Abstract
Gout is a common disease which results from hyperuricemia. We have reported that the dysfunction of urate exporter ABCG2 is the major cause of renal overload (ROL) hyperuricemia, but its involvement in renal underexcretion (RUE) hyperuricemia, the most prevalent subtype, is not clearly explained so far. In this study, the association analysis with 644 hyperuricemia patients and 1,623 controls in male Japanese revealed that ABCG2 dysfunction significantly increased the risk of RUE hyperuricemia as well as overall and ROL hyperuricemia, according to the severity of impairment. ABCG2 dysfunction caused renal urate underexcretion and induced hyperuricemia even if the renal urate overload was not remarkable. These results show that ABCG2 plays physiologically important roles in both renal and extra-renal urate excretion mechanisms. Our findings indicate the importance of ABCG2 as a promising therapeutic and screening target of hyperuricemia and gout.
Gout is a common disease which causes severe acute arthritis, and results from persistent hyperuricemia. Hyperuricemia shows elevated serum uric acid (SUA) levels and most of them are asymptomatic. So far, three urate transporters, URAT1/SLC22A121, GLUT9/SLC2A92,3, and ABCG2/BCRP4,5,6, have been reported to play important roles in the regulation of SUA, and their dysfunctions cause urate transport disorders. Among them, common dysfunction of ABCG2 exporter has proved to be a major cause of hyperuricemia and gout4,5. Recently, we have provided a new mechanism for hyperuricemia that the decrease in extra-renal (intestinal) urate excretion by ABCG2 dysfunction induces renal urate overload, thereby causing hyperuricemia7. This mechanism, however, does not give a sufficient explanation for all ABCG2 dysfunction cases as a major cause of hyperuricemia and gout because the most prevalent type of hyperuricemia is not renal urate overload but renal urate underexcretion (Supplementary Fig. S1). In this study, we first focused on the involvement of ABCG2 dysfunction in renal underexcretion (RUE) hyperuricemia.
Results
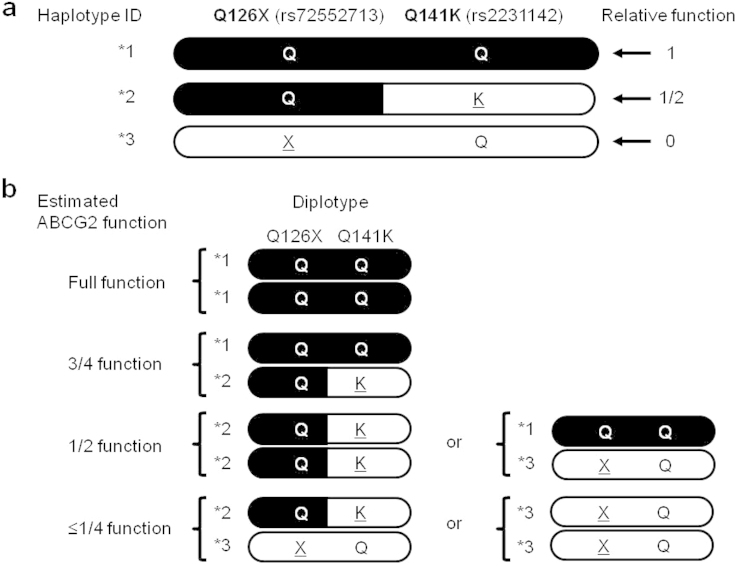
Genotyping was performed for 2,267 Japanese male participants, who consisted of 644 hyperuricemia cases (SUA>7.0 mg/dl) and 1,623 controls. Their functional ABCG2 activities were estimated from their genotype combinations of its two dysfunctional missense variants, Q126X (rs72552713) and Q141K (rs2231142). Because there is no simultaneous presence of the minor alleles of non-functional variant Q126X and half-functional variant Q141K in one haplotype5,7, we defined three haplotype IDs as *1, *2, and *3, as shown in Figure 1a. Thus, all participants were divided into four functional groups; i.e. full function (*1/*1), 3/4 function (*1/*2), 1/2 function (*2/*2 or *1/*3), and ≤1/4 function (*2/*3 or *3/*3) (Fig. 1b, Table 1)5,6,7. From the patients' fractional excretion of urate (FEUA) and urinary urate excretion (UUE), all cases were then classified into two groups, RUE hyperuricemia and renal overload (ROL) hyperuricemia (Supplementary Fig. S1).
Figure 1. Estimation of ABCG2 function from diplotype of Q126X and Q141K alleles.
