Abstract
Background: E. faecalis is the predominant micro-organism recovered from root canal of the teeth where previous endodontic treatment has failed. Thorough debridement and complete elimination of micro-organisms are objectives of an effective endodontic treatment. For many years, intracanal irrigants have been used as an adjunct to enhance antimicrobial effect of cleaning and shaping in endodontics. The constant increase in antibiotic-resistant strains and side-effects of synthetic drugs has promoted researchers to look for herbal alternatives. For thousands of years humans have sought to fortify their health and cure various illnesses with herbal remedies, but only few have been tried and tested to withstand modern scientific scrutiny. The present study was aimed to evaluate alternative, inexpensive simple and effective means of sanitization of the root canal systems. The antimicrobial efficacy of herbal alternatives as endodontic irrigants is evaluated and compared with the standard irrigant sodium hypochlorite. Materials & Methods: Neem leaf extracts, grape seed extracts, 3% Sodium hypochlorite, absolute ethanol, Enterococcus faecalis (ATCC 29212) cultures, Brain heart infusion media. The agar diffusion test was performed in brain heart infusion media and broth. The agar diffusion test was used to measure the zone of inhibition. Results: Neem leaf extracts and grape seed extracts showed zones of inhibition suggesting that they had anti-microbial properties. Neem leaf extracts showed significantly greater zones of inhibition than 3% sodium hypochlorite. Also interestingly grape seed extracts showed zones of inhibition but were not as significant as of neem extracts. Conclusion: Under the limitations of this study, it was concluded that neem leaf extract has a significant antimicrobial effect against E. faecalis. Microbial inhibition potential of neem leaf extract observed in this study opens perspectives for its use as an intracanal medication. How to cite this article: Ghonmode WN, Balsaraf OD, Tambe VH, Saujanya KP, Patil AK, Kakde DD. Comparison of the antibacterial efficiency of neem leaf extracts, grape seed extracts and 3% sodium hypochlorite against E. feacalis – An in vitro study. J Int Oral Health 2013; 5(6):61-6 .
Key words: : Agar diffusion test, E. feacalis, endodontic irrigants, grape seed extracts, neem leaf extracts, zone of inhibition.
Introduction
The objective of root canal treatment is to thoroughly clean the root canal system debride it, so that it can be sealed without microleakge. This process is also called as chemico-mechanical process in which the canal is derided with the help of chemical irrigants and mechanical instruments. 1 Most widely used irrigant is sodium hypochlorite which is used in different concentrations varying from 1-6%. 2 Other than sodium hypochlorite various other substances are used to to remove the smear layer which is formed. 3 The use of smear layer-dissolving agents can also remove the bacterial toxins and reduce the chances of survival and multiplication of microbes. 4 EDTA is most commonly used for removing the smear layer. NaOCl has many draw-backs like unpleasant taste, cytotoxicity and inability to dissolve the smear layer. Also various studies have shown that NaOCl has is unable to penetrate at greater depths in dentin and long-term exposure of dentin to high concentrations of sodium hypochlorite can have a detrimental effect on dentin elasticity and flexural strength, thereby predisposing the tooth to vertical fracture. 5 , 6 The constant increase in antibiotic resistant strains and side effects caused by synthetic drugs has prompted researchers to look for herbal alternatives.
Recently, much attention has been focused in the literature regarding antimicrobial regimens against pathogens associated with endodontic retreatment. 7 , 8 However, the great majority of endodontic disease is primary in nature. The role of bacteria in the development in apical periodontitis has been well established. 9 Numerous studies have shown that the bacterial flora in endodontic infections is polymicrobial with a predominance of anaerobic species. 10 , 11 Using advanced anaerobic bacteriological techniques, Lana et al. showed a polymicrobial environment in necrotic teeth that consisted of obligate and facultative anaerobes, microaerophilic bacteria and yeast. 12 Eradication of these microorganisms in the root canal space is paramount in preventing them from reaching the periapical tissues. With such a complex and dynamic microbial environment in the root canal system, selection of an effective antibacterial agent to use during treatment is critical.
