Abstract
Proliferative diabetic retinopathy is a consequence of retinal ischemia due to capillary occlusion resulting from damage to the retinal microvascular endothelium. Recent evidence suggests that high levels of bone-marrow derived circulating endothelial progenitor cells (EPCs) contribute to the pathological neovascularization of ischemic tissues and are a critical risk factor for the development of these complications. In the absence of a consensus definition of a circulating EPC and its surface markers in humans we evaluated the functional properties of CD34+ CD45− endothelial colony forming cells (ECFCs) in patients with proliferative diabetic retinpathy (PDR). Higher levels of circulating CD34+ CD45− cells were observed in patients with PDR compared to controls. However, ECFCs from patients with PDR were impaired in their ability to migrate toward SDF-1 and human serum, incorporate into and form vascular tubes with human retinal endothelial cells. The results from these pilot studies suggest that ECFCs from patients with PDR are mobilized into the circulation but may be unable to migrate and repair damaged capillary endothelium. This suggests that ECFCs may be a potential therapeutic target in the prevention and treatment of diabetic vascular complications.
Introduction
Proliferative diabetic retinopathy (PDR) is a complication of diabetes and results in severe vision loss. The exact patho-physiological mechanisms that are involved in this process remain to be elucidated. The role of high blood glucose has been suggested to be the primary catalyst for the bio-molecular and cellular changes seen in the retina(Group., 1993; Stratton et al., 2000). It is likely that the retinal neovascularization in proliferative diabetic retinopathy (PDR) is a consequence of retinal ischemia caused by damage to the retinal microvascular endothelium. The tissue hypoxia that ensues may induce the subsequent release of local hypoxic and pro-angiogenic factors such as VEGF(Funatsu et al., 2001), hypoxia-inducible factors(Arjamaa and Nikinmaa, 2006) and erythropoietin(Watanabe et al., 2005). These factors may stimulate abnormal vessel growth extending into the vitreous of the eye, leading to hemorrhage and visual loss.
Since the discovery of bone-marrow derived circulating endothelial progenitor cells (EPC)(Asahara et al., 1997) several studies have determined their potential to differentiate into endothelial cells at sites of post-natal retinal neovascularization(Grant et al., 2003; Grant et al., 2002; Lee et al., 2006; Otani et al., 2002; Takahashi et al., 1999). These EPCs have also been shown to have the capability of homing to sites of tissue ischemia and contribute to vascular repair(Rafii and Lyden, 2003). While there is increasing evidence that circulating endothelial progenitor cells (EPCs) contribute significantly to both normal and pathological angiogenesis there has been little consensus on the markers that should be used to identify these cells (Ingram et al., 2005a; Ingram et al., 2004; Yoder, 2009; Yoder and Ingram, 2009a; b). Since the initial discovery by Asahara and colleagues of a method to isolate bone marrow-derived endothelial progenitor cells from blood(Asahara et al., 1997), there have been studies indicating that there are various subpopulations of endothelial progenitor cells in the adult with the ability to form vessels when cultured ex vivo. These circulating EPC populations may be derived from bone marrow(Shi et al., 1998), peripheral blood(Ingram et al., 2004), and the vessel wall itself(Alessandri et al., 2001; Ingram et al., 2005b). Many studies have examined the implications of colony-forming unit endothelial cells (CFU-Hill) initially isolated by Asahara’s group, and demonstrate an inverse relationship between the number and function of circulating FU-ECs with the risk of cardiovascular disease(Hill et al., 2003) and peripheral diabetic vasculopathy(Fadini, 2008; Fadini et al., 2005; Fadini et al., 2006c; Loomans et al., 2004). However, Yoder and colleagues have recently determined that the “endothelial colony forming cell (ECFC)” and not the CFU-EC have a robust proliferative potential and exhibit the ability to form functional vessels in vivo(Yoder et al., 2007). ECFCs cultured from peripheral blood appear 14–21 days after initial plating and have been shown to be positive for CD34 and negative for CD45 (leukocyte common antigen)(Smadja et al., 2007; Yoder et al., 2007). While increased levels of circulating CD34+/c-Kit+, CD34+/KDR+ and CD34+/CD133+ CFU-ECs have been reported in patients with non-proliferative and proliferative diabetic retinopathy(Fadini et al., 2006b; Lee et al., 2006; Liu et al.), none of these studies examined the role of ECFCs. An increased expansion potential of ECFCs was recently reported in patients at risk or affected by neovascular AMD(Thill et al., 2008). In this study, we sought to determine the role of peripheral blood-derived ECFCs in the pathogenesis of PDR by evaluating the relative number of these cells in the circulation of patients with PDR and evaluating their angiogenic function.
