Abstract
Changes in glutamatergic transmission in the nucleus accum-bens play a key role in mediating reward-related behaviors and addiction to psychostimulants. Glutamatergic inputs to the accumbens originate from multiple sources, including the prefrontal cortex, basolateral amygdala, and midline thalamus. The group I metabotropic glutamate receptors (mGluRs) are found throughout the core and shell of the nucleus accumbens, but their localization and function at specific glutamatergic synapses remain unknown. To further characterize the substrate that underlies group I mGluR functions in the accumbens, we combined anterograde tract tracing method with electron microscopy immunocytochemistry to study the ultrastructural relationships between specific glutamatergic afferents and mGluR1a- or mGluR5-containing neurons in the rat nucleus accumbens. Although cortical, thalamic, and amygdala glutamatergic terminals contact both mGluR1a- and mGluR5-immunoreactive dendrites and spines in the shell and core of the accumbens, they do so to varying degrees. Overall, glutamatergic terminals contact mGluR1a–positive spines about 30% of the time, whereas they form synapses twice as frequently with mGluR5-labeled spines. At the subsynaptic level, mGluR5 is more frequently expressed perisynaptically and closer to the edges of glutamatergic axospinous synapses than mGluR1a, suggesting a differential degree of activation of the two group I mGluRs by transmitter spillover from glutamatergic synapses in the rat accumbens. These results lay the foundation for a deeper understanding of group I mGluR-mediated effects in the ventral striatum, and their potential therapeutic benefits in drug addiction and other neuropsychiatric changes in reward-related behaviors.
INDEXING TERMS: nucleus accumbens, group I mGluRs, tract tracing, electron microscopy
Glutamate is a key neurotransmitter of accumbens-mediated functions in reward and addictive behaviors (Robinson and Berridge, 2003). The nucleus accumbens, which is divided into the shell and core, receives glutamatergic innervation from various brain structures, including the prefrontal cortex, thalamus, and amygdala (Groenewegen et al., 1987; Berendse and Groenewegen, 1990; McDonald, 1991, Berendse et al., 1992; French and Totterdell, 2003). Specific regions of these glutamatergic nuclei have segregated projections to the different areas of the accumbens. For example, the dorsal region of the limbic prefrontal cortex (PFC) projects to the core of the accumbens, whereas the ventral region projects to the shell (Berendse et al, 1992). The intermediodorsal (IMD) and central medial (CM) nuclei of the midline thalamus project to the core, whereas the paraventricular nucleus (PVN) targets the shell (Berendse and Groenewegen, 1990). Finally, the same divisions hold true for the amygdala, the anterior basolateral amygdala (BLA) sending afferents to the core, whereas the posterior BLA targets the shell (McDonald, 1991).
The group I metabotropic glutamate receptors are G-protein-coupled receptors (GPCRs) widely expressed throughout the accumbens (Testa et al, 1994; Mitrano and Smith, 2007a), where they play an important role in the neural mechanisms involved in addiction to psychostimulants (Swanson et al., 2001; Chiamulera et al., 2001; Mc-Geehan and Olive, 2003). However, their main source(s) of activation and function at major glutamatergic synapses in the accumbens remains unknown. The first goal of this study was, therefore, to compare the degree of synaptic innervation of group I mGluRs-containing neurons by cortical, amygdala, or thalamic inputs in the core and shell of the rat nucleus accumbens.
In the rat nucleus accumbens, mGluR1a and mGluR5 are mainly found extrasynaptically on the plasma membrane of both dendrites and spines (Mitrano and Smith, 2007a), suggesting that glutamate spillover from neighboring synapses or glia are likely to be their main sources of activation (Danbolt, 2001; Baker et al., 2002; Oliet et al., 2004). The dynamic activation of these receptors is, therefore, highly dependent on their relative proximity to synaptic release sites of glutamate. In order to characterize the potential neuronal sources of activation of these receptors, another goal of this study was to determine the spatial relationships between plasma membrane-bound mGluR1a and mGluR5 immunoreactivity and cortical, amygdala, or thalamic axospinous synapses. To achieve these goals, the anterograde labeling of glutamatergic projections from the PFC, BLA or midline thalamus was combined with high-resolution electron microscopic immunocytochemistry for mGluR1a and mGluR5 in the shell and core of the rat nucleus accumbens.
Some of these data have been previously presented in abstract form (Mitrano and Smith, 2007b).
MATERIALS AND METHODS
Animals and treatments
All procedures were approved by the animal care and use committee of Emory University and conform to the U.S. National Institutes of Health guidelines. A total of 20 male, adult Sprague-Dawley rats (weighing between 200 and 300 g) were used in this study. Rats were anesthetized with isoflurane and fixed in a stereotaxic frame (Kopf, Tujunga, CA), and a glass micropipette containing the anterograde tracer biotinylated dextran amine (BDA; 10,000MW, Invitrogen, Carlsbad, CA) was placed in the limbic PFC, PVN, or CM/IMD of the thalamus, and the BLA. The coordinates used were as follows (in mm): PFC: +3.2 A-P, +0.5 M-L, −4.0 D-V; CM/IMD: −2.8 A-P, −1.6 M-L, −6.4 D-V, 15° lateral angle; PVN: −1.6 A-P, −1.2 M-L, −5.43 D-V, 13° lateral angle; BLA: −2.0 A-P, +5.0 M-L, −8.0 D-V, all based on coordinates from the stereotaxic atlas of Paxinos and Watson (1998). Because the CM and IMD are in close proximity to each other and both project to the accumbens core, injections into these two nuclei were grouped together. The iontophoretic delivery of BDA was performed with a 7-µA positive current for 20 minutes via a 7-seconds on/7-seconds off cycle.
After 7 days’ survival, the animals were deeply anesthetized with a cocktail of ketamine (60 –100 mg/kg, i.p.) and medetomidine (Dormitor, Pfizer, Groton, CT, 0.1 mg/kg, i.p.) and then were transcardially perfused with cold oxygenated Ringer’s solution followed by a fixative containing 4% paraformaldehyde and 0.1% glutaraldehyde in phosphate buffer (0.1 M; pH 7.4). Following perfusion, brains were removed from the skull and postfixed in 4% paraformaldehyde for 24 hours.
Tissue processing
Following fixation, all brains were cut at 60 µm on a vibrating microtome and processed for light and electron microscopy immunoperoxidase or immunogold localization of BDA, calbindin-D28k, mGluR1a, and mGluR5. Prior to immunocytochemical processing, all sections were put into a 1% sodium borohydride solution for 20 minutes and then washed with phosphate-buffered saline (PBS).
Primary antibodies (Table 1)
TABLE 1.
Antibodies Used in This Study
| Antigens | Immunogen | Manufacturer data | Dilution used |
|---|---|---|---|
| Calbindin-D28k | Purified bovine kidney calbindin-D28k | Sigma, mouse monoclonal, #C-9848 | 1:5,000 |
| Metabotropic glutamate receptor 1a | C-terminus of rat mGluR1a (PNVTYASVILRDYKQSSSTL) |
Millipore, rabbit polyclonal, #AB1551 | 1:1,000 |
| Metabotropic glutamate receptor 5 | C-terminus of synthetic mGluR5 with lysine added to N-terminal (KSSPKYDTLIIRDYTNSSSSL) |
Millipore, rabbit polyclonal, #06–451 | 1:5,000 |
A commercially available monoclonal antibody against calbindin-D28k (Sigma, St. Louis, MO; cat. no. C-9848, lot no. 082k4879) was used at a concentration of 1:5,000 to distinguish between the accumbens shell and core. The calbindin-D28k antibody is derived from CB-955 hybridomas produced by fusion of mouse myeloma cells and splenocytes from BALB/c mice that were immunized with purified bovine kidney calbindin-D28k. The specificity of this antibody has been demonstrated through preadsorption immunohistochemical assays that abolish calbindin labeling (Celio, 1990), through Western blot analysis of rat brain tissue, which shows a distinct band at 28 kDa (Miyata et al., 2000), and through immunohistochemistry, which shows calbindin immunostaining in brain regions known to express a significant level of calbindin-D28k mRNA (Winsky et al., 1989; Celio, 1990; Miyata et al., 2000).
To localize mGluR1a, an affinity-purified rabbit polyclonal antibody against the C-terminus of rat mGluR1a (PNVTYASVILRDYKQSSSTL) conjugated to keyhole limpet hemocyanin (KLH) with glutaraldehyde was used at a concentration of 1:1,000 (Millipore, Temecula, CA; cat no. AB1551). In Western blot analysis by the manufacturer, this antibody labels a single band of ~ 140 kDa. Previous studies from our laboratory and others have used a combination of knockout mice, transfected HEK-293 cells, and preadsorption to determine the specificity of this mGluR1a antiserum. These studies showed that brain tissue from mGluR1a knockout mice did not display any specific mGluR1a labeling compared with wild-type. In addition, immunoblotting of cells transfected with mGluR1a, but not mGluR5, labeled a band of 140 kDa (Kuwajima et al., 2004). Preadsorption studies in rat retina cells abolished mGluR1a labeling (Koulen et al., 1997).
An affinity-purified synthetic rabbit polyclonal antibody against the C-terminus of mGluR5 with a lysine added to the N-terminus (KSSPKYDTLIIRDYTNSSSSL) at a concentration of 1:5,000 (Millipore; cat. no. 06–451) was used to label mGluR5-containing elements. According to the manufacturer’s immunoblot analysis, the mGluR5 antibody labels a band of ~ 130 kDa. Specificity of the mGluR5 antibody has been shown in previous studies from our laboratory using knockout mice, transfected cells, and homogenates of rat brain. These studies showed that brain tissue from mGluR5 knockout mice do not stain for mGluR5, and HEK-293 cells transfected with mGluR5 label a band of the correct molecular weight (Kuwajima et al., 2004). Furthermore, immunoblot analysis on proteins isolated from various brain regions labels a band that corresponds to the size of mGluR5 in regions known to express mGluR5 protein and mRNA (Mannaioni et al., 2001).
