Abstract
In a flexible nanocomposite-based nanogenerator, in which piezoelectric nanostructures are mixed with polymers, important parameters to increase the output power include using long nanowires with high piezoelectricity and decreasing the dielectric constant of the nanocomposite. Here, we report on piezoelectric power generation from a lead-free LiNbO3 nanowire-based nanocomposite. Through ion exchange of ultra-long Na2Nb2O6-H2O nanowires, we synthesized long (approximately 50 μm in length) single-crystalline LiNbO3 nanowires having a high piezoelectric coefficient (d33 approximately 25 pmV-1). By blending LiNbO3 nanowires with poly(dimethylsiloxane) (PDMS) polymer (volume ratio 1:100), we fabricated a flexible nanocomposite nanogenerator having a low dielectric constant (approximately 2.7). The nanogenerator generated stable electric power, even under excessive strain conditions (approximately 105 cycles). The different piezoelectric coefficients of d33 and d31 for LiNbO3 may have resulted in generated voltage and current for the e33 geometry that were 20 and 100 times larger than those for the e31 geometry, respectively. This study suggests the importance of the blending ratio and strain geometry for higher output-power generation in a piezoelectric nanocomposite-based nanogenerator.
PACS
77.65.-j; 77.84.-s; 73.21.Hb
Keywords: High and stable electric power, Lead-free LiNbO3 nanowire, Nanocomposite nanogenerator
Background
Lead-based piezoelectric materials, such as Pb(Zr,Ti)O3 and Pb(Mg,Nb)O3-PbTiO3, have been utilized for the last several decades in actuators, transducers, and sensor applications [1]. As the restriction of hazardous substances becomes an emerging issue, however, much attention has been paid to lead-free piezoelectric materials having a perovskite structure [2]. Among the candidates to replace toxic lead-based piezoelectric materials, alkaline niobates, such as (K,Na,Li)NbO3, are regarded as one of the most appropriate materials due to their high Curie temperature, piezoelectric coefficient, and electromechanical coupling coefficient [3,4].
In addition to nanoelectromechanical system (NEMS) applications, one of the most challenging applications of nanosize lead-free piezoelectric materials is the nanogenerator, which can effectively convert ubiquitous mechanical vibrations into electricity [5]. Due to the low power consumption of modern devices, lead-free piezoelectric nanostructure-based nanogenerators could be a powerful alternative to batteries. Until recently, several nanogenerators have been reported using BaTiO3, ZnSnO3, Pb(Zr,Ti)O3, Pb(Mg,Nb)O3-PbTiO3, and (K,Na)NbO3[6-11]. In particular, piezoelectric nanocomposite devices, in which piezoelectric nanostructures are mixed with flexible polymers, have exhibited relatively easy, cost-effective fabrication, and high-power generation [9-13]. In a flexible nanocomposite-based nanogenerator, important parameters to increase the output power include using long nanowires with high piezoelectricity and decreasing the dielectric constant of the nanocomposite [9].
In this paper, we report on piezoelectric power generation from a lead-free LiNbO3 nanowire-based composite device. As for the nanogenerator applications, LiNbO3 has several merits such as small dielectric constant, relatively high piezoelectric constant, and thermal stability [14,15]. Through successful ion exchange in micro-porous Na2Nb2O6-H2O nanowires, we synthesized long (approximately 50 μm) LiNbO3 nanowires having high piezoelectricity (approximately 25 pmV-1). By mixing LiNbO3 and poly(dimethylsiloxane) (PDMS) (in a volume ratio of 1:100, respectively), we fabricated a flexible nanogenerator having a low dielectric constant for the e33 and e31 geometries. For a similar value of strain, we note that the open-circuit voltage and closed-circuit current for the e33 geometry were 20 and 100 times larger than those for the e31 geometry, respectively. For up to 105 cycles of strain, we observed that the generated power was quite stable; the dielectric constant and electric loss did not change significantly.
