Summary
Neonatal alloimmune thrombocytopenia, (NAIT) is caused by maternal antibodies raised against alloantigens carried on fetal platelets. Although many cases are mild, NAIT is a significant cause of morbidity and mortality in newborns and is the most common cause of intracranial haemorrhage in full-term infants. In this report, we review the pathogenesis, clinical presentation, laboratory diagnosis and prenatal and post-natal management of NAIT and highlight areas of controversy that deserve the attention of clinical and laboratory investigators.
Keywords: neonatal thrombocytopenia, platelet antigens, alloimmune thrombocytopenia
In 1953, two infants born with severe thrombocytopenia to mothers with normal platelet counts were described (Harrington et al, 1953). Despite severe bleeding and other complications, recovery occurred after 2 and 8 weeks, respectively. Although definitive serological studies were not feasible at the time, this appears to be the first formal report describing the condition now designated neonatal alloimmune thrombocytopenia (NAIT). Shulman et al (1962) first implicated a maternal antibody raised against a defined platelet alloantigen as the cause of platelet destruction in an infant with this condition. In two of Shulman’s cases, placental transfer of maternal antibodies specific for a platelet alloantigen designated PlA1 was the cause of platelet destruction in the newborn. PlA1 was later found to be identical to an antigen designated Zwa by Dutch workers (Van Loghem et al, 1959) and is now known as ‘HPA-1a’ (human platelet antigen 1a). In subsequent years, numerous other platelet-specific antigens were shown to be capable of inducing maternal immunization during pregnancy and causing fetal platelet destruction. NAIT is now recognized as an important complication of pregnancy that can present difficult diagnostic and therapeutic challenges. In this review, we will consider NAIT pathogenesis, diagnosis and management and will highlight areas of controversy that deserve the attention of clinical and laboratory investigators.
Incidence of NAIT
Several large prospective studies of women negative for HPA-1a, the most common trigger for antibodies causing NAIT, showed that between one in 1000 and one in 2000 HPA-1a-positive infants had neonatal thrombocytopenia caused by maternal antibodies (Blanchette et al, 1990; Williamson, 1998; Kjeldsen-Kragh et al, 2007). The incidence of the HPA-1a-negative (HPA-1bb) phenotype in Caucasian populations is about 2.5%. One third of these individuals are positive for the HLA-DR antigen B3*0101 and are at high risk to become immunized against HPA-1a when they carry an HPA-1a positive fetus (L’Abbe et al, 1992; Williamson, 1998); this occurs in about 35% of cases. Of these, about one in three will deliver an HPA-1a-positive child with significant thrombocytopenia (less than 50 × 109 platelets/l). In contrast to maternal immunization against fetal red cell antigens, it is common for immunization against platelet alloantigens to occur during a first pregnancy and for a firstborn infant to be affected by NAIT. However, most instances of maternal immunization may be triggered by exposure to fetal blood at the time of delivery, setting the stage for an infant to be born subsequently with thrombocytopenia (Stuge et al, 2011).
Clinical manifestations
NAIT is the leading cause of severe thrombocytopenia in the fetus and neonate (Sainio et al, 2000), can produce serious bleeding, intracranial haemorrhage and death (Pearson et al, 1964; Mueller-Eckhardt et al, 1989b; Bonacossa & Jocelyn, 1996), and is the leading cause of intracranial haemorrhage in full term infants (Bussel, 2009). A severely affected infant will present with florid petechial haemorrhages and purpura and a profoundly low platelet count. Typically, no other explanation for thrombocytopenia is discovered after evaluation for bacterial and viral infection, disseminated intravascular coagulation and other congenital conditions associated with thrombocytopenia. A similar, but usually less serious condition can be caused by passive transfer of platelet autoantibodies; in such cases the mother will usually have a low platelet count and/or a history of autoimmune thrombocytopenia.
Prospective studies (Blanchette et al, 1990; Williamson, 1998; Kjeldsen-Kragh et al, 2007) have shown that the degree of thrombocytopenia in neonates at risk for NAIT (mother immunized against HPA-1a) can be quite variable. A recent report that ‘severe’ thrombocytopenia is more than twice as common in infants born to a group A mother than in those born to a mother whose blood group is O (Ahlen et al, 2012) requires confirmation. An infant born to a mother who previously gave birth to an infant with NAIT tends to have more severe disease than its older sibling (Fenichel et al, 1984; Bussel et al, 1997a; Bussel & Sola-Visner, 2009).
The most serious complication of NAIT is intracranial haemorrhage, which occurs in 10–20% of symptomatic infants (Mueller-Eckhardt et al, 1989b; Bussel et al, 1991, 2005; Kaplan et al, 1991). Up to 80% of these bleeds occur prenatally (Spencer & Burrows, 2001). After delivery, the greatest risk of bleeding is in the first 96 h of life (Mueller-Eckhardt et al, 1989b; Bussel et al, 1991, 2005; Kaplan et al, 1991; Spencer & Burrows, 2001). Untreated, the thrombocytopenia in the neonate typically resolves within the first two weeks of life. For reasons not well understood, a low count sometimes persists for longer periods of time, occasionally for several months.
Antigens implicated in NAIT
Platelet-specific (HPA) antigens
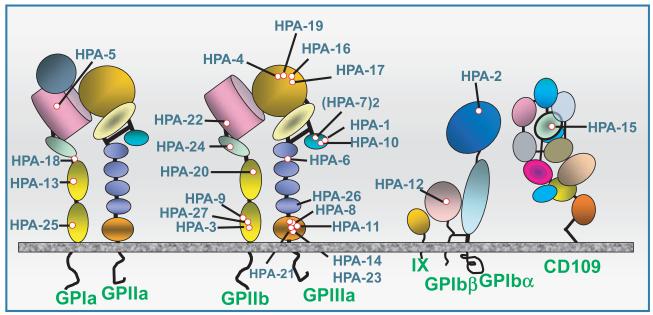
Antigens capable of triggering NAIT are carried on platelet membrane glycoproteins (GPs) GPIb-V-IX (von Willebrand receptor), GPIIb/IIIa (αIIb/β3 integrin, fibrinogen receptor) GPIa/IIa (a collagen receptor) and CD109, a glycosylphosphatidylinositol (GPI)-anchored protein of uncertain function. Together, these platelet GPs interact with proteins of the extracellular matrix and coagulation factors to facilitate haemostasis (Clemetson, 2012). Genetic polymorphisms resulting from at least 27 single amino acid substitutions located in six different glycoproteins (GPIIb, GPIIIa, GPIbα, GPIbβ, GPIa, CD109) have been shown to cause maternal immunization during pregnancy resulting in NAIT (Fig 1). When it became apparent that platelet GPs are remarkably polymorphic, a system of Human Platelet Antigen (HPA) nomenclature was developed by international consensus under which antigen systems are designated HPA-1, HPA-2, etc., more or less in the order of their discovery (von dem Borne et al, 1995; Metcalfe et al, 2003). In each system, the more common and less common alleles are designated ‘a’ and ‘b’ respectively. An HPA database is maintained by several groups in the United Kingdom and can be reached at http://www.ebi.ac.uk/ipd/hpa/index.html.
Fig 1.
Antigens known to trigger maternal sensitization leading to neonatal alloimmune thrombocytopenia are carried on four different platelet membrane glycoproteins (GP) and glycoprotein complexes. Structural domains identified by crystallographic studies are shown schematically. Adapted from (Peterson et al, 2012a).
The HPA-1 antigen system
The first human platelet antigen (HPA) implicated in NAIT, HPA-1a (originally called PlA1) (Shulman et al, 1962), results from a leucine/proline substitution at position 33 of the PSI (plextrin-semaphorin-integrin) homology domain of the GPIIIa subunit of the GPIIb/IIIa complex (αIIb/β3 integrin) (Newman et al, 1989). Maternal-fetal incompatibility for HPA-1a is by far the most common cause of NAIT in families of Caucasian and African ancestry, accounting for about 85% of cases in which an HPA-specific antibody is identified (Davoren et al, 2004; McQuilten et al, 2011) despite the fact that only 2% of women are HPA-1a negative and at risk to make antibodies with this specificity. More than 90% of HPA-1a antibodies are made by women positive for the Class II histocompatibility antigen DRB3*0101 (DR52a), present in about one-third of the general population (L’Abbe et al, 1992; Braud et al, 1994; Williamson et al, 1998; Kjeldsen-Kragh et al, 2007). This correlation appears to be related to high affinity of a GPIIIa peptide containing Leu33 for the peptide binding groove of DRB3*0101 (Maslanka et al, 1996; Rayment et al, 2009). The history of HPA-1a, from its first discovery to characterization of its molecular basis and elucidation of its role in disease was recently reviewed (Aster & Newman, 2007).
HPA-1a sensitization in surrogate pregnancies
Four instances have been described in which HPA-1a-positive fertilized eggs were implanted into HPA-1a-negative woman, two of whom had previously given birth to children with NAIT (Curtis et al, 2005). Among a total of seven infants carried by these women, four had profound thrombocytopenia at birth, two had antenatal intracranial haemorrhage and one died in utero. The severity of NAIT in these cases could in part be related to fact that the infants were homozygous for HPA-1a, a condition that is normally impossible when the mother is HPA-1a-negative. It was recommended that women being considered as surrogate mothers be typed routinely for HPA-1a (Curtis et al, 2005).