Antimicrobial solutions must possess many qualities such as the ability to penetrate the infected site, to suppress or destroy microbial growth, and to avoid the possible development of resistance to the agent. 13 Sodium hypochlorite [NaOCl] is a commonly used irrigating solution that has been shown to have both antimicrobial and tissue dissolving properties. 14 , 15 However, there is concern about its possible toxic effect on the periapical tissues at higher concentrations. At lower concentrations, however, not only is its tissue dissolving ability reduced, but its antimicrobial effectiveness as well. 16 , 17
The purpose of this study was to compare the in vitro effectiveness of neem leaf extracts, grape seed extracts and 3% sodium hypochlorite against E. feacalis by agar diffusion testing.
Aim and Objectives
The purpose of this in-vitro study was to compare the antimicrobial activity of neem leaf extracts, grape seed extracts and 3% sodium hypochlorite against E. feacalis using agar disc diffusion tests.
Methods and Materials
Neem leaf extracts, grape seed extracts, 3% Sodium hypochlorite, absolute ethanol, normal saline, Enterococcus faecalis (ATCC 29212) cultures, Brain heart infusion media were the materials used in the study.
Preparation of neem leaf extracts
Mature fresh Azadirachta indica leaves were collected from the medicinal garden of SMBT dental college & hospital & taxonomic identification of the plant was performed. Leaves were washed in sterilized distilled water and weighed in sterile disposable cup. 25 gms of fresh neem leaves were added to 50 ml of absolute ethanol. Mixture as macerated for 1-2 min, extract was filtered with muslin cloth for coarse residue. Extraction process was repeatedagain using coarse residue and 25 ml ethanol.
Both the extracts were pooled together and filtered through fast filter paper. Alcohol part was removed from the extract on water bath till the volume was about 25ml. Extract was kept ready and stored in airtight container.
Preparation of grape seed extracts
Commercially available grape seed extracts were used in the study and were mixed with 25 ml absolute ethanol. The extract was then filtered through muslin cloth for coarse residue. Extraction process was repeated using coarse residue and 25 ml. Alcohol
part was removed from the extract on water bath till the volume was about 25 ml. Extract was prepared and stored in airtight colored container.
All the samples were divided into 5 groups as follows:
Group 1: Neem leaf extracts
Group 2: Grape seed extracts
Group 3: 3% Sodium Hypochlorite
Group 4: Ethanol (Control)
Group 5: Saline (Control)
Agar- diffusion test
Cultures of E. faecalis were maintained on brain heart infusion (BHI) broth and agar. Cultures grown overnight at 37 0 C in brain heart infusion (BHI) broth on a rotary shaker (150 rpm) and bacterial growth was checked by changes in turbidity after 24hrs.
To check the antimicrobial efficacy of neem leaf extract, grape seed extracts 3% NaOCl, ethanol and saline; agar well diffusion method was performed. BHI agar plates were prepared and cultures (200μl) were spread on agar plates. Wells of 6mm diameter were made in the agar surfaces. Neem leaf extract, grape seed extracts 3% NaOCl and controls, each 50μl, were added to the respective wells and the plates were incubated for 24hrs at 37 0 C in an incubator. Controls used in the study were ethanol and saline. After incubation period, plates were removed and zones of inhibition were recorded. The inhibition zones were recorded and the experiment was carried out simultaneously on three culture plates.