Light microscopic observations
Single immunoperoxidase for light microscopy
To reveal the injection site and anterograde tracer labeling in the accumbens, one of every six sections of the entire brain was rinsed in PBS and then incubated for 90 minutes with the avidin-biotin-peroxidase complex (ABC) at a dilution of 1:100 (Vector, Burlingame, CA). The sections were then washed in PBS and Tris buffer (50 mM; pH 7.6) and transferred to a solution containing 0.025% 3,3′-diaminobenzidine tetrahydrochloride (DAB; Sigma), 10 mM imidazole, and 0.005% hydrogen peroxide in Tris buffer for 10 minutes. Sections were rinsed in PBS, mounted onto gelatin-coated slides, dehydrated, and then coverslipped with Permount. The tissue was examined with a Leica DMRB microscope (Leica Microsystems, Bannockburn, IL), and images were taken by using a CCD camera (Leica DC500), which was controlled by Leica IM50 software. Another series of sections was Nissl stained to help delineate thalamic nuclei borders and determine the exact location of injection sites aimed at CM/IMD.
In the striatum, one of every six sections was stained for calbindin-D28k to distinguish the core-shell boundaries for subsequent EM studies. Following sodium borohydride treatment, sections were incubated for 1 hour at RT in PBS containing normal horse serum (NHS), 1% bovine serum albumin (BSA), and 0.3% Triton X-100, followed by the primary antibody solution containing 1% NHS, 1% BSA, and 0.3% Triton X-100 in PBS for 24 hours at RT. After three rinses in PBS, sections were incubated in secondary biotinylated horse antimouse IgGs at a concentration of 1:200 (Vector) for 90 minutes. The sections were then rinsed in PBS and exposed to the ABC complex as described above.
Electron microscopic observations
Double pre-embedding peroxidase labeling for BDA, mGluR1a, and mGluR5
Following sodium borohydride treatment, sections were placed in a cryoprotectant solution for 20 minutes (PB 0.05 M, pH 7.4, 25% sucrose, and 10% glycerol), frozen at − 80°C for 20 minutes, returned to a decreasing gradient of cryoprotectant solutions, and rinsed in PBS. Sections were then incubated for 1 hour at RT in PBS containing 10% normal goat serum (NGS), and 1% BSA, followed by the primary antibody solution containing 1% NGS and 1% BSA in PBS for 48 hours at 4°C. After three rinses in PBS, sections were incubated in secondary biotinylated goat anti-rabbit IgGs at a concentration of 1:200 (Vector) for 90 minutes, rinsed in PBS, and then exposed to ABC and DAB as described above. After the DAB reaction, the tissue was rinsed in PB (0.1 M, pH 7.4) and treated with 1% OsO4 for 20 minutes. It was then returned to PB and dehydrated with increasing concentrations of ethanol. When exposed to 70% ETOH, 1% uranyl acetate was added to the solution for 35 minutes to increase the contrast of the tissue at the electron microscope.
Following dehydration, sections were treated with propylene oxide and embedded in epoxy resin for 12 hours (Durcupan ACM, Fluka, Buchs, Switzerland), mounted onto slides, and placed in a 60°C oven for 48 hours. Separate samples of the nucleus accumbens core and medial shell (depending on the initial injection site) were cut out of the larger sections, mounted onto resin blocks, and cut into 60-nm sections by using an ultramicrotome (Leica Ultracut T2). The 60-nm sections were collected on Pioloform-coated copper grids, stained with lead citrate for 5 minutes to enhance tissue contrast, and examined on the Zeiss EM-10C electron microscope. Electron micrographs were taken and saved with a CCD camera (DualView 300W, Gatan, Pleasanton, CA) controlled by Digital Micrograph software (version 3.10.1, Gatan).
Double pre-embedding immunoperoxidase (BDA) and immunogold (mGluR1a and mGluR5)
Following sodium borohydride treatment, sections were placed in a cryoprotectant solution for 20 minutes (PB 0.05 M, pH 7.4, 25% sucrose, and 10% glycerol), frozen at −80°C for 20 minutes, returned to a decreasing gradient of cryoprotectant solutions, and rinsed in PBS. The sections were then incubated for 30 minutes in PBS containing 5% dry milk at RT and then rinsed in TBS-gelatin buffer (0.02 M, 0.1% gelatin at pH 7.6). Sections were then transferred to primary antibody solutions (i.e., rabbit antimGluR1a or anti-mGluR5 IgGs) with 1% dry milk in TBS-gelatin buffer for 24 hours at room temperature and then rinsed again in TBS-gelatin. After rinsing, sections were treated for 2 hours at RT with secondary goat anti-rabbit IgGs conjugated with 1.4-nm gold particles at a concentration of 1:100 (Nanoprobes, Yaphank, NY) diluted with 1% dry milk in TBS-gelatin. Sections were rinsed in TBS-gelatin and 2% sodium acetate buffer before gold particles were silver intensified to 30–50 nm by using the HQ silver kit (Nanoprobes) for approximately 10 minutes. Following silver intensification, ABC and DAB procedures similar to those described above were used to localize BDA.
Immediately following the DAB reaction, the sections were rinsed with PB (0.1 M, pH 7.4) and then treated according to the same protocol of osmification, dehydration, embedding, and tissue selection described above for the immunoperoxidase procedure including the following changes: 1) the tissue was kept in 0.5% OsO4 for 10 minutes instead of 20; and 2) the tissue was stained with 1% uranyl acetate for 10 minutes instead of 35.
Analysis of material
Double immunoperoxidase labeling for BDA and group I mGluRs
Data were collected from four animals injected in the PFC or PVN, and three animals injected in the CM/IMD or BLA. This yielded a total of eight blocks from the core and eight blocks from the shell of the accumbens from animals injected in the PFC, six blocks from the core and six blocks from the shell of animals injected in the BLA, eight blocks from the shell of animals injected in the PVN, and six blocks from the core of animals injected in the CM/IMD. Serial ultrathin sections from the surface of each block were taken and examined in the electron microscope for positively labeled axon terminals (based on ultrastructural features described by Peters et al., 1991). Electron micrographs of labeled terminals (regardless of labeling in the postsynaptic element) were digitized at 31,500×.
Approximately 20–30 labeled terminals were photographed per animal, and the proportion of these terminals in contact with mGluR1a- or mGluR5-immunoreactive (IR) spines and dendrites was calculated for each animal; then the mean percentage of terminals forming synapses on either population of mGluR-containing elements was averaged across the number of animals and compared between injection sites and receptors by using a series of t-tests and one-way ANOVAs followed by Tukey’s post hoc tests in Sigma Stat software (version 2.03, Systat Software, San Jose, CA).
Double pre-embedding immunoperoxidase for BDA and immunogold for mGluR1a or mGluR5
In order to assess the spatial relationships between mGluR1a and mGluR5 and the different glutamatergic synapses, the receptors were revealed with immunogold, whereas the tracer was localized with immunoperoxidase. Positively labeled terminals in contact with either mGluR1a-or mGluR5-IR spines or dendrites were followed through three to six serial sections to examine the pattern of immunogold labeling at individual synapses. Serial sections were used in this analysis to ensure that the bulk of immunogold labeling was detected, as its abundance and location can vary from section to section. Data were collected for both mGluR1a- and mGluR5-immunostained tissue from three animals injected in the PFC, PVN, CM/IMD, and BLA. This yielded a total of 12 blocks taken from both the core and shell of the accumbens from animals injected in the PFC, 11 blocks from both core and shell from animals injected in the BLA, 6 blocks from the nucleus accumbens shell of animals injected in the PVN, and 6 blocks from the nucleus accumbens core of animals injected in the CM/IMD.
Serial ultrathin sections were taken from each of the blocks and examined in the electron microscope for positively labeled axon terminals. Electron micrographs of labeled terminals in contact with gold-containing postsynaptic elements were digitized at 31,500×. Approximately 20–30 terminals were photographed per animal and followed through three to six serial sections. The pattern of gold labeling was classified as intracellular or plasma membrane-bound based on criteria defined in previous studies (Galvan et al., 2006; Mitrano and Smith, 2007a; Mitrano et al., 2008). Taking into consideration the size of the primary and secondary antibodies, the maximum distance between the 1.4-nm gold particle and the epitope would be about 17 nm (Blackstad et al., 1990). Based on this figure, the plasma membrane-bound gold particles were further classified into three categories: perisynaptic (touching or within a 20-nm range of the edges of postsynaptic specializations); synaptic (in contact with the main body of postsynaptic specializations); or extrasynaptic (on the plasma membrane, but not associated with synapses). The proportion of spines that expressed perisynaptic labeling (out of all gold-labeled spines) when contacted by a labeled terminal was determined and averaged across the number of animals and compared between input nuclei and receptors by using one-way ANOVAs in Sigma Stat software (version 2.03, Systat Software). In addition, to assess potential differences in the localization of extrasynaptic receptors in relation to cortical, thalamic, or amygdala inputs, the distance between the closest extrasynaptic gold particles and the edges of the postsynaptic density was measured by using Image J software (National Institutes of Health) and then averaged across the number of animals and compared between regions and receptors as described for the perisynaptic labeling.
The digitally acquired electron micrographs (for all figures) were adjusted only for brightness or contrast by using either the Digital Micrograph software or Adobe Photoshop software (version 8.0, Adobe Systems, San Jose, CA). Micrographs were then compiled into figures by using Adobe Illustrator (version 11.0, Adobe Systems).