Other HPA antigens
More than 95% of serologically confirmed NAIT cases in Caucasian families are caused by maternal immunization against antigens belonging to five antigen systems (HPA-1, -2, -3, -5, and -15) consisting of two alleles, each of which is relatively common in most populations (Table I). Studies performed in cases of apparent NAIT in which maternal antibodies specific for one of these ‘common’ HPA antigens are not detected have led to the identification of mutations encoding a series of less common HPA antigens. At this writing, a total of 23 such mutations have been identified, each encoding a low frequency antigen. Some of these have been described only in a single case report. Low frequency antigens shown to be capable of triggering NAIT are listed in Table II. The most immunogenic of these appears to be HPA-9b, found in about one of 400 normal individuals and located close to the HPA-3 antigen site in the calf-2 domain of GPIIb (Fig 1). HPA-4b (Kupatawintu et al, 2005), HPA-6b (Tanaka et al, 1996) and HPA-21b (Peterson et al, 2012b) are significantly more common in Asian than in Caucasian populations. The number of identified low frequency HPA antigens is likely to increase in the future. On average, however, maternal sensitization against this group of antigens probably accounts for only a small fraction of all NAIT cases (Ghevaert et al, 2009).
Table I.
Common human platelet antigens.
| Antigen | Alias | GP | AA | Reference |
|---|---|---|---|---|
| HPA-1a/b | Pla/b | GPIIIa | L33P | Newman et al (1989) |
| HPA-2a/b | Kob/a | GPIba | T145M | Kuijpers et al (1992) |
| HPA-3a/b | Baka/b | GPIIb | I843S | Lyman et al (1990) |
| HPA-5a/b | Brb/a | GPIa | E505K | Santoso et al (1993) |
| HPA-15a/b | Zav, Gova/b | CD109 | S703Y | Schuh et al (2002) |
GP, glycoprotein; AA, amino acid.
Table II.
Low frequency human platelet antigens.
| Antigen | Alias | GP | AA | Validated cases (n) |
Index reference |
|---|---|---|---|---|---|
| *HPA-4a/b | (Yuka/b) Pena/b | GPIIIa | R143Q | a:5; b:9 | Wang et al (1992) |
| *HPA-6b | Ca/Tua | GPIIIa | R489Q | 5 | Wang et al (1993) |
| HPA-7b | Moa | GPIIIa | P407A | 1 | Kuijpers et al (1993) |
| HPA-7c | Hit | GPIIIa | P407S | 1 | Koh et al (2010) |
| HPA-8b | Sra | GPIIIa | R636C | 3 | Santoso et al (1994) |
| HPA-9b | Maxa | GPIIb | V837M | 14 | Noris et al (1995) |
| HPA-10b | Laa | GPIIIa | R62Q | 2 | Peyruchaud et al (1997) |
| HPA-11b | Groa | GPIIIa | R633H | 3 | Simsek et al (1997) |
| HPA-12b | Iya | GPIbb | G15E | 2 | Sachs et al (2000) |
| HPA-13b | Sita | GPIa | T799M | 2 | Santoso et al (1999) |
| HPA-14b | Oea | GPIIIa | K611del | 1 | Santoso et al (2002) |
| HPA-16b | Duva | GPIIIa | T140I | 1 | Jallu et al (2002) |
| HPA-17b | Vaa | GPIIIa | T195M | 1 | Stafford et al (2008b) |
| HPA-18b | Caba | GPIa | Q716H | 1 | Bertrand et al (2009) |
| HPA-19b | Sta | GPIIIa | K137Q | 1 | Peterson et al (2010) |
| HPA-20b | Kno | GPIIb | T619M | 1 | Peterson et al (2010) |
| *HPA-21b | Nos | GPIIIa | E628K | 3 | Peterson et al (2010) |
| HPA-22b | She, Sey | GPIIb | K164T | 1 | Peterson et al (2012a) |
| HPA-23b | Hug2 | GPIIIa | R622W | 1 | Peterson et al (2012a) |
| HPA-24b | Cab2, In | GPIIb | S472N | 1 | Jallu et al (2011) |
| HPA-25b | Swia | GPIa | T1087M | 1 | Kroll et al (2011) |
| HPA-26b | Seca | GPIIIa | K580N | 1 | Sachs et al (2012) |
| HPA-27b | Cab3, Ak | GPIIb | L841M | 3 | Jallu et al (2012) |
GP, glycoprotein; AA, amino acid.
*HPA-4b, HPA-6b and HPA-21b are more common in Asian populations.
ABO antigens
It has been known for many years that platelets normally express small quantities of A and B antigens on their surface (Moreaux & Andre, 1954). Ogasawara et al (1993) first showed that about 5% of normal subjects positive for blood groups A or B possess platelets that carry unusually large numbers of A and B antigen sites and showed that such platelets survive poorly when transfused to an ABO incompatible recipient. Curtis et al (2000) confirmed these findings and showed that in a subset of individuals (‘Type 2 high-expressers’), platelet A1 and B antigen levels are extremely high, ranging up to 20 000 antigen sites per platelet. These findings raised the possibility that some infants possessing the Type 2 high expresser trait could be at risk for thrombocytopenia if born to an ABO incompatible mother. This prediction was borne out in a study of a family in which two infants who were Type 2 high expressers of blood group B were born with thrombocytopenia (one requiring platelet transfusions) and low grade ABO haemolytic disease to a group O mother whose serum contained high titre IgG anti-B (Curtis et al, 2008). The platelet-reactive antibody in maternal serum was completely absorbed with group B but not group O red cells. A third infant whose blood type was A2 was borne to the same mother with a normal platelet count. These findings indicate that NAIT can be caused by maternal IgG anti-B (and presumably anti-A) antibodies under some circumstances. Typing of a father’s platelets for the high expresser trait can be helpful in some cases of apparent NAIT not resolved on the basis of HPA incompatibility.
Glycoprotein IV (CD36, Nak)
Glycoprotein IV (CD36) is a member of the class B scavenger receptor family of proteins expressed on platelets, red cells, endothelial cells and other tissues (Silverstein & Febbraio, 2009). About 5% of persons of African or Asian ancestry have inherited mutations that lead to failure of CD36 expression (Type 2 CD36 deficiency) and are at risk to become immunized if exposed to the protein by transfusion or pregnancy (Curtis et al, 2002). The resulting antibodies were originally considered to be specific for an alloantigen designated ‘Nak’ (Ikeda et al, 1989). With the recognition that the antibodies probably recognize multiple epitopes on the target protein, it is more appropriate to designate them as isoantibodies having CD36 specificity. The clinical picture of NAIT associated with maternal immunization against CD36 is similar to that seen in infants affected by HPA-specific antibodies (Curtis et al, 2002). It is of particular interest that affected infants can have severe thrombocytopenia without evidence of other tissue damage despite the fact that CD36 is expressed on endothelium and other blood cells (Curtis et al, 2002). NAIT caused by anti-CD36 antibodies has not been reported in persons of Caucasian ancestry, presumably because of the rarity of the CD36-negative phenotype in that group.
Human leucocyte antigen (HLA) antigens
Human platelets carry at least 20 000 copies of class I HLA antigens (Janson et al, 1986; Pereira et al, 1988) and account for the majority of HLA antigens present in circulating blood (Pereira et al, 1988). Given that about one-third of multiparous women are sensitized to Class I HLA (King et al, 1996), the possibility exists that class I HLA antibodies could cause NAIT in some newborns. However, women sensitized to Class I HLA antigens routinely give birth to infants with normal platelet counts and at least one formal study failed to show any correlation between neonatal platelet counts and the presence or absence of such antibodies in the mother (King et al, 1996). Nonetheless, the literature contains many anecdotal reports of infants born with apparent NAIT possibly caused by maternal anti-HLA (Saito et al, 2003; Moncharmont et al, 2004; Thude et al, 2006). Accordingly, whether Class I HLA antibodies cause some cases of NAIT not accounted for by maternal-fetal HPA incompatibility is presently unresolved. Maternal HLA antibodies vary greatly in potency; the possibility that high titre maternal antibodies or those specific for HLA antigens that are strongly expressed on platelets (Szatkowski et al, 1978; Szatkowski & Aster, 1980) can cause neonatal thrombocytopenia or contribute to its severity deserves investigation.
Laboratory diagnosis of NAIT
As noted, sensitization to fetal platelet antigens can occur during a first pregnancy. Norwegian investigators typed women pregnant for the first time for HPA-1a and screened those found to be negative for HPA-1a antibody at various times during gestation in an attempt to identify infants at risk for NAIT (Kjeldsen-Kragh et al, 2007; Killie et al, 2008; Skogen et al, 2010). Some have argued that it may be cost-effective to perform such screening routinely and offer special case management to the 10% of HPA-1a-negative women who produce antibody (Husebekk et al, 2009) but at the present time this is not practiced in the absence of a family history of NAIT, e.g., in a sister. Therefore, the first suspicion of NAIT usually arises when a newborn exhibits petechial haemorrhages, echymoses or other bleeding symptoms. As time is required for serological studies to be performed, the initial diagnosis is made and treatment is started on the basis of the platelet count and other clinical findings. Even in mildly affected infants, however, serological investigation is indicated because results can be critical for effective management of future pregnancies. Proper laboratory diagnosis of NAIT requires sophisticated testing, a thorough understanding of platelet serology and, often, personal communication between the testing laboratory and the attending physician and should be undertaken only by laboratories that possess these capabilities. For the most informative evaluation, it is important to study blood samples from both mother and father.
Here, we will describe the approach used by the Platelet and Neutrophil Immunology Laboratory of BloodCenter of Wisconsin to investigate newly referred cases of suspected NAIT. Approaches used by other laboratories may differ in some details but are likely to be generally similar. Readers interested in a more detailed description of antibody detection and platelet typing methods can refer to a recently published review (Curtis & McFarland, 2009).
