Abstract
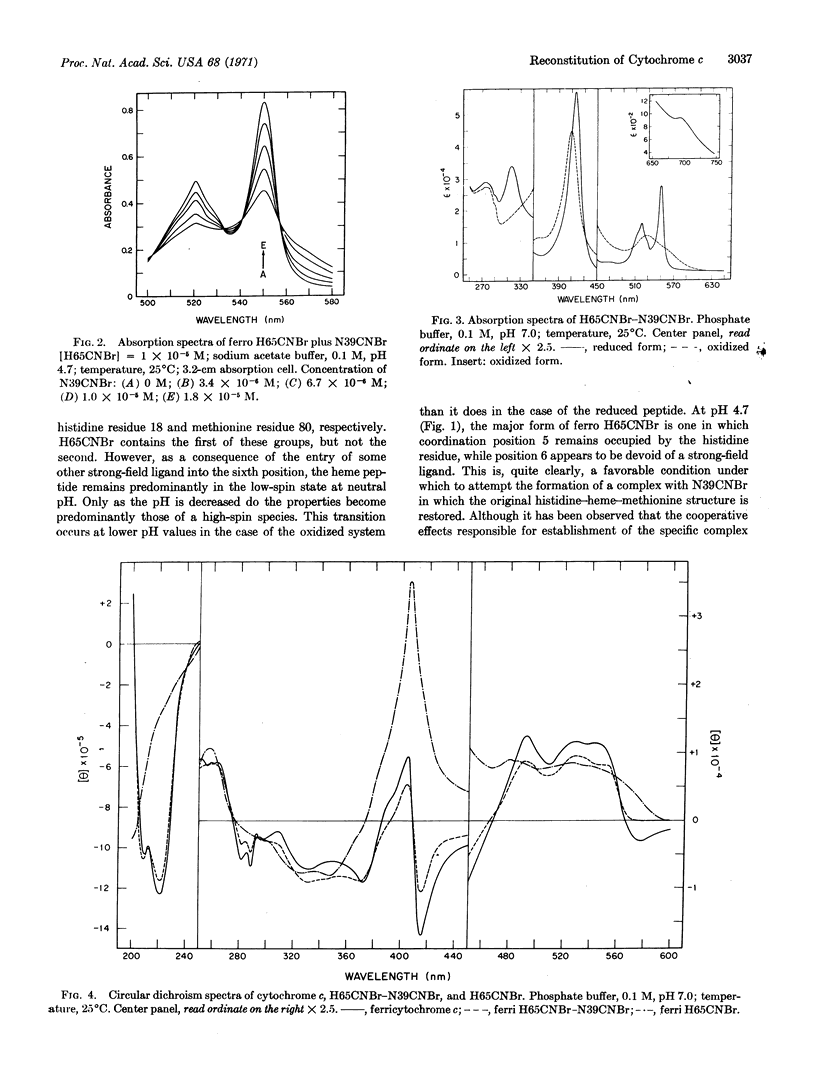
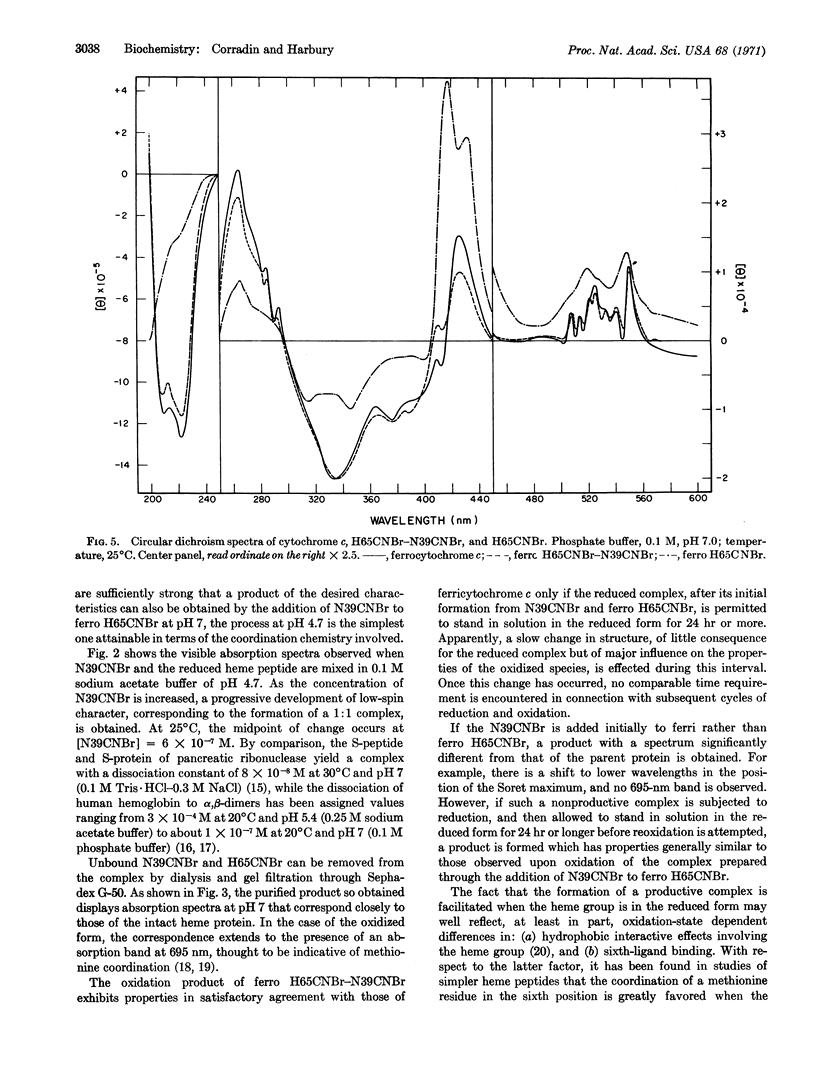
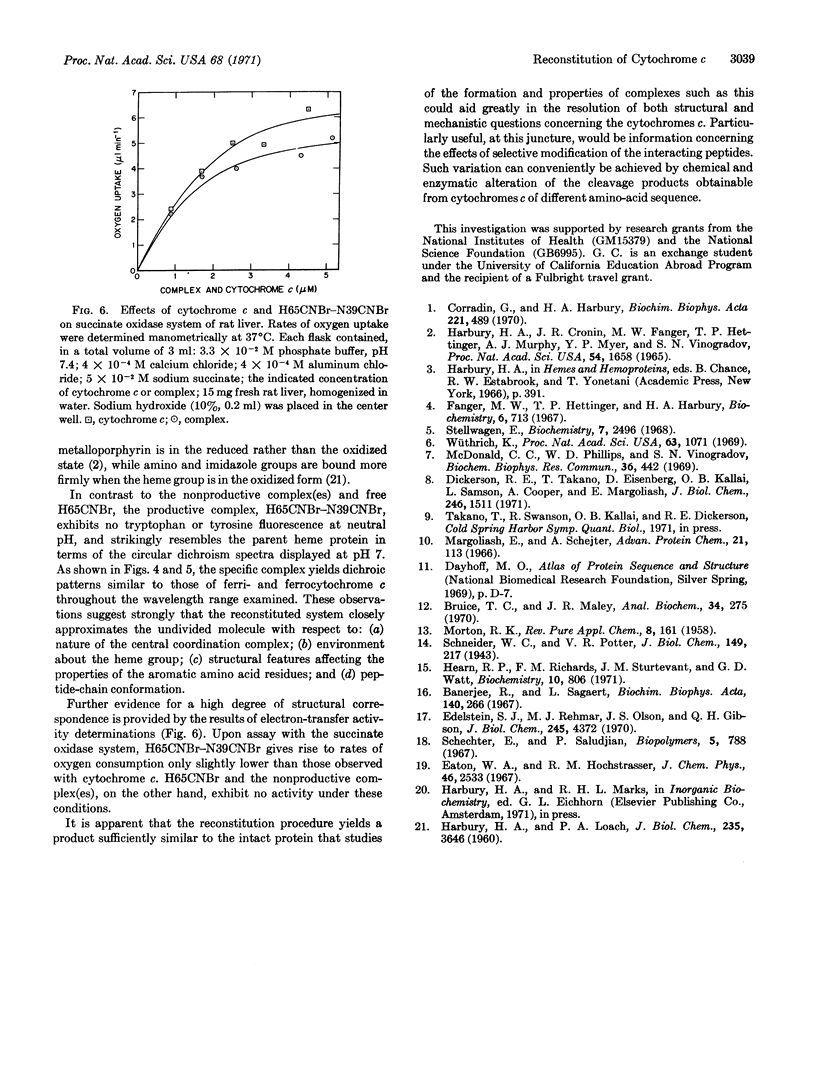
Horse heart cytochrome c can be divided into a heme peptide of 65 residues (H65CNBr) and a nonheme peptide of 39 residues (N39CNBr) by treatment of the molecule with cyanogen bromide. Upon mixture of the two peptides in aqueous solution, a 1: 1 complex with properties closely resembling those of the parent heme protein can be formed. The reaction can conveniently be effected in sodium acetate buffer of pH 4.7, with the H65CNBr in the reduced form. The heme peptide is predominantly in the high-spin state under these conditions, and, upon the addition of N39CNBr, is converted to a complex with absorption and circular dichroism spectra which correspond closely to those of ferrocytochrome c. If N39CNBr is added to the oxidized form of H65CNBr, the spectral properties of the product differ appreciably from those of the parent protein. A complex with absorption and circular dichroism spectra comparable to those of ferricytochrome c can readily be obtained, however, through oxidation of the product of the reaction of N39CNBr with H65CNBr in