Abstract
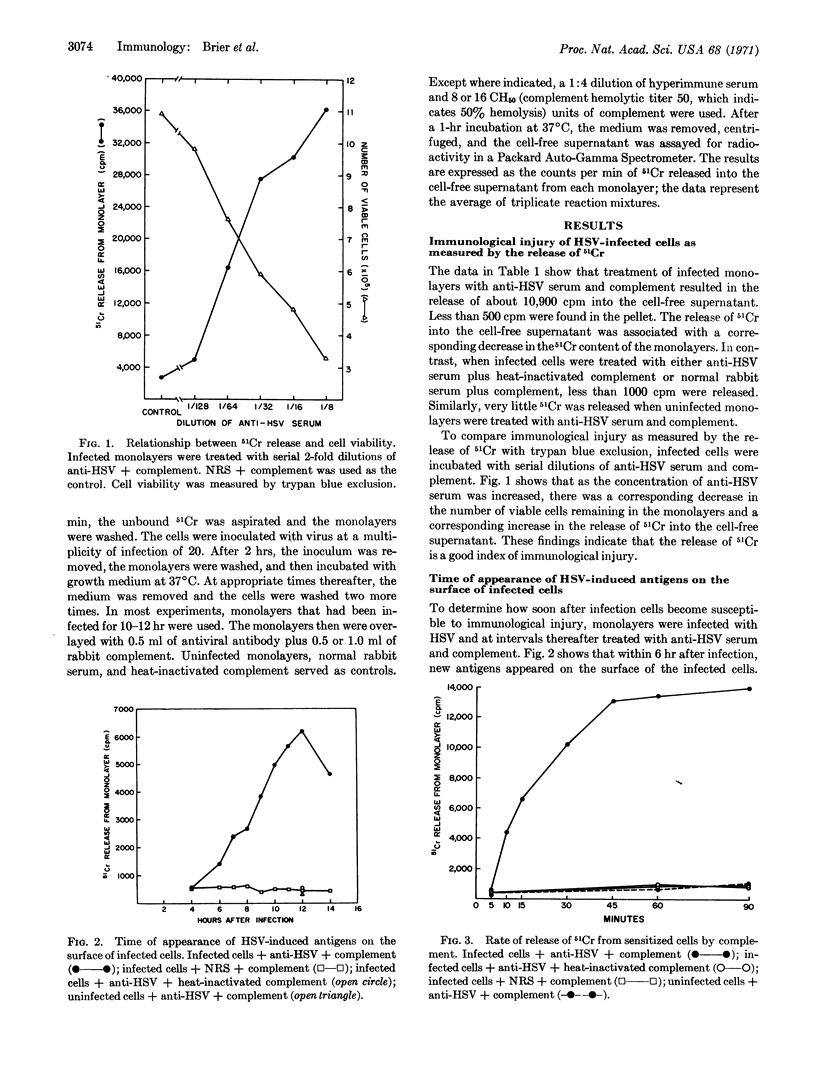
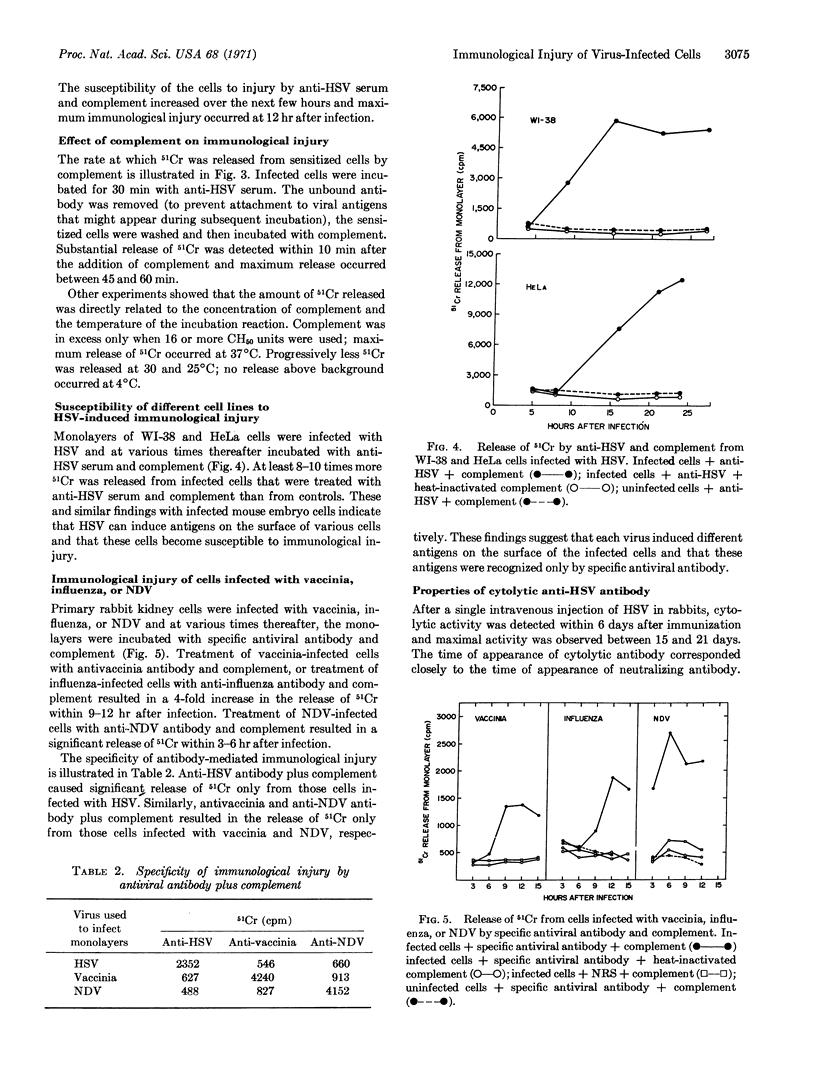
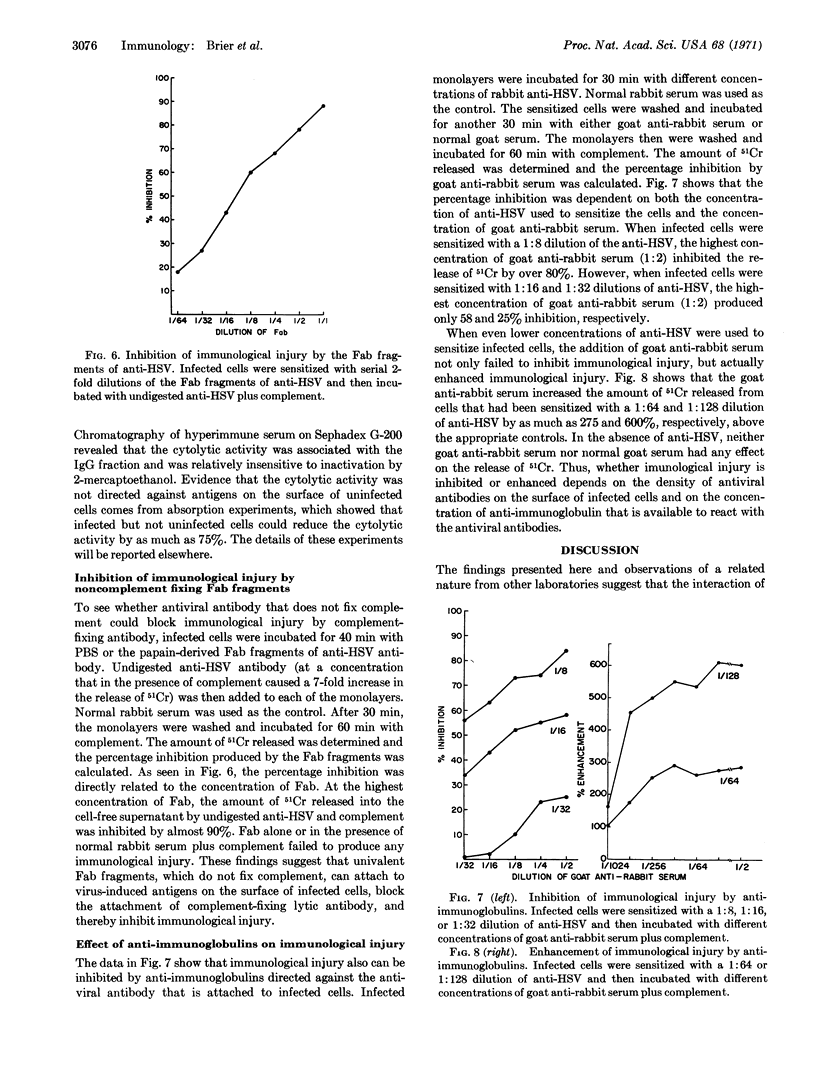
Within hours after infection of cells with herpes simplex, vaccinia, influenza, or Newcastle disease virus, new antigens appeared on the surface of infected cells. The interaction of specific antiviral antibody and complement with these antigens resulted in cell destruction, which was quantitated by the release of 51Cr. A number of factors can influence the degree of cell destruction, including the density of viral antigens on the surface of infected cells, the nature of the antiviral antibody, and the presence of anti-immunoglobulins. The immunological destruction of virus-infected cells may on the one hand serve as a defense mechanism against certain viral infections, while on the other hand it may contribute to the pathology of the host.