Results
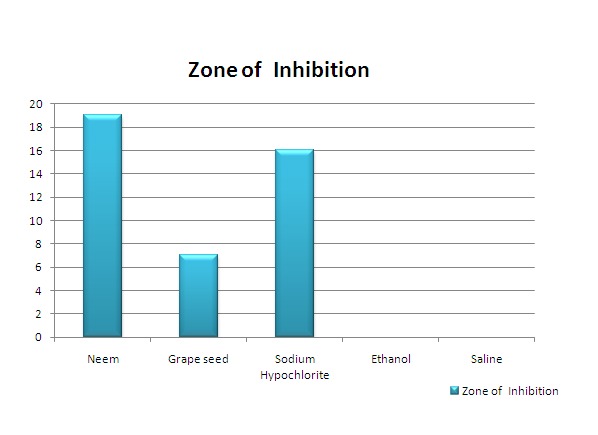
The zones of inhibition were recorded as shown in Figure No. 1 . The results were tabulated and statistically analyzed using analysis of variance (ANOVA). In Table No. 1 , ANOVA shows that there is significant difference between the zone diameters of neem leaf extracts, grape seed extracts and 3% sodium hypochlorite against E. faecalis (p<0.05). Significant difference was observed between zone of inhibition for Neem leaf extract and 3% NaOCl.
Figure: 1A and 1B: Agar disc diffusion test demonstrating the culture plates before and after completion of the diffusion test.
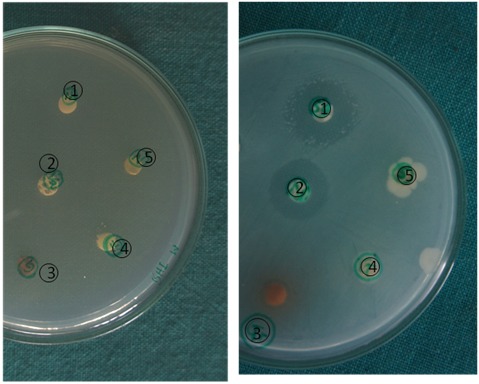
Table 1: Statistical analysis of the zone of inhibitions.(Mean values)
| Group | Extracts | Mean +/- S.D. |
| 1 | Neem leaf extracts | 19.57+/- 1.93 |
| 2 | Grape seed extracts | 7.34 +/- 0.27 |
| 3 | 3% NaOCl | 16.34 +/- 0.27 |
| 4 | Ethanol | 0mm |
| 5 | Saline | 0mm |
| 6. | Statistical Analysis [p value] |
p < 0.05 Significant |
Discussion
Several studies on the antimicrobial activity of irrigation solutions in endodontics, such as 0.5%,1%, 2.5%, 5% NaOCl are found in literature. 16 , 17 On the other hand, lack of studies on phytotherapic substances such as chlorophyll, propolis, morinda citrifolia juice, neem and grape seed extracts do not permit more objective conclusions about their use. Several pharmacological activities and medicinal
Graph 1: Graphs shows the statistical analysis of the zones of inhibition in disc diffusion tests.
applications of various parts of neem are well known. Interest on this substance is based on its properties like antibacterial, antifungal, antiviral, antioxidant, antiinfl-ammatory, antipyretic, analgesic, immunostimulant. 18 - 20 Inclusion of E. faecalis in this study was based on the literature that relates these micro-organisms to pulp infections, mainly in recalcitrant infections after endodontic treatment. 21 , 22 Methodology of this study followed the standard established for agar dilution tests. Study design presented in this paper is more consistent with other studies testing ability of antimicrobial action. 22 - 24 The use of best possible irrigant during chemicomechanical preparation is of great importance. 25 Also Botelho et al and Singhal et al in their experiments concluded that Azadirachta indica is highly efficacious in the treatment of periodontal disease thus exhibiting its biocompatibility with human PDL fibroblasts. 26 , 27 Ideal irrigant should combine antimicrobial action and a capacity to dissolve organic and inorganic remnants. NaOCl in full concentration is well known for its bactericidal action and cytotoxicity. 23 , 28 , 29 Moreover, its anti-adherence activity by altering bacterial adhesion and ability of organism to colonize, also stimulated the study of this substance. 30 , 31 Use of neem as an endodontic irrigant might be advantageous because it is a biocompatible antioxidant and thus not likely to cause severe injuries to patients that might occur via NaOCl accidents. Bitter taste associated with this plant can be altered by different formulations due to addition of sweeteners and flavors to increase the patient compliance and acceptability. 27 , 31
The results obtained in this in vitro study showed that neem leaf extract is a viable medicament against E. faecalis . Although 3% NaOCl showed comparatively less antimicrobial effect than neem, it still had an observable effectiveness against test organisms. Also grape seed extracts showed some amount of anti-bacterial activity but was not as significant as neem or sodium hypochlorite.