RESULTS
Light microscopic observations
To make sure that the injection sites were properly located, we first examined tissue from every sixth section through the rostrocaudal extent of the injection site and compared the pattern of anterograde labeling generated in cortical, thalamic, or amygdala and striatal regions with that described in previous studies using the same or other anterograde tracers (Berendse and Groenewegen, 1990; McDonald, 1991; Berendse et al., 1992; Raju et al., 2006). The accumbens tissue was only used for further study if the injection sites were confined to the targeted brain structure and the corresponding area of the accumbens contained anterograde labeling. Figure 1 shows examples of injection sites in the cortex, thalamus, and amygdala, and the resulting anterograde labeling in the shell and/or core of the nucleus accumbens. Table 2 provides the approximate anteroposterior stereotaxic level (using interaural coordinates from Paxinos and Watson, 1998) with blocks of the shell and core of the accumbens taken for each of the injection sites used in the electron microscopic observations.
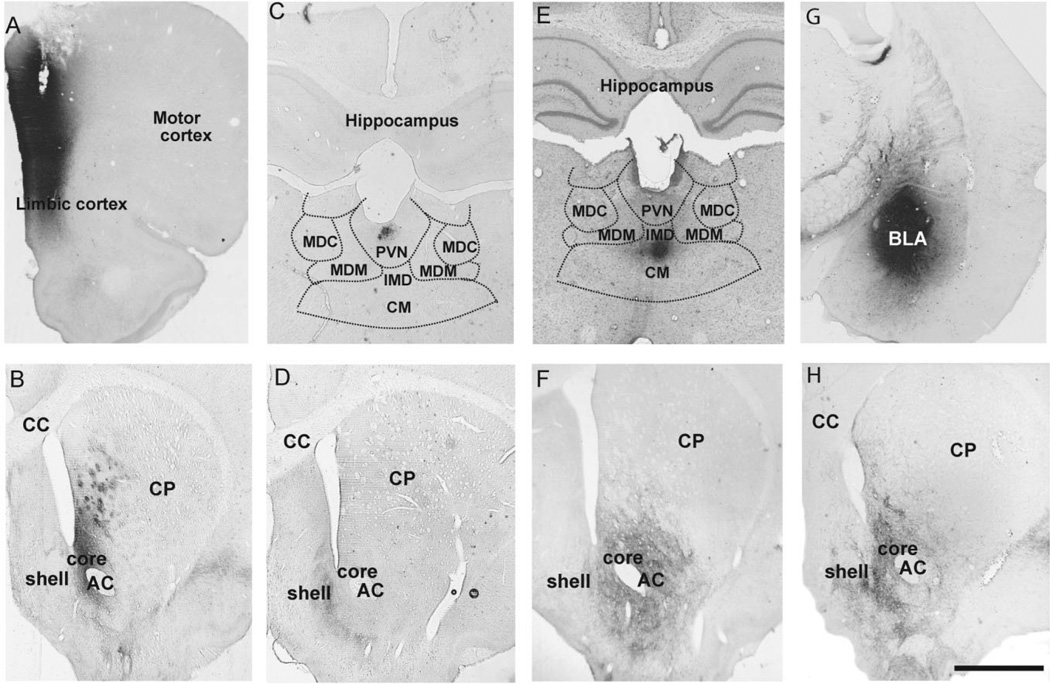
Figure 1.
BDA injection sites in the PFC (A), thalamus (C,E), and BLA (G) with resulting anterograde labeling in the shell and core of the nucleus accumbens (B,D,F,H). A: BDA injection site in the dorsal and ventral limbic prefrontal cortex. B: Anterograde labeling in the core of the nucleus accumbens and the ventral medial caudate-putamen after PFC injection shown in A. C: BDA injection site in the PVN of the thalamus. The limits of surrounding thalamic nuclei are schematically drawn based on adjacent Nissl-stained sections to better illustrate the specificity of the PVN injection. D: Anterograde labeling confined to the shell of the accumbens following the PVN injection shown in C. E,F: Injection in the CM/IMD(E) and the anterograde labeling (F) in the core of the nucleus accumbens that resulted from this injection. G,H: BDA injection site in both the anterior and posterior BLA (G), with the corresponding anterograde labeling in both the shell and core of the accumbens (H). Abbreviations: CP, caudate-putamen; CC, corpus callosum; AC, anterior commissure; MDC, mediodorsal thalamic nuclei, central part; MDM, mediodorsal thalamic nuclei, medial part. Scale bar = 0.5 mm in H (applies to A-H).
TABLE 2.
Anteroposterior (A–P) Stereotaxic Coordinates Showing Approximate Locations of Accumbens Core and Shell Tissue Blocks Selected for Electron Microscopic Analyses1
| Injection site | Core/shell | A-P coordinates |
|---|---|---|
| PFC | Core | + 1.70-+ 1.00 |
| Shell | + 1.70-+ 1.00 | |
| CM/IMD | Core | + 1.60 |
| PVN | Shell | + 1.20 |
| BLA | Core | + 1.60-+ 1.00 |
| Shell | + 1.60-+ 1.00 |
Abbreviations: PFC, prefrontal cortex; CM/IMD, central medial/intermedial dorsal nuclei; PVN, paraventricular nucleus; BLA, basolateral amygdala.
Electron microscopic observations
Glutamatergic inputs onto mGluR1a- or mGluR5-containing neurons in the accumbens
In order to determine the proportion of terminals from the cortex, thalamus, and amygdala in contact with mGluR1a- or mGluR5-IR postsynaptic elements, we used a double immunoperoxidase method. We could use such method in the present study because BDA and group I mGluRs labeling was largely segregated into different neuronal elements, i.e., the BDA staining was exclusively found in axon terminals, whereas the group I mGluRs immunoreactivity was almost exclusively associated with postsynaptic dendrites and spines, as described in detail in our previous study using the same mGluR antibodies and immunocytochemical procedures (Mitrano and Smith, 2007a). In this material, fewer than 1 mGluR1a- or mGluR5-immunostained axon terminal was found per 100 µm2 of both the shell and core of the accumbens compared with 15–25 labeled postsynaptic elements in the same regions (Mitrano and Smith, 2007a). Because of its high sensitivity and comparable tissue penetration of the immunogenic reaction product in both pre- and postsynaptic elements, the use of the double immunoperoxidase technique in these experiments also provides more accurate measurements of the incidence of synaptic connections between BDA-positive boutons and group I mGluR-labeled postsynaptic targets.
Examples of tissue dually stained for BDA and either group I mGluRs are depicted in Figure 2. Most labeled boutons from the PFC (95.1%), PVN (93.5%), CM/IMD (84.7%), or BLA (81.8%) formed asymmetric axospinous synapses, whereas far fewer displayed axodendritic synaptic specializations (PFC: 8.8%; PVN: 11.2%; CM/IMD: 14.6%; BLA: 21.5%). The percentages of axodendritic and axospinous synapses add up to more than 100% because some terminals formed two synapses.
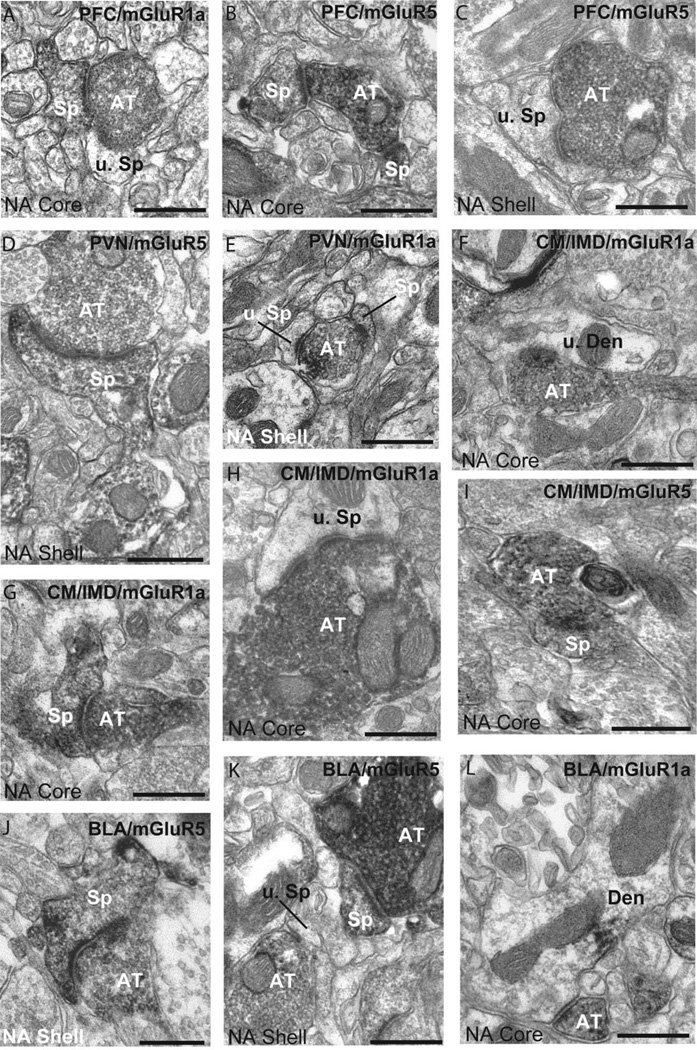
Figure 2.
Double immunoperoxidase for BDA and group I mGluRs. The location of injection sites and the receptor subtype the tissue is immunostained for are indicated in the upper right corner of each micrograph. A,B: Electron micrographs of labeled cortical terminals from the dorsal limbic PFC in the core of the nucleus accumbens. C: Cortical terminal from the ventral limbic PFC in the accumbens shell. D,E: Micrographs of labeled terminals from the PVN in contact with mGluR5- (D) or mGluR1a- (E) containing spines in the shell of the nucleus accumbens. F: Example of a terminal arising from the CM/IMD in contact with an unlabeled dendrite in the core of the accumbens. G–I: CM/IMD terminals in contact with labeled or unlabeled spines in mGluR1a–and mGluR5-immunostained accumbens tissue. J,K: BLA terminals in contact with unlabeled and labeled spines in mGluR1a- and mGluR5-immunostained tissue in the core and shell. L: Example of a BLA terminal in contact with an mGluR1a–IR dendrite in the core. Abbreviations: AT, labeled axon terminal; Sp, labeled spine; u. Sp, unlabeled spine; Den, labeled dendrite; u. Den, unlabeled dendrite. Scale bar = 0.2 µm in A-L.