Serological studies
Flow cytometry using secondary probes specific for IgG and IgM immunoglobulin isotypes (Curtis & McFarland, 2009) provides a rapid and sensitive means of detecting platelet-reactive antibodies and is used to test maternal serum against washed paternal and maternal platelets and a small panel of platelets from normal group O donors typed for selected common HPA antigens. A screen for class I HLA antibodies is also performed and paternal and maternal red cells are typed for ABO. The glycoprotein for which maternal antibody is specific can often be identified by performing one or more solid phase assays. In one approach, designated MACE (modified antigen capture enzyme-linked immunosorbent assay [ELISA]), target platelets are incubated with maternal serum, washed and lysed with a detergent, such as triton X-100. The glycoprotein of interest is then captured on a solid surface to which a monoclonal antibody specific for it has been fixed. After washing, maternal antibody bound to the captured GP is detected by ELISA (Curtis & McFarland, 2009). MACE is used routinely to detect maternal antibodies reactive with HPA antigens carried on GPIIb/IIIa and GPIa/IIa using paternal platelets and a small platelet panel as targets. In addition, an antigen capture ELISA (ACE) assay is used to screen maternal serum for antibodies against GPIb/IX, the carrier for HPA-2a/b, CD36 (GPIV) and class I HLA. In selected cases, MACE is used to detect HPA antibodies reactive with HPA-2a/b. A slightly different approach, designated monoclonal antibody immobilization of platelet antigens (MAIPA) is widely used in Europe (Kaplan et al, 2007). MACE and MAIPA are probably equivalent in sensitivity and specificity. Other solid phase assays suitable for HPA antibody detection are reviewed by Curtis and McFarland (2009). Detection of maternal antibodies specific for HPA-15a and -15b requires MAIPA using fresh target platelets because the carrier protein, CD109, is only weakly expressed (about 1000 copies per platelet) and is relatively labile (Ertel et al, 2005; Maslanka et al, 2012).
Platelet typing
Development of DNA-based methods has made serological typing for HPA antigens obsolete. The nucleotide changes that encode individual antigens can be rapidly and accurately identified with allelic discrimination assays using 5′ fluorescently-labelled hydrolysis probes that anneal to specific alleles (Ruan et al, 2007). A quencher that inhibits fluorescence is attached to the 3′ end of the probe. When specific probe annealing and extension occurs, the quencher is removed and the resulting fluorescence reports the presence of the targeted allele (Arinsburg et al, 2012). Other polymerase chain reaction (PCR)-based techniques, such as those that use PCR-sequence-specific primer amplification (Skogen et al, 1994; Curtis, 2008) followed by electrophoresis and visualization of DNA bands, are used by some laboratories. Our practice is to type paternal and maternal DNA routinely for antigens of the HPA-1 through 6, -9 and -15 systems.
Interpretation of test results
A reaction of maternal IgG with paternal but not maternal platelets and a negative screening result for class I HLA antibodies suggests that an HPA antibody may be present. Reaction of maternal serum with paternal platelets but not with any member of the normal panel suggests that the antibody could be specific for a low frequency HPA antigen. However, if the father is incompatible with mother’s serum for blood group A1 or B, this reaction can be due to a blood group antibody. If the reaction is strong, paternal platelets are tested with monoclonal antibodies specific for blood groups A or B and the strength of this reaction is compared with that of ‘normal’ group A1 or B platelets to identify fathers who are Type 2 high expressers of A1 or B (Curtis et al, 2008). If a high expresser state is identified, maternal serum is absorbed with washed group A1 or B red cells. If the reaction with paternal platelets is abolished, it is assumed to be a result of ABO incompatibility.
In 20–35% of cases, an antibody specific for an HPA antigen present in the father but not the mother is found in maternal serum (Mueller-Eckhardt et al, 1989b; Davoren et al, 2004; Ghevaert et al, 2007; McQuilten et al, 2011). HPA-1a is targeted in 75–90% of these ‘resolved’ cases, HPA-5b in 8–15%, HPA-1b in 1–4% HPA-3a in 1–2% and HPA-5a in about 1% (McQuilten et al, 2011). In a recent study, antibodies specific for HPA-15b were found in 8 of 200 cases (4%) (Ghevaert et al, 2007). Occasionally, two HPA antibodies are present in the same mother (Davoren et al, 2004; McQuilten et al, 2011). HPA-4b, -6b and -21b in mothers of Asian descent and GPIV (CD36) in African American women are targeted much more often than in Caucasian women.
As noted, reaction of a maternal antibody only with paternal platelets in flow cytometry that is not accounted for by ABO incompatibility suggests maternal immunization against a low frequency HPA antigen (Table II). In such cases, identification of the carrier protein in a solid phase assay using paternal platelets as targets can be helpful. Given that panel cells carrying low frequency HPA antigens are not readily available, sequencing of relevant exons encoding known low frequency HPA antigens in paternal DNA may be required to identify the probable sensitizing antigen. Transfected cell lines expressing low frequency antigens (Kroll et al, 2003) or recombinant glycoprotein fragments engineered to carry relevant epitopes (Stafford et al, 2008a,b) that can be used to identify such antibodies may become available in the future. Although many low frequency antigens have been described (Table II), sensitization to these markers probably accounts for no more than a few percent of all cases of NAIT (Ghevaert et al, 2009).
Unresolved cases: possible explanations
As noted, about two-thirds of suspected NAIT cases referred for study are not resolved on the basis of maternal-fetal incompatibility for one of the antigens listed in Tables I and II. In some of these, neonatal thrombocytopenia is undoubtedly a consequence of one of the many non-immune conditions that can lower the platelet count in a newborn (Arnold et al, 2008; Chakravorty & Roberts, 2012). If the mother is thrombocytopenic or has a history of autoimmune thrombocytopenia (ITP), maternal autoantibodies not readily detected in laboratory assays may be responsible.
In the remaining cases, a cause of thrombocytopenia other than NAIT is usually not identified. In many such instances, class I HLA antibodies, sometimes very high titre, are present in the mother. As already noted, anecdotal reports claim that HLA antibodies can cause NAIT. In fact, the very first report of NAIT caused by HPA-1a immunization described two additional cases associated with maternal immunization against a newly identified antigen designated ‘PlB1’, which was later found to be the class I HLA antigen HLA-A2 (Shulman et al, 1962). Although there is no consensus as to whether class I HLA antibodies can cause NAIT, further studies to define whether such antibodies cause the condition or contribute to its severity are needed.
Antibodies specific for HPA-3a and HPA-3b can be extremely difficult to detect in standard serological assays for reasons not yet fully understood (Lin et al, 1995; Harrison et al, 2003; Socher et al, 2008). Zhu et al (2008) recently found that the region of the GPIIb calf-2 domain where these antigens are located is not resolved in the crystal structure of the GPIIb/IIIa ectodomain, suggesting that this region is not rigidly constrained. The resulting lability of the antigen structure could explain difficulties encountered in serological assays. The low frequency antigens HPA-9b and HPA-27b are located in calf-2 very close to HPA-3a/b and antibodies specific for these markers can also be difficult to detect (Kaplan et al, 2005; Peterson et al, 2005; Jallu et al, 2012), perhaps for the same reason. Maternal-fetal incompatibility for HPA-3a and -3b is common; perhaps improved assays for HPA-3 antibody detection will show that HPA-3 incompatibility causes NAIT more often than has been suspected.
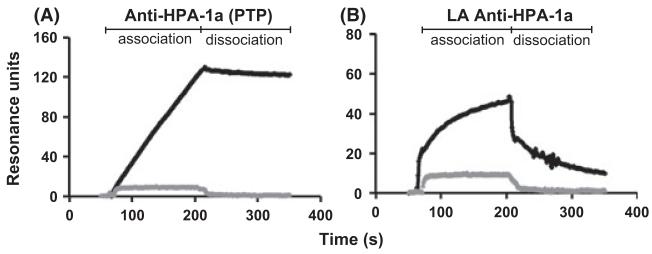
Recent studies suggest yet another explanation for the failure of laboratory studies to resolve apparent cases of NAIT – that thrombocytopenia is caused by low avidity HPA antibodies not detected in standard assays that require washing of the target antigen (Socher et al, 2009; Bakchoul et al, 2011; Peterson et al, 2012c). Antibodies of this type can be detected using surface plasmon resonance (SPR) analysis in which a signal translated into ‘resonance units’ is generated in real time as antibody (ligand) binds to immobilized antigen (GPIIb/IIIa). A typical tracing obtained with IgG from an HPA-1a negative, ‘antibody-negative’ mother who gave birth to an infant with NAIT is shown in Fig 2 where it can be seen that perfused antibody accumulated on the HPA-1a-positive but not the HPA-1a-negative target and then dissociated rapidly when perfusion with buffer was begun. In contrast, a ‘conventional’ HPA-1a antibody dissociated very slowly. Potential pathogenicity of low avidity HPA-1a antibodies can be demonstrated by showing that they cause destruction of circulating human platelets in a non-obese diabetic severe combined immunodeficiency (NOD/SCID) mouse model (Bakchoul et al, 2011; Peterson et al, 2012c). SPR analysis is technically demanding and is presently unsuitable for routine antibody detection. However, it is important that further studies be done to define the extent to which low avidity HPA antibodies cause ‘antibody-negative’ NAIT.
Fig 2.
Detection of HPA-1a antibodies by surface plasmon resonance analysis (SPR). Equal quantities of GPIIb/IIIa purified from HPA-1a-positive and HPA-1a-negative platelets were fixed to CM5 Biacore chips using standard linkage chemistry and were perfused with IgG containing HPA-1a antibodies from a patient with post-transfusion purpura (PTP) (A) and from an HPA-1a-negative woman who delivered an infant with apparent neonatal alloimmune thrombocytopenia (NAIT) but had no HPA-1a antibody detectable by conventional serological methods (B). The progressive increase in SPR signal during the first 200 s of perfusion reflects binding of IgG to HPA-1a-positive GPIIb/IIIa (dark line). IgG from a healthy individual is depicted by the grey line. During subsequent perfusion with buffer, the HPA-1a antibody from the PTP case remained associated with its target but the low avidity (LA) antibody from the NAIT case dissociated almost completely. HPA-1a antibodies from NAIT cases that could be detected by standard serology produce intermediate tracings (not shown). Adapted from (Peterson et al, 2012c).
