Abstract
Objective
To investigate the risk profiles, treatment utilization, and outcomes of myocardial infarction (MI) patients with rheumatoid arthritis (RA) and matched MI patients without RA.
Methods
We utilized a population-based cohort of Olmsted County, Minnesota, residents with MI from 1979–2009. Among these, we identified 77 patients who fulfilled the American College of Rheumatology (ACR) 1987 criteria for RA and 154 age, sex, and calendar year matched MI patients without RA. Data collection from medical records included RA and MI characteristics, antirheumatic and cardioprotective medications, and reperfusion therapy as well as outcomes (mortality, heart failure, and recurrent ischemia).
Results
The mean age at MI was 72.4 and 55% were female in both cohorts. Cardiovascular risk factor profiles, MI characteristics, and treatment with reperfusion therapy or cardioprotective medications were similar in MI patients with and without RA. However, patients with RA experienced poorer long-term outcomes compared to patients without RA (for mortality: hazard ratio [HR]: 1.47; 95% confidence interval [CI]: 1.04, 2.08, and for recurrent ischemia: HR: 1.51; 95% CI: 1.04, 2.18).
Conclusion
MI patients with RA receive similar treatment with reperfusion therapy and cardioprotective medications, and have similar short-term outcomes compared to patients without RA. However, patients with RA have poorer long-term outcomes. Thus, despite similar treatment, MI patients with RA have worse long-term outcomes than MI patients without RA.
Key Indexing Terms: Rheumatoid arthritis, myocardial infarction
Introduction
Cardiovascular (CV) disease is implicated as a major cause of morbidity and mortality in rheumatoid arthritis (RA) (1–9). RA is associated with systemic inflammation, contributing to the increased CV risk in patients with RA (10, 11). Recently, a well-powered observational study demonstrated impaired prognosis after acute coronary events in patients with RA compared to the general population (12). Subsequent studies have indicated that patients with RA may be less likely to receive reperfusion therapy and may also be less likely to receive appropriate medical management (13). In addition to short-term mortality, long-term mortality in MI patients with RA may be worse than in MI patients without RA. Two small studies have demonstrated that despite similarity in short-term mortality outcomes after MI, patients with RA have poorer long-term outcomes than patients without RA (14, 15). The aim of this study was to further elucidate these findings by assessing the CV risk profile, treatment utilization (e.g., reperfusion therapy and cardioprotective medication use), and outcomes (mortality, heart failure and recurrent ischemia) after MI in patients with and without RA.
Patients and Methods
Study Population
This study utilized the resources of the Rochester Epidemiology Project (REP), a diagnostic indexing linkage system that allows access to the complete (inpatient and outpatient) medical records of all health care providers for the population of Olmsted County, Minnesota, USA (16).
The study population was comprised of a retrospectively identified cohort of Olmsted County, Minnesota residents with incident MI between January 1, 1979 and January 1, 2010. MI was defined using standardized epidemiologic criteria, and Minnesota coding of the electrocardiogram (ECG) (17–19). All patients with RA were identified within the MI cohort using the 1987 American College of Rheumatology classification criteria for RA (20). The RA incidence date was defined as the first date of fulfillment of 4 of the 7 ACR diagnostic criteria. For patients with incident MI who moved to Olmsted County with prevalent RA, the incidence date of RA was estimated from medical record documentation. For each patient with RA, two MI patients without RA were randomly selected from all patients with MI of similar age and sex with an MI in the same calendar year. The inpatient and outpatient medical records were reviewed longitudinally from the onset of MI until the subject’s death, migration from Olmsted County, or September 1, 2011 for collection of CV data (as described below). A separate abstractor reviewed the medical records of patients with RA beginning with the date of diagnosis of RA to collect data on RA disease characteristics.
Data Collection
Clinical diagnoses were used to ascertain hypertension, diabetes mellitus, and hyperlipidemia. Previous physician diagnoses of cerebrovascular disease and peripheral vascular disease were also collected. Body mass index (BMI) was calculated using the height and weight at onset of MI, and obesity was defined as BMI ≥ 30 kg/m2. MI characteristics and severity were classified using the Killip class II–IV. ECG findings were documented and categorized into anterior MI, ST-segment elevation MI, and presence of Q waves. Heart failure (HF) was defined based on the Framingham Criteria (21). Recurrent ischemia was defined as hospitalization for recurrent MI or unstable angina using physicians’ diagnosis. Reperfusion or revascularization therapy was defined as the use of thrombolytic therapy, percutaneous coronary intervention (PCI), or coronary artery bypass surgery (CABG) within the same hospitalization. Medical treatment during hospitalization and at discharge was documented and included aspirin, angiotensin converting enzyme (ACE) inhibitors/angiotensin II receptor blockers, beta blockers, statins, other lipid lowering medications, aspirin, and other anti-platelet agents.
All subjects (irrespective of residency status) were tracked nationally to ascertain vital status, and death certificates were obtained from the respective states for subjects who died outside Minnesota. The underlying cause of death was coded from national mortality statistics and grouped according to International Classification of Diseases, 9th Revision (ICD-9) and ICD-10 chapters. Cardiovascular death was defined as ICD-9 codes 390–459, and ICD-10 codes I00–I99 according to the American Heart Association classification (22).