Group I mGluR axospinous synapses
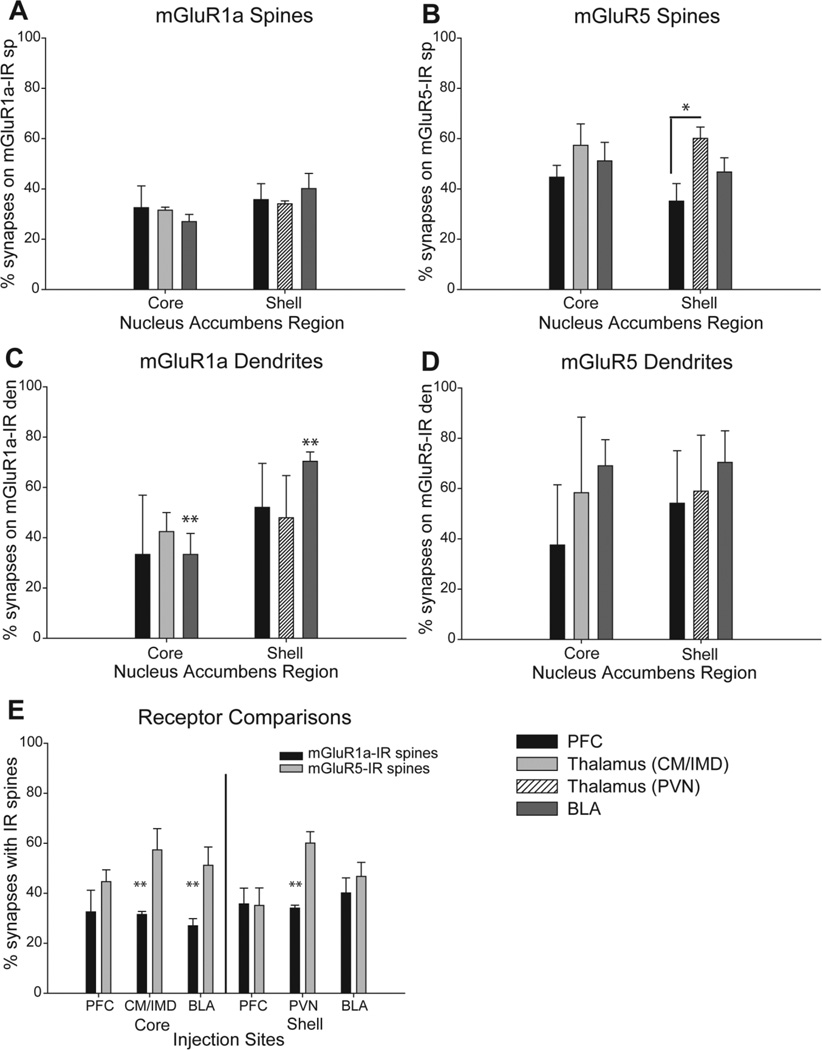
In a first series of experiments, we determined the proportion of axospinous synapses from the various glutamatergic sources that involved mGluR1a- or mGluR5-immunoreactive spines. Figure 3A and B shows the average percentages (+SEM) of labeled terminals in contact with spines immunoreactive for either receptor subtype. Overall, there was no significant difference in the relative percentage of cortical, thalamic, or amygdala terminals in contact with mGluR1a-IR spines in the core or shell of the accumbens. For each source of glutamatergic inputs, about one-third of labeled boutons was in contact with mGluR1a-positive spines (PFC: 32.5 + 8.7% in the core; 35.7 + 6.3% in the shell; CM/IMD: 31.5 + 1.2% in the core; PVN: 34.1 + 1.2% in the shell; BLA: 27.0 + 2.9% in the core; 40.1 + 6.0% in the shell).
Figure 3.
Histograms showing the percentage of labeled terminals in contact with mGluR1a- or mGluR5-IR spines and dendrites in the accumbens. The legend in the lower right corner applies to A-D. A,B: Summary of the mean percentage ( + SEM) of labeled terminals from the cerebral cortex, the basolateral amygdala, or the different thalamic nuclei in contact with mGluR1a–IR (A) or mGluR5-IR (B) spines in the core and shell of the nucleus accumbens. The total numbers of animals used and terminals examined in each of these experiments are: PFC injections: n = 4 animals; 122 terminals in the core, 87 in the shell of mGluR1a–labeled tissue; 94 terminals in the core, 85 in the shell of the mGluR5-labeled tissue. PVN injections: n = 4 animals; 97 terminals in the mGluR1a–labeled tissue, 104 in the mGluR5-labeled tissue. CM/IMD injections: n = 3 animals; 79 terminals in the mGluR1a–labeled tissue, 54 in the mGluR5-labeled tissue). BLA injections: n = 3 animals; 87 terminals in the core, 51 in the shell of mGluR1a–labeled tissue; 83 terminals in the core, 80 in the shell of mGluR5-labeled tissue). In B, the single asterisk indicates a significantly higher percentage of positive terminals from the PVN than the PFC in contact with mGluR5-IR spines in the shell of the accumbens (F2, 10 = 4.9, P< 0.05). C,D: The percent of labeled terminals in contact with mGluR1a–IR (C) or mGluR5-IR (D) dendrites in the core and shell of the accumbens. The total number of animals used from each group was the same as for the axospinous synapses, whereas the total number of terminals examined was as follows: from the PFC: 5 in the core, 18 in the shell of mGluR1a–labeled tissue; 4 in the core, 9 in the shell of mGluR5-labeled tissue; from the PVN: 11 in mGluR1a–labeled tissue, 17 in mGluR5-immunostained tissue; from the CM/IMD: 17 in mGluR1a–labeled tissue, 6 in mGluR5-labeled tissue; and from the BLA: 12 in the core, 24 in the shell of mGluR1a–labeled tissue; 20 in the core, 23 in the shell of mGluR5-immunostained tissue. The double asterisks indicate a significant difference between the percent of mGluR1a–IR dendrites contacted by the BLA in the shell compared with the core (C, t = 4.1, P < 0.05). E: Comparison of the relative percentages of mGluR1a- and mGluR5-IR spines contacted by the different glutamatergic afferents to the core and shell of the nucleus accumbens. The double asterisks indicate a significantly lower percentage of terminals from the CM/IMD (t = 3.0, P < 0.05), BLA (t = 3.1, P < 0.05), and PVN (t = 5.5, P = 0.001) in contact with mGluR1a–IR spines compared with mGluR5-IR spines in the accumbens core and shell, respectively.
The pattern of glutamatergic inputs to mGluR5-IR spines was more heterogeneous. Significant differences were found between the proportion of cortical versus thalamic boutons (from the PVN) in contact with mGluR5-IR spines in the accumbens shell (35.1 + 7.0% for cortex vs. 60.1 + 4.5% for thalamus; F2,10 = 4.9, n = 4, P<0.05; Fig. 3B). Although the proportion of mGluR5-positive structures contacted by other glutamatergic inputs was slightly variable, these differences did not reach significance (PFC: 44.7 + 4.7% in the core; 35.1 + 7.0% in the shell; CM/IMD: 57.3 + 8.5% in the core; PVN: 60.1 + 4.5% in the shell; BLA: 51.2 + 7.3% in the core; 46.7 + 5.6% in the shell; Fig. 3).
To better illustrate the comparison between receptor subtypes, data presented in Figure 3A and B were reorganized in Figure 3E to compare the proportions of labeled terminals from different sources in contact with mGluR1a- or mGluR5-IR spines. This analysis revealed a significant difference between the prevalence of CM/IMD thalamic terminals in contact with mGluR5-IR spines (57.3 + 8.5%) versus mGluR1a-IR spines (31.5 + 1.2%; t = 3.0, n = 3, P < 0.05) in the accumbens core. Similarly, there were significantly more BLA terminals in contact with mGluR5-IR spines (51.2 + 7.3%) than mGluR1a-IR spines (27.0 + 2.9%; t = 3.1, n = 3, P< 0.05) in the accumbens core. A preferential targeting of mGluR5-labeled spines was also found for PVN thalamic inputs in the accumbens shell (60.1 + 4.5% for mGluR5-IR spines vs. 34.1 + 1.2% for mGluR1a-IR spines, t = 5.5, n = 4, P = 0.001).
To make sure the preferential targeting of mGluR5-labeled spines was not simply the result of a higher proportion of accumbens spines labeled for this receptor, we counted the number of labeled and unlabeled spines in a series of micrographs from the same accumbal tissue that was used in the double immunoperoxidase experiments, labeled with either of the two group I mGluR antibodies, and found that approximately 20 labeled spines/100 µm2 were present in both the shell and core of accumbens tissue immunostained for mGluR1a or mGluR5. Thirty micrographs were used from each animal used to generate the results presented in Figure 3. To make sure the density of group I mGluR-labeled spines remained constant across all animals used in this study, statistical tests were performed to compare the density of mGluR1a-or mGluR5-labeled spines in each injection group. For instance, the mean density (per 100 µm2 of accumbens tissue) of mGluR1a- versus mGluR5-labeled spines was 23.0 + 0.3 versus 26.0 + 3.2 in the core and 15.5 + 2.9 versus 20.4 + 3.3 in the shell for rats injected in the CM/IMD or PVN of the thalamus. A one-way ANOVA revealed no statistical difference between the density of labeled spines for either receptor subtype in the core or shell (F3,10 = 2.3, n = 14, P = 0.134). Similar findings were obtained for other injection groups.