Keywords: herpes simplex, cell-surface antigens, cytolytic antibody, complement, immunopathology
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashe W. K., Mage M., Mage R., Notkins A. L. Neutralization and sensitization of herpes simplex virus with antibody fragments from rabbits of different allotypes. J Immunol. 1968 Sep;101(3):500–504. [PubMed] [Google Scholar]
- Borsos T., Rapp H. J. Complement fixation on cell surfaces by 19S and 7S antibodies. Science. 1965 Oct 22;150(3695):505–506. doi: 10.1126/science.150.3695.505. [DOI] [PubMed] [Google Scholar]
- Cikes M., Friberg S., Jr Expression of H-2 and Moloney leukemia virus-determined cell-surface antigens in synchronized cultures of a mouse cell line. Proc Natl Acad Sci U S A. 1971 Mar;68(3):566–569. doi: 10.1073/pnas.68.3.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton M. D., Scala A. R. Species source of complement in viral-immune and other cytolytic reactions. Proc Soc Exp Biol Med. 1970 Feb;133(2):615–619. doi: 10.3181/00379727-133-34529. [DOI] [PubMed] [Google Scholar]
- Glasgow L. A. Cellular immunity in host resistance to viral infections. Arch Intern Med. 1970 Jul;126(1):125–134. [PubMed] [Google Scholar]
- Hampar B., Notkins A. L., Mage M., Keehn M. A. Heterogeneity in the properties of 7 S and 19S rabbit-neutralizing antibodies to herpes simplex virus. J Immunol. 1968 Mar;100(3):586–593. [PubMed] [Google Scholar]
- Hellström K. E., Hellström I. Immunological enhancement as studied by cell culture techniques. Annu Rev Microbiol. 1970;24:373–398. doi: 10.1146/annurev.mi.24.100170.002105. [DOI] [PubMed] [Google Scholar]
- Holmes K. V., Klenk H. D., Choppin P. W. A comparison of immune cytolysis and virus-induced fusion of sensitive and resistant cell types. Proc Soc Exp Biol Med. 1969 Jun;131(2):651–657. doi: 10.3181/00379727-131-33945. [DOI] [PubMed] [Google Scholar]
- Linscott W. D. Effect of cell surface antigen density on immunological enhancement. Nature. 1970 Nov 28;228(5274):824–827. doi: 10.1038/228824a0. [DOI] [PubMed] [Google Scholar]
- Notkins A. L., Mergenhagen S. E., Howard R. J. Effect of virus infections on the function of the immune system. Annu Rev Microbiol. 1970;24:525–538. doi: 10.1146/annurev.mi.24.100170.002521. [DOI] [PubMed] [Google Scholar]
- Oldstone M. B., Dixon F. J. Immune complex disease in chronic viral infections. J Exp Med. 1971 Sep 1;134(3 Pt 2):32s–40s. [PubMed] [Google Scholar]
- Wiktor T. J., Kuwert E., Koprowski H. Immune lysis of rabies virus-infected cells. J Immunol. 1968 Dec;101(6):1271–1282. [PubMed] [Google Scholar]