Serological confirmation of an NAIT diagnosis is particularly important in guiding the management of subsequent pregnancies, where fetal genotyping for the implicated incompatible antigen can be done if indicated. However, there are cases of ‘true’ NAIT for which serological confirmation is not available due to one of the scenarios mentioned above, or to the lack of an appropriately timed maternal serum sample, i.e., one obtained before antibody became detectable or after it disappeared. Such cases need to be assessed on clinical grounds alone, particularly if multiple pregnancies have been affected in a family. Although the absence of a serological diagnosis adds more uncertainty to management of a subsequent pregnancy, the clinician may well offer empiric antenatal therapy as discussed below if the clinical suspicion of NAIT is particularly high.
Treatment
A first affected neonate with NAIT in a family is normally identified when clinical signs of bleeding are evident at or shortly after birth and a platelet count confirms isolated thrombocytopenia. The immediate treatment for very severe thrombocytopenia (<30 × 109/l), especially if serious bleeding signs are evident (petechiae, ecchymoses, gastrointestinal, gentio-urinary or intracranial haemorrhage), is platelet transfusion (Sola-Visner et al, 2008). Random donor platelets appropriate for neonates (ABO compatible; volume reduced, if indicated; cytomegalovirus negative; and irradiated) will usually elevate the platelet count at least transiently and reduce the likelihood of bleeding even when they are incompatible with the maternal antibody. In addition, intravenous immunoglobulin (IVIG) at 0.4–1.0 g/kg/d for 2-5 d can be given to potentially prolong the survival of the incompatible platelets and lessen the overall period of thrombocytopenia (Mueller-Eckhardt et al, 1989a; Bussel, 2009; McQuilten et al, 2011). Compatible, HPA-1b/b platelets may be available from some blood providers who have HPA-typed donors available (Verran et al, 2000).
HPA-compatible platelets can also be obtained by performing platelet pheresis on the mother of the affected infant and can be helpful in unusual cases requiring transfusion support over an extended period of time. If maternal platelets are used, it is absolutely essential that they be washed to remove antibody-containing maternal plasma. Methods of plasma removal include simple volume reduction by centrifugation with resuspension in normal saline, or more exhaustive washing of platelets in saline or normal AB plasma. Maternal platelets should always undergo gamma irradiation prior to transfusion to prevent transfusion-induced graft-versus-host disease in the infant (McFarland, 2008). While it may seem obvious that maternal antibody should be removed before maternal platelets are transfused to an affected infant, we are aware of several instances in which failure to observe this precaution led to prolonged thrombocytopenia in the infant, in one case for more than two months.
Moderately severe thrombocytopenia (e.g. between 50 and 30 × 109/l (Williamson et al, 1998) without obvious haemorrhage can be managed with IVIG treatment alone. Typically, total doses of 2 g/kg are given over 2–5 d (Mueller-Eckhardt et al, 1989a; Bussel, 2009).
Management of subsequent pregnancies
As noted, NAIT tends to be more severe in infants born subsequently to a mother who previously gave birth to an infant with this condition. Accordingly, later pregnancies should be managed in consultation with physicians experienced in NAIT diagnosis and management. Several steps should be considered in such cases. One is to determine whether the infant being carried is incompatible with the maternal alloantibody previously demonstrated, whether or not it is still detectable. A second (if the infant is incompatible) is to estimate the degree of fetal thrombocytopenia so as to gauge the risk of antenatal intracranial haemorrhage. A third is to offer risk-stratified antenatal therapy to the mother to ameliorate fetal thrombocytopenia and reduce the likelihood of prenatal and postnatal bleeding. The HPA genotype of the father can be used to determine whether the infant has a 100% (father homozygous) or 50% (father heterozygous) chance of possessing the implicated antigen. If the father is homozygous all subsequent fetuses will be obligate heterozygotes and will be incompatible with the maternal antibody. If the father is heterozygous the fetus will have a 50% chance of inheriting the marker. In the latter case, fetal genotyping can be performed on amniotic fluid or chorionic villus material to determine whether the fetus is at risk (McFarland et al, 1991; Skogen et al, 1994). The former material can be typed at 18–20 weeks gestation and the latter as early as 8–10 weeks.
When a fetus has been determined to be at risk for NAIT, an estimate should be made of its likely severity. The most direct way to accomplish this is to perform a platelet count on a fetal blood sample. However, this procedure carries significant risk, particularly if the fetus happens to have a severely depressed platelet count (Paidas et al, 1995; Bussel, 2009). Non-invasive methods for estimating the severity of NAIT during pregnancy include testing of the mother’s serum for the strength of the anti-HPA antibody and considering the severity of disease in previously affected siblings. Several investigators found that there is a roughly inverse relationship between the strength of maternal antibody measured serologically and the platelet count in the newborn (Jaegtvik et al, 2000; Killie et al, 2007, 2008; Bessos et al, 2009). While this approach may hold some promise, there are many examples of an infant incompatible with a very strong maternal antibody having only mild thrombocytopenia at birth and, conversely, an infant being born with severe thrombocytopenia to a mother whose antibody is quite weak. The severity of NAIT in previously affected siblings can be a helpful predictor, especially if an older sibling experienced intracranial haemorrhage (Christiaens et al, 1997; Bussel, 2009).
From earlier antenatal treatment trials in which serial fetal blood sampling was done prior to and after administering treatment to mothers, it is clear that, without intervention, fetuses at risk for NAIT who had previously affected older siblings usually experience a progressive drop in platelet count as pregnancy progresses (Bussel et al, 1997a). Beginning in 1997, trials of antenatal maternal treatment using high dose IVIG with or without corticosteroids have shown that both the degree of thrombocytopenia in the fetus and the risk of intracranial haemorrhage can be reduced by such therapy (Bussel et al, 1988, 1991, 1997b,b; Bussel, 2009). It is now recognized that prenatal treatment can be stratified. More intense and earlier therapy should be given if a previous untreated fetus experienced an early (i.e. prior to 28 weeks gestation) in utero haemorrhage (Bussel et al, 2010).
A treatment algorithm for stratifying risk (Table III) and customizing antenatal therapy on the basis of prior clinical trials and expert opinion has been proposed (Pacheco et al, 2011). The authors recommend monitoring of women in Stratum 1 with serial testing to detect anti-HPA antibodies, including serological crossmatches with paternal platelets to detect rare specificities at 12 weeks, 24 weeks and 30 weeks gestation, and withholding antenatal therapy unless an HPA antibody is detected. (However, as noted above, if the clinical suspicion for NAIT is particularly high, empiric therapy could be considered in this group without serological confirmation.) Stratum 2 pregnancies, known to have an at-risk fetus either from paternal zygosity or fetal genotyping, are offered antenatal therapy at approximately 20 weeks gestation with IVIG (1 g/kg/week and prednisone (0.5 mg/kg/d) or IVIG at 2 g/kg/week, and therapy is increased to IVIG 2 g/kg/week plus prednisone at 32 weeks gestation without fetal blood sampling. Caesarean delivery is done electively at 37–38 weeks. Stratum 3 mothers with at-risk fetuses are offered IVIG at 1 g/kg/week at 12 weeks gestation and therapy is increased (doubling the IVIG dose or adding prednisone) at 20 weeks and again at 28 weeks (all mothers receiving IVIG 2 g/kg/week plus prednisone) with elective delivery as per Group 2. Stratum 4 mothers are given IVIG at 2 g/kg/week beginning at week 12 with prednisone added at week 20 and further acceleration of treatment as per Group 3 at week 28 with elective delivery as per Groups 2 and 3.
Table III.
Stratification of NAIT cases according to risk of intracranial haemorrhage *.
| Stratum | Definition | Risk |
|---|---|---|
| 1 | History of previous fetus or newborn with thrombocytopenia or intracranial haemorrhage of unknown aetiology; no HPA antibody detected. |
Unknown |
| 2 | History of previous fetus or newborn with serologically confirmed fetal or neonatal alloimmune thrombocytopenia having only thrombocytopenia and no evidence of an intracranial haemorrhage. |
Standard |
| 3 | History of serologically confirmed fetal or neonatal alloimmune thrombocytopenia and previous fetus or newborn with intracranial haemorrhage at 28 weeks of gestation or more (includes peripartum and neonatal intracranial haemorrhage). |
High |
| 4 | History of serologically confirmed fetal or neonatal alloimmune thrombocytopenia and previous fetus with intracranial haemorrhage at less than 28 weeks. |
Very High |
Adapted from Pacheco et al, 2011.
Based on previous clinical trials in which at risk pregnancies were classified on the basis of severity of a previously affected sibling, (Berkowitz et al, 2006, 2007; Bussel et al, 2010), this approach seeks to minimize the risk of antenatal intracranial haemorrhage while avoiding fetal blood sampling, which has been shown to have a high complication rate, especially for at- risk fetuses with extremely low platelet counts (Paidas et al, 1995).
Footnotes
Authorship contributions
J Peterson, J McFarland, BR Curtis and R Aster have each for many years been engaged in the study of NAIT. Dr. McFarland was the primary author of sections dealing with incidence, clinical presentation and management. Dr Curtis (Director of the Platelet and Neutrophil Immunology Laboratory of BloodCenter of Wisconsin) edited the section dealing with laboratory evaluation of NAIT. Dr Peterson wrote sections concerning HPA antigen systems and edited all sections of the paper. Dr Aster provided oversight and edits to all sections of the paper.
Conflicts of interest
The authors have no disclaimers to make or conflicts to disclose.