the reduced state. The complex formed in this manner has an absorption band at 695 nm, exhibits no tryptophan or tyrosine fluorescence, and is active in the succinate oxidase system.
Keywords: heme protein, heme peptide
Full text
PDF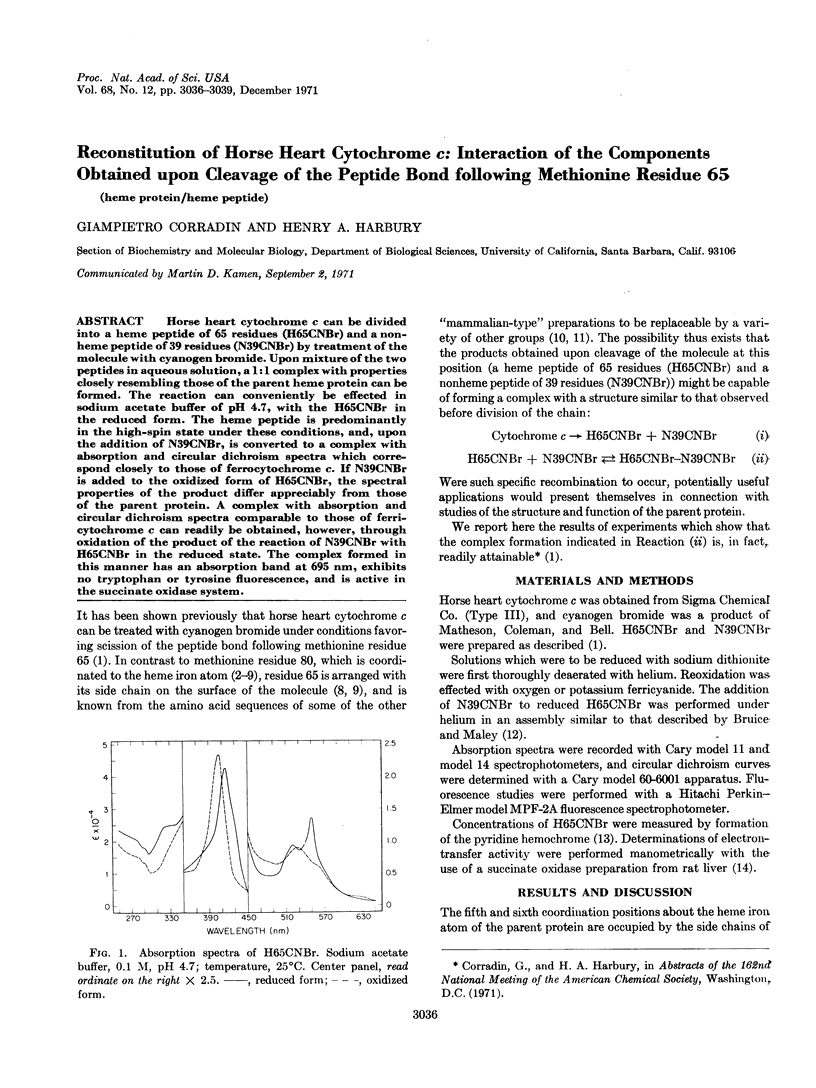
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banerjee R., Sagaert L. Dissociation de l'hémoglobine humaine en milieu acide. Biochim Biophys Acta. 1967 Jun 27;140(2):266–273. [PubMed] [Google Scholar]
- Bruice T. C., Maley J. R. Equilibrium titration and pH-stat cell for a Cary 15 spectrophotometer. Anal Biochem. 1970 Mar;34:275–278. doi: 10.1016/0003-2697(70)90105-3. [DOI] [PubMed] [Google Scholar]
- Corradin G., Harbury H. A. Cleavage of cytochrome c with cyanogen bromide. Biochim Biophys Acta. 1970 Dec 22;221(3):489–496. doi: 10.1016/0005-2795(70)90219-9. [DOI] [PubMed] [Google Scholar]
- Dickerson R. E., Takano T., Eisenberg D., Kallai O. B., Samson L., Cooper A., Margoliash E. Ferricytochrome c. I. General features of the horse and bonito proteins at 2.8 A resolution. J Biol Chem. 1971 Mar 10;246(5):1511–1535. [PubMed] [Google Scholar]