Methods
High-quality LiNbO3 nanowires were synthesized using a three-step procedure. First, we obtained microporous Na2Nb2O6-H2O nanowires by a hydrothermal method. NaOH (12 M) was dissolved in 20 mL of distilled water; 0.113 M of Nb2O5 was then added to the NaOH solution. The solution was stirred and transferred into a 25-mL Teflon lining in a stainless steel autoclave to undergo a hydrothermal reaction at 120°C for 5 h. In the second step, we obtained Li2Nb2O6-H2O nanowires using the ion-exchange method. LiCl (20 M) was dissolved in 20 mL of distilled water. Na2Nb2O6-H2O nanowires were added to the LiCl solution. After stirring for 20 h, the stirred solution was filtered, washed with distilled water, and dried at 80°C for 12 h. In the third step, LiNbO3 nanowires were obtained after heating the ion-exchanged Li2Nb2O6-H2O nanowires at 500°C for 2 h.
The crystalline structure of the nanowires was characterized by high-resolution X-ray diffraction (HR-XRD), field-emission scanning electron microscopy (FE-SEM), and field-emission transmission electron microscopy (FE-TEM) measurements. To characterize the detailed crystal structure and symmetry, we performed neutron diffraction measurements and a Rietveld analysis. We used piezoresponse force microscopy (PFM) to investigate the piezoelectricity and piezoelectric/ferroelectric domains of the LiNbO3 nanowires. The PFM measurements were performed using an atomic force microscope at 1 V and 73 kHz. To scan the surface, we used Pt/Ir-coated tips and a force constant of 3 Nm-1. Before scanning, we thoroughly dispersed and tightly attached the nanowires to the Pt-coated Si substrate using a polymer (5 wt.% poly(vinylpyrrolidone) dissolved in ethanol). The LiNbO3 nanowires were then coated with 10-nm-thick Pt to obtain a uniform electric field and to minimize electrostatic effects.
To fabricate the nanocomposite nanogenerator, the LiNbO3 nanowires were thoroughly mixed with PDMS at a volume ratio of 1:100. (We noted that LiNbO3 nanowires were not mixed well with PDMS for an increased volume ratio of 2:100.) Small amounts of the mixture were spin-coated onto an Au/Cr-coated Kapton polyimide film at 500 rpm for 10 s. The 25-nm-thick Au and 10-nm-thick Cr films were deposited onto the Kapton film by thermal evaporation. Another Au/Cr-coated Kapton film was attached to the top surface of the spin-coated LiNbO3-PDMS composite for the electrode. Finally, polyester (PS) film was attached to the bottom Kapton film. The thicknesses of the Kapton and PS films were 125 and 500 μm, respectively. We applied an electric field of approximately 100 kV · cm-1 for electric poling at room temperature [16].
To measure the Young’s modulus of the LiNbO3-PDMS composite, we used a nanoindenter with a Berkovich tip, and applied the continuous stiffness measurement option. A linear motor was used to periodically apply and release compressive force at a frequency of 0.8 Hz. The pushing and bending amplitudes were varied over the course of the measurement. The output signal of the piezoelectric device was recorded by low-noise voltage and current preamplifiers.
Results and discussion
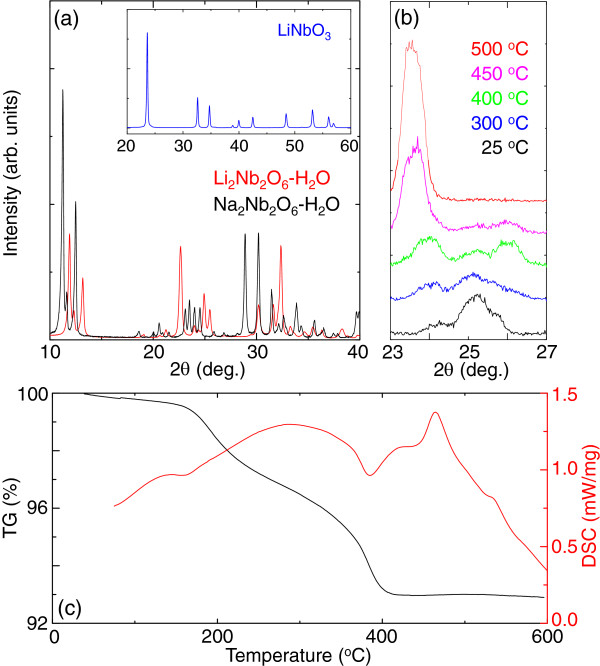
Microporous Na2Nb2O6-H2O nanowires seem to be an excellent template for ion exchange [17]. Due to the smaller ionic size of the lithium ion (Li+) compared with the sodium ion (Na+), as well as the excessive amount of LiCl (i.e., approximately 20 M), all of the Na+ appeared to be involved in the exchange with Li+ in Na2Nb2O6-H2O. Figure 1a compares the XRD pattern of Li2Nb2O6-H2O and Na2Nb2O6-H2O. The overall XRD pattern of Li2Nb2O6-H2O was quite different from that of Na2Nb2O6-H2O. From an inductive-coupled plasma (ICP) measurement of Li2Nb2O6-H2O, we did not find any trace of Na+ within the experimental limits. These results imply that crystalline Li2Nb2O6-H2O could be obtained from Na2Nb2O6-H2O through an ion exchange process.