Conclusion
Under the limitations of this study, it was concluded that neem leaf extract has a significant antimicrobial effect against E. faecalis . Microbial inhibition potential of neem leaf extracts and grape seed extracts observed in this study opens new perspectives for its use as an intracanal medication. However, preclinical and clinical trials are needed to evaluate biocompatibility & safety before neem leafextracts and grape seed extracts can conclusively be recommended as an intracanal irrigating solution, but in vitro observation of both shows promising effectiveness. As the global scenario is now changing towards the use of non toxic plant products that have traditional medicinal use, extensive research and developmental work therefore should be undertaken on neem leaf extracts and grape seed extracts and its products for their better economic and therapeutic utilization.
Footnotes
Source of Support: Nil
Conflict of Interest: None Declared
Contributor Information
Wasudeo Namdeo Ghonmode, Department of Conservative Dentistry & Endodontics, SMBT Dental College & Hospital, Sangamner, Maharashtra, India.
Omkar D Balsaraf, Department of Conservative Dentistry & Endodontics, SMBT Dental College & Hospital, Sangamner, Maharashtra, India.
Varsha H Tambe, Department of Conservative Dentistry & Endodontics, SMBT Dental College & Hospital, Sangamner, Maharashtra, India.
K P Saujanya, Department of Conservative Dentistry & Endodontics, SMBT Dental College & Hospital, Sangamner, Maharashtra, India.
Ashishkumar K Patil, Department of Conservative Dentistry & Endodontics, SMBT Dental College & Hospital, Sangamner, Maharashtra, India.
Deepak D Kakde, Department of Conservative Dentistry & Endodontics, SMBT Dental College & Hospital, Sangamner, Maharashtra, India.
References
- 1.JF Siqueira, Jr, IN Rocas, FN Riche, JC Provenzano. Clinical outcome of the endodontic treatment of teeth with apical periodontitis using an antimicrobial protocol. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;106:757–762. doi: 10.1016/j.tripleo.2008.06.007. [DOI] [PubMed] [Google Scholar]
- 2.JF Siqueira, Jr., IN Rocas, A Favieri, KC Lima. Chemomechanical reduction of the bacterial population in the root canal after instrumentation and irrigation with 1%, 2.5% and 5.25% sodium hypochlorite. J Endod. 2002;26:331–334. doi: 10.1097/00004770-200006000-00006. [DOI] [PubMed] [Google Scholar]
- 3.S Lottanti, H Gautschi, B Sener, M Zehnder. Effects of ethylenediaminetetraacetic, etidronic and peracetic acid irrigation on human root dentine and the smear layer. Int Endod J. 2009;42:335–343. doi: 10.1111/j.1365-2591.2008.01514.x. [DOI] [PubMed] [Google Scholar]
- 4.M Torabinejad, R Handysides, AA Khandesi, LK Bakland. Clinical implications of the smear layer in endodontics: a review. Oral Surg Oral Med Oral Pathol Oral Radiol Endodontol. 2002;94:658–666. doi: 10.1067/moe.2002.128962. [DOI] [PubMed] [Google Scholar]
- 5.L Giardino, E Ambu, E Savoldi, R Rimondini, C Cassanelli, EA Debbia. Comparative evaluation of antimicrobial efficacy of sodium hypochlorite, MTAD, and Tetraclean against Enterococcus faecalis biofilm. J Endod. 2007;33:852–855. doi: 10.1016/j.joen.2007.02.012. [DOI] [PubMed] [Google Scholar]