Group I mGluR axodendritic synapses
Whereas most glutamatergic terminals in the accumbens formed axospinous synapses, 10–20% contacted dendritic shafts (Fig. 2F,L). Because of the relatively small number of axodendritic synapses formed by the different glutamatergic inputs, the degree of variability across animals was larger than for axospinous synapses, which hampered considerably the power of statistics applied to these data. In spite of this limiting sampling problem, a statistically significant difference was found between the proportions of mGluR1a-labeled dendrites contacted by BLA terminals in the core (33.3 + 8.3%) versus the shell (70.4 + 3.7%) (t = 4.1, n = 3,P<0.05; Fig. 3C,D). In contrast, both cortical and thalamic inputs displayed comparable frequencies of interactions with mGluR1a-containing dendrites in the core or shell of the accumbens (Fig. 3C,D). None of the three sources of glutamatergic inputs showed any significant difference in their pattern of innervation of mGluR5-labeled dendrites between core and shell (Fig. 3C,D).
Subsynaptic localization of mGluR1a and mGluR5 at specific glutamatergic synapses
Because group I mGluRs in the accumbens are extrasynaptic (Mitrano and Smith, 2007a), their activation largely relies on spillover of glutamate from synaptic sites. Thus, to further characterize the potential neuronal sources of glutamate to mGluR1a and mGluR5 in the shell and core of the accumbens, we used the pre-embedding immunogold method combined with the anterograde labeling of specific glutamatergic terminals to determine the spatial relationships between mGluR1a and mGluR5 and the postsynaptic densities of axospinous synapses formed by the different glutamatergic terminals onto accumbens neurons. Taking into consideration that the pattern of receptor distribution along a specific synapse may not be homogeneous, we examined each individual synaptic contact through serial ultrathin sections to get a more accurate view of these relationships. Figure 4 shows examples of group I mGluRs labeling through serial sections of various glutamatergic synapses in the core and shell of the accumbens.
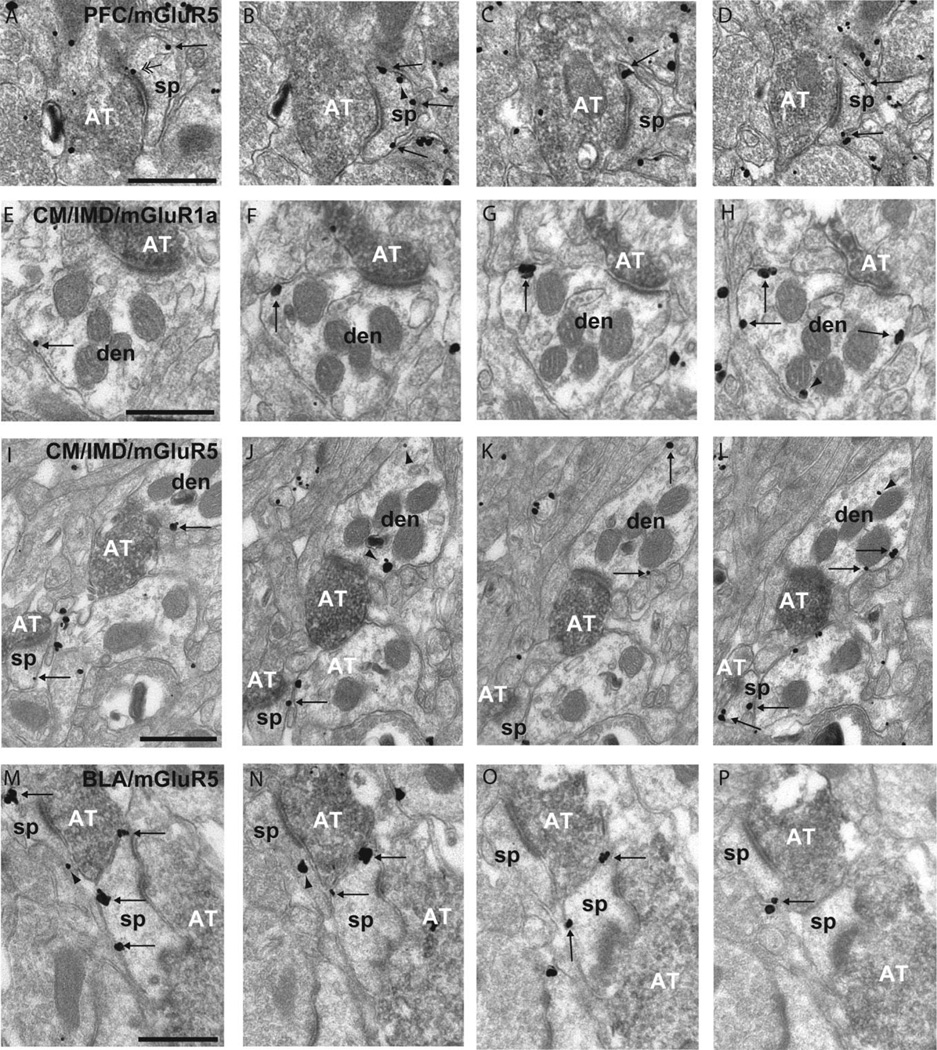
Figure 4.
Serial sections of rat accumbens tissue double immunostained for BDA (immunoperoxidase) and group I mGluRs (immunogold). A-D: Series of micrographs of a positively labeled axon terminal (AT) from the PFC forming an asymmetric synapse with a mGluR5-labeled spine (sp) in the accumbens core. Note that the majority of plasma membrane-bound labeling is extrasynaptic (single arrows), except for A, which shows an example of perisynaptic mGluR5 labeling (double arrowhead). E-H: Four micrographs of extrasynaptic mGluR1a labeling on a dendrite (den) contacted by an AT from theCM/IMD. I-L: Example of extrasynaptic mGluR5-IR in a dendrite and spine contacted by a positively labeled AT from the CM/IMD. There is also intracellular labeling in the dendrite (arrowheads). M-P: Example of mGluR5-IR spines in the accumbens shell contacted by labeled ATs from the posterior BLA with both extrasynaptic plasma membrane-bound labeling and intracellular labeling. Scale bar = 0.2 µm in A (applies to A-D), E (applies to E-H), I (applies to I-L), and M (applies to M-P).
The histograms in Figure 5 show the patterns of perisynaptic (A) or extrasynaptic (B) labeling in spines contacted by positively labeled terminals. The percentage of synaptic labeling was also quantified, but because less than 10% of spines contacted by cortical, thalamic, or amygdala terminals displayed synaptic group I mGluRs labeling in the rat accumbens, data are not presented in these figures. The same is the case for axodendritic glutamatergic synapses, which accounted for a very low percentage of total synaptic innervation of group I mGluR-containing neurons by the glutamatergic inputs labeled in this study.
Figure 5.
Histograms showing the subsynaptic distribution of group I mGluRs in relation to asymmetric postsynaptic specializations of cortical, amygdale, and thalamic axospinous synapses. A: Mean percent of spines (+SEM) that showed perisynaptic labeling for either mGluR1a or mGluR5 in the core and shell when contacted by a positively labeled terminal from the PFC, thalamus, or BLA. The asterisk indicates that perisynaptic mGluR5 labeling at PFC synapses is significantly more frequent than mGluR1a in the shell of the nucleus accumbens (one-way ANOVA and Tukey’s post hoc test, P < 0.05). The total number of terminals followed through three to six serial sections of three animals each (two for BLA/mGluR1a shell): PFC/mGluR1a Core = 64; PFC/mGluR1a Shell = 60; PFC/mGluR5 Core = 73; PFC/mGluR5 Shell = 75; CM/IMD/mGluR1aCore = 64; PVN/mGluR1a Shell = 58; CM/IMD/mGluR5 Core = 63; PVN/mGluR5 Shell = 61; BLA/mGluR1a Core = 60; BLA/mGluR1a Shell = 37; BLA/mGluR5 Core = 60; BLA/mGluR5 Shell = 62. B: Average distance of the closest extrasynaptic gold particle (µm + SEM) from the edge of the postsynaptic density (PSD) in mGluR1a- or mGluR5-labeled spines in asymmetric contact with an anterogradely labeled terminal from the PFC, BLA, or thalamus. The asterisks indicate that mGluR5 is closer to the edges of PVN synapses compared with mGluR1a in the shell of the accumbens (one-way ANOVA and Tukey’s post hoc test; P < 0.05).
As shown in Figure 5A, the degree of perisynaptic labeling associated with all glutamatergic inputs analyzed was higher for mGluR5 than mGluR1a in the core or shell of the rat accumbens (mGluR1a vs. mGluR5 in: PFC core: 15.7 + 1.7% vs. 28.7 + 11.7%; PFC shell: 11.6 + 3.4% vs. 47.7 + 9.1%; CM/IMD core: 6.7 + 6.7% vs. 31.6 + 10.3%; PVN shell: 7.0 + 7.0% vs. 28.5 + 5.5%; BLA core: 6.1 + 3.4% vs. 12.3 + 4.6%; BLA shell: 6.7 + 6.7% vs. 17.6 + 4.3%). Although there was a general trend toward a higher degree of perisynaptic immunoreactivity for all inputs, the difference between mGluR1a and mGluR5 was significant only for the perisynaptic labeling at cortical synapses in the shell of the accumbens (11.6 + 3.4% for mGluR1a vs. 47.7 + 9.1% for mGluR5; one-way ANOVA and Tukey’s post hoc text (F3, 11 = 4.392, n = 3, P < 0.05).
The relative distance between receptors and their sources of activating neurotransmitter contributes to the dynamic regulation of receptor activation (Triller and Choquet, 2005, 2008). Therefore, a second set of experiments was performed to compare the distance between the closest extrasynaptic gold particle labeling for mGluR1a or mGluR5 and the edges of the postsynaptic densities in spines contacted by glutamatergic inputs. Data from this analysis are shown in Figure 5B. As was the case for the prevalence of perisynaptic labeling, extrasynaptic mGluR5 immunoreactivity was closer to the edges of asymmetric synapses than mGluR1a for all inputs studied in both the shell and core of the rat accumbens (mGluR1a vs. mGluR5 in: PFC core: 0.30 + 0.06 µm vs. 0.21 + 0.02 µm; PFC shell: 0.38 + 0.07 µm vs. 0.18 + 0.03 µm; CM/IMD core: 0.32 + 0.04 µm vs. 0.23 + 0.03 µm; PVN shell: 0.33 + 0.02µm vs. 0.19 + 0.01µm; BLA core: 0.31 + 0.07 µm vs. 0.21 + 0.01 µm; BLA shell: 0.40 + 0.20 µmvs. 0.23 + 0.02 µm). Using a one-way ANOVA and Tukey’s post hoc tests, a significant difference was found between mGluR5 (0.19 µm + 0.01) and mGluR1a (0.33 µm + 0.02) extrasynaptic labeling associated with thalamic PVN inputs into the shell (F3,11 = 5.3, n = 3, P < 0.05).