RA Characteristics
Data was collected on RA disease characteristics present on or before the date of onset of MI including rheumatoid factor (RF), anti-citrullinated protein antibodies (ACPA), presence of rheumatoid nodules, erosions/destructive changes on radiographs, and severe extra articular disease. Severe extra articular disease was defined, according to the Malmö criteria, as the presence of pericarditis, pleuritis, Felty’s syndrome, vasculitis, neuropathy, scleritis, episcleritis, and glomerulonephritis (23). Data was collected on antirheumatic medication use at the time of onset of MI including corticosteroids, disease-modifying antirheumatic drugs (DMARDs), and biologic agents.
Statistical analysis
Descriptive statistics (percentages, means, etc.) were used to summarize the data. Characteristics of patients with and without RA at the time of MI were compared using conditional logistic regression models, accounting for the matched nature of the cohorts. In addition, short-term (30-day) outcomes were analyzed using conditional logistic regression with results summarized using odds ratios, instead of hazard ratios from Cox models, because censoring was not an issue for short-term outcomes. Kaplan-Meier methods were used to estimate the rate of development of each outcome after MI to account for censoring. Log rank tests were used to compare the rate of development of each outcome between those with and without RA. Cox proportional hazards models were used to examine the association between RA disease characteristics at the time of MI and the development of the outcomes of interest during follow-up, and the results were summarized using hazard ratios. In these analyses, patients who had HF prior to MI were excluded from the analyses of the development of HF during follow-up. The proportional hazards assumption was examined using the Schoenfeld residuals, and tested using methods by Therneau and Grambsch (24). No violations of the proportional hazards assumption were found. Analyses were conducted using R (R Project for Statistical Computing, Vienna, Austria) and SAS version 9.2 (SAS Institute Inc., Cary, North Carolina).
Results
Characteristics of the Study Population
The study population consisted of 77 MI patients with RA and 154 MI patients without RA. The mean age at MI was 72.4 and 55% were female in both cohorts (Table 1). CV risk factors including hypertension, dyslipidemia, diabetes mellitus, smoking status, obesity, previous revascularization procedures, cerebrovascular disease and peripheral vascular disease were assessed and no significant differences between cohorts were found. At the time of MI, there were fewer current smokers among the patients with RA than in those without RA, but this difference did not reach statistical significance (12% vs 21%, p=0.06). MI characteristics and severity were similar in both groups with Killip class II–IV in 36% of patients with RA and 35% of patients without RA (p=0.92). ECG findings were also similar among patients with and without RA.
Table 1.
Characteristics of 77 patients with rheumatoid arthritis (RA) and 154 patients without RA at onset of myocardial infarction (MI)
| Characteristic | MI patients with RA (N = 77) |
MI patients without RA (N = 154) |
P value |
|---|---|---|---|
| Age, years, mean ± SD | 72.4 ± 12.2 | 72.4 ± 12.2 | 1.0 |
| Women, n (%) | 42 (55%) | 84 (55%) | 1.0 |
| Medical history, n (%) | |||
| Hypertension | 55 (71%) | 99 (63%) | 0.26 |
| Dyslipidemia | 31 (40%) | 62 (40%) | 0.91 |
| Diabetes mellitus | 21 (27%) | 41 (26%) | 0.92 |
| Current smoking | 9 (12%) | 32 (21%) | 0.06 |
| Obesity (Body mass index ≥ 30 kg/m2) | 26 (33%) | 45 (29%) | 0.48 |
| Revascularization procedures | 21 (27%) | 41 (27%) | 0.91 |
| Cerebrovascular disease | 18 (23%) | 33 (21%) | 0.73 |
| Peripheral vascular disease | 16 (21%) | 39 (25%) | 0.45 |
| MI characteristics & severity, n (%) | |||
| Killip class II-IV | 28 (36%) | 55 (35%) | 0.92 |
| Electrocardiogram, n (%) | |||
| Anterior MI | 25 (32%) | 50 (32%) | 0.92 |
| ST-segment elevation MI | 16 (21%) | 43 (28%) | 0.24 |
| Presence of Q waves | 34 (44%) | 68 (44%) | 0.96 |
Treatment with Reperfusion Therapy and Cardioprotective Medications
Treatment of patients with and without RA was similar (Table 2). Reperfusion therapy occurred at similar rates in both groups. When subcategorized by type of reperfusion therapy (i.e., thrombolytics, CABG, and PCI), there were no significant differences in treatment between the two groups. Treatment with cardioprotective medications (aspirin, ACE inhibitors, beta blockers, and statins) was assessed both during hospitalization and at discharge from the hospital. During hospitalization, there was a trend toward lower rates of patients with RA receiving each treatment, but these differences did not reach statistical significance. At the time of discharge, there were no significant differences between patients with and without RA. The duration of hospitalization after MI shortened over time in both groups with no evidence of differences in patients with RA compared to those without RA (8.3 ± 7.2 days vs. 7.7 ± 7.7 days, respectively; p=0.55).