References
- Ahlen MT, Husebekk A, Killie MK, Kjeldsen-Kragh J, Olsson ML, Skogen B. The development of severe neonatal alloimmune thrombocytopenia due to anti-HPA-1a antibodies is correlated to maternal ABO genotypes. Clinical & Developmental Immunology. 2012;2012:156867. doi: 10.1155/2012/156867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arinsburg SA, Shaz BH, Westhoff C, Cushing MM. Determination of human platelet antigen typing by molecular methods: importance in diagnosis and early treatment of neonatal alloimmune thrombocytopenia. American Journal of Hematology. 2012;87:525–528. doi: 10.1002/ajh.23111. [DOI] [PubMed] [Google Scholar]
- Arnold DM, Smith JW, Kelton JG. Diagnosis and management of neonatal alloimmune thrombocytopenia. Transfusion Medicine Reviews. 2008;22:255–267. doi: 10.1016/j.tmrv.2008.05.003. [DOI] [PubMed] [Google Scholar]
- Aster RH, Newman PJ. HPA-1a/b (PlA1/A2, Zwa/b): the odyssey of an alloantigen system. Immunohematology. 2007;23:2–8. [PubMed] [Google Scholar]
- Bakchoul T, Kubiak S, Krautwurst A, Roderfeld M, Siebert HC, Bein G, Sachs UJ, Santoso S. Low-avidity anti-HPA-1a alloantibodies are capable of antigen-positive platelet destruction in the NOD/SCID mouse model of alloimmune thrombocytopenia. Transfusion. 2011;51:2455–2461. doi: 10.1111/j.1537-2995.2011.03171.x. [DOI] [PubMed] [Google Scholar]
- Berkowitz RL, Kolb EA, McFarland JG, Wissert M, Primani A, Lesser M, Bussel JB. Parallel randomized trials of risk-based therapy for fetal alloimmune thrombocytopenia. Obstetrics and Gynecology. 2006;107:91–96. doi: 10.1097/01.AOG.0000192404.25780.68. [DOI] [PubMed] [Google Scholar]
- Berkowitz RL, Lesser ML, McFarland JG, Wissert M, Primiani A, Hung C, Bussel JB. Antepartum treatment without early cordocentesis for standard-risk alloimmune thrombocytopenia: a randomized controlled trial. Obstetrics and Gynecology. 2007;110:249–255. doi: 10.1097/01.AOG.0000270302.80336.dd. [DOI] [PubMed] [Google Scholar]
- Bertrand G, Jallu V, Saillant D, Kervran D, Martageix C, Kaplan C. The new platelet alloantigen Cab a: a single point mutation Gln 716 His on the alpha 2 integrin. Transfusion. 2009;49:2076–2083. doi: 10.1111/j.1537-2995.2009.02240.x. [DOI] [PubMed] [Google Scholar]
- Bessos H, Killie MK, Seghatchian J, Skogen B, Urbaniak SJ. The relationship of anti-HPA-1a amount to severity of neonatal alloimmune thrombocytopenia - Where does it stand? Transfusion and Apheresis Science. 2009;40:75–78. doi: 10.1016/j.transci.2009.01.010. [DOI] [PubMed] [Google Scholar]
- Blanchette VS, Chen L, de Friedberg ZS, Hogan VA, Trudel E, Decary F. Alloimmunization to the PlA1 platelet antigen: results of a prospective study. British Journal of Haematology. 1990;74:209–215. doi: 10.1111/j.1365-2141.1990.tb02567.x. [DOI] [PubMed] [Google Scholar]
- Bonacossa IA, Jocelyn LJ. Alloimmune thrombocytopenia of the newborn: neurodevelopmental sequelae. American Journal of Perinatology. 1996;13:211–215. doi: 10.1055/s-2007-994366. [DOI] [PubMed] [Google Scholar]
- von dem Borne AE, Kaplan C, Minchinton R. Nomenclature of human platelet alloantigens. Blood. 1995;85:1409–1410. [PubMed] [Google Scholar]
- Braud V, Chevrier D, Cesbron A, Bignon JD, Kaplan C, Valentin N, Muller JY. Susceptibility to alloimmunization to platelet HPA-1a antigen involves TAP1 polymorphism. Human Immunology. 1994;41:141–145. doi: 10.1016/0198-8859(94)90007-8. [DOI] [PubMed] [Google Scholar]
- Bussel J. Diagnosis and management of the fetus and neonate with alloimmune thrombocytopenia. Journal of Thrombosis and Haemostasis. 2009;7(Suppl. 1):253–257. doi: 10.1111/j.1538-7836.2009.03380.x. [DOI] [PubMed] [Google Scholar]
- Bussel JB, Sola-Visner M. Current approaches to the evaluation and management of the fetus and neonate with immune thrombocytopenia. Seminars in Perinatology. 2009;33:35–42. doi: 10.1053/j.semperi.2008.10.003. [DOI] [PubMed] [Google Scholar]
- Bussel JB, Berkowitz RL, McFarland JG, Lynch L, Chitkara U. Antenatal treatment of neonatal alloimmune thrombocytopenia. New England Journal of Medicine. 1988;319:1374–1378. doi: 10.1056/NEJM198811243192103. [DOI] [PubMed] [Google Scholar]
- Bussel J, Kaplan C, McFarland J. Recommendations for the evaluation and treatment of neonatal autoimmune and alloimmune thrombocytopenia. The working party on neonatal immune thrombocytopenia of the neonatal hemostasis subcommittee of the scientific and standardization committee of the ISTH. Thrombosis and Haemostasis. 1991;65:631–634. [PubMed] [Google Scholar]
- Bussel JB, Zabusky MR, Berkowitz RL, McFarland JG. Fetal alloimmune thrombocytopenia. New England Journal of Medicine. 1997a;337:22–26. doi: 10.1056/NEJM199707033370104. [DOI] [PubMed] [Google Scholar]
- Bussel JB, Berkowitz RL, McFarland JG. Maternal IVIG in neonatal alloimmune thrombocytopenia. British Journal of Haematology. 1997b;98:493–494. [PubMed] [Google Scholar]
- Bussel JB, Zacharoulis S, Kramer K, McFarland JG, Pauliny J, Kaplan C. Clinical and diagnostic comparison of neonatal alloimmune thrombocytopenia to non-immune cases of thrombocytopenia. Pediatric Blood & Cancer. 2005;45:176–183. doi: 10.1002/pbc.20282. [DOI] [PubMed] [Google Scholar]
- Bussel JB, Berkowitz RL, Hung C, Kolb EA, Wissert M, Primiani A, Tsaur FW, Macfarland JG. Intracranial hemorrhage in alloimmune thrombocytopenia: stratified management to prevent recurrence in the subsequent affected fetus. American Journal of Obstetrics and Gynecology. 2010;203:e131–114. doi: 10.1016/j.ajog.2010.03.011. [DOI] [PubMed] [Google Scholar]
- Chakravorty S, Roberts I. How I manage neonatal thrombocytopenia. British Journal of Haematology. 2012;156:155–162. doi: 10.1111/j.1365-2141.2011.08892.x. [DOI] [PubMed] [Google Scholar]
- Christiaens GC, Nieuwenhuis HK, Bussel JB. Comparison of platelet counts in first and second newborns of mothers with immune thrombocytopenic purpura. Obstetrics and Gynecology. 1997;90:546–552. doi: 10.1016/s0029-7844(97)00349-9. [DOI] [PubMed] [Google Scholar]
- Clemetson KJ. Platelets and primary haemostasis. Thrombosis Research. 2012;129:220–224. doi: 10.1016/j.thromres.2011.11.036. [DOI] [PubMed] [Google Scholar]
- Curtis BR. Genotyping for human platelet alloantigen polymorphisms: applications in the diagnosis of alloimmune platelet disorders. Seminars in Thrombosis and Hemostasis. 2008;34:539–548. doi: 10.1055/s-0028-1103365. [DOI] [PubMed] [Google Scholar]
- Curtis BR, McFarland JG. Detection and identification of platelet antibodies and antigens in the clinical laboratory. Immunohematology. 2009;25:125–135. [PubMed] [Google Scholar]
- Curtis BR, Edwards JT, Hessner MJ, Klein JP, Aster RH. Blood group A and B antigens are strongly expressed on platelets of some individuals. Blood. 2000;96:1574–1581. [PubMed] [Google Scholar]
- Curtis BR, Ali S, Glazier AM, Ebert DD, Aitman TJ, Aster RH. Isoimmunization against CD36 (glycoprotein IV): description of four cases of neonatal isoimmune thrombocytopenia and brief review of the literature. Transfusion. 2002;42:1173–1179. doi: 10.1046/j.1537-2995.2002.00176.x. [DOI] [PubMed] [Google Scholar]
- Curtis BR, Bussel JB, Manco-Johnson MJ, Aster RH, McFarland JG. Fetal and neonatal alloimmune thrombocytopenia in pregnancies involving in vitro fertilization: a report of four cases. American Journal of Obstetrics and Gynecology. 2005;192:543–547. doi: 10.1016/j.ajog.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Curtis BR, Fick A, Lochowicz AJ, McFarland JG, Ball RH, Peterson J, Aster RH. Neonatal alloimmune thrombocytopenia associated with maternal-fetal incompatibility for blood group B. Transfusion. 2008;48:358–364. doi: 10.1111/j.1537-2995.2007.01531.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davoren A, Curtis BR, Aster RH, McFarland JG. Human platelet antigen-specific alloantibodies implicated in 1162 cases of neonatal alloimmune thrombocytopenia. Transfusion. 2004;44:1220–1225. doi: 10.1111/j.1537-2995.2004.04026.x. [DOI] [PubMed] [Google Scholar]
- Ertel K, Al-Tawil M, Santoso S, Kroll H. Relevance of the HPA-15 (Gov) polymorphism on CD109 in alloimmune thrombocytopenic syndromes. Transfusion. 2005;45:366–373. doi: 10.1111/j.1537-2995.2005.04281.x. [DOI] [PubMed] [Google Scholar]