Figure 1.
Phase transformation from Li2Nb2O6-H2O to LiNbO3. High-resolution X-ray diffraction (HR-XRD) patterns of Li2Nb2O6-H2O at (a) room temperature and (b) elevated temperatures. In (a), we show the XRD patterns of Na2Nb2O6-H2O and LiNbO3 for comparison. (c) Thermogravimetric (TG) and differential scanning calorimetry (DSC) results for Li2Nb2O6-H2O.
In Figure 1b, we show in-situ XRD patterns of Li2Nb2O6-H2O at elevated temperatures. The diffraction patterns of Li2Nb2O6-H2O were significantly modified with an increase in temperature, especially above 400°C, and exhibited an irreversible phase transformation. In the inset of Figure 1a, we show the XRD pattern after heat treatment of Li2Nb2O6-H2O. We note that the XRD pattern obtained after heat treatment was well indexed by LiNbO3. To the best of our knowledge, this is the first report for the synthesis of LiNbO3 nanowire through ion exchange and subsequent heat treatment.
To gain insight into the phase transformation from Li2Nb2O6-H2O to LiNbO3, we show the thermogravimetric (TG) and differential scanning calorimetry (DSC) results in Figure 1c. The mass of Li2Nb2O6-H2O changed significantly near 400°C and was accompanied by endothermic reactions at the same temperature. After the endothermic reactions, an exothermic reaction occurred near 460°C without a noticeable change in the mass. Comparing the well-known phase transformation mechanism from Na2Nb2O6-H2O to NaNbO3[18], the peaks at 400°C and 460°C corresponded well to the dehydration of H2O from Li2Nb2O6-H2O and the structural transformation from Li2Nb2O6 to LiNbO3, respectively. (The broad change in the mass near 220°C seems to have originated from the desorption of surface/lattice-absorbed hydroxyl defects [19]).
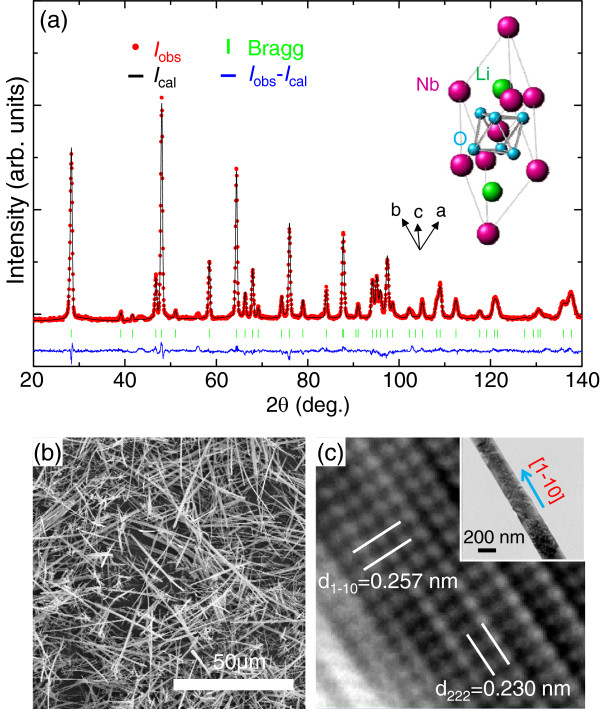
Due to the light Li ions, we used neutrons rather than X-rays to determine the detailed crystal structure of LiNbO3. Figure 2a shows a Rietveld analysis of the neutron diffraction pattern of LiNbO3. The neutron diffraction pattern of LiNbO3 was well-fit by the trigonal structure (a = 5.488 Å, α = 55.89°) with R3c symmetry. The resulting lattice constant (angle) of the LiNbO3 nanostructure was slightly smaller (larger) than that of the LiNbO3 single crystal (a = 5.492 Å, α = 55.53°) [20]. Based on the Rietveld analysis, we show the crystal structure of LiNbO3 in the inset of Figure 2a. The Nb ions in the NbO6 octahedra shifted toward the [111] direction, hence initiating the spontaneous formation of electric polarization without applying an electric field.