(a) ABCG2*2 or *3 represents a haplotype with Q141K or Q126X variant, respectively. ABCG2*1 indicates a haplotype with neither Q141K nor Q126X variant. Since Q141K is a half-functional variant and Q126X is a nonfunctional variant, relative function of ABCG2*1, *2, and *3 is 1, 1/2, and 0, respectively, which is visualized by black-indicated areas. Substituted residues are underlined. (b) Each participant's function of urate exporter ABCG2 can be estimated from the diplotype, and can be also divided into four functional groups; i.e., ≤1/4 function, 1/2 function, 3/4 function, and full function.
Table 1. ABCG2 functions of participants.
| Case† | Control | ||||
|---|---|---|---|---|---|
| Estimated transport activity | Diplotype of Q126X (rs72552713) and Q141K (rs2231142) alleles** | N | % | N | % |
| ≤1/4 function | *3/*3 or *2/*3 | 29 (26) | 4.5 (4.7) | 22 | 1.3 |
| 1/2 function | *1/*3 or *2/*2 | 151 (135) | 23.4 (23.5) | 190 | 11.7 |
| 3/4 function | *1/*2 | 307 (277) | 47.7 (48.2) | 600 | 37.0 |
| Full function | *1/*1 | 157 (136) | 24.4 (23.7) | 811 | 50.0 |
| Total | 644 (575) | 100.0 (100.0) | 1,887 | 100.0 | |
**Haplotypes “Q-Q”, “Q-K”, and “X-Q” of two SNPs (Q126X and Q141K) are referred to as *1, *2, and *3, respectively. Risk alleles are X for Q126X, and K for Q141K. The relative functional activities of these haplotypes are 1, 1/2, and 0, respectively, and visualized as Figure 1.
†The numbers in parentheses show the numbers and percentages of gout cases only (cases without asymptomatic hyperuricemia).
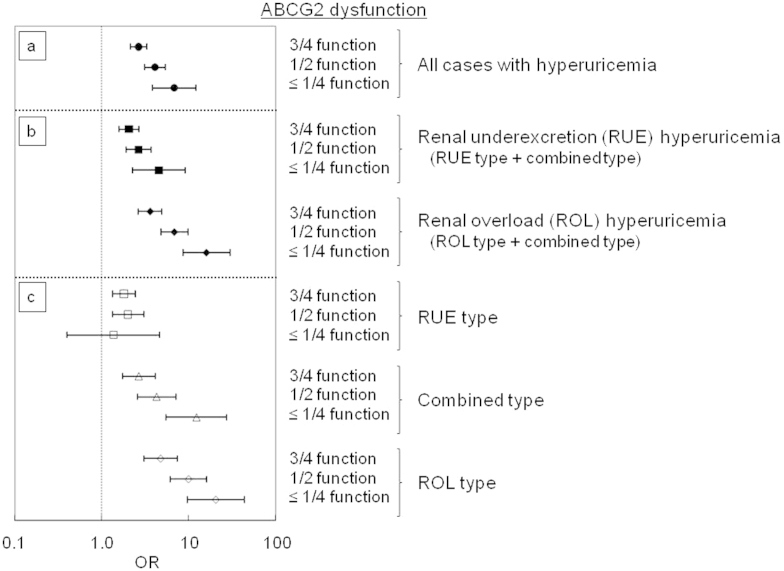
The association analysis revealed that ABCG2 dysfunction increased the risk of overall hyperuricemia according to the severity of its impairment (Fig. 2a, Supplementary Table S1); the odds ratios (ORs) in 3/4, 1/2 and ≤1/4 function were 2.64, 4.11 and 6.81, respectively. In RUE hyperuricemia that represents the dysfunction of renal urate excretion, the ORs also increased as the ABCG2 dysfunction became more severe; the ORs in 3/4, 1/2 and ≤1/4 function were 2.05, 2.66 and 4.53, respectively (Fig. 2b, Supplementary Table S1). In ROL hyperuricemia in which extra-renal (mainly intestinal) urate excretion plays an important role, contributions of ABCG2 dysfunction to the increase of ORs were more obvious; the ORs in 3/4, 1/2 and ≤1/4 function were 3.60, 6.83 and 16.0, respectively (Fig. 2b, Supplementary Table S1). Furthermore, Q126X homozygote signifying complete deficiency of ABCG2 was identified in one case with gout in the ROL hyperuricemia group. This fact is consistent with our previous report on the homozygous Abcg2 knockout mice having characteristics of ROL hyperuricemia7.
Figure 2. Risk of hyperuricemia by ABCG2 dysfunction.
The risk of hyperuricemia is calculated based on the estimated ABCG2 dysfunction, i.e., 3/4 function (mild dysfunction), 1/2 function (moderate dysfunction), and ≤1/4 function (severe dysfunction). All bars show odds ratio (OR) ± 95% confidence interval (CI).