- Fenichel GM, Webster DL, Wong WK. Intracranial hemorrhage in the term newborn. Archives of Neurology. 1984;41:30–34. doi: 10.1001/archneur.1984.04050130036018. [DOI] [PubMed] [Google Scholar]
- Ghevaert C, Campbell K, Walton J, Smith GA, Allen D, Williamson LM, Ouwehand WH, Ranasinghe E. Management and outcome of 200 cases of fetomaternal alloimmune thrombocytopenia. Transfusion. 2007;47:901–910. doi: 10.1111/j.1537-2995.2007.01208.x. [DOI] [PubMed] [Google Scholar]
- Ghevaert C, Rankin A, Huiskes E, Porcelijn L, Javela K, Kekomaki R, Bakchoul T, Santoso S, Nutland S, Smyth DJ, Smith GA, McBride S, Watkins NA, Ouwehand WH. Alloantibodies against low-frequency human platelet antigens do not account for a significant proportion of cases of fetomaternal alloimmune thrombocytopenia: evidence from 1054 cases. Transfusion. 2009;49:2084–2089. doi: 10.1111/j.1537-2995.2009.02246.x. [DOI] [PubMed] [Google Scholar]
- Harrington WJ, Sprague CC, Minnich V, Moore CV, Aulvin RC, Dubach R. Immunologic mechanisms in idiopathic and neonatal thrombocytopenic purpura. Annals of Internal Medicine. 1953;38:433–469. doi: 10.7326/0003-4819-38-3-433. [DOI] [PubMed] [Google Scholar]
- Harrison CR, Curtis BR, McFarland JG, Huff RW, Aster RH. Severe neonatal alloimmune thrombocytopenia caused by antibodies to human platelet antigen 3a (Bak(a)) detectable only in whole platelet assays. Transfusion. 2003;43:1398–1402. doi: 10.1046/j.1537-2995.2003.00533.x. [DOI] [PubMed] [Google Scholar]
- Husebekk A, Killie MK, Kjeldsen-Kragh J, Skogen B. Is it time to implement HPA-1 screening in pregnancy? Current Opinion in Hematology. 2009;16:497–502. doi: 10.1097/MOH.0b013e3283317be9. [DOI] [PubMed] [Google Scholar]
- Ikeda H, Mitani T, Ohnuma M, Haga H, Ohtzuka S, Kato T, Nakase T, Sekiguchi S. A new platelet-specific antigen, Naka, involved in the refractoriness of HLA-matched platelet transfusion. Vox Sanguinis. 1989;57:213–217. doi: 10.1111/j.1423-0410.1989.tb00826.x. [DOI] [PubMed] [Google Scholar]
- Jaegtvik S, Husebekk A, Aune B, Oian P, Dahl LB, Skogen B. Neonatal alloimmune thrombocytopenia due to anti-HPA 1a antibodies; the level of maternal antibodies predicts the severity of thrombocytopenia in the newborn. BJOG. 2000;107:691–694. doi: 10.1111/j.1471-0528.2000.tb13315.x. [DOI] [PubMed] [Google Scholar]
- Jallu V, Meunier M, Brement M, Kaplan C. A new platelet polymorphism Duv(a+), localized within the RGD binding domain of glycoprotein IIIa, is associated with neonatal thrombocytopenia. Blood. 2002;99:4449–4456. doi: 10.1182/blood.v99.12.4449. [DOI] [PubMed] [Google Scholar]
- Jallu V, Dusseaux M, Kaplan C. A new Ser472Asn (Cab2(a+)) polymorphism localized within the alphaIIb “thigh” domain is involved in neonatal thrombocytopenia. Transfusion. 2011;51:393–400. doi: 10.1111/j.1537-2995.2010.02815.x. [DOI] [PubMed] [Google Scholar]
- Jallu V, Bertrand G, Bianchi F, Chenet C, Poulain P, Kaplan C. The alphaIIb p. Leu841Met (Cab3(a+)) polymorphism results in a new human platelet alloantigen involved in neonatal alloimmune thrombocytopenia. Transfusion. 2012 doi: 10.1111/j.1537-2995.2012.03762.x. doi: 10.1111/j.1537-2995.2012.03762.x. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Janson M, McFarland J, Aster RH. Quantitative determination of platelet surface alloantigens using a monoclonal probe. Human Immunology. 1986;15:251–262. doi: 10.1016/0198-8859(86)90001-7. [DOI] [PubMed] [Google Scholar]
- Kaplan C, Daffos F, Forestier F, Morel MC, Chesnel N, Tchernia G. Current trends in neonatal alloimmune thrombocytopenia: diagnosis and therapy. In: Kaplan-Gouet C, Schlegel N, Salmon C, McGregor J, editors. Platelet Immunology: Fundamental and Clinical Aspects. Vol. 206. Colloque INSERM/John Libbey Eurotext; London: 1991. pp. 267–278. [Google Scholar]
- Kaplan C, Porcelijn L, Vanlieferinghen P, Julien E, Bianchi F, Martageix C, Bertrand G, Jallu V. Anti-HPA-9bw (Maxa) fetomaternal alloimmunization, a clinically severe neonatal thrombocytopenia: difficulties in diagnosis and therapy and report on eight families. Transfusion. 2005;45:1799–1803. doi: 10.1111/j.1537-2995.2005.00606.x. [DOI] [PubMed] [Google Scholar]
- Kaplan C, Freedman J, Foxcroft Z, Husebekk A, Metcalfe P, Muniz-Diaz E, Ouwehand W, Panzer S, Rozman P, Skogen B. Monoclonal platelet antigen capture assays (MA-IPA) and reagents: a statement. Vox Sanguinis. 2007;93:298–299. doi: 10.1111/j.1423-0410.2007.00943.x. [DOI] [PubMed] [Google Scholar]
- Killie MK, Husebekk A, Kaplan C, Taaning E, Skogen B. Maternal human platelet antigen-1a antibody level correlates with the platelet count in the newborns: a retrospective study. Transfusion. 2007;47:55–58. doi: 10.1111/j.1537-2995.2007.01063.x. [DOI] [PubMed] [Google Scholar]
- Killie MK, Husebekk A, Kjeldsen-Kragh J, Skogen B. A prospective study of maternal anti-HPA 1a antibody level as a potential predictor of alloimmune thrombocytopenia in the newborn. Haematologica. 2008;93:870–877. doi: 10.3324/haematol.12515. [DOI] [PubMed] [Google Scholar]
- King KE, Kao KJ, Bray PF, Casella JF, Blakemore K, Callan NA, Kennedy SD, Kickler TS. The role of HLA antibodies in neonatal thrombocytopenia: a prospective study. Tissue Antigens. 1996;47:206–211. doi: 10.1111/j.1399-0039.1996.tb02542.x. [DOI] [PubMed] [Google Scholar]
- Kjeldsen-Kragh J, Killie MK, Tomter G, Golebiowska E, Randen I, Hauge R, Aune B, Oian P, Dahl LB, Pirhonen J, Lindeman R, Husby H, Haugen G, Gronn M, Skogen B, Husebekk A. A screening and intervention program aimed to reduce mortality and serious morbidity associated with severe neonatal alloimmune thrombocytopenia. Blood. 2007;110:833–839. doi: 10.1182/blood-2006-08-040121. [DOI] [PubMed] [Google Scholar]
- Koh Y, Taniue A, Ishii H, Matsuyama N, Amakishi E, Hayashi T, Furuta RA, Fukumori Y, Hirayama F, Yoshimura K, Nagamine T, Tamai S, Nakano S. Neonatal alloimmune thrombocytopenia caused by an antibody specific for a newly identified allele of human platelet antigen-7. Transfusion. 2010;50:1276–1284. doi: 10.1111/j.1537-2995.2009.02557.x. [DOI] [PubMed] [Google Scholar]
- Kroll H, Yates J, Santoso S. Cell lines expressing recombinant platelet membrane glycoprotein isoforms as tools for the characterization of antibodies against low frequency alloantigens in neonatal alloimmune thrombocytopenia. Thrombosis and Haemostasis. 2003;(Suppl. 1) Abstract P1155. [Google Scholar]
- Kroll H, Feldmann K, Zwingel C, Hoch J, Bald R, Bein G, Bayat B, Santoso S. A new platelet alloantigen, Swi(a), located on glycoprotein Ia identified in a family with fetal and neonatal alloimmune thrombocytopenia. Transfusion. 2011;51:1745–1754. doi: 10.1111/j.1537-2995.2010.03038.x. [DOI] [PubMed] [Google Scholar]
- Kuijpers RW, Faber NM, Cuypers HT, Ouwehand WH, von dem Borne AE. NH2-terminal globular domain of human platelet glycoprotein Ib alpha has a methionine 145/threonine145 amino acid polymorphism, which is associated with the HPA-2 (Ko) alloantigens. The Journal of Clinical Investigation. 1992;89:381–384. doi: 10.1172/JCI115596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuijpers RW, Simsek S, Faber NM, Goldschmeding R, van Wermerkerken RK, dem Borne AE. Single point mutation in human glycoprotein IIIa is associated with a new platelet-specific alloantigen (Mo) involved in neonatal alloimmune thrombocytopenia. Blood. 1993;81:70–76. [PubMed] [Google Scholar]
- Kupatawintu P, Nathalang O, O-Charoen R, Patmasiriwat P. Gene frequencies of the HPA-1 to 6 and Gov human platelet antigens in Thai blood donors. Immunohematology. 2005;21:5–9. [PubMed] [Google Scholar]
- L’Abbe D, Tremblay L, Filion M, Busque L, Goldman M, Decary F, Chartrand P. Alloimmunization to platelet antigen HPA-1a (PIA1) is strongly associated with both HLA-DRB3*0101 and HLA-DQB1*0201. Human Immunology. 1992;34:107–114. doi: 10.1016/0198-8859(92)90036-m. [DOI] [PubMed] [Google Scholar]
- Lin M, Shieh SH, Liang DC, Yang TF, Shibata Y. Neonatal alloimmune thrombocytopenia in Taiwan due to an antibody against a labile component of HPA-3a (Baka) Vox Sanguinis. 1995;69:336–340. doi: 10.1111/j.1423-0410.1995.tb00369.x. [DOI] [PubMed] [Google Scholar]