- Eaton W. A., Hochstrasser R. M. Electronic spectrum of single crystals of ferricytochrome-c. J Chem Phys. 1967 Apr 1;46(7):2533–2539. doi: 10.1063/1.1841081. [DOI] [PubMed] [Google Scholar]
- Edelstein S. J., Rehmar M. J., Olson J. S., Gibson Q. H. Functional aspects of the subunit association-dissociation equilibria of hemoglobin. J Biol Chem. 1970 Sep 10;245(17):4372–4381. [PubMed] [Google Scholar]
- Fanger M. W., Hettinger T. P., Harbury H. A. Pseudomonas cytochrome c. II. Effect of modification of the methionine residues. Biochemistry. 1967 Mar;6(3):713–720. doi: 10.1021/bi00855a010. [DOI] [PubMed] [Google Scholar]
- HARBURY H. A., LOACH P. A. Interaction of nitrogenous ligands with heme peptides from mammalian cytochrome c. J Biol Chem. 1960 Dec;235:3646–3653. [PubMed] [Google Scholar]
- Harbury H. A., Cronin J. R., Fanger M. W., Hettinger T. P., Murphy A. J., Myer Y. P., Vinogradov S. N. Complex formation between methionine and a heme peptide from cytochrome c. Proc Natl Acad Sci U S A. 1965 Dec;54(6):1658–1664. doi: 10.1073/pnas.54.6.1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hearn R. P., Richards F. M., Sturtevant J. M., Watt G. D. Thermodynamics of the binding of S-peptide to S-protein to form ribonuclease S.. Biochemistry. 1971 Mar 2;10(5):806–817. doi: 10.1021/bi00781a013. [DOI] [PubMed] [Google Scholar]
