Abstract
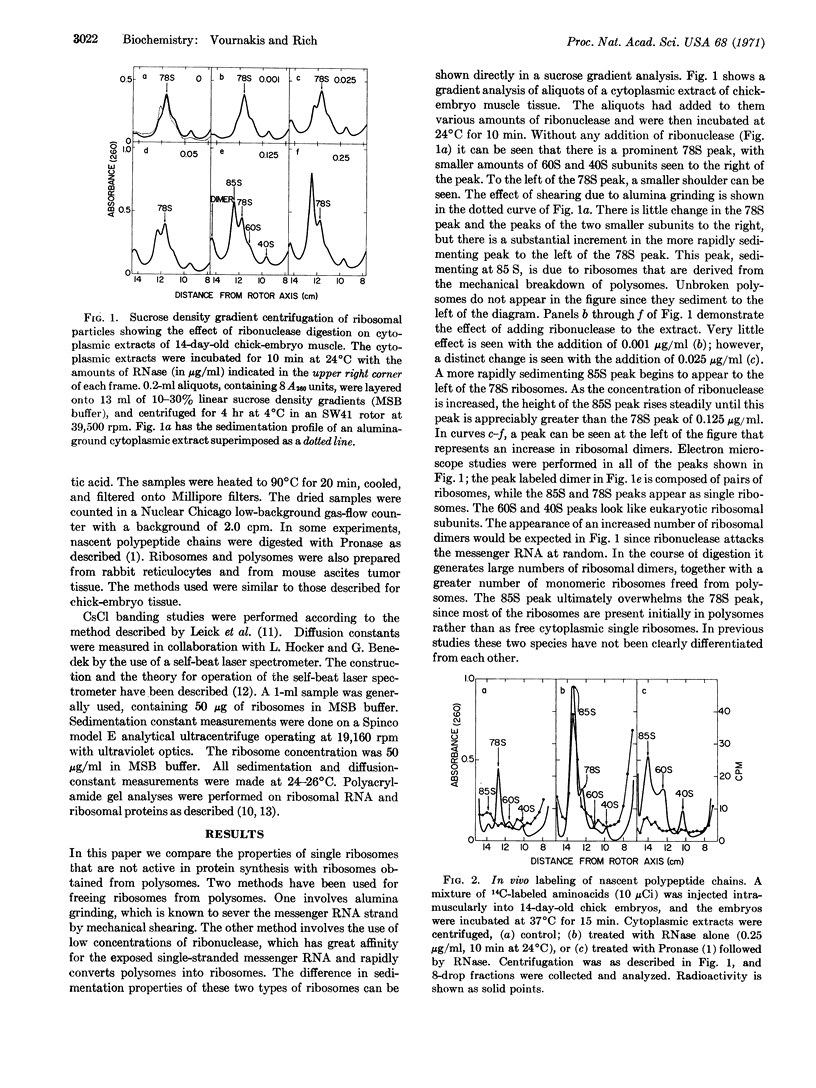
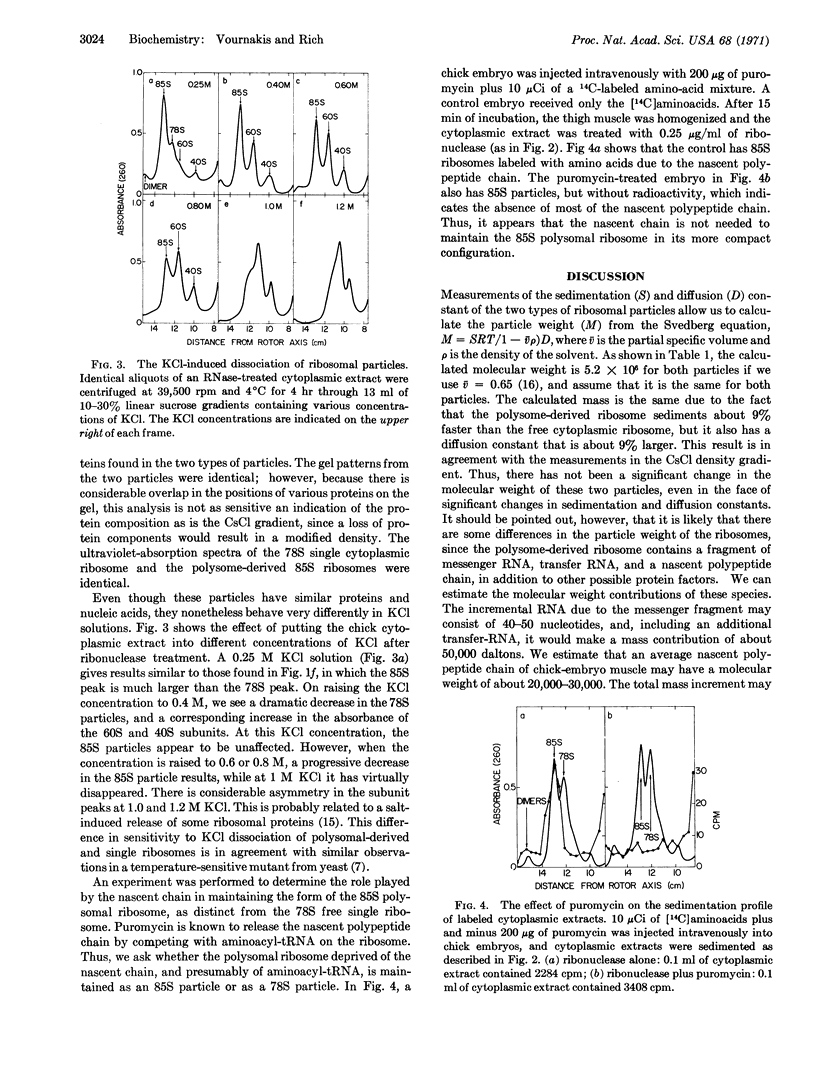
Evidence is presented that ribosomes active in protein synthesis and attached to messenger RNA on polysomes have a smaller diameter than free cytoplasmic single ribosomes. Measurements have been made on these two types of ribosomes of differences in sedimentation velocity and diffusion constant. Differences in these quantities suggest about a 20-Å decrease in the diameter of the ribosomes from chick embryo muscles when they are attached to messenger RNA. Similar differences are also observed in rabbit reticulocytes and mouse ascites tumor cells. These two ribosomal states have different sensitivity to Pronase digestion and dissociate into ribosomal subunits at different KCl concentrations. This size difference is not associated with a significant difference in overall ribosomal mass and appears not to be dependent upon the presence of nascent polypeptide chains.
Keywords: diffusion constant, sedimentation constant, ribosomal dissociation, chick embryo
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chuang D., Simpson M. V. A translocation-associated ribosomal conformational change detected by hydrogen exchange and sedimentation velocity. Proc Natl Acad Sci U S A. 1971 Jul;68(7):1474–1478. doi: 10.1073/pnas.68.7.1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubin S. B., Benedek G. B., Bancroft F. C., Freifelder D. Molecular weights of coliphages and colip- hage DNA. II. Measurement of diffusion coefficients using optical mixing spectroscopy, and measurement of sedimentation coefficients. J Mol Biol. 1970 Dec 28;54(3):547–556. doi: 10.1016/0022-2836(70)90125-7. [DOI] [PubMed] [Google Scholar]