- Lyman S, Aster RH, Visentin GP, Newman PJ. Polymorphism of human platelet membrane glycoprotein IIb associated with the Baka/Bakb alloantigen system. Blood. 1990;75:2343–2348. [PubMed] [Google Scholar]
- Maslanka K, Yassai M, Gorski J. Molecular identification of T cells that respond in a primary bulk culture to a peptide derived from a platelet glycoprotein implicated in neonatal alloimmune thrombocytopenia. The Journal of Clinical Investigation. 1996;98:1802–1808. doi: 10.1172/JCI118980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maslanka K, Michur H, Guz K, Wrobel A, Uhrynowska M, Misiak A, Ejduk A, Brojer E, Zupanska B. The relevance of HPA-15 antigen expression for anti-HPA-15 antibody detection. International Journal of Laboratory Hematology. 2012;34:65–69. doi: 10.1111/j.1751-553X.2011.01358.x. [DOI] [PubMed] [Google Scholar]
- McFarland J. Platelet and granulocyte antigens and antibodies. In: Roback J, editor. Technical Manual. American Association of Blood Banks; Bethesda: 2008. pp. 525–546. [Google Scholar]
- McFarland JG, Aster RH, Bussel JB, Gianopoulos JG, Derbes RS, Newman PJ. Prenatal diagnosis of neonatal alloimmune thrombocytopenia using allele-specific oligonucleotide probes. Blood. 1991;78:2276–2282. [PubMed] [Google Scholar]
- McQuilten ZK, Wood EM, Savoia H, Cole S. A review of pathophysiology and current treatment for neonatal alloimmune thrombocytopenia (NAIT) and introducing the Australian NAIT registry. Australian and New Zealand Journal of Obstetrics and Gynaecology. 2011;51:191–198. doi: 10.1111/j.1479-828X.2010.01270.x. [DOI] [PubMed] [Google Scholar]
- Metcalfe P, Watkins NA, Ouwehand WH, Kaplan C, Newman P, Kekomaki R, De Haas M, Aster R, Shibata Y, Smith J, Kiefel V, Santoso S. Nomenclature of human platelet antigens. Vox Sanguinis. 2003;85:240–245. doi: 10.1046/j.1423-0410.2003.00331.x. [DOI] [PubMed] [Google Scholar]
- Moncharmont P, Dubois V, Obegi C, Vignal M, Merieux Y, Gebuhrer L, Rigal D. HLA antibodies and neonatal alloimmune thrombocytopenia. Acta Haematologica. 2004;111:215–220. doi: 10.1159/000077569. [DOI] [PubMed] [Google Scholar]
- Moreaux P, Andre A. Blood groups of human platelets. Nature. 1954;174:88–92. [Google Scholar]
- Mueller-Eckhardt C, Kiefel V, Grubert A. High-dose IgG treatment for neonatal alloimmune thrombocytopenia. Blut. 1989a;59:145–146. doi: 10.1007/BF00320268. [DOI] [PubMed] [Google Scholar]
- Mueller-Eckhardt C, Kiefel V, Grubert A, Kroll H, Weisheit M, Schmidt S, Mueller-Eckhardt G, Santoso S. 348 cases of suspected neonatal alloimmune thrombocytopenia. Lancet. 1989b;1:363–366. doi: 10.1016/s0140-6736(89)91733-9. [DOI] [PubMed] [Google Scholar]
- Newman PJ, Derbes RS, Aster RH. The human platelet alloantigens, PlA1 and PlA2, are associated with a leucine33/proline33 amino acid polymorphism in membrane glycoprotein IIIa, and are distinguishable by DNA typing. The Journal of Clinical Investigation. 1989;83:1778–1781. doi: 10.1172/JCI114082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noris P, Simsek S, Bruijne-Admiraal LG, Porcelijn L, Huiskes E, van der Vlist GJ, van Leeuwen EF, van der Schoot CE, dem Borne AE. Max(a), a new low-frequency platelet-specific antigen localized on glycoprotein IIb, is associated with neonatal alloimmune thrombocytopenia. Blood. 1995;86:1019–1026. [PubMed] [Google Scholar]
- Ogasawara K, Ueki J, Takenaka M, Furihata K. Study on the expression of ABH antigens on platelets. Blood. 1993;82:993–999. [PubMed] [Google Scholar]
- Pacheco LD, Berkowitz RL, Moise KJ, Jr, Bussel JB, McFarland JG, Saade GR. Fetal and neonatal alloimmune thrombocytopenia: a management algorithm based on risk stratification. Obstetrics and Gynecology. 2011;118:1157–1163. doi: 10.1097/AOG.0b013e31823403f4. [DOI] [PubMed] [Google Scholar]
- Paidas MJ, Berkowitz RL, Lynch L, Lockwood CJ, Lapinski R, McFarland JG, Bussel JB. Alloimmune thrombocytopenia: fetal and neonatal losses related to cordocentesis. American Journal of Obstetrics and Gynecology. 1995;172:475–479. doi: 10.1016/0002-9378(95)90559-6. [DOI] [PubMed] [Google Scholar]
- Pearson HA, Shulman NR, Marder VJ, Cone TE., Jr Isoimmune neonatal thrombocytopenic purpura. Clinical and therapeutic considerations. Blood. 1964;23:154–177. [PubMed] [Google Scholar]
- Pereira J, Cretney C, Aster RH. Variation of class I HLA antigen expression among platelet density cohorts: a possible index of platelet age? Blood. 1988;71:516–519. [PubMed] [Google Scholar]
- Peterson JA, Balthazor SM, Curtis BR, McFarland JG, Aster RH. Maternal alloimmunization against the rare platelet-specific antigen HPA-9b (Max a) is an important cause of neonatal alloimmune thrombocytopenia. Transfusion. 2005;45:1487–1495. doi: 10.1111/j.1537-2995.2005.00561.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson JA, Gitter ML, Kanack A, Curtis B, McFarland J, Bougie D, Aster R. New low-frequency platelet glycoprotein polymorphisms associated with neonatal alloimmune thrombocytopenia. Transfusion. 2010;50:324–333. doi: 10.1111/j.1537-2995.2009.02438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson J, Pechauer S, Gitter M, Kanack A, Curtis B, Reese J, Vasudeva K, McFarland J, Aster R. New platelet glycoprotein polymorphisms causing maternal immunization and neonatal alloimmune thrombocytopenia. Transfusion. 2012a;52:1117–1124. doi: 10.1111/j.1537-2995.2011.03428.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson JA, Pechauer SM, Gitter ML, Szabo A, Curtis BR, Aster RH. The human platelet antigen-21bw is relatively common among Asians and is a potential trigger for neonatal alloimmune thrombocytopenia. Transfusion. 2012b;52:915–916. doi: 10.1111/j.1537-2995.2011.03508.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson JA, Kanack A, Nayak D, Bougie DW, McFarland JG, Curtis BR, Aster RH. Prevalence and clinical significance of low-avidity HPA-1a antibodies in women exposed to HPA-1a during pregnancy. Transfusion. 2012c doi: 10.1111/j.1537-2995.2012.03903.x. doi: 10.1111/j.1537-2995.2012.03903.x. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyruchaud O, Bourre F, Morel-Kopp MC, Reviron D, Mercier P, Nurden A, Kaplan C. HPA-10w(b) (La(a)): genetic determination of a new platelet-specific alloantigen on glycoprotein IIIa and its expression in COS-7 cells. Blood. 1997;89:2422–2428. [PubMed] [Google Scholar]
- Rayment R, Kooij TW, Zhang W, Siebold C, Murphy MF, Allen D, Willcox N, Roberts DJ. Evidence for the specificity for platelet HPA-1a alloepitope and the presenting HLA-DR52a of diverse antigen-specific helper T cell clones from alloimmunized mothers. The Journal of Immunology. 2009;183:677–686. doi: 10.4049/jimmunol.0801473. [DOI] [PubMed] [Google Scholar]
- Ruan L, Pei B, Li Q. Multicolor real-time polymerase chain reaction genotyping of six human platelet antigens using displacing probes. Transfusion. 2007;47:1637–1642. doi: 10.1111/j.1537-2995.2007.01335.x. [DOI] [PubMed] [Google Scholar]
- Sachs UJ, Kiefel V, Bohringer M, Afshar-Kharghan V, Kroll H, Santoso S. Single amino acid substitution in human platelet glycoprotein Ibbeta is responsible for the formation of the platelet-specific alloantigen Iy(a) Blood. 2000;95:1849–1855. [PubMed] [Google Scholar]
- Sachs UJ, Bakchoul T, Eva O, Giptner A, Bein G, Aster RH, Gitter M, Peterson J, Santoso S. A point mutation in the EGF-4 domain of beta(3) integrin is responsible for the formation of the Sec(a) platelet alloantigen and affects receptor function. Thrombosis and Haemostasis. 2012;107:80–87. doi: 10.1160/TH11-08-0542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sainio S, Jarvenpaa AL, Renlund M, Riikonen S, Teramo K, Kekomaki R. Thrombocytopenia in term infants: a population-based study. Obstetrics and Gynecology. 2000;95:441–446. doi: 10.1016/s0029-7844(99)00543-8. [DOI] [PubMed] [Google Scholar]
- Saito S, Ota M, Komatsu Y, Ota S, Aoki S, Koike K, Tokunaga I, Tsuno T, Tsuruta G, Kubo T, Fukushima H. Serologic analysis of three cases of neonatal alloimmune thrombocytopenia associated with HLA antibodies. Transfusion. 2003;43:908–917. doi: 10.1046/j.1537-2995.2003.00429.x. [DOI] [PubMed] [Google Scholar]