- 6.M Zehnder. Root canal irrigants. J Endod. 2006;32:389–398. doi: 10.1016/j.joen.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 7.HH Hancock, A Sigurdsson, M Trope, J Moiseiwitsch. Bacteria isolated after unsuccessful endodontic treatment in a North American population. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2001;91:579–586. doi: 10.1067/moe.2001.113587. [DOI] [PubMed] [Google Scholar]
- 8.AJ Moller, L Fabricius, G Dahlen, AE Ohman, G Heyden. Influence on periapical tissues of indigenous oral bacteria and necrotic pulp tissue in monkeys. Scand J Dent Res. 1981;89:475–484. doi: 10.1111/j.1600-0722.1981.tb01711.x. [DOI] [PubMed] [Google Scholar]
- 9.S Kakehashi, HR Stanley, RJ Fitzgerald. The effects of surgical exposures of dental pulps in germ-free and conventional laboratory rats. Oral Surg Oral Med Oral Pathol. 1965;20:340–349. doi: 10.1016/0030-4220(65)90166-0. [DOI] [PubMed] [Google Scholar]
- 10.G Sundqvist, E Johansson, U Sjogren. Prevalence of black-pigmented bacteroides species in root canal infections. J Endod. 1989;15:13–19. doi: 10.1016/S0099-2399(89)80092-5. [DOI] [PubMed] [Google Scholar]
- 11.M Haapasalo, H Ranta, K Ranta, H Shah. Black-pigmented Bacteroides sp. In human apical periodontitis. Infect Immun. 1986;53:149–153. doi: 10.1128/iai.53.1.149-153.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.MA Lana, AP Ribeiro-Sobrinho, R Stehling, GD Garcia, BK Silva, JS Hamdan, JR Nicoli, MA Carvalho, M Farias Lde. Microorganisms isolated from root canals presenting necrotic pulp and their drug susceptibility in vitro. Oral Microbiol Immunol. 2001;16:100–105. doi: 10.1034/j.1399-302x.2001.016002100.x. [DOI] [PubMed] [Google Scholar]
- 13.C Estrela, CR Estrela, EL Barbin, JC Spano, MA Marchesan, JD Pecora. Mechanism of action of sodium hypochlorite. Braz Dent J. 2002;13:113–117. doi: 10.1590/s0103-64402002000200007. [DOI] [PubMed] [Google Scholar]
- 14.A Bystrom, G Sundqvist. Bacteriologic evaluation of the effect of 0.5 percent sodium hypochlorite in endodontic therapy. Oral Surg Oral Med Oral Pathol. 1983;55:307–312. doi: 10.1016/0030-4220(83)90333-x. [DOI] [PubMed] [Google Scholar]
- 15.G Hasselgren, B Olsson, M Cvek. Effects of calcium hydroxide and sodium hypochlorite on the dissolution of necrotic porcine muscle tissue. J Endod. 1988;14:125–127. doi: 10.1016/S0099-2399(88)80212-7. [DOI] [PubMed] [Google Scholar]
- 16.RE Hand, ML Smith, JW Harrison. Analysis of the effect of dilution on the necrotic tissue dissolution property of sodium hypochlorite. J Endod. 1978;4:60–64. doi: 10.1016/S0099-2399(78)80255-6. [DOI] [PubMed] [Google Scholar]
- 17.JW Harrison, RE Hand. The effect of dilution and organic matter on the anti-bacterial property of 5.25% sodium hypochlorite. J Endod. 1981;7:128–132. doi: 10.1016/S0099-2399(81)80127-6. [DOI] [PubMed] [Google Scholar]
- 18.K Biswas, I Chattopadhyay, RK Banerjee, U Bandyopadhyay. Biological activities and medicinal properties of neem (Azadirachta indica) Curr Sci. 2002;82(11):1336–1345. [Google Scholar]