When hyperuricemia was divided into three distinct types (i.e., RUE type, combined type, and ROL type as shown in Supplementary Fig. S1), severe ABCG2 dysfunction (≤1/4 function) significantly raised the risk of combined and ROL types but not that of RUE type (P = 0.62) (Fig. 2c, Supplementary Table S1). Nevertheless, moderate and mild dysfunction (3/4 and 1/2 functions) still contributed to increase the risk of RUE type hyperuricemia, conferring ORs of 1.80 and 2.00, respectively. These data imply that ABCG2 dysfunction under certain conditions causes renal urate underexcretion and leads to hyperuricemia even without renal urate overload.
Discussion
We previously reported a new mechanism by which ABCG2 dysfunction leads to the blockade of intestinal urate excretion (extra-renal underexcretion, Supplementary Fig. S1), thereby inducing hyperuricemia with renal urate overload (i.e., ROL hyperuricemia) and its overflow into the kidney7. ROL hyperuricemia consists of urate overproduction and extra-renal underexcretion, while most ROL hyperuricemia is supposed to be induced by extra-renal underexcretion due to ABCG2 dysfunction7 (Supplementary Fig. S1). However, about two-thirds of uric acid is known to be excreted from kidney in humans8,9,10, and RUE hyperuricemia consists of approximately 70–90% of all hyperuricemia cases10,11,12. Therefore, the elucidation of ABCG2 involvement in the pathogenesis of RUE hyperuricemia is of great importance.
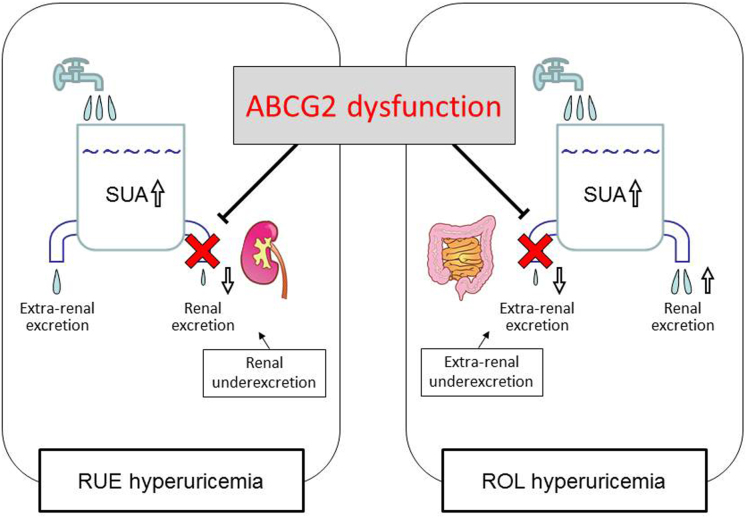
The present study showed that ABCG2 dysfunction also had a great influence on renal urate underexcretion, and thus strongly involved in the pathogenesis of two hyperuricemia groups, RUE and ROL hyperuricemia, through two different mechanisms; i.e., one is retention of urate in the blood stream because of the blockade of urate excretion from the kidney, and the other is renal urate overload because of the blockade of urate excretion from the intestine (Fig. 3). Our results are consistent with the fact that urate exporter ABCG2 expresses in both kidney and intestine in humans13,14. Severe ABCG2 dysfunction did not increase the risk of RUE type (Fig. 2c), and this type involved only a very small number of patients (n = 3) (Supplementary Table S1). This result indicates that severe ABCG2 dysfunction (≤1/4 function) causes either ROL type or combined type rather than RUE type because of renal urate overload. Furthermore, our data show that moderate and mild ABCG2 dysfunction (1/2 and 3/4 function) significantly increase the risk of RUE type (Fig. 2c). These findings support our idea that ABCG2 dysfunction caused renal urate underexcretion and induced hyperuricemia even without renal urate overload. Importantly, the present study is the first to show that mild to severe ABCG2 dysfunction also causes RUE hyperuricemia (Fig. 2b), suggesting its pathophysiological involvement in decreased renal urate excretion (Fig. 3).
Figure 3. Pathophysiology of hyperuricemia due to ABCG2 dysfunction.
The dysfunction of urate exporter ABCG2 is revealed to cause RUE hyperuricemia as well as ROL hyperuricemia due to blockade of urate excretion from the kidney and intestine, respectively. Abbreviation: SUA, serum uric acid. RUE, renal underexcretion. ROL, renal overload. (This figure, and the images contained therein, were produced by the authors).