- Santoso S, Kalb R, Walka M, Kiefel V, Mueller-Eckhardt C, Newman PJ. The human platelet alloantigens Br(a) and Brb are associated with a single amino acid polymorphism on glycoprotein Ia (integrin subunit alpha 2) The Journal of Clinical Investigation. 1993;92:2427–2432. doi: 10.1172/JCI116849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santoso S, Kalb R, Kroll H, Walka M, Kiefel V, Mueller-Eckhardt C, Newman PJ. A point mutation leads to an unpaired cysteine residue and a molecular weight polymorphism of a functional platelet beta 3 integrin subunit. The Sra alloantigen system of GPIIIa. Journal of Biological Chemistry. 1994;269:8439–8444. [PubMed] [Google Scholar]
- Santoso S, Amrhein J, Hofmann HA, Sachs UJ, Walka MM, Kroll H, Kiefel V. A point mutation Thr(799)Met on the alpha(2) integrin leads to the formation of new human platelet alloantigen Sit(a) and affects collagen-induced aggregation. Blood. 1999;94:4103–4111. [PubMed] [Google Scholar]
- Santoso S, Kiefel V, Richter IG, Sachs UJ, Rahman A, Carl B, Kroll H. A functional platelet fibrinogen receptor with a deletion in the cysteine-rich repeat region of the beta(3) integrin: the Oe(a) alloantigen in neonatal alloimmune thrombocytopenia. Blood. 2002;99:1205–1214. doi: 10.1182/blood.v99.4.1205. [DOI] [PubMed] [Google Scholar]
- Schuh AC, Watkins NA, Nguyen Q, Harmer NJ, Lin M, Prosper JY, Campbell K, Sutherland DR, Metcalfe P, Horsfall W, Ouwehand WH. A tyrosine703serine polymorphism of CD109 defines the Gov platelet alloantigens. Blood. 2002;99:1692–1698. doi: 10.1182/blood.v99.5.1692. [DOI] [PubMed] [Google Scholar]
- Shulman NR, Aster RH, Pearson HA, Hiller MC. Immunoreactions involving platelet. VI. Reactions of maternal isoantibodies responsible for neonatal purpura. Differentiation of a second platelet antigen system. The Journal of Clinical Investigation. 1962;41:1059–1069. doi: 10.1172/JCI104556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverstein RL, Febbraio M. CD36, a scavenger receptor involved in immunity, metabolism, angiogenesis, and behavior. Science Signaling. 2009;2(72):1–8. doi: 10.1126/scisignal.272re3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simsek S, Folman C, van der Schoot CE, dem Borne AE. The Arg633His substitution responsible for the private platelet antigen Gro(a) unravelled by SSCP analysis and direct sequencing. British Journal of Haematology. 1997;97:330–335. doi: 10.1046/j.1365-2141.1997.502696.x. [DOI] [PubMed] [Google Scholar]
- Skogen B, Bellissimo DB, Hessner MJ, Santoso S, Aster RH, Newman PJ, McFarland JG. Rapid determination of platelet alloantigen genotypes by polymerase chain reaction using allele-specific primers. Transfusion. 1994;34:955–960. doi: 10.1046/j.1537-2995.1994.341195065032.x. [DOI] [PubMed] [Google Scholar]
- Skogen B, Killie MK, Kjeldsen-Kragh J, Ahlen MT, Tiller H, Stuge TB, Husebekk A. Reconsidering fetal and neonatal alloimmune thrombocytopenia with a focus on screening and prevention. Expert Review of Hematology. 2010;3:559–566. doi: 10.1586/ehm.10.49. [DOI] [PubMed] [Google Scholar]
- Socher I, Zwingel C, Santoso S, Kroll H. Heterogeneity of HPA-3 alloantibodies: consequences for the diagnosis of alloimmune thrombocytopenic syndromes. Transfusion. 2008;48:463–472. doi: 10.1111/j.1537-2995.2007.01550.x. [DOI] [PubMed] [Google Scholar]
- Socher I, Andrei-Selmer C, Bein G, Kroll H, Santoso S. Low-avidity HPA-1a alloantibodies in severe neonatal alloimmune thrombocytopenia are detectable with surface plasmon resonance technology. Transfusion. 2009;49:943–952. doi: 10.1111/j.1537-2995.2008.02065.x. [DOI] [PubMed] [Google Scholar]
- Sola-Visner M, Saxonhouse MA, Brown RE. Neonatal thrombocytopenia: what we do and don’t know. Early Human Development. 2008;84:499–506. doi: 10.1016/j.earlhumdev.2008.06.004. [DOI] [PubMed] [Google Scholar]
- Spencer JA, Burrows RF. Feto-maternal alloimmune thrombocytopenia: a literature review and statistical analysis. Australian and New Zealand Journal of Obstetrics and Gynaecology. 2001;41:45–55. doi: 10.1111/j.1479-828x.2001.tb01293.x. [DOI] [PubMed] [Google Scholar]
- Stafford P, Garner SF, Huiskes E, Kaplan C, Kekomaki R, Santoso S, Tsuno NH, Watkins NA, Ouwehand WH. Three novel beta3 domain-deletion peptides for the sensitive and specific detection of HPA-4 and six low frequency beta3-HPA antibodies. Journal of Thrombosis and Haemostasis. 2008a;6:376–383. doi: 10.1111/j.1538-7836.2008.02843.x. [DOI] [PubMed] [Google Scholar]
- Stafford P, Garner SF, Rankin A, Kekomaki R, Watkins NA, Ouwehand WH. A single-nucleotide polymorphism in the human ITGB3 gene is associated with the platelet-specific alloantigen Va (HPA-17bw) involved in fetal maternal alloimmune thrombocytopenia. Transfusion. 2008b;48:1432–1438. doi: 10.1111/j.1537-2995.2008.01737.x. [DOI] [PubMed] [Google Scholar]
- Stafford P, Ghevaert C, Campbell K, Proulx C, Smith G, Williamson LM, Ranasinghe E, Watkins NA, Huntington JA, Ouwehand WH. Immunologic and structural analysis of eight novel domain-deletion beta3 integrin peptides designed for detection of HPA-1 antibodies. Journal of Thrombosis and Haemostasis. 2008c;6:366–375. doi: 10.1111/j.1538-7836.2008.02858.x. [DOI] [PubMed] [Google Scholar]
- Stuge TB, Skogen B, Ahlen MT, Husebekk A, Urbaniak SJ, Bessos H. The cellular immunobiology associated with fetal and neonatal alloimmune thrombocytopenia. Transfusion and Apheresis Science. 2011;45:53–59. doi: 10.1016/j.transci.2011.06.003. [DOI] [PubMed] [Google Scholar]
- Szatkowski NS, Aster RH. HLA antigens of platelets. IV. Influence of “private” HLA–B locus specificities on the expression of Bw4 and Bw6 on human platelets. Tissue Antigens. 1980;15:361–368. doi: 10.1111/j.1399-0039.1980.tb00196.x. [DOI] [PubMed] [Google Scholar]
- Szatkowski N, Duquesnoy RJ, Aster RH. Further studies of the expression of Bw4, Bw6, and other antigens of the HLA-B locus on human platelets. Transplantation Proceedings. 1978;10:739–740. [PubMed] [Google Scholar]
- Tanaka S, Taniue A, Nagao N, Tomita T, Ohnoki S, Shibata H, Okubo Y, Yamaguchi H, Shibata Y. Genotype frequencies of the human platelet antigen, Ca/Tu, in Japanese, determined by a PCR-RFLP method. Vox Sanguinis. 1996;70:40–44. doi: 10.1111/j.1423-0410.1996.tb00995.x. [DOI] [PubMed] [Google Scholar]
- Thude H, Schorner U, Helfricht C, Loth M, Maak B, Barz D. Neonatal alloimmune thrombocytopenia caused by human leucocyte antigen-B27 antibody. Transfusion Medicine (Oxford, England) 2006;16:143–149. doi: 10.1111/j.1365-3148.2006.00634.x. [DOI] [PubMed] [Google Scholar]
- Van Loghem J, Jr, Dorfmeijer H, Van Hart M, Schreuder F. Serological and genetical studies on a platelet antigen (Zw) Vox Sanguinis. 1959;4:161–169. doi: 10.1111/j.1423-0410.1959.tb04032.x. [DOI] [PubMed] [Google Scholar]
- Verran J, Grey D, Bennett J, Lown JA, Erber WN. HPA-1, 3, 5 genotyping to establish a typed platelet donor panel. Pathology. 2000;32:89–93. doi: 10.1080/003130200104295. [DOI] [PubMed] [Google Scholar]
- Wang R, Furihata K, McFarland JG, Friedman K, Aster RH, Newman PJ. An amino acid polymorphism within the RGD binding domain of platelet membrane glycoprotein IIIa is responsible for the formation of the Pena/Penb alloantigen system. The Journal of Clinical Investigation. 1992;90:2038–2043. doi: 10.1172/JCI116084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R, McFarland JG, Kekomaki R, Newman PJ. Amino acid 489 is encoded by a mutational “hot spot” on the beta 3 integrin chain: the CA/TU human platelet alloantigen system. Blood. 1993;82:3386–3391. [PubMed] [Google Scholar]
- Williamson LM. Screening programmes for foetomaternal alloimmune thrombocytopenia. Vox Sanguinis. 1998;74(Suppl. 2):385–389. doi: 10.1111/j.1423-0410.1998.tb05446.x. [DOI] [PubMed] [Google Scholar]
- Williamson LM, Hackett G, Rennie J, Palmer CR, Maciver C, Hadfield R, Hughes D, Jobson S, Ouwehand WH. The natural history of fetomaternal alloimmunization to the platelet-specific antigen HPA-1a (PlA1, Zwa) as determined by antenatal screening. Blood. 1998;92:2280–2287. [PubMed] [Google Scholar]
- Zhu J, Luo BH, Xiao T, Zhang C, Nishida N, Springer TA. Structure of a complete integrin ectodomain in a physiologic resting state and activation and deactivation by applied forces. Molecular Cell. 2008;32:849–861. doi: 10.1016/j.molcel.2008.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]