DISCUSSION
Because of the multifarious origins of extrinsic glutamatergic afferents to the nucleus accumbens, attempts at understanding the specific roles of these different inputs to the complex regulation of accumbens neurons rely on a detailed characterization of the functional microcircuits that mediate these effects. Although there is compelling evidence for AMPA- and NMDA-mediated glutamatergic effects in the nucleus accumbens (Schmidt et al., 2005; Missale et al., 2006; Del Arco and Mora, 2008; Feltenstein and See, 2008; Kalivas et al., 2009), much less is known about the role of mGluRs in the functional glutamatergic microcircuitry of this brain region. However, data obtained over the past decade have clearly demonstrated that group I mGluRs play key roles in glutamate-mediated regulation of accumbens neurons following administration of psychostimulants (Vezina and Kim, 1999; Fellin et al., 2007; Uys and Lalumiere, 2008). Because the substrates through which these receptors regulate accumbens neurons’ activity remain poorly understood, the physiological mechanisms underlying the functional properties of group I mGluRs in the accumbens are merely empirical.
In order to further address this issue, the results of this study provide a detailed comparison of the synaptic relationships between mGluR1a and mGluR5 and the main glutamatergic inputs to the shell and core of the rat nucleus accumbens. Overall, the two group I mGluRs are located to subserve postsynaptic modulatory influences of cortical, thalamic, and amygdala glutamatergic inputs to the nucleus accumbens. However, based on the prevalence of synaptic relationships between specific inputs and postsynaptic striatal targets containing mGluR1a or mGluR5, two key conclusions can be drawn from this study: 1) both the amygdala and thalamic afferents preferentially target mGluR5-containing spines, whereas cortical inputs are evenly distributed between mGluR1a- and mGluR5-containing elements in either the core or shell of the nucleus accumbens; and (2) mGluR5 displays closer relationships with the postsynaptic densities of the main glutamatergic inputs to the nucleus accumbens than mGluR1a, thereby suggesting a differential degree of sensitivity of the two receptor subtypes to extrasynaptic spillover of glutamate from specific synaptic sites. Together, these findings provide a basic framework moving toward a deeper understanding of the functional anatomy and possible therapeutic-mediated effects of the two group I mGluRs in the nucleus accumbens.
Activation and physiology of group I mGluRs in the nucleus accumbens
The nucleus accumbens receives glutamatergic inputs from the cerebral cortex, hippocampus, thalamus, and amygdala (Heimer et al., 1997; Haber and McFarland, 1999; Zahm, 1999). However, the specific localization and function of group I mGluRs at these various synapses remain poorly understood. Earlier in vitro electrophysiology studies combined with microdialysis provided evidence for mGluR-mediated glutamate and dopamine release in the accumbens following stimulation of the rat prefrontal cortex (Taber and Fibiger, 1995). However, because only the broad-spectrum mGluR agonist (trans (1S, 3R)−1-aminocyclopentane−1, 3-dicarboxylic acid [ACPD]) was used, the respective role of mGluR1a or mGluR5 in mediating these effects could not be assessed (Taber and Fibiger, 1995). Recent data demonstrated that activation of both mGluR1 and mGluR5 is necessary to induce long-term potentiation (LTP) in mice accumbal neurons (Schotanus and Chergui, 2008; Anwyl, 2009); a phenomenon reminiscent of previous observations in the dorsal striatum (Calabresi et al., 2007). Because the high-frequency stimulation applied to induce LTP was delivered in the accumbens per se, it was not possible to determine which of the main glutamatergic synapses were potentiated in this study (Schotanus and Chergui, 2008).
Additional findings showed that group I mGluRs are involved in both LTP and long-term depression (LTD) of glutamatergic synapses in the ventral striatum (Robbe et al., 2002; Fourgeaud et al., 2004; Schotanus and Chergui, 2008). There is also evidence that mGluR5 mediates endocannabinoid-induced striatal LTD following stimulation of the prelimbic cortex in mice (Robbe et al., 2002; Fourgeaud et al., 2004).
Based on our ultrastructural localization data, it is reasonable to believe that both group I mGluRs are located to subserve LTP induction at cortical, thalamic, and amygdala synapses onto accumbens neurons. Because of the closer relationships between mGluR5 and glutamatergic synapses, it is possible that mGluR5 is recruited by extrasynaptic glutamate spillover prior to mGluR1 following activation of specific glutamatergic afferents (Galvan et al., 2006). However, this interpretation may be oversimplistic, taking into consideration that a significant proportion of spines in the nucleus accumbens co-express both group I mGluR subtypes (Mitrano and Smith, 2007a; Mitrano et al., 2008), and that mGluR1a and mGluR5 functionally interact to regulate their desensitization in other neuronal populations (Poisik et al., 2003; Bonsi et al., 2005).
One must also keep in mind that group I mGluRs activation do not solely rely on spillover of glutamate from synapses, but also on transmitter release from astrocytes, a major source of nonsynaptic glutamate in various parts of the brain, including the nucleus accumbens (Danbolt, 2001; Oliet et al., 2004; Galvan et al., 2006; Fellin et al., 2007; Haydon et al., 2009). It is also important to consider that extrasynaptic group I mGluRs could mediate their physiological effects in the accumbens through receptor-receptor interactions with various receptor subtypes including NMDA (Martin et al., 1997), dopamine-D1 (Schotanus and Chergui, 2008), and endocannabinoid-CB1 receptors (Robbe et al., 2002; Fourgeaud et al., 2004). Future studies looking at the degree of co-localization of mGluR1a and mGluR5 with these receptors could also add to our understanding of group I mGluRs function in the nucleus accumbens. Another piece of information missing from our study is the localization of group I mGluRs at hippocampal synapses. Knowing that the subiculum and other hippocampal areas provide strong inputs to the accumbens, future studies aimed at characterizing the relationships between specific hippocampal inputs and the two group I mGluRs are warranted.
Although the aforementioned studies provide some insights into the functions of group I mGluRs in relation to glutamatergic inputs, the in vitro preparations used did not allow a clear assessment of the physiology of mGluR1 and mGluR5 at cortical versus subcortical glutamatergic afferents to the accumbens because it is technically challenging to maintain the integrity of subcortical glutamatergic inputs to the accumbens. However, recent successful attempts at developing a plane of slice sectioning that partly preserves the thalamostriatal connection to the caudate-putamen complex in mice are encouraging (Smeal et al., 2007; Ding et al., 2008), and could possibly be used to study the physiology and pharmacology of thalamic synapses in the ventral striatum. The combination of intra-accumbens delivery of group I mGluR-related drugs with single-unit recordings following thalamic stimulation should also be considered as an in vivo approach to address this problem (Kliem and Wichmann, 2004; Galvan et al., 2005; Kita et al., 2007). Based on our data, it appears that mGluR5 could be the prime source of thalamic-induced excitatory effects on accumbens neurons, and that these effects may differ depending on the exact origin of thalamic afferents. However, without the use of the appropriate in vitro or in vivo preparations and proper electrophysiological methods using specific mGluR1- and mGluR5-related pharmacological agents, these suggestions remain empirical.
Psychostimulant addiction and group I mGluRs
Both group I mGluRs, but most particularly mGluR5, play a significant role in the rewarding effects of psychostimulants (Chiamulera et al., 2001; Swanson et al., 2001; Backstrom and Hyytia, 2006; Dravolina et al., 2006; Iso et al., 2006). Because of their anti-addictive properties, mGluR5 antagonists have generated significant interest as potential therapeutic targets for drug addiction (Carroll, 2008; Gasparini et al., 2008; Uys and Lalumiere, 2008; Kalivas et al., 2009). The results of the present study suggest that the effects of group I mGluR antagonists on drug intake could be mediated through modulation of cortical and subcortical glutamatergic inputs to the nucleus accumbens. The importance of prefrontal cortical inputs to the accumbens as a major source of glutamate-related changes associated with drug addiction is well established (Xi et al., 2003; McFarland et al., 2003; Kalivas, 2007; Del Arco and Mora, 2008). Although not as extensively studied, the amygdala has also been implicated in the reinstatement and spontaneous recovery of cocaine self-administration (Fuchs et al., 2007; Peters et al., 2008).
In contrast, very little is known about the role of the thalamostriatal system in drug addiction, but it is worth noting that both cocaine and amphetamine induce Fos expression in the PVN (Brown et al., 1992; Deutch et al., 1998). Additionally, animals with PVN lesion have enhanced locomotion following acute exposure to cocaine (Young and Deutch, 1998). Although much remains to be discovered about the functional interactions through which these different glutamatergic afferents may contribute to various aspects of addictive behaviors, our findings provide clear anatomical evidence that they are all endowed with the necessary machinery to participate in group I mGluR-mediated effects in the nucleus accumbens.
Because of its modulatory nature, pharmacological specificity for bioavailable agents, and restricted brain localization, mGluR5 has been the target of choice for preclinical studies of therapeutic efficacy in various brain diseases including Parkinson’s disease, drug addiction, pain, schizophrenia, and Fragile X syndrome (Carroll, 2008; Gasparini et al., 2008; Conn et al., 2009). The successful development of any pharmacotherapy relies on a detailed knowledge of the basic localization and function of the brain targets. The results of the present study are a step forward in that direction, laying the foundation for a deeper understanding of group I mGluR-mediated physiological effects in the ventral striatum and potential therapeutic benefits in drug addiction and other brain diseases.