We wish to emphasize here that the present study was performed as a subtype analysis based on participants' clinical information of SUA-related parameters. This approach could be applicable for other research on common diseases; i.e., the results of genetic analysis also indicate both the molecular function and localization of their gene products. For instance, we have reported that a common variant of transporter gene MCT9 (also known as SLC16A9) increases the risk of ROL gout15, which suggests the intestinal expression of MCT9 and its association with intestinal urate excretion. Likewise, common variants in URAT1/SLC22A12 and GLUT9/SLC2A9 are reported to have an association with SUA16,17. We previously showed that URAT1/SLC22A12 and GLUT9/SLC2A9 are causative genes of renal hyporucemia type 1 and type 2, respectively, and encode renal urate reabsorption transporters. Thus, it is probable that changes in the function of these two transporters associate with RUE hyperuricemia. Because our previous study showed that renal expression levels of Urat1 are markedly decreased in Abcg2 knockout mice which represent ROL hyperuricemia7, urate reabsorption transporter URAT1/SLC22A12 also should be involved in the pathogenesis of ROL hyperuricemia by ABCG2 dysfunction.
Taken together, we first indicated that ABCG2 physiologically mediates renal urate excretion as well as extra-renal (intestinal) urate excretion, and its dysfunctional mutations are involved in all types of hyperuricemia as their major genetic causes (Fig. 3). Besides our previous reports6,7, the present study showed that ABCG2 genotyping in combination with FEUA and UUE tests is sufficient for screening high-risk individuals with hyperuricemia and gout. Our findings will therefore serve to build up the health of people predisposed to hyperuricemia and gout.
Methods
All procedures involved in this study were performed in accordance with the Declaration of Helsinki and were approved by the institutional ethical committees (National Defense Medical College and Jikei University School of Medicine). Written informed consent was obtained from all subjects participating in this study. 644 male outpatients with hyperuricemia (SUA>7.0 mg/dl) including 575 gout patients were registered at the gout clinics of either Jikei University Hospital (Tokyo, Japan) or Midorigaoka Hospital (Osaka, Japan) as previously described7. As a control group, 1,623 male individuals with normal SUA (≤7.0 mg/dl) were collected from the Japan Multi-Institutional Collaborative Cohort Study (J-MICC Study)18. Genotyping of ABCG2 Q126X (rs72552713) and Q141K (rs2231142) was performed by high-resolution melting analysis with a LightCycler 480 (Roche Diagnostics)19. From the haplotype analyses reported in the previous studies5,7, there is no simultaneous presence of the minor alleles (risk alleles) of non-functional variant Q126X and half-functional variant Q141K in one haplotype. In this study, their haplotype IDs, *1, *2, and *3, were defined as Figure 1a; the combination of wild-type Q126X and Q141K alleles (“Q-Q”) was designated as ABCG2*1, which corresponds to the cDNA sequence of GenBank (accession number NM_004827). “Q-K” and “X-Q” were also named as ABCG2*2 and *3, respectively. Based on the diplotype of Q126X and Q141K alleles (Fig. 1b)5,7, ABCG2 function was estimated and divided into four groups5,6,7; i.e., full function, 3/4 function, 1/2 function, and ≤1/4 function (Table 1). As previously described7, FEUA and UUE were measured and used as markers for renal and extra-renal urate excretion function, respectively. Hyperuricemia patients were then classified into two groups, RUE hyperuricemia and ROL hyperuricemia; the former was characterized by low FEUA (< 5.5%) and the latter was defined by high UUE (> 25 mg/hr/1.73 m2) (Supplementary Fig. S1)7. RUE type, the combined type, and ROL type, were also defined as shown in Supplementary Fig. S1. Association analysis with χ2 test was performed by SPSS software (version 17.0J) to estimate the risk of each type of hyperuricemia.
Author Contributions
H.M., A.N. and N.S. designed the experiment. H.M., A.N., M.S., T.C., S.S., K.W., S.K., Y.G., H. Nakagawa, T.H., K.I. and T.S. collected samples and analyzed clinical data. H.M., A.N., M.S., T.C., S.S., Y.K., Y.T., Y.O., J.A., H.I., K.N., K.Y. and K.I. performed genetic analysis. H. Nakashima, T.N., H. Nakaoka and Y.S. performed statistical analysis. M.S., T.T., H. Nakaoka, T.I., K.Y., H.S., K.I., T.S. and N.S. provided intellectual input and assisted with the preparation of the manuscript. H.M., A.N. and N.S. wrote the paper. H.M. and A.N. contributed equally to this work.