Figure 2.
Structural characterization of LiNbO3. (a) Rietveld analysis of neutron diffraction patterns of LiNbO3. The red dots represent the observed intensity. The black lines represent the calculated intensity. The blue line corresponds to the difference between the observed and calculated intensities. The green line shows the Bragg reflection. In the inset of (a), we show the crystal structure of LiNbO3. (b) Field-emission scanning electron microscopy (FE-SEM) and (c) high-resolution transmission electron microscopy (HR-TEM) images of LiNbO3. In the inset of (c), we show a medium-resolution TEM image of a LiNbO3 nanowire.
Figure 2b,c shows FE-SEM and HR-TEM images of LiNbO3, respectively. All of the LiNbO3 samples had nanowire morphology, with a high aspect ratio of 160 to 600 (width 100 to 250 nm; length 40 to 60 μm). Note that the LiNbO3 nanowires, synthesized using the molten salt method, had a relatively short length (<10 μm) [21]. The clear lattice fringe indicated the single-crystalline quality of the LiNbO3 nanowires. Based on the Rietveld analysis, the LiNbO3 nanowires appeared to grow along the [1-10] direction.
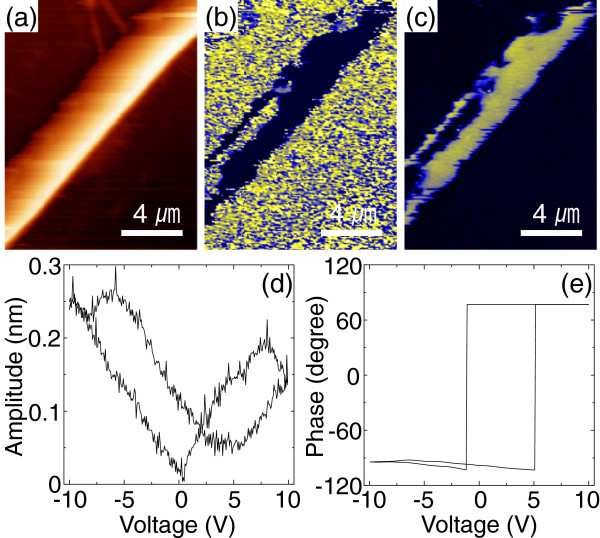
To investigate the piezoelectricity of the LiNbO3 nanowires, we used PFM. Figure 3a,b,c shows the topography, amplitude, and phase of the piezoelectric response of a single LiNbO3 nanowire, respectively. The brightness of the amplitude map represents the strength of the piezoelectric response; the contrast of the phase map corresponds to the direction of the electric polarization in the nanowire. From Figure 3b,c, the piezoelectric domains in the LiNbO3 nanowire were clearly evident.
Figure 3.
Piezoelectricity/ferroelectricity of the LiNbO3 nanowire. (a) Topography, (b) piezoelectric amplitude, and (c) piezoelectric phase for a LiNbO3 nanowire. Applied voltage dependences of (d) piezoelectric amplitude and (e) piezoelectric phase.
Figure 3d,e shows the switching of the piezoelectric/ferroelectric amplitude and phase with the application of direct-current (dc) voltage. An abrupt change in the phase suggests the switching of domains in LiNbO3, which is generally associated with ferroelectric behavior [22]. We estimated the piezoelectric coefficient d33 value from the linear portion of the piezoresponse amplitude signal as approximately 25 pmV-1.