- 19.R Pritima, R Pandian, A Antimicrobial potency of crude extracts of Azadirachta indica A. Juss (leaf) against microbes causing reproductive tract infections among women, Curr Biotica. 2008;2(2):193–199. [Google Scholar]
- 20.R Subapriya, S Nagini. Medicinal properties of neem leaves: a review. Curr Med Chem Anti Cancer Agent. 2005;5:146–149. doi: 10.2174/1568011053174828. [DOI] [PubMed] [Google Scholar]
- 21.L Maekawa, R Lamping, M Marcacci, M Maekawa, M Nassri, C Koga-Ito. Antimicrobial activity of Chlorophyll based solutions on candida albicans and E. faecalis. Rev Sul Bras Odontol. 2007;4(2):36–40. [Google Scholar]
- 22.J Davis, J Maki, J Babcall. In vitro comparison of antimicrobial effects of various endodontic medicaments on Enterococcus faecalis. J Endod. 2007;33(5):567–569. doi: 10.1016/j.joen.2007.01.015. [DOI] [PubMed] [Google Scholar]
- 23.H Ayhan, N Sultan, M Cirak, M Ruhi, H Bodur. Antimicrobial effects of various endodontic medicaments on selected microorganisms. Int Endod J. 1999;32:99–102. doi: 10.1046/j.1365-2591.1999.00196.x. [DOI] [PubMed] [Google Scholar]
- 24.P Murray, R Farber, K Namerow, S Kuttler, F Garcia-Godoy. Evaluation of Morinda citrifolia as an endodontic irrigant. J Endod. 2008;34(1):65–70. doi: 10.1016/j.joen.2007.09.016. [DOI] [PubMed] [Google Scholar]
- 25.Z Mohammadi. Sodium Hypochlorite in Endodontics: An Update Review. Int Dent J. 2008;58:329–341. doi: 10.1111/j.1875-595x.2008.tb00354.x. [DOI] [PubMed] [Google Scholar]
- 26.S Anurag, G Anuraag, G Chandrawati. Comparison of antimicrobial efficacy of conventional irrigants and herbal products alone and with calcium hydroxide against entercoccus faecalis. Guident. 2012;5(2):77–79. [Google Scholar]
- 27.M Botelho, AD Santos, J Martins, C Carvalho, M Paz, C Azenha, R Ruela, D Queiroz, W Ruela, G Marino, F Ruela. Efficacy of a mouthrinse based on leaves of neem in the treatment of patients with chronic gingivitis. J Med Plants Res. 2008;2(11):341–346. [Google Scholar]
- 28.J Pereira, D Bergamo, S Franca, R Pietro, Y Silva-Sousa. Antimicrobial activity of articum lappa against microorganisms commonly found in endodontic infections. Braz Dent J. 2005;16(3):192–196. doi: 10.1590/s0103-64402005000300004. [DOI] [PubMed] [Google Scholar]
- 29.CR Gernhardt, K Eppendorf, A Kozlowski, M Brandt. Toxicity of concentrated sodium hypochlorite used as an endodontic irrigant. Int Endod J. 2004;37:272–280. doi: 10.1111/j.0143-2885.2004.00804.x. [DOI] [PubMed] [Google Scholar]
- 30.S Balasenthil, S Arivazhagan, CR Ramachandran, V Ramachandran, S Nagini. Chemopreventive potential of neem (Azadirachta indica) on 7,12- dimethylbenz [a] anthracene (DMBA) induced hamster buccal pouch carcinogenesis. J Ethnopharmacol. 1999;67(2):189–195. doi: 10.1016/s0378-8741(99)00015-x. [DOI] [PubMed] [Google Scholar]
- 31.S Polaquini, T Svidzinski, C Kemmelmeier, A Gasparetto. Effect of aqueous extract from Neem (Azadirachta indica A. Juss) on hydrophobicity, biofilm formation and adhesion in composite resin by Candida albicans. Arch Oral Biol. 2006;51(6):482–490. doi: 10.1016/j.archoralbio.2005.11.007. [DOI] [PubMed] [Google Scholar]