Supplementary Material
SUPPLEMENTARY INFORMATION
Acknowledgments
We would like to thank all the participants involved in this study. We are also indebted to K. Gotanda, Y. Utsumi, S. Terashige, and H. Ogata for genetic analysis, R. Funatsu and H. Kasuga for composing figures, and I. Inoue for helpful discussion. This study was supported by grants from the Ministry of Education, Culture, Sports, Science and Technology (MEXT) of Japan including the MEXT KAKENHI (grant number 221S0002, 25293145, 22689021, 25670307), the Ministry of Health, Labor and Welfare of Japan, the Ministry of Defense of Japan, the Japan Society for the Promotion of Science, the Takeda Science Foundation, the AstraZeneca VRI Research Grant, the Kawano Masanori Memorial Foundation for Promotion of Pediatrics, and the Gout Research Foundation of Japan.
References
- Enomoto A. et al. Molecular identification of a renal urate anion exchanger that regulates blood urate levels. Nature 417, 447–452 (2002). [DOI] [PubMed] [Google Scholar]
- Matsuo H. et al. Mutations in glucose transporter 9 gene SLC2A9 cause renal hypouricemia. Am. J. Hum. Genet. 83, 744–751 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinour D. et al. Homozygous SLC2A9 mutations cause severe renal hypouricemia. J. Am. Soc. Nephrol. 21, 64–72 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward O. M. et al. Identification of a urate transporter, ABCG2, with a common functional polymorphism causing gout. Proc. Natl. Acad. Sci. U. S. A. 106, 10338–10342 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuo H. et al. Common defects of ABCG2, a high-capacity urate exporter, cause gout: a function-based genetic analysis in a Japanese population. Sci. Transl. Med. 1, 5ra11 (2009). [DOI] [PubMed] [Google Scholar]
- Matsuo H. et al. Common dysfunctional variants in ABCG2 are a major cause of early-onset gout. Sci. Rep. 3, 2014 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichida K. et al. Decreased extra-renal urate excretion is a common cause of hyperuricemia. Nat. Commun. 3, 764 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorensen L. B. Role of the intestinal tract in the elimination of uric acid. Arthritis Rheum. 8, 694–706 (1965). [DOI] [PubMed] [Google Scholar]
- Sica D. A. & Schoolwerth A. Brenner and Rector's The Kidney. Brenner, B. M. (ed.), 645–649 (Saunders, Philadelphia, 2004). [Google Scholar]
- Wortmann R. L. Harrison's Principles of Internal Medicine. Fauci, A. S. et al. (eds.), 2444–2449 (McGraw-Hill, New York, 2008). [Google Scholar]
- Becker M. A. The Metabolic & Molecular Bases of Inherited Disease. Scriver, C. R., Childs, B., Kinzler, K. W. & Vogelstein, B. (eds.), 2513–2535 (McGraw-Hill, New York, 2001). [Google Scholar]
- The guideline revising committee of the Japanese Society of Gout and Nucleic Acid Metabolism. Guideline for the Management of Hyperuricemia and Gout. The guideline revising committee of the Japanese Society of Gout and Nucleic Acid Metabolism (ed.), (Medical Review, Osaka, 2010). [DOI] [PubMed] [Google Scholar]
- Maliepaard M. et al. Subcellular localization and distribution of the breast cancer resistance protein transporter in normal human tissues. Cancer Res. 61, 3458–3464 (2001). [PubMed] [Google Scholar]
- Huls M. et al. The breast cancer resistance protein transporter ABCG2 is expressed in the human kidney proximal tubule apical membrane. Kidney Int. 73, 220–225 (2008). [DOI] [PubMed] [Google Scholar]
- Nakayama A. et al. A common missense variant of monocarboxylate transporter 9 (MCT9/SLC16A9) gene is associated with renal overload gout, but not with all gout susceptibility. Hum. Cell 26, 133–136 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolz M. et al. Meta-analysis of 28,141 individuals identifies common variants within five new loci that influence uric acid concentrations. PLoS Genet. 5, e1000504 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamatani Y. et al. Genome-wide association study of hematological and biochemical traits in a Japanese population. Nat. Genet. 42, 210–215 (2010). [DOI] [PubMed] [Google Scholar]
- Hamajima N. & J-MICC Study Group. The Japan Multi-Institutional Collaborative Cohort Study (J-MICC Study) to detect gene-environment interactions for cancer. Asian Pac. J. Cancer Prev. 8, 317–323 (2007). [PubMed] [Google Scholar]
- Margraf R. L., Mao R. & Wittwer C. T. Rapid diagnosis of MEN2B using unlabeled probe melting analysis and the LightCycler 480 instrument. J. Mol. Diagn. 10, 123–128 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
SUPPLEMENTARY INFORMATION