- Margoliash E., Schejter A. Cytochrome c. Adv Protein Chem. 1966;21:113–286. doi: 10.1016/s0065-3233(08)60128-x. [DOI] [PubMed] [Google Scholar]
- McDonald C. C., Phillips W. D., Vinogradov S. N. Proton magnetic resonance evidence for methionine-iron coordination in mammalian-type ferrocytochrome c. Biochem Biophys Res Commun. 1969 Aug 7;36(3):442–449. doi: 10.1016/0006-291x(69)90584-1. [DOI] [PubMed] [Google Scholar]
- Shechter E., Saludjian P. Conformation of ferricytochrome c. IV. Relationship between optical absorption and protein conformation. Biopolymers. 1967;5(8):788–790. doi: 10.1002/bip.1967.360050812. [DOI] [PubMed] [Google Scholar]
- Stellwagen E. Carboxymethylation of horse heart ferricytochrome c and cyanferricytochrome c. Biochemistry. 1968 Jul;7(7):2496–2501. doi: 10.1021/bi00847a008. [DOI] [PubMed] [Google Scholar]
- Wüthrich K. High-resolution proton nuclear magnetic resonance spectroscopy of cytochrome. Proc Natl Acad Sci U S A. 1969 Aug;63(4):1071–1078. doi: 10.1073/pnas.63.4.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]