Table 2.
Treatment of myocardial infarction (MI) patients with and without rheumatoid arthritis (RA)
| Treatment | MI patients with RA (N = 77) |
MI patients without RA (N = 154) |
Odds ratio (95% CI) |
|---|---|---|---|
| Reperfusion therapy, n (%) | 39 (50%) | 73 (47%) | 1.19 (0.63, 2.23) |
| Medications during hospitalization, n (%) | |||
| Aspirin | 56 (72%) | 127 (81%) | 0.44 (0.18, 1.09) |
| ACE Inhibitor | 25 (32%) | 58 (37%) | 0.78 (0.41, 1.48) |
| Beta Blocker | 54 (69%) | 115 (74%) | 0.83 (0.40, 1.71) |
| Statin | 24 (31%) | 61 (39%) | 0.58 (0.25, 1.30) |
| Other lipid-lowering agents | 0 (0%) | 5 (3%) | 0.99 |
| Other anti-platelet agents | 31 (40%) | 71 (46%) | 0.31 |
| Medications at discharge, n (%) | |||
| Aspirin | 49 (63%) | 99 (63%) | 0.93 (0.45, 1.95) |
| ACE Inhibitor | 23 (29%) | 51 (33%) | 0.91 (0.46, 1.78) |
| Beta Blocker | 48 (62%) | 92 (59%) | 1.45 (0.67, 3.13) |
| Statin | 29 (37%) | 58 (37%) | 1.12 (0.50, 2.48) |
| Other lipid-lowering agents | 0 (0%) | 3 (2%) | 0.99 |
| Other anti-platelet agents | 29 (40%) | 55 (39%) | 0.82 |
Outcomes
Mortality
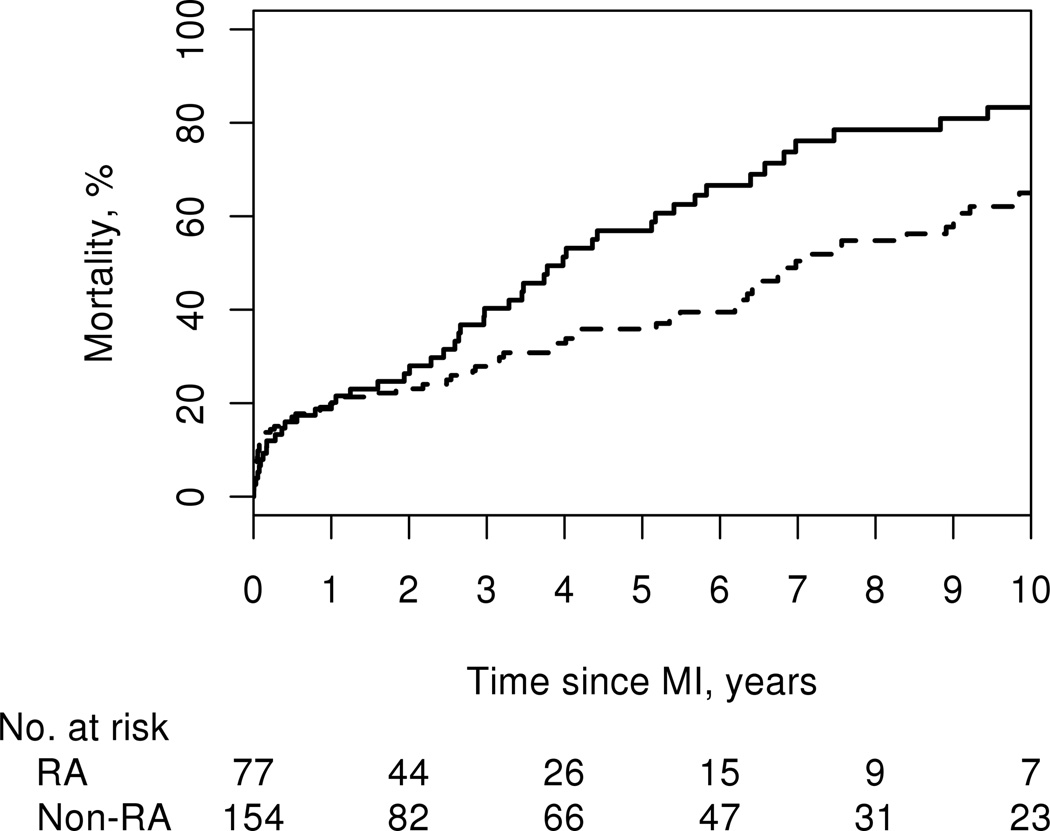
We assessed outcomes of death, recurrent ischemia, and heart failure following MI. During hospitalization, 4 (5%) patients with RA and 13 (8%) patients without RA died (p=0.37). During a median follow-up of 2.6 years among the patients with RA (interquartile range [IQR]: 1.0–5.6 years] and a median follow-up of 2.7 years among the patients without RA (IQR: 0.9, 6.6 years), 55 of the RA and 85 of those without RA died, corresponding to a higher death rate among the patients with RA compared to those without RA (hazard ratio [HR]: 1.47; 95% CI: 1.04, 2.08). Although the short-term mortality was somewhat, but not significantly, lower among patients with RA than those without RA with 30-day case fatality rates of 6% among patients with RA and 12% among patients without RA (OR: 0.41; 95% CI: 0.13, 1.31; p=0.13), by 1 year after MI, the mortality rates were similar in both groups (19% vs.20%). By 5 years mortality rates were significantly higher among patients with RA (57% ± 6%) compared to those without RA (36% ± 4%) (logrank p=0.036; Figure 1).