ACKNOWLEDGMENTS
The authors thank Susan Jenkins for her valuable technical assistance.
Grant sponsor: National Institutes of Health; Grant numbers:R01 NS037423 (to Y.S.), 5F31DA020301 (to D.A.M.), and RR00165 (base grant of the Yerkes Primate Center).
LITERATURE CITED
- Anwyl R. Metabotropic glutamate receptor-dependent long-term potentiation. Neuropharmacology. 2009;56:735–740. doi: 10.1016/j.neuropharm.2009.01.002. [DOI] [PubMed] [Google Scholar]
- Backstrom P, Hyytia P. Ionotropic and metabotropic glutamate receptor antagonism attenuates cue-induced cocaine seeking. Neuropsychopharmacology. 2006;31:778–786. doi: 10.1038/sj.npp.1300845. [DOI] [PubMed] [Google Scholar]
- Baker DA, Xi ZX, Shen H, Swanson CJ, Kalivas PW. The origin and neuronal function of in vivo nonsynaptic glutamate. J Neurosci. 2002;22:9134–9141. doi: 10.1523/JNEUROSCI.22-20-09134.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berendse HW, Groenewegen HJ. Organization of the thalamostriatal projections in the rat, with special emphasis on the ventral striatum. J Comp Neurol. 1990;299:187–228. doi: 10.1002/cne.902990206. [DOI] [PubMed] [Google Scholar]
- Berendse HW, Galis-de Graaf Y, Groenewegen HJ. Topographical organization and relationship with ventral striatal compartments of prefrontal corticostriatal projections in the rat. J Comp Neurol. 1992;316:314–347. doi: 10.1002/cne.903160305. [DOI] [PubMed] [Google Scholar]
- Blackstad TW, Karagulle T, Ottersen OP. Morforel, a computer program for two-dimensional analysis of micrographs of biological specimens, with emphasis on immunogold preparations. Comput Biol Med. 1990;20:15–34. doi: 10.1016/0010-4825(90)90041-m. [DOI] [PubMed] [Google Scholar]
- Bonsi P, Cuomo D, De Persis C, Centonze D, Bernardi G, Calabresi P, Pisani A. Modulatory action of metabotropic glutamate receptor (mGluR) 5 on mGluR1 function in striatal cholinergic interneurons. Neuropharmacology. 2005;49(Suppl 1):104–113. doi: 10.1016/j.neuropharm.2005.05.012. [DOI] [PubMed] [Google Scholar]
- Brown EE, Robertson GS, Fibiger HC. Evidence for conditional neuronal activation following exposure to a cocaine-paired environment: role of forebrain limbic structures. J Neurosci. 1992;12:4112–4121. doi: 10.1523/JNEUROSCI.12-10-04112.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabresi P, Picconi B, Tozzi A, Di Filippo M. Dopamine-mediated regulation of corticostriatal synaptic plasticity. Trends Neurosci. 2007;30:211–219. doi: 10.1016/j.tins.2007.03.001. [DOI] [PubMed] [Google Scholar]
- Carroll FI. Antagonists at metabotropic glutamate receptor subtype 5: structure activity relationships and therapeutic potential for addiction. Ann N Y Acad Sci. 2008;1141:221–232. doi: 10.1196/annals.1441.015. [DOI] [PubMed] [Google Scholar]
- Celio MR. Calbindin D-28k and parvalbumin in the rat nervous system. Neuroscience. 1990;35:375–475. doi: 10.1016/0306-4522(90)90091-h. [DOI] [PubMed] [Google Scholar]
- Chiamulera C, Epping-Jordan MP, Zocchi A, Marcon C, Cottiny C, Tacconi S, Corsi M, Orzi F, Conquet F. Reinforcing and locomotor stimulant effects of cocaine are absent in mGluR5 null mutant mice. Nat Neurosci. 2001;4:873–874. doi: 10.1038/nn0901-873. [DOI] [PubMed] [Google Scholar]
- Conn PJ, Lindsley CW, Jones CK. Activation of metabotropic glutamate receptors as a novel approach for the treatment of schizophrenia. Trends Pharmacol Sci. 2009;30:25–31. doi: 10.1016/j.tips.2008.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danbolt NC. Glutamate uptake. Prog Neurobiol. 2001;65:1–105. doi: 10.1016/s0301-0082(00)00067-8. [DOI] [PubMed] [Google Scholar]
- Del Arco A, Mora F. Prefrontal cortex-nucleus accumbens interaction: in vivo modulation by dopamine and glutamate in the prefrontal cortex. Pharmacol Biochem Behav. 2008;90:226–235. doi: 10.1016/j.pbb.2008.04.011. [DOI] [PubMed] [Google Scholar]
- Deutch AY, Bubser M, Young CD. Psychostimulant-induced Fos protein expression in the thalamic paraventricular nucleus. J Neurosci. 1998;18:10680–10687. doi: 10.1523/JNEUROSCI.18-24-10680.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding J, Peterson JD, Surmeier DJ. Corticostriatal and thalamostriatal synapses have distinctive properties. J Neurosci. 2008;28:6483–6492. doi: 10.1523/JNEUROSCI.0435-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dravolina OA, Danysz W, Bespalov AY. Effects of group I metabotropic glutamate receptor antagonists on the behavioral sensitization to motor effects of cocaine in rats. Psychopharmacology (Berl) 2006;187:397–404. doi: 10.1007/s00213-006-0440-1. [DOI] [PubMed] [Google Scholar]
- Fellin T, D’Ascenzo M, Haydon PG. Astrocytes control neuronal excitability in the nucleus accumbens. Scientific World J. 2007;7:89–97. doi: 10.1100/tsw.2007.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feltenstein MW, See RE. The neurocircuitry of addiction: an overview. Br J Pharmacol. 2008;154:261–274. doi: 10.1038/bjp.2008.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fourgeaud L, Mato S, Bouchet D, Hemar A, Worley PF, Manzoni OJ. A single in vivo exposure to cocaine abolishes endocannabinoid-mediated long-term depression in the nucleus accumbens. J Neurosci. 2004;24:6939–6945. doi: 10.1523/JNEUROSCI.0671-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French SJ, Totterdell S. Individual nucleus accumbens-projection neurons receive both basolateral amygdala and ventral subicular afferents in rats. Neuroscience. 2003;119:19–31. doi: 10.1016/s0306-4522(03)00150-7. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Eaddy JL, Su ZI, Bell GH. Interactions of the basolateral amygdala with the dorsal hippocampus and dorsomedial prefrontal cortex regulate drug context-induced reinstatement of cocaine-seeking in rats. Eur J Neurosci. 2007;26:487–498. doi: 10.1111/j.1460-9568.2007.05674.x. [DOI] [PubMed] [Google Scholar]
- Galvan A, Villalba RM, West SM, Maidmont NT, Ackerson LC, Smith Y, Wichmann T. GABAergic modulation of the activity of globus pallidus neurons in primates: in vivo analysis of the functions of GABA receptors and GABA transporters. J Neurophysiol. 2005;94:990–1000. doi: 10.1152/jn.00068.2005. [DOI] [PubMed] [Google Scholar]
- Galvan A, Kuwajima M, Smith Y. Glutamate and GABA receptors and transporters in the basal ganglia: what does their subsynaptic localization reveal about their function? Neuroscience. 2006;143:351–375. doi: 10.1016/j.neuroscience.2006.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasparini F, Bilbe G, Gomez-Mancilla B, Spooren W. mGluR5 antagonists: discovery, characterization and drug development. Curr Opin Drug Discov Dev. 2008;11:655–665. [PubMed] [Google Scholar]
- Groenewegen HJ, Vermeulen-Van der Zee E, te Kortschot A, Witter MP. Organization of the projections from the subiculum to the ventral striatum in the rat. A study using anterograde transport of Phaseolus vulgaris leucoagglutinin. Neuroscience. 1987;23:103–120. doi: 10.1016/0306-4522(87)90275-2. [DOI] [PubMed] [Google Scholar]
- Haber SN, McFarland NR. The concept of the ventral striatum in nonhuman primates. Ann N Y Acad Sci. 1999;877:33–48. doi: 10.1111/j.1749-6632.1999.tb09259.x. [DOI] [PubMed] [Google Scholar]
- Haydon PG, Blandy J, Moss SJ, Rob Jackson F. Astrocytic control of synaptic transmission and plasticity: a target for drugs of abuse? Neuropharmacology. 2009;56(Suppl 1):83–90. doi: 10.1016/j.neuropharm.2008.06.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heimer L, Alheid GF, de Olmos JS, Groenewegen HJ, Haber SN, Harlan RE, Zahm DS. The accumbens: beyond the coreshell dichotomy. J Neuropsychiatry Clin Neurosci. 1997;9:354–381. doi: 10.1176/jnp.9.3.354. [DOI] [PubMed] [Google Scholar]
- Iso Y, Grajkowska E, Wroblewski JT, Davis J, Goeders NE, Johnson KM, Sanker S, Roth BL, Tueckmantel W, Kozikowski AP. Synthesis and structure-activity relationships of 3-[(2-methyl-1,3-thiazol-4-yl)ethynyl]pyridine analogues as potent, non-competitive metabotropic glutamate receptor subtype 5 antagonists; search for cocaine medications. J Med Chem. 2006;49:1080–1100. doi: 10.1021/jm050570f. [DOI] [PubMed] [Google Scholar]
- Kalivas PW. Cocaine and amphetamine-like psychostimulants: neurocircuitry and glutamate neuroplasticity. Dialogues Clin Neurosci. 2007;9:389–397. doi: 10.31887/DCNS.2007.9.4/pkalivas. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW, Lalumiere RT, Knackstedt L, Shen H. Glutamate transmission in addiction. Neuropharmacology. 2009;56(Suppl 1):169–173. doi: 10.1016/j.neuropharm.2008.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kita H, Chiken S, Tachibana Y, Nambu A. Serotonin modulates pallidal neuronal activity in the awake monkey. J Neurosci. 2007;27:75–83. doi: 10.1523/JNEUROSCI.4058-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kliem MA, Wichmann T. A method to record changes in local neuronal discharge in response to infusion of small drug quantities in awake monkeys. J Neurosci Methods. 2004;38:45–49. doi: 10.1016/j.jneumeth.2004.03.015. [DOI] [PubMed] [Google Scholar]