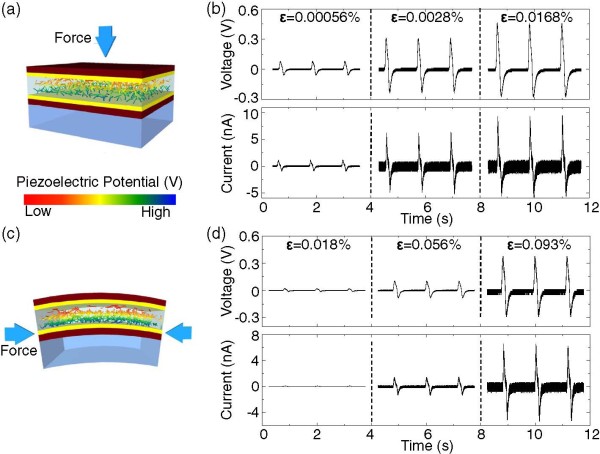
After confirming the piezoelectricity/ferroelectricity of the LiNbO3 nanowire, we fabricated a composite nanogenerator for the e33 and e31 geometries, as schematically shown in Figure 4a,c, respectively. Even though the LiNbO3 nanowires were randomly distributed inside the PDMS polymer, the piezoelectric/ferroelectric domains could be vertically aligned after applying a strong electric field for poling. If we were to apply stress, then the nanowires would be subjected to compressive strain, which would induce a piezoelectric potential due to the piezoelectricity of LiNbO3. To screen the piezoelectric potential, positive and negative charges would accumulate at the top and bottom electrodes, respectively. Once the strain is released, the piezoelectric potential should diminish and the accumulated charges should move back in the opposite direction. Therefore, the continuous application and release of the strain will result in an alternating voltage and current [23].
Figure 4.
Schematic diagram and power generation for the LiNbO3-PDMS composite nanogenerator. Schematic diagram of the LiNbO3-PDMS composite nanogenerator for (a)e33 and (c)e31 geometries. Dark brown, yellow, and light blue represent the Kapton film, Au/Cr electrode, and PS film, respectively. The rainbow color of the LiNbO3 nanowires represents the piezoelectric potential after the stress application. The open-circuit voltage (V) and closed-circuit current (I) at selected strains for (b)e33 and (d)e31 geometries.
To quantify the strain (ϵ), we used Young’s modulus, Y, of the LiNbO3-PDMS, Kapton, and PS films, having values of 0.87, 2.5, and 3.25 GPa, respectively [24]. The strain for the e33 geometry was then calculated using the equation ϵ = P/Y, where P represents the applied pressure. To quantify the strain for the e31 geometry, we calculated the strain neutral line from the equation ΣY i t i y i = 0 (for i = 1 to 4), where t and y represent the thickness of each layer and the distance from the strain neutral line to the center of each layer, respectively. The strain for the e31 geometry was obtained using the equation ϵ = 2 t′ × h/(a2 + h2), where a, h, and t′ represent the half-width of the arc, the height of the arc, and the distance from the strain neutral line to the center of the LiNbO3-PDMS composite layer, respectively [25].
Figure 4b,d shows the open-circuit voltage and closed-circuit current obtained for the e33 and e31 geometries, respectively. Through the polarity reversal test, we confirmed that the signals originated from the piezoelectricity of LiNbO3. With an increase in the strain, both the voltage and current increased as well. We note that the obtained voltage (current) for the e33 geometry was almost 20 times (100 times) larger than that for the e31 geometry for a similar value of the strain. For example, the open-circuit voltage and closed-circuit current (current density) for e33 with ϵ = 0.0168% were 0.46 V and 9.11 nA (4.64 nA · cm-2), respectively; whereas, for e31 with ϵ = 0.018%, values of 0.02 V and 0.09 nA (0.044 nA · cm-2) were obtained, respectively. Note that due to the low output voltage and current for e31, we could not detect a signal for strain lower than ϵ = 0.018%. The electric power generated from the piezoelectric nanostructures was affected by the piezoelectric coefficient, dielectric constant, and strained length of the nanowire [9]. All of the other parameters were the same for both the e33 and e31 geometries, which implied that the significant difference in power generation was related to the different piezoelectric coefficients of d33 = 27 pmV-1 and d31 = 4.3 pmV-1 for LiNbO3[26].