- Dubin S. B., Lunacek J. H., Benedek G. B. Observation of the spectrum of light scattered by solutions of biological macromolecules. Proc Natl Acad Sci U S A. 1967 May;57(5):1164–1171. doi: 10.1073/pnas.57.5.1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falvey A. K., Staehelin T. Structure and function of mammalian ribosomes. I. Isolation and characterization of active liver ribosomal subunits. J Mol Biol. 1970 Oct 14;53(1):1–19. doi: 10.1016/0022-2836(70)90042-2. [DOI] [PubMed] [Google Scholar]
- Heywood S. M., Dowben R. M., Rich A. A study of muscle polyribosomes and the coprecipitation of polyribosomes with myosin. Biochemistry. 1968 Sep;7(9):3289–3296. doi: 10.1021/bi00849a036. [DOI] [PubMed] [Google Scholar]
- Heywood S. M., Dowben R. M., Rich A. The identification of polyribosomes synthesizing myosin. Proc Natl Acad Sci U S A. 1967 Apr;57(4):1002–1009. doi: 10.1073/pnas.57.4.1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan B. L., Korner A. The role of ribosomal subunits and 80-S monomers in polysome formation in an ascites tumour cell. Biochim Biophys Acta. 1968 Nov 20;169(1):139–149. doi: 10.1016/0005-2787(68)90015-4. [DOI] [PubMed] [Google Scholar]
- Huang A. S., Balitmore D. Initiation of polyribosome formation in poliovirus-infected HeLa cells. J Mol Biol. 1970 Feb 14;47(3):275–291. doi: 10.1016/0022-2836(70)90302-5. [DOI] [PubMed] [Google Scholar]
- Infante A. A., Baierlein R. Pressure-induced dissociation of sedimenting ribosomes: effect on sedimentation patterns. Proc Natl Acad Sci U S A. 1971 Aug;68(8):1780–1785. doi: 10.1073/pnas.68.8.1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabat D., Rich A. The ribosomal subunit--polyribosome cycle in protein synthesis of embryonic skeletal muscle. Biochemistry. 1969 Sep;8(9):3742–3749. doi: 10.1021/bi00837a038. [DOI] [PubMed] [Google Scholar]