- Koulen P, Kuhn R, Wassle H, Brandstatter JH. Group I metabotropic glutamate receptors mGluR1alpha and mGluR5a: localization in both synaptic layers of the rat retina. J Neurosci. 1997;17:2200–2211. doi: 10.1523/JNEUROSCI.17-06-02200.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwajima M, Hall RA, Aiba A, Smith Y. Subcellular and subsynaptic localization of group I metabotropic glutamate receptors in the monkey subthalamic nucleus. J Comp Neurol. 2004;474:589–602. doi: 10.1002/cne.20158. [DOI] [PubMed] [Google Scholar]
- Mannaioni G, Marino MJ, Valenti O, Traynelis SF, Conn PJ. Metabotropic glutamate receptors 1 and 5 differentially regulate CA1 pyramidal cell function. J Neurosci. 2001;21:5925–5934. doi: 10.1523/JNEUROSCI.21-16-05925.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin G, Nie Z, Siggins GR. Metabotropic glutamate receptors regulate N-methyl-D-aspartate-mediated synaptic transmission in nucleus accumbens. J Neurophysiol. 1997;78:3028–3038. doi: 10.1152/jn.1997.78.6.3028. [DOI] [PubMed] [Google Scholar]
- McDonald AJ. Topographical organization of amygdaloid projections to the caudatoputamen, nucleus accumbens, and related striatal-like areas of the rat brain. Neuroscience. 1991;44:15–33. doi: 10.1016/0306-4522(91)90248-m. [DOI] [PubMed] [Google Scholar]
- McFarland K, Lapish CC, Kalivas PW. Prefrontal glutamate release into the core of the nucleus accumbens mediates cocaine-induced reinstatement of drug-seeking behavior. J Neurosci. 2003;23:3531–3537. doi: 10.1523/JNEUROSCI.23-08-03531.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeehan AJ, Olive MF. The mGluR5 antagonist MPEP reduces the conditioned rewarding effects of cocaine but not other drugs of abuse. Synapse. 2003;47:240–242. doi: 10.1002/syn.10166. [DOI] [PubMed] [Google Scholar]
- Missale C, Fiorentini C, Busi C, Collo G, Spano PF. The NMDA/D1 receptor complex as a new target in drug development. Curr Top Med Chem. 2006;6:801–808. doi: 10.2174/156802606777057562. [DOI] [PubMed] [Google Scholar]
- Mitrano DA, Smith Y. Comparative analysis of the subcellular and subsynaptic localization of mGluR1a and mGluR5 metabotropic glutamate receptors in the shell and core of the nucleus accumbens in rat and monkey. J Comp Neurol. 2007a;500:788–806. doi: 10.1002/cne.21214. [DOI] [PubMed] [Google Scholar]
- Mitrano DA, Smith Y. 9th. The Netherlands: Triennial Meeting of the International Basal Ganglia Society; 2007b. Differential glutamatergic innervation of mGluR1a- and mGluR5-containing neurons in the rat nucleus accumbens. Program No. P-024. [Google Scholar]
- Mitrano DA, Arnold C, Smith Y. Subcellular and subsynaptic localization of group I metabotropic glutamate receptors in the nucleus accumbens of cocaine-treated rats. Neuro-science. 2008;154:653–666. doi: 10.1016/j.neuroscience.2008.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyata S, Nakai S, Kiyohara T, Hatton GI. Calbindin-D28k and calretinin in the rat posterior pituitary; light and electron microscopic localization and upregulation with dehydration. J Neurocytol. 2000;29:5–17. doi: 10.1023/a:1007180328597. [DOI] [PubMed] [Google Scholar]
- Oliet SH, Piet R, Poulain DA, Theodosis DT. Glial modulation of synaptic transmission: insights from the supraoptic nucleus of the hypothalamus. Glia. 2004;47:258–267. doi: 10.1002/glia.20032. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. London: Academic Press; 1998. [DOI] [PubMed] [Google Scholar]
- Peters A, Palay S, Webster HD. The fine structure of the nervous system: neurons and their supporting cells. New York: Oxford University Press; 1991. [Google Scholar]
- Peters J, Vallone J, Laurendi K, Kalivas P. Opposing roles for the ventral prefrontal cortex and the basolateral amygdala on the spontaneous recovery of cocaine-seeking rats. Psycho-pharmacology. 2008;197:319–326. doi: 10.1007/s00213-007-1034-2. [DOI] [PubMed] [Google Scholar]
- Poisik OV, Mannaioni G, Traynelis S, Smith Y, Conn PJ. Distinct functional roles of the metabotropic glutamate receptors 1 and 5 in the rat globus pallidus. J Neurosci. 2003;23:122–130. doi: 10.1523/JNEUROSCI.23-01-00122.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raju DV, Ahern TD, Shah DJ, Wright TM, Standaert DG, Hall RA, Smith Y. Differential synaptology of vGluT2-containing thalamostriatal afferents between the patch and matrix compartments in rats. J Comp Neurol. 2006;499:231–243. doi: 10.1002/cne.21099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbe D, Alonso G, Chaumont S, Bockaert J, Manzoni OJ. Role of p/q-Ca2+ channels in metabotropic glutamate receptor 2/3-dependent presynaptic long-term depression at nucleus accumbens synapses. J Neurosci. 2002;22:4346–4356. doi: 10.1523/JNEUROSCI.22-11-04346.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. Addiction. Annu Rev Psychol. 2003;54:25–53. doi: 10.1146/annurev.psych.54.101601.145237. [DOI] [PubMed] [Google Scholar]
- Schmidt HD, Anderson SM, Famous KR, Kumaresan V, Pierce RC. Anatomy and pharmacology of cocaine priming-induced reinstatement of drug seeking. Eur J Pharmacol. 2005;526:65–76. doi: 10.1016/j.ejphar.2005.09.068. [DOI] [PubMed] [Google Scholar]
- Schotanus SM, Chergui K. Dopamine D1 receptors and group I metabotropic glutamate receptors contribute to the induction of long-term potentiation in the nucleus accum-bens. Neuropharmacology. 2008;54:837–844. doi: 10.1016/j.neuropharm.2007.12.012. [DOI] [PubMed] [Google Scholar]
- Smeal RM, Gaspar RC, Keefe KA, Wilcox KS. A rat brain slice preparation for characterizing both thalamostriatal and corticostriatal afferents. J Neurosci Methods. 2007;159:224–235. doi: 10.1016/j.jneumeth.2006.07.007. [DOI] [PubMed] [Google Scholar]
- Swanson CJ, Baker DA, Carson D, Worley PF, Kalivas PW. Repeated cocaine administration attenuates group I metabotropic glutamate receptor-mediated glutamate release and behavioral activation: a potential role for Homer. J Neurosci. 2001;21:9043–9052. doi: 10.1523/JNEUROSCI.21-22-09043.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taber MT, Fibiger HC. Electrical stimulation of the prefrontal cortex increases dopamine release in the nucleus accumbens of the rat: modulation by metabotropic glutamate receptors. J Neurosci. 1995;15:3896–3904. doi: 10.1523/JNEUROSCI.15-05-03896.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Testa CM, Standaert DG, Young AB, Penney JB., Jr Metabotropic glutamate receptor mRNA expression in the basal ganglia of the rat. J Neurosci. 1994;14:3005–3018. doi: 10.1523/JNEUROSCI.14-05-03005.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triller A, Choquet D. Surface trafficking of receptors between synaptic and extrasynaptic membranes: and yet they do move! Trends Neurosci. 2005;28:133–139. doi: 10.1016/j.tins.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Triller A, Choquet D. New concepts in synaptic biology derived from single-molecule imaging. Neuron. 2008;59:359–374. doi: 10.1016/j.neuron.2008.06.022. [DOI] [PubMed] [Google Scholar]
- Uys JD, LaLumiere RT. Glutamate: the new frontier in pharmacotherapy for cocaine addiction. CNS Neurol Disord Drug Targets. 2008;7:482–491. doi: 10.2174/187152708786927868. [DOI] [PubMed] [Google Scholar]
- Vezina P, Kim JH. Metabotropic glutamate receptors and the generation of locomotor activity: interactions with midbrain dopamine. Neurosci Biobehav Rev. 1999;23:577–589. doi: 10.1016/s0149-7634(98)00055-4. [DOI] [PubMed] [Google Scholar]
- Winsky L, Nakata H, Martin BM, Jacobowitz DM. Isolation, partial amino acid sequence, and immunohistochemical localization of a brain-specific calcium-binding protein. Proc Natl Acad Sci U S A. 1989;86:10139–10143. doi: 10.1073/pnas.86.24.10139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi ZX, Shen H, Baker DA, Kalivas PW. Inhibition of nonvesicular glutamate release by group III metabotropic glutamate receptors in the nucleus accumbens. J Neurochem. 2003;87:1204–1212. doi: 10.1046/j.1471-4159.2003.02093.x. [DOI] [PubMed] [Google Scholar]
- Young CD, Deutch AY. The effects of thalamic paraventricular nucleus lesions on cocaine-induced locomotor activity and sensitization. Pharmacol Biochem Behav. 1998;60:753–758. doi: 10.1016/s0091-3057(98)00051-3. [DOI] [PubMed] [Google Scholar]
- Zahm DS. Functional-anatomical implications of the nucleus accumbens core and shell subterritories. Ann N Y Acad Sci. 1999;877:113–128. doi: 10.1111/j.1749-6632.1999.tb09264.x. [DOI] [PubMed] [Google Scholar]