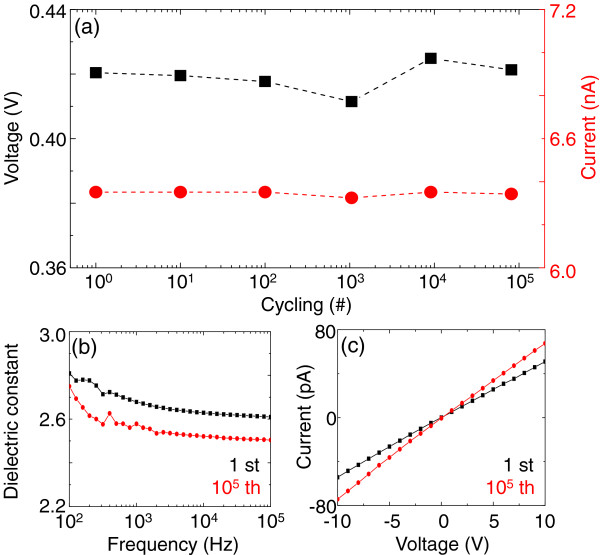
The LiNbO3-PDMS-based composite nanogenerator for the e33 geometry generates stable power even for excessive strain. In Figure 5a, we show the push-pull cycling number dependence of the open-circuit voltage and closed-circuit current. Over a period of 22 h, we continuously applied a compressive strain of up to 105 cycles. Within ±1%, the open-circuit voltage and closed-circuit current were quite stable. The stability of the dielectric constant and electric loss are shown in Figure 5b,c, respectively. The dielectric constant and current–voltage (I-V) characteristics were similar before and after the application of excessive strain (approximately 105 cycles).
Figure 5.
Stability of the LiNbO3-PDMS composite nanogenerator. (a) Cycling number-dependent open-circuit voltage and closed-circuit current of the LiNbO3-PDMS composite nanogenerator. (b) Dielectric constant and (c) current–voltage (I-V) characteristics before and after 105 cycles of excessive strain.
In the LiNbO3-PDMS composite nanogenerator, stable power generation depended on the mixing ratio. LiNbO3 has high piezoelectricity, but is fragile and lossy. In contrast, PDMS has flexibility and a low dielectric constant, but no piezoelectricity. Nearly the same power generation, dielectric constant, and loss after excessive strain suggest that our LiNbO3-PDMS composite nanogenerator was quite stable; this was attributed to the low volume ratio of LiNbO3 inside the PDMS (approximately 1%). If the volume ratio of LiNbO3 were to increase, then the power generation would increase as well at the expense of a larger dielectric constant; however, the composite devices may become fragile and lossy. Therefore, we suggest that optimization of the mixing ratio is crucial for the application of a lead-free piezoelectric composite nanogenerator.
Conclusions
We report a lead-free LiNbO3 nanowire-based nanocomposite for piezoelectric power generation. Through the ion exchange of Na2Nb2O6-H2O, we synthesized long (approximately 50 μm) single-crystalline LiNbO3 nanowires having a high piezoelectric coefficient (approximately 25 pmV-1). By blending LiNbO3 and PDMS polymer at a volume ratio of 1:100, we fabricated a flexible nanocomposite nanogenerator. For a similar strain, the piezoelectric power generation for the e33 geometry was significantly larger than that for the e31 geometry due to the difference in the d33 and d31 piezoelectric coefficients of LiNbO3. For up to 105 cycles of excessive strain, we observed that the output power, dielectric constant, and loss were quite stable. Optimization of the mixing ratio between lead-free piezoelectric materials and flexible polymers is an important factor to consider in the application of an energy-harvesting nanogenerator.
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
BKY and YKP prepared the nanowire and performed the XRD, TG, DSC, SEM, and TEM measurements. BKY and ML fabricated the nanocomposite nanogenerator and tested the performance. NL and WJ carried out the PFM measurements and analysis. BKY and SL performed neutron diffraction measurements and the Rietveld analysis. JHJ designed the work and wrote the manuscript. All authors read and approved the final manuscript.