- Kaempfer R. Dissociation of ribosomes on polypeptide chain termination and origin of single ribosomes. Nature. 1970 Nov 7;228(5271):534–537. doi: 10.1038/228534a0. [DOI] [PubMed] [Google Scholar]
- Leick V., Engberg J., Emmersen J. Nascent subribosomal particles in Tetrahymena pyriformis. Eur J Biochem. 1970 Apr;13(2):238–246. doi: 10.1111/j.1432-1033.1970.tb00923.x. [DOI] [PubMed] [Google Scholar]
- Malkin L. I., Rich A. Partial resistance of nascent polypeptide chains to proteolytic digestion due to ribosomal shielding. J Mol Biol. 1967 Jun 14;26(2):329–346. doi: 10.1016/0022-2836(67)90301-4. [DOI] [PubMed] [Google Scholar]
- Martin T. E., Hartwell L. H. Resistance of active yeast ribosomes to dissociation by KCl. J Biol Chem. 1970 Mar 25;245(6):1504–1506. [PubMed] [Google Scholar]
- Perry R. P., Kelley D. E. Buoyant densities of cytoplasmic ribonucleoprotein particles of mammalian cells: distinctive character of ribosome subunits and the rapidly labeled components. J Mol Biol. 1966 Apr;16(2):255–268. doi: 10.1016/s0022-2836(66)80171-7. [DOI] [PubMed] [Google Scholar]
- Schreier M. H., Noll H. Conformational changes in ribosomes during protein synthesis. Proc Natl Acad Sci U S A. 1971 Apr;68(4):805–809. doi: 10.1073/pnas.68.4.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spirin A. S. On the equilibrium of the association-dissociation reaction of ribosomal subparticles and on the existance of the so-called '60 S intermediate' ('swollen 70 S') during centrifugation of the equilibrium mixture. FEBS Lett. 1971 May 20;14(5):349–353. doi: 10.1016/0014-5793(71)80298-3. [DOI] [PubMed] [Google Scholar]
- Vesco C., Colombo B. Effect of sodium fluoride on protein synthesis in HeLa cells: inhibition of ribosome dissociation. J Mol Biol. 1970 Feb 14;47(3):335–352. doi: 10.1016/0022-2836(70)90306-2. [DOI] [PubMed] [Google Scholar]