Figure 1.
Cumulative incidence of mortality after myocardial infarction among 77 patients with (solid line) and 154 patients without (dashed line) rheumatoid arthritis (55 deaths in patients with RA and 85 deaths in patients without RA; logrank p=0.036).
Cause of death information was available in all but 5 of the deceased patients. Cardiovascular causes were found in 28 (53% of 52) deaths among patients with RA and in 40 (49% of 82) deaths among patients without RA. In both groups, the early deaths were predominately cardiovascular, and the later deaths were predominantly non-cardiovascular. However, there was no apparent difference between groups in the proportion of deaths due to CV causes.
Recurrent Ischemia
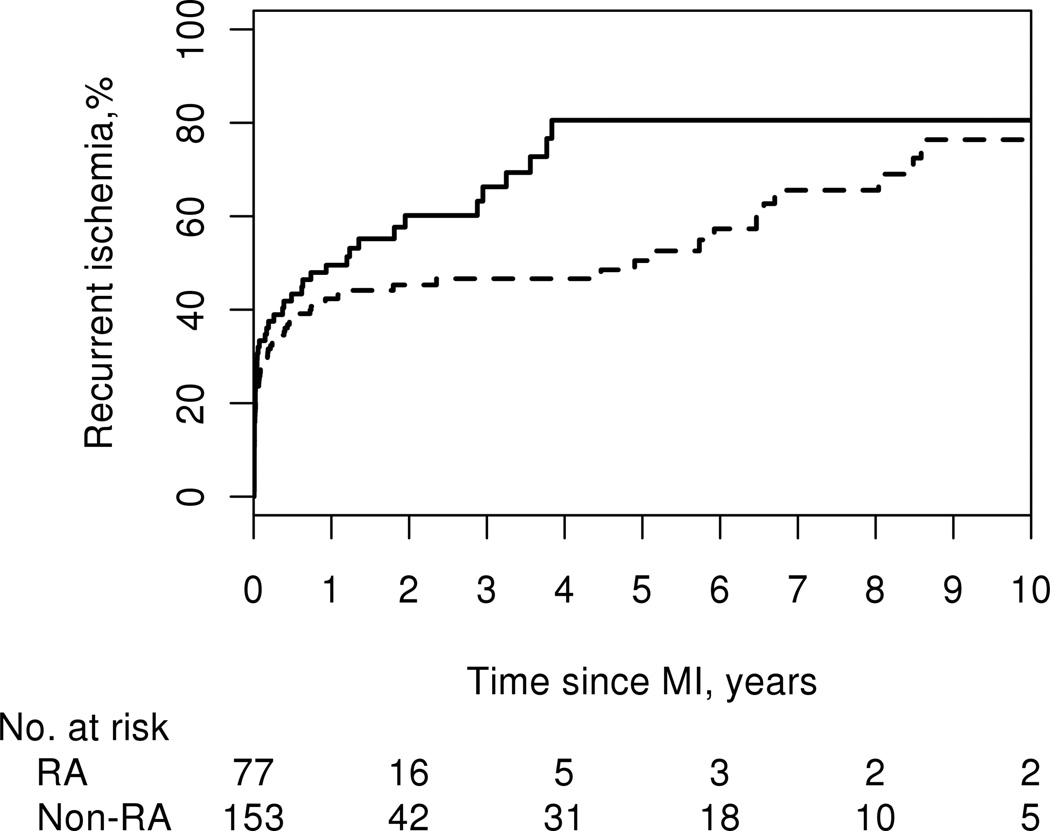
Recurrent ischemia was more common among patients with RA compared with patients without RA (HR: 1.51; 95% CI: 1.04, 2.18). A total of 48 patients with RA and 76 patients without RA developed recurrent ischemia during follow-up with no difference in the 30-day rates of recurrent ischemia (32% among patients with RA vs. 25% among patients without RA; p=0.24). There were minimal differences in the cumulative incidence of recurrent ischemia at 1 year after MI; 50% (± 6%) among patients with RA and 42% (± 4%) among patients without RA. By 5 years after MI, this difference in cumulative incidence of recurrent ischemia was more pronounced with 80% (± 7%) in patients with RA and 50% (± 5%) in patients without RA (logrank p=0.043; Figure 2).
Figure 2.