Contributor Information
Byung Kil Yun, Email: nitrogen@hanmail.net.
Yong Keun Park, Email: pyk071865@naver.com.
Minbaek Lee, Email: mlee@inha.ac.kr.
Nuri Lee, Email: nurilee@ewhain.net.
William Jo, Email: wmjo@ewha.ac.kr.
Seongsu Lee, Email: seongsulee@kaeri.re.kr.
Jong Hoon Jung, Email: jhjung@inha.ac.kr.
Acknowledgements
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korean government (MEST) (No. 2012M2B2A4029408).
References
- Jaffe B, Cook WR, Jaffe H. Piezoelectric Ceramics. New York: Academic; 1971. [Google Scholar]
- Saito Y, Takao H, Tani T, Nonoyama T, Takatori K, Homma T, Nagaya T, Nakamura M. Lead-free piezoceramics. Nature. 2004;9:84–87. doi: 10.1038/nature03028. [DOI] [PubMed] [Google Scholar]
- Rödel J, Jo W, Seifert KTP, Anton E-M, Granzow T, Damjanovic D. Perspective on the development of lead-free piezoceramics. J Am Ceram Soc. 2009;9:1153–1177. doi: 10.1111/j.1551-2916.2009.03061.x. [DOI] [Google Scholar]
- Choi S-Y, Jeong S-J, Lee D-S, Kim M-S, Lee J-S, Cho JH, Kim BI, Ikuhara Y. Gigantic electrostrain in duplex structured alkaline niobates. Chem Mater. 2012;9:3363–3369. doi: 10.1021/cm301324h. [DOI] [Google Scholar]
- Wang ZL, Song JH. Piezoelectric nanogenerators based on zinc oxide nanowire arrays. Science. 2006;9:242–246. doi: 10.1126/science.1124005. [DOI] [PubMed] [Google Scholar]
- Park K-I, Xu S, Liu Y, Hwang GT, Kang SJL, Wang ZL, Lee KJ. Piezoelectric BaTiO3 thin film nanogenerator on plastic substrates. Nano Lett. 2010;9:4939–4943. doi: 10.1021/nl102959k. [DOI] [PubMed] [Google Scholar]
- Wu JM, Xu C, Zhang Y, Yang Y, Zhou Y, Wang ZL. Flexible and transparent nanogenerators based on a composite of lead-free ZnSnO3 triangular-belts. Adv Mater. 2012;9:6094–6099. doi: 10.1002/adma.201202445. [DOI] [PubMed] [Google Scholar]
- Xu S, Hansen BJ, Wang ZL. Piezoelectric-nanowire-enabled power source for driving wireless microelectronics. Nature Comms. 2010;9:93. doi: 10.1038/ncomms1098. [DOI] [PubMed] [Google Scholar]
- Xu S, Yeh Y-W, Poirier G, McAlpine MC, Register RA, Yao N. Flexible piezoelectric PMN-PT nanowire-based nanocomposite and device. Nano Lett. 2013;9:2393–2398. doi: 10.1021/nl400169t. [DOI] [PubMed] [Google Scholar]
- Jung JH, Lee M, Hong J-I, Ding Y, Chen C-Y, Chou L-J, Wang ZL. Lead-free NaNbO3 nanowires for a high output piezoelectric nanogenerator. ACS Nano. 2011;9:10041–10046. doi: 10.1021/nn2039033. [DOI] [PubMed] [Google Scholar]
- Jung JH, Chen C-Y, Yun BK, Lee N, Zhou Y, Jo W, Chou L-J, Wang ZL. Lead-free KNbO3 ferroelectric nanorod based flexible nanogenerators and capacitors. Nanotechnology. 2012;9:375401. doi: 10.1088/0957-4484/23/37/375401. [DOI] [PubMed] [Google Scholar]
- Park K-I, Lee M, Liu Y, Moon S, Hwang G-T, Zhu G, Kim JE, Kim SO, Kim DK, Wang ZL, Lee KJ. Flexible nanocomposite generator made of BaTiO3 nanoparticles and graphitic carbons. Adv Mater. 2012;9:2999–3004. doi: 10.1002/adma.201200105. [DOI] [PubMed] [Google Scholar]