Research Design and Methods
Patients and adult peripheral blood samples
The Institutional Review Board at the Cleveland Clinic approved all protocols, and informed consent was obtained from all patients who agreed to participate in the study in accordance with the Declaration of Helsinki. Patients who were being seen for regular visits in the ophthalmology clinics at the Cole Eye Institute and in the endocrinology clinics at the Cleveland Clinic were recruited and were designated as either healthy controls without any diabetic eye disease or diabetics with documented proliferative diabetic retinopathy (active or quiescent) by an ophthalmologist at the Cole Eye Institute. Patients with other co-morbid ocular diseases involving the retina including age-related macular degeneration and vein/artery occlusions were excluded. Approximately twenty-five milliliters of peripheral blood was obtained from the antecubital vein of 45 volunteer donors.
Preparation of plasma
Plasma isolated from whole blood samples collected in EDTA on ice was treated with 2,6-di-tert-butyl-4-methyl-phenol (BHT) (Sigma B1378, St. Louis, MO) in ethanol (22 mg/ml) to protect samples from in vitro oxidation. A protease inhibitor cocktail (Sigma P8340, St. Louis, MO) was added, and the sample was centrifuged using a Sorvall RTH-750 rotor at 2000 × g for 20 min at 4°C. Samples were flash frozen in liquid nitrogen and stored at −80°C until used for ELISA.
Measurement of vasculogenic and homing factors in plasma and vitreous
Human vitreous was obtained from whole eyes donated through a local eye bank. Levels of VEGF and stromal derived factor (SDF)-α in human plasma and vitreous were determined using a human VEGF and SDF-α ELISA kit (all from R&D Systems, Minneapolis, MN). All samples were assayed in duplicate and performed according to the manufacturer’s instructions.
Preparation of mononuclear cells
Plasma-free blood was diluted 1:1 with PBS supplemented with 2% fetal bovine serum (FBS) and overlaid onto Ficoll-Paque PLUS (GE Healthcare Corp, Piscataway, NJ). Cells were subjected to density gradient centrifugation at 740 × g for 30 min at 19 degrees C. Buffy-coat mononuclear cells (MNCs) were isolated and washed three times in PBS with 2% FBS at a lower speed (100 × g) to remove platelets and other contaminant cells.
Culture of Endothelial Colony-Forming Cells
MNCs from each patient were plated into one well of a 6-well tissue culture plate coated with type I rat tail collagen (BD Biosciences, San Jose, CA) and were cultured in endothelial growth medium (EGM-2) with bullet kit (Lonza, Basel, Switzerland) supplemented with 10% FBS, 2% penicillin-streptomycin (Invitrogen, Carlsbad, CA) and 0.25 µg/mL amphoterocin B (Invitrogen, Carlsbad, CA). Cells were maintained in a 37°C, 5% CO2 humidified incubator, and medium was changed every other day for three weeks or until cobblestone-appearing endothelial colonies appeared, with careful attention to minimize dislodging of adherent cells. If colonies had not appeared within 35 days, the initial culture was discarded. After initial appearance of ECFC colonies, cells were transferred to a new well of a 6-well plate and further passaged in 25-cm2 flasks. All studies were done using patient ECFCs at passages 3–5 at 85–95% confluence.
Culture of Human Retinal Endothelial Cells
Primary human retinal microvascular endothelial cells (ACBRI-181) were obtained from Cell Systems (Kirkland, WA) and seeded on to a 25-cm2 flask coated with Attachment Factor™ and further passaged in 75-cm2 flasks with Passage Reagent Group™, according to the manufacturer’s instructions. Cells at passages 5–8 at approximately 90% confluence were used in all studies.
Immunophenotyping of MNCs
MNCs isolated from density gradient centrifugation were washed in PBS supplemented with 1%BSA and 0.02% sodium azide, and stained (1 ×106 cells/tube) for 30 min at 4°C with antibodies to human CD34 conjugated to fluoroscein isothiocyanate (FITC) (BD Biosciences, San Jose, CA), and with antibodies to CD45 conjugated to biotin (eBioscience, San Diego, CA). Phycoerythrin (PE) conjugated to streptavidin (eBioscience, San Diego, CA) was used as a second step for CD45. Following each incubation, cells were washed with PBS supplemented with 1% BSA and 0.02% sodium azide. Isotype-matched (IgG) non-specific antibodies were used as negative controls. Samples were analyzed on a FACScan flow cytometer (BD Biosciences) and stored as hardware compensated list-mode files. Data were analyzed using Cell-Quest 3.3 software. Lymphoblastoid cells were gated on FCS/SSC scatter plots and percentage CD34+CD45− cells were defined on CD34/CD45 dot plots. Samples that demonstrated a high non- specific binding (>5%background) on the CD34+ subset were not used for subsequent analysis.