Cumulative incidence of recurrent ischemia after myocardial infarction among 77 patients with (solid line) and 153 patients without (dashed line) rheumatoid arthritis (recurrent ischemia occurred in 48 patients with RA and 76 patients without RA; logrank p=0.043).
Heart Failure
Among the 64 patients with RA and 127 patients without RA who did not have heart failure at the time of MI, the development of HF after MI was similar among patients with and without RA (HR: 1.30; 95% CI: 0.81, 2.10). During follow-up, 28 patients with RA and 45 patients without RA developed HF, and there was no difference in 30-day event rates (31% in both groups). By 5 years after MI, the cumulative incidence of developing HF was higher among patients with RA (57% ± 8%) than those without RA (35% ± 4%), but this difference did not reach statistical significance (logrank p=0.24). As our study spans several decades, calendar trends in outcomes (mortality, heart failure and recurrent ischemia) were also examined. Improvements in outcomes over calendar time were similar in both groups, with no evidence that the rate of improvement in outcomes after MI differed in patients with RA compared to patients without RA (p>0.7 for all outcomes).
RA disease characteristics and association with outcomes
Associations between RA disease characteristics and outcomes (mortality, recurrent ischemia, and HF) were assessed in MI patients with RA (Table 3). No significant associations were found for any of the RA disease characteristics examined (presence of RF and/or ACPA, duration of RA, presence of severe extra-articular disease, or DMARDs and corticosteroids). There were several hazard ratios in the 1.5 –2 range, but confidence intervals were wide, indicating our study may have been underpowered to detect associations between RA disease characteristics and MI outcomes. The use of biologic agents could not be assessed as only 2 patients with RA in this cohort were exposed to biologic agents.
Table 3.
Risk factors for mortality, heart failure and recurrent ischemia among 77 patients with rheumatoid arthritis and myocardial infarction
| RA characteristics at onset of MI |
N (%) or mean ± SD |
Mortality Hazard Ratio (95% CI) |
Heart Failure† Hazard Ratio (95% CI) |
Recurrent Ischemia Hazard Ratio (95% CI) |
|---|---|---|---|---|
| RF and/or ACPA positive | 54 (70%) | 1.36 (0.73, 2.54) | 1.19 (0.52, 2.73) | 0.96 (0.51, 1.80) |
| Duration of RA, years | 14.0 ± 9.5 | 1.23** (0.92, 1.65) | 1.27** (0.86, 1.88) | 0.95 (0.69, 1.32) |
| Rheumatoid Nodules | 63 (82%) | 1.29 (0.56, 2.97) | 1.11 (0.40, 3.07) | 0.72 (0.35, 1.49) |
| Erosions/ destructive changes | 65 (84%) | 1.76 (0.71, 4.35) | 1.84 (0.54, 6.30) | 1.60 (0.66, 3.85) |
| Severe extra-articular disease | 63 (82%) | 1.42 (0.63, 3.20) | 0.99 (0.36, 2.69) | 0.98 (0.46, 2.08) |
| Methotrexate | 17 (22%) | 0.83 (0.42, 1.66) | 0.93 (0.37, 2.38) | 0.91 (0.43, 1.93) |
| Hydroxychloroquine | 9 (12%) | 1.75 (0.67, 4.59) | 0.67 (0.15, 2.88) | 0.82 (0.28, 2.37) |
| Other non-biologic DMARDs | 10 (13%) | 2.36 (0.84, 6.62) | 0.45 (0.06, 3.52) | 1.09 (0.40, 2.95) |
| Corticosteroids | 25 (32%) | 1.24 (0.68, 2.27) | 0.75 (0.32, 1.73) | 1.47 (0.79, 2.72) |
RA: rheumatoid arthritis; MI: myocardial infarction; RF: rheumatoid factor; ACPA: anti-citrullinated protein antibodies; DMARD: disease modifying antirheumatic drugs MId myocardial infarctionnt. Ref 31 not neededt ischemia) were also compared
Models adjusted for age at MI, sex and year of MI
hazard ratio per 10 year increase in disease duration
among 64 patients without heart failure prior to myocardial infarction
Discussion
In this population-based study of MI patients with and without RA, we found no significant differences in risk factor profile, MI treatment, or use of cardioprotective medications, but there was a trend toward lower short-term mortality in patients with RA than in those without RA. Patients with RA experienced significantly poorer long-term outcomes compared to patients without RA and there was no evidence that this disparity between groups had improved over time. Despite this, although underpowered, we did not find an association between RA disease characteristics and mortality, recurrent ischemia, or heart failure. These findings indicate that despite having similar treatment, MI patients with RA have worse long-term outcomes than MI patients without RA.
Several studies have recently demonstrated a disparity in treatment of MI patients with RA compared to those without RA. A recent observational study demonstrated that MI patients with RA were less likely than MI patients without RA to be treated with reperfusion therapy and were also less likely to receive beta blockers or lipid-lowering agents (13). Another study found that fewer patients with RA received treatment with cardioprotective drugs during and after hospitalization, although this difference did not reach statistical significance (14). In contrast, other studies have demonstrated increased rates of PCI in MI patients with RA (25). Our study did not demonstrate a significant difference in utilization of reperfusion therapy during hospitalization or cardioprotective medication use after discharge from the hospital. This may be explained by institutional variation in treatment of patients with and without RA. Rates of PCI and CABG as well as outcomes vary based on geographic location (26–28). Our results may not be generalizable to other geographic regions. Others have postulated that treatment of MI patients with RA has changed over time, affecting outcomes (29).