- Momeni K, Odegard GM, Yassar RS. Nanocomposite electrical generator based on piezoelectric zinc oxide nanowires. J Appl Phys. 2010;9:114303. doi: 10.1063/1.3517095. [DOI] [Google Scholar]
- Fluck D, Günter P. Second-harmonic generation in potassium niobate waveguides. IEEE J Sel Topics Quantum Electron. 2000;9:122–131. [Google Scholar]
- Zhao L, Steinhart M, Yosef M, Lee SK, Schlecht S. Large-scale template-assisted growth of LiNbO3 one-dimensional nanostructures for nano-sensors. Sens Actuators B. 2005;9:86–90. doi: 10.1016/j.snb.2005.03.093. [DOI] [Google Scholar]
- Simoes AZ, Zaghete MA, Stojanovic BD, Gonzalez AH, Riccardi CS, Cantoni M, Varela JA. Influence of oxygen atmosphere on crystallization and properties of LiNbO3 thin films. J Eur Ceram Soc. 2004;9:1607–1613. doi: 10.1016/S0955-2219(03)00453-9. [DOI] [Google Scholar]
- Zhu H, Zheng Z, Gao X, Huang Y, Yan Z, Zou J, Yin H, Zou Q, Kable SH, Zhao J, Xi Y, Martens WN, Frost RL. Structural evolution in a hydrothermal reaction between Nb2O5 and NaOH solution: from Nb2O5 grains to microporous Na2Nb2O6 · 2/3H2O fibers and NaNbO3 cubes. J Am Chem Soc. 2006;9:2373–2384. doi: 10.1021/ja056301w. [DOI] [PubMed] [Google Scholar]
- Xu H, Nyman M, Nenoff TM, Navrotsky A. Prototype Sandia octahedral molecular sieve (SOMS) Na2Nb2O6 H2O: Synthesis, structure and thermodynamic stability. Chem Mater. 2004;9:2034–2040. doi: 10.1021/cm035066i. [DOI] [Google Scholar]
- Goh GKL, Lange FF, Haile SM, Levi CG. Hydrothermal synthesis of KNbO3 and NaNbO3 powders. J Mater Res. 2003;9:338–345. doi: 10.1557/JMR.2003.0044. [DOI] [Google Scholar]
- Shinozaki Y, Mitsui T. Powder neutron diffraction study of LiNbO3. J Phys Chem Solids. 1963;9:1057–1061. doi: 10.1016/0022-3697(63)90012-X. [DOI] [Google Scholar]
- Santulli AC, Zhou H, Berweger S, Raschke MB, Sutter E, Wong SS. Synthesis of single-crystalline one-dimensional LiNbO3 nanowires. Cryst Eng Comm. 2010;9:2675–2678. doi: 10.1039/c005318j. [DOI] [Google Scholar]
- Jesse S, Baddorf AP, Kalinin SV. Switching spectroscopy piezoresponse force microscopy of ferroelectric materials. Appl Phys Lett. 2006;9:062908. doi: 10.1063/1.2172216. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Liu Y, Wang ZL. Fundamental theory of piezotronics. Adv Mater. 2011;9:3004–3013. doi: 10.1002/adma.201100906. [DOI] [PubMed] [Google Scholar]
- Zhou J, Gu YD, Fei P, Mai WJ, Gao YF, Yang RS, Bao G, Wang ZL. Flexible piezotronic strain sensor. Nano Lett. 2008;9:3035–3040. doi: 10.1021/nl802367t. [DOI] [PubMed] [Google Scholar]
- Lee M, Chen C-Y, Wang S, Cha SN, Park YJ, Kim JM, Chou L-J, Wang ZL. A hybrid piezoelectric structure for wearable nanogenerators. Adv Mater. 2012;9:1759–1764. doi: 10.1002/adma.201200150. [DOI] [PubMed] [Google Scholar]
- Miller RC, Nordland WA, Bridenbaugh PM. Dependence of second-harmonic-generation coefficients of LiNbO3 on melt composition. J Appl Phys. 1971;9:4145–4147. doi: 10.1063/1.1659746. [DOI] [Google Scholar]