Microarray analysis
RNA from ECFCs growing in 25-cm2 flasks (at passage 2) was extracted from two PDR patients (one type 1 diabetic patient and one type 2 diabetic patient) and two healthy patients using a Qiagen RNeasy kit according to the manufacturer’s instructions (Qiagen, Valencia, CA). RNA concentration and purity was measured using absorbance values at 260 and 280 nm on a spectrophotometer. A stable aliquot of RNA from each patient sample (> 5 µg) was sent to the Gene Expression and Genotyping Facility Core at Case Western Reserve University for microarray analysis using a U133 A 2.0 human genome Affymetrix chip containing over 55,000 transcripts. Four pair-wise binary comparisons between the four patient samples (healthy ECFCs vs PDR ECFCs) were made and analyzed for statistical significance using GC robust multiarray (RMA) normalization of CEL signals on GeneSpring GX 9.0.5 (Agilent, Santa Clara, CA). A criterion of ≥ 2 fold change in at least 3 out of 4 binary comparisons was deemed to be a “credible change” in transcript expression.
Western blot analyses
Cell lysates from 80% confluent ECFCs at passages 2–4 were prepared using a cold 1× radio immuno-precipitation assay (RIPA) buffer that was prepared from a 10× stock solution (Millipore, Billerica, MA). Protein concentration was measured using a standard bicinchoninic acid (BCA) kit (Thermoscientific, Rockford, IL), and equal amounts of protein were loaded onto a 10% bis/tris polyacrylamide gel and subjected to SDS-PAGE. Semi-dry transfer of separated proteins on to nitrocellulose membrane was performed at constant 100 V for 1 h at 4°C. After blocking with 5% milk, membranes were probed for thrombospondin-1 levels using a mouse anti-human TSP-1 Ab-4 monoclonal antibody (Lab Vision, Fremont, CA). To detect TIMP-3 levels, membranes were probed using a mouse anti-human TIMP-3 clone 136-13H4 monoclonal antibody (Millipore, Billerica, MA) followed by anti-mouse IgG conjugated to horseradish peroxidase and ECL+ chemiluminescence detection (GE Healthcare, Piscataway, NJ).
Cell Migration Assays
ECFCs from PDR patients were expanded from one type 1 diabetic patient and one type 2 diabetic patient and tested against ECFCs from healthy controls in separate experiments. An 8.0-µm pore polycarbonate membrane (Neuroprobe, Gaithersburg, MD) was coated with rat tail type I collagen overnight. ECFCs at 85% confluence were harvested and resuspended in serum-free endothelial basal medium (EBM-2) at a concentration of 3 × 105 cells/ml. Human VEGF (20 ng/ml), recombinant SDF-1 (R&D Systems, Minneapolis, MN) (20 ng/ml), 0.1% patient serum, or serum-free EBM-2 medium was placed into each well of a 48-well Boyden chamber transwell apparatus. 1.5 × 104 cells in suspension was applied on top of the membrane in each well. Cells were allowed to migrate over 5 h in a 37°C, 5% CO2 humidified incubator. After migration, cells that had not migrated were scraped and migrated cells on the lower side were fixed in formalin and stained with hematoxylin. The membrane was mounted on a slide and examined using the Olympus BX40 microscope under 10× magnification. All migrated cells were counted.
Tube Formation Assay
Patient ECFCs in culture were harvested at 90% confluence and incubated with Qdot®-655 nm cell tracking-nanocystals (Invitrogen, Carlsbad, CA) and allowed to equilibrate for 1 h at 37°C. Cultured primary human retinal endothelial cells (HRECs) were harvested at 90% confluence and incubated with Qdot®-525 nm cell tracking-nanocrystals for 1 h at 37°C. To assess incorporation of ECFCs and HRECs into vascular tubes, ECFCs were mixed with HRECs at a 1:1 ratio and a total of 5.0 × 104 cells were plated onto ECMatrix supplied by the In Vitro Angiogenesis Assay kit (Millipore, Billerica, MA) within an 8-well culture slide (BD Biosciences, San Jose, CA). Cells were incubated at 37°C for 18–20 h overnight in complete EGM-2 medium (supplemented with 10% FBS), and vascular tube structures were visualized using a Leica Confocal Microscope at 10× magnification using Ar/Kr laser excitation and emission detection at 633 nm. Gain settings were fixed at 543 V, 600 V, and 590 V for PMTs 1, 2, and 3, respectively, and remained unchanged