There is evidence from a prospective observational study that patients who developed RA after 1980 were less likely to experience an MI or to die of a fatal MI than patients who developed RA before 1980, and the rate of fatal MI in patients who developed RA after 1980 was not statistically different from the general population (29). We found no differences in the relative improvement of outcomes (mortality, heart failure and recurrent ischemia) over calendar time in patients with RA compared to patients without RA. This may be explained by a difference in timing of the cohorts. In the aforementioned study, differences were found in patients who developed RA after 1980 compared to those who developed it earlier. Our study included patients who had an MI in 1979 or later.
We found that 30-day mortality was somewhat lower among MI patients with RA compared to MI patients without RA, although this finding was not statistically significant. 30-day recurrent ischemia and heart failure were similar between the two groups. We found no differences in rates of reperfusion therapy after MI, but other reports suggest that short term mortality may be dependent on therapy choice while hospitalized. In one report, MI patients with RA were less likely to receive reperfusion therapy than MI patients without RA, and an increased 30-day case fatality rate was found in the patients with RA (12, 13). In contrast, another report showed that MI patients with RA were more likely to have PCI than MI patients without RA, and a decreased in-hospital mortality rate was found in patients with RA (25). Yet another cross-sectional study performed on all patients with RA who underwent revascularization found decreased in-hospital mortality in patients with RA (30). The disparity of these results might be explained by differences in study design and in case-mix which may have led to differences in the use of PCI in these cohorts.
Additionally, we found increased long-term mortality and recurrent ischemic events in patients with RA compared to patients without RA. These findings correspond to previous studies demonstrating increased long-term mortality in MI patients with RA (1, 31). In a recent study of post-MI patients, patients with RA who received similar treatment to patients without RA had increased case fatality at 10 years, but no difference in short-term survival (14). Similar treatment between patients with and without RA may result in similar short-term outcomes; however long-term outcomes between patients with and without RA remain disparate. This difference in long-term outcomes suggests RA disease characteristics may play a role in determining the long-term mortality after MI in RA. We were unable to demonstrate significant associations between RA disease characteristics and mortality, recurrent ischemia, or HF after MI, likely due to limited statistical power. Previous studies have demonstrated associations between RA severity and cardiovascular mortality, but none have examined predictors of mortality after MI in RA (32, 33). If RA disease characteristics do indeed play a role in determining long-term survival after MI in persons with RA, this would argue for a more personalized, disease-specific approach to post-MI management for patients with RA similar to post-MI management in patients with Diabetes Mellitus.
Our study has several potential limitations. In this retrospective study, we were limited to information available from medical records for evaluation of risk factors and outcomes. Risk factors and outcomes were not measured at regular intervals and were dependent on physician documentation. This limitation was minimized using the REP and standardized case ascertainment. Data on treatment therapy of modifiable risk factors was not collected due to diagnosis and treatment guideline changes over the time period of our study. Data on non-steroidal antiinflammatory agents was not collected because use of this over-the-counter medication is frequently not documented in medical records. Finally, we were unable to obtain data on the disease activity of patients at the time of MI. Erythrocyte sedimentation rate (ESR) was documented in fewer than half of the cohort in the months prior to MI, and no other measure of disease activity was consistently used or documented over the study time period.
The statistical power of this study was adequate for comparisons between cohorts, but limited when examining associations within the RA cohort, such as associations between RA disease characteristics and outcomes after MI. It may have been underpowered to detect associations between RA disease characteristics and MI outcomes.
As an observational study, we cannot rule out the possibility of confounding by indications/contraindications of medication use with the risk of fatality and HF Furthermore, the population of Olmsted County, Minnesota is predominately white, so our conclusions may not be generalizable to other more diverse populations (34). Strengths of this study include the population-based longitudinal design with long-term follow-up of both cohorts.
In conclusion, this study did not demonstrate a significant difference in treatment of MI patients with and without RA, including reperfusion therapy and use of cardioprotective medications. 30-day case fatality was somewhat lower among patients with RA compared to those without RA, but 30-day rates of recurrent ischemia and heart failure rate were similar between MI patients with and without RA.
The increase in long-term mortality in patients with RA when compared to patients without RA indicates that early similarity in post-MI treatment changes outcomes over time. MI patients with RA may benefit from closer monitoring and treatment of their rheumatologic disease as well as their cardiovascular disease to optimize their CVD outcomes. More research is needed to understand the determinants of long-term outcomes after MI in patients with RA, in particular, the role of RA disease characteristics.
Acknowledgments
Funding: This work was funded by the National Institutes of Health, NIAMS (R01 AR46849) and NHLBI (R01 HL 59205), and made possible by the Rochester Epidemiology Project (R01 AG034676 from the National Institute on Aging).
REFERENCES
- 1.Maradit-Kremers H, Crowson C, Nicola P, Baliman K, Roger V, Jacobsen S, et al. Increased unrecognized coronary heart disease and sudden deaths in rheumatoid arthritis: a population-based cohort study. Arthritis Rheum. 2005;52(2):402–411. doi: 10.1002/art.20853. [DOI] [PubMed] [Google Scholar]
- 2.Van Doornum S, McGoll G, Wicks I. Accelerated atherosclerosis: an extraarticular feature of rheumatoid arthritis? Arthritis Rheum. 2002;46:862–873. doi: 10.1002/art.10089. [DOI] [PubMed] [Google Scholar]
- 3.Kaplan M, McCune W. New evidence for vascular disease in patients with early rheumatoid arthritis. Lancet. 2003;361:1068–1069. doi: 10.1016/S0140-6736(03)12901-7. [DOI] [PubMed] [Google Scholar]
- 4.Wolfe F, Freundlich B, Straus W. Increase in cardiovascular and cerebrovascular disease prevalence in rheumatoid arthritis. J Rheumatol. 2003;30:36–40. [PubMed] [Google Scholar]
- 5.Wallberg-Jonsson S, Ohman M, Dahlqvist S. Cardiovascular morbidity and mortality in patients with seropositive rheumatoid arthritis in Northern Sweden. J Rheumatol. 1997;24:445–451. [PubMed] [Google Scholar]
- 6.Wallberg-Jonsson S, Johansson H, Ohman M, Rantapaa-Dahlqvist S. Extent of inflammation predicts cardiovascular disease and overall mortality in seropositive rheumatoid arthritis: a retrospective cohort study from disease onset. J Rheumatol. 1999;26:2562–2571. [PubMed] [Google Scholar]
- 7.Solomon D, Karlson E, Rimm E, Cannuscio C, Mandl L, Manson J, et al. Cardiovascular morbidity and mortality in women diagnosed with rheumatoid arthritis. Circulation. 2003;107(9):1303–1307. doi: 10.1161/01.cir.0000054612.26458.b2. [DOI] [PubMed] [Google Scholar]
- 8.Libby P, Ridker P, Maseri A. Inflammation and atherosclerosis. Circulation. 2002;105:1135–1143. doi: 10.1161/hc0902.104353. [DOI] [PubMed] [Google Scholar]
- 9.Del Rincon I, Williams K, Stern M, Freeman G, Escalante A. High incidence of cardiovascular events in a rheumatoid arthritis cohort not explained by traditional cardiac risk factors. Arthritis Rheum. 2001;44:2737–2745. doi: 10.1002/1529-0131(200112)44:12<2737::AID-ART460>3.0.CO;2-%23. [DOI] [PubMed] [Google Scholar]
- 10.Gonzalez-Gay M, Gonzalez-Juanatey C, Pineiro A, Garcia-Porrua C, Testa A, Llorca J. High-grade C-reactive protein elevation correlates with accelerated atherogenesis in patients with rheumatoid arthritis. J Rheumatol. 2005;32:1219–1223. [PubMed] [Google Scholar]
- 11.Sattar N, McCarey D, Capell H, McInnes I. Explaining how "high-grade" systemic inflammation accelerates vascular risk in rheumatoid arthritis. Circulation. 2003;108:2957–2963. doi: 10.1161/01.CIR.0000099844.31524.05. [DOI] [PubMed] [Google Scholar]
- 12.Van Doornum S, Brand C, King B, Sundararajan V. Increased case fatality rates following a first acute cardiovascular event in patients with rheumatoid arthritis. Arthritis Rheum. 2006;54(7):2061–2068. doi: 10.1002/art.21932. [DOI] [PubMed] [Google Scholar]
- 13.Van Doornum S, Brand C, Sundararajan V, Ajani AE, Wicks IP. Rheumatoid arthritis patients receive less frequent acute reperfusion and secondary prevention therapy after myocardial infarction compared with the general population. Arthritis Res Ther. 2010;12(5):R183. doi: 10.1186/ar3151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sodergren A, Stegmayr B, Lundberg V, Ohman M, Wallberg-Jonsson S. Increased incidence of and impaired prognosis after acute myocardial infarction among patients with seropositive rheumatoid arthritis. Ann Rheum Dis. 2007;66(2):263–266. doi: 10.1136/ard.2006.052456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Douglas K, Pace A, Treharne G, Saratzis A, Nightingale P, Erb N, et al. Excess recurrent cardiac events in rheumatoid arthritis patients with acute coronary syndrome. Ann Rheum Dis. 2006;65:348–353. doi: 10.1136/ard.2005.037978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Melton L. History of the Rochester epidemiology project. Mayo Clin Proc. 1996;71:266–274. doi: 10.4065/71.3.266. [DOI] [PubMed] [Google Scholar]
- 17.Gillum R, Fortmann S, Prineas R, Kottke T. International diagnostic criteria for acute myocardial infarction and acute stroke. Am Heart J. 1984;108:150–158. doi: 10.1016/0002-8703(84)90558-1. [DOI] [PubMed] [Google Scholar]
- 18.Jabre P, Jouven X, Adnet F, Thabut G, Bielinski S, Weston S, et al. Atrial fibrillation and death after myocardial infarction: a community study. Circulation. 2011;123(19):2094–2100. doi: 10.1161/CIRCULATIONAHA.110.990192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Prineas R, Crow R, Blackburn H. Minnesota code manual of electrocardiographic findings: Standards and procedures for measurement and classification. Littleton, MA: Wright-PSG; 1982. [Google Scholar]
- 20.Arnett F, Edworthy S, Block D, McShane D, Fries J, Cooper N, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 21.Ho K, Pinsky J, Kannel W, Levy D. The epidemiology of heart failure: the Framingham Study. J Am Coll Cardiol. 1993;22:6A–13A. doi: 10.1016/0735-1097(93)90455-a. [DOI] [PubMed] [Google Scholar]
- 22.Thom T, Haase N, Rosamond W, Howard VJ, Rumsfeld J, Manolio T, et al. Heart Disease and Stroke Statistics—2006 Update: a report from the American heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2006 Feb 14;113(6):e85–e151. doi: 10.1161/CIRCULATIONAHA.105.171600. 2006. [DOI] [PubMed] [Google Scholar]
- 23.Turesson C, O'Fallon W, Crowson C, Gabriel S, Matteson E. Extra-articular disease manifestations in rheumatoid arthritis: incidence trends and risk factors over 46 years. Ann Rheum Dis. 2003;62:236–241. doi: 10.1136/ard.62.8.722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grambsch P, Therneau T. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika. 1994;81:515–526. [Google Scholar]
- 25.Francis ML, Varghese JJ, Mathew JM, Koneru S, Scaife SL, Zahnd WE. Outcomes in patients with Rheumatoid Arthritis and Myocardial Infarction. Am J Med. 2010;123(10):922–928. doi: 10.1016/j.amjmed.2010.05.017. [DOI] [PubMed] [Google Scholar]
- 26.Beller GA. President’s page: geographic variations in delivery of cardiovascular care: an issue of great importance to cardiovascular specialists. J Am Coll Cardiol. 2000;36(2):652–655. doi: 10.1016/s0735-1097(00)00832-9. [DOI] [PubMed] [Google Scholar]
- 27.Anderson HV, Shaw RE, Brindis RG, Hewitt K, Krone RJ, Block PC, et al. A contemporary overview of percutaneous coronary interventions: The American College of Cardiology–National Cardiovascular Data Registry (ACC–NCDR) J Am Coll Cardiol. 2002;39(7):1096–1103. doi: 10.1016/s0735-1097(02)01733-3. [DOI] [PubMed] [Google Scholar]
- 28.The Center for the Evaluative Clinical Sciences, Dartmouth Medical School. The Dartmouth Atlas of Cardiovascular Health Care. Chicago: AHA Press; 1999. The Center for Outcomes Research and Evaluation, Maine Medical Center. [Google Scholar]
- 29.Krishnan E, Lingala VB, Sing G. Declines in Mortality From Acute Myocardial Infarction in Successive Incidence and Birth Cohorts of Patients with Rheumatoid Arthritis. Circulation. 2004;110:1774–1779. doi: 10.1161/01.CIR.0000142864.83780.81. [DOI] [PubMed] [Google Scholar]
- 30.Varghese JJ, Sushma K, Scaife SL, Zahnd WE, Francis ML. Mortality after coronary artery revascularization of patients with rheumatoid arthritis. J Thoracic Cardiovas Surg. 2010;140(1):91–96. doi: 10.1016/j.jtcvs.2009.09.036. [DOI] [PubMed] [Google Scholar]
- 31.Gabriel S. Cardiovascular morbidity and mortality in rheumatoid arthritis. Am J Med. 2008;121(10 Suppl 1):S9–S14. doi: 10.1016/j.amjmed.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maradit-Kremers H, Nicola P, Crowson C, Ballman K, Gabriel S. Cardiovascular death in rheumatoid arthritis: A population-based study. Arthritis Rheum. 2005;52(3):722–732. doi: 10.1002/art.20878. [DOI] [PubMed] [Google Scholar]
- 33.Goodson N, Symmons D, Scott D, Bunn D, Lunt M, Silman A. Baseline levels of C-reactive protein and prediction of death from cardiovascular disease in patients with inflammatory polyarthritis: a ten-year followup study of a primary care-based inception cohort. Arthritis Rheum. 2005;52(8):2293–2299. doi: 10.1002/art.21204. [DOI] [PubMed] [Google Scholar]
- 34.St Sauver J, Grossardt B, Leibson C, Yawn B, Melton Lr, Rocca W. Generalizability of epidemiological findings and public health decisions: an illustration from the Rochester Epidemiology Project. Mayo Clin Proc. 2012;87(2):151–160. doi: 10.1016/j.mayocp.2011.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]