatin is identified.

Immunohistochemically both granular and tall cells lining the follicle were positive for cytokeratin indicating their epithelial origin. These cells were negative for vimentin, desmin and S-100. Granular cells stained positive for CD-68 but the tall columnar lining cells were negative.
Staining with Annexin V by immunoflurescence:
Viable cells maintained an asymmetric distribution of different phospholipids between outer and inner leaflets of plasma membrane. Loss of plasma membrane asymmetry is a important event in apoptosis, independent of the cell type. This resulted
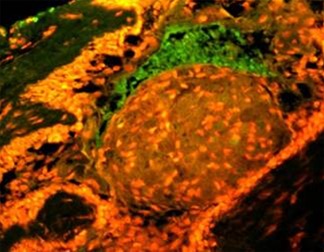
in exposure of phospholipid from the inner phase to outer phase of plasma membrane. Phosphotidyl serine an aminophospholipid present in inner phase of the plasma membrane is exposed to the outer leaflet during apoptosis. This acts as a tag for its specific recognition by macrophages and for phagocytosis of the dying cell [ Figure 6 ]. Annexin V is a calcium dependent protein that binds with phospholipid and this FITC tagged annexin V floresed as it stained positive for apoptotic bodies [Figure 7 & 8 ].
Figure 6: Phosphotidyl serine an aminophospholipid present in inner phase of the plasma membrane is exposed to the outer leaflet during apoptosis. This acts as a tag for its specific recognition by macrophages and for phagocytosis of the dying cell.
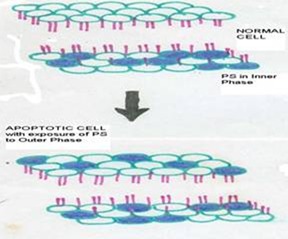
Figure 7: Annexin V is a calcium dependent protein that binds with phospholipid and this FITC tagged annexin V floresed as it stained positive for apoptotic bodies.
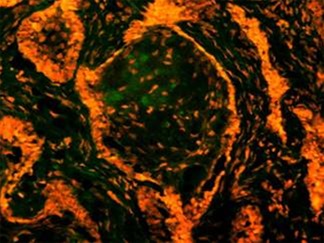
Figure 8: Annexin V is a calcium dependent protein that binds with phospholipid and this FITC tagged annexin V floresed as it stained positive for apoptotic bodies.
Discussion
Ameloblastoma sometimes exhibits granular transformation of cytoplasm usually occurring in central stellate reticulam like cells and this change often extends to peripheral columnar or cuboidal cells. 1 - 3 On routine histological examinations of the specimens, we found various quantities of granular cells in the neoplastic epithelial tissues. Also few apoptotic nuclear fragments were recognized in granular cell clusters.
Ultrastructural studies of granular cells ameloblatoma have revealed cytoplasmic granularity is associated with high content of lysosomes. 5 - 7 Our EM examination also showed cytoplasmic lysosomal aggregation in both the cases. We also detected numerous apoptotic cell fragments with condensed nuclei in granular cell clusters and most of these fragments were phagocytosed by adjacent neoplastic cells.
Both granular and tall columnar cells lining the follicles were positive for cytokeratine and negative for vimentin, desmin & S-100 which confirms epithelial origin of granular cells. In addition granular cells stained positive for CD-68.
Annexin V is a sensitive marker to detect early apoptosis. Apoptotic bodies identified in histopathological and EM sections were confirmed by staining with annexin V by immunoflurescence. Fluorescence in granular cell clusters showed that apoptotic cell death is higher in granular cells. Both early and late events of apoptosis were identified in Annexin V staining and EM study respectively. Thus our study confirms that it might be increased apoptotic cell death and subsequent phagocytosis, that is responsible for granular appearance of cells in granular cell ameloblastoma compared with that of conventional ameloblastoma.
Conclusion
Annexin V is a sensitive marker to detect early apoptosis. Apoptotic bodies identified in histopathological and EM sections were confirmed by staining with Annexin V by immunoflurescence. Though earlier studies have been conducted with quest of search for the nature of granularity of granular cell ameloblastoma, whether there is any possible correlation between the granularity of the tumour and prognosis, has to be yet answered. Further studies involving larger sample size would help in establishing the fact, which in turn, can be an aid in better therapeutic intervention.
Footnotes
Source of Support: Nil
Conflict of Interest: None Declared
Contributor Information
N Balaji, Department of Oral and Maxillofacial Pathology, Teerthankar Mahaveer Dental College and Research Centre, Teerthankar Mahaveer University, Moradabad, Uttar Pradesh, India.
A Santha Devy, Department of Oral and Maxillofacial Pathology, Indira Gandhi Institute of Dental Sciences, Pondicherry, India.
M K Sumathi, Department of Oral and Maxillofacial Pathology, Teerthankar Mahaveer Dental College and Research Centre, Teerthankar Mahaveer University, Moradabad, Uttar Pradesh, India.
S Vidyalakshmi, Department of Oral and Maxillofacial Pathology, Indira Gandhi Institute of Dental Sciences, Pondicherry, India.
G Sathish Kumar, Department of Oral and Maxillofacial Pathology, Indira Gandhi Institute of Dental Sciences, Pondicherry, India.
Shaloom D’Silva, Doha, Qatar.
References
- 1.S Huffman, JR Jacoway, SO Krolls. Intraosseous and parosteal tumours of the Jaws. Washington, DC: Armed forces institute of pathology. 1987:94–101. [Google Scholar]
- 2.RH Kramer, JJ Pindborg, M Shear. WHO histological typing of Odontogenic tumours. Berlin: Springer - Verlag. 1992:11–14. [Google Scholar]
- 3.KS Hartman. Granular-cell ameloblastoma. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1974;38(2):241–253. doi: 10.1016/0030-4220(74)90063-2. [DOI] [PubMed] [Google Scholar]
- 4.R Dina, C Marchetti, G Vallania, G Corinaldesi, V Eusebi. Granular cell ameloblastoma. An immunocytochemical study. Pathol Res Pract. 1996;192(6):541–546. doi: 10.1016/S0344-0338(96)80103-8. [DOI] [PubMed] [Google Scholar]
- 5.AR Navarrette, M Smith. Ultrastructure of granular cell ameloblastoma. Cancer. 1971;27(4):948–955. doi: 10.1002/1097-0142(197104)27:4<948::aid-cncr2820270430>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 6.B Tandler, EP Rossi. Granular cell ameloblastoma: Electron microscopic observations. J Oral Pathol. 1977;6(6):401–412. doi: 10.1111/j.1600-0714.1977.tb01807.x. [DOI] [PubMed] [Google Scholar]
- 7.M Nasu, M Takagi, H Yamamoto. Ultrastructural and histochemical studies of granular-cell ameloblastoma. J Oral Pathol. 1984;13(4):448–456. doi: 10.1111/j.1600-0714.1984.tb01445.x. [DOI] [PubMed] [Google Scholar]
- 8.M Mori. Histochemical evaluation of enzymes in ameloblastic tumors--acanthomatous and granular-cell ameloblastoma. J Oral Surg. 1970;28(11):825–831. [PubMed] [Google Scholar]
- 9.P Slootweg, P de Wilde, P Vooijs, F Ramaekers. Oral granular cell lesions. An immunohistochemical study with emphasis on intermediate-sized filaments proteins. Virchows Arch A Pathol Anat Histopathol. 1983;402(1):35–45. doi: 10.1007/BF00695047. [DOI] [PubMed] [Google Scholar]
- 10.CM Stewart, RE Watson, LR Eversole, W Fischlschweiger, AS Leider. Oral granular cell tumors: a clinicopathologic and immunocytochemical study. Oral Surg Oral Med Oral Pathol. 1988;65(4):427–435. doi: 10.1016/0030-4220(88)90357-x. [DOI] [PubMed] [Google Scholar]
- 11.GH Ruhl, E Akuamoa-Boateng. Granular cells in odontogenic and non-odontogenic tumours. Virchows Arch A Pathol Anat Histopathol. 1989;415(5):403–409. doi: 10.1007/BF00747741. [DOI] [PubMed] [Google Scholar]
- 12.JF Kerr, AH Wyllie, AR Currie. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972;26(4):239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.DA Carson, JM Ribeiro. Apoptosis and disease. Lancet. 1993;341(8855):1251–1254. doi: 10.1016/0140-6736(93)91154-e. [DOI] [PubMed] [Google Scholar]
- 14.H Kumamoto. Detection of apoptosis-related factors and apoptotic cells in ameloblastomas: analysis by immunohistochemistry and an in situ DNA nick end-labelling method. J Oral Pathol Med. 1997;26(9):419–425. doi: 10.1111/j.1600-0714.1997.tb00242.x. [DOI] [PubMed] [Google Scholar]
- 15.PJ Slootweg, RA de Weger. Immunohistochemical demonstration of bcl-2 protein in human tooth germs. Arch Oral Biol. 1994;39(7):545–550. doi: 10.1016/0003-9969(94)90129-5. [DOI] [PubMed] [Google Scholar]
- 16.S Nishikawa, F Sasaki. DNA localization in nuclear fragments of apoptotic ameloblasts using anti-DNA immunoelectron microscopy: programmed cell death of ameloblasts. Histochem Cell Biol. 1995;104(2):151–159. doi: 10.1007/BF01451574. [DOI] [PubMed] [Google Scholar]
- 17.A Vaahtokari, T Aberg, I Thesleff. Apoptosis in the developing tooth: association with an embryonic signaling center and suppression by EGF and FGF-4. Development. 1996;122(1):121–129. doi: 10.1242/dev.122.1.121. [DOI] [PubMed] [Google Scholar]
- 18.T Mitsuyasu, H Harada, Y Higuchi, K Kimura, N Nakamura, T Katsuki, E Kubota, K Toyoshima, M Ohishi. Immunohistochemical demonstration of bcl-2 protein in ameloblastoma. J Oral Pathol Med. 1997;26(8):345–348. doi: 10.1111/j.1600-0714.1997.tb00228.x. [DOI] [PubMed] [Google Scholar]
- 19.H Kumamoto, K Ooya. Immunohistochemical analysis of bcl-2 family proteins in benign and malignant ameloblastomas. J Oral Pathol Med. 1999;28(8):343–349. doi: 10.1111/j.1600-0714.1999.tb02051.x. [DOI] [PubMed] [Google Scholar]
- 20.N Inaba, N Sato, M Ijichi, I Fukazawa, A Nito, H Takamizawa, G Luben, H Bohn. The immunocytochemical location of two membrane-associated placental tissue proteins in human and cynomolgus monkey placentae. Tumour Biol. 1984;5(2):75–85. [PubMed] [Google Scholar]
- 21.CP Reutelingsperger, G Hornstra, HC Hemker. Isolation and partial purification of a novel anticoagulant from arteries of human umbilical cord. Eur J Biochem. 1985;151(3):625–629. doi: 10.1111/j.1432-1033.1985.tb09150.x. [DOI] [PubMed] [Google Scholar]
- 22.A Iwasaki, M Suda, H Nakao, T Nagoya, Y Saino, K Arai, et al. Structure and expression of cDNA for an inhibitor of blood coagulation isolated from human placenta: a new lipocortin-like protein. J Biochem. 1987;102(5):1261–1273. doi: 10.1093/oxfordjournals.jbchem.a122165. [DOI] [PubMed] [Google Scholar]
- 23.R Kaplan, M Jaye, WH Burgess, DD Schlaepfer, HT Haigler. Cloning and expression of cDNA for human endonexin II, a Ca2+ and phospholipid binding protein. J Biol Chem. 1988;263(17):8037–8043. [PubMed] [Google Scholar]
- 24.I Maurer-Fogy, CP Reutelingsperger, J Pieters, G Bodo, C Stratowa, R Hauptmann. Cloning and expression of cDNA for human vascular anticoagulant, a Ca2+-dependent phospholipid-binding protein. Eur J Biochem. 1988;174(4):585–592. doi: 10.1111/j.1432-1033.1988.tb14139.x. [DOI] [PubMed] [Google Scholar]
- 25.G Koopman, CP Reutelingsperger, GA Kuijten, RM Keehnen, ST Pals, MH van Oers. Annexin V for flow cytometric detection of phosphatidyl -serine expression on B cells undergoing apoptosis. Blood. 1994;84(5):1415–1420. [PubMed] [Google Scholar]
- 26.M van Engeland, LJ Nieland, FC Ramaekers, B Schutte, CP Reutelingsperger. Annexin V-affinity assay: a review on an apoptosis detection system based on phosphatidylserine exposure. Cytometry. 1998;31(1):1–9. doi: 10.1002/(sici)1097-0320(19980101)31:1<1::aid-cyto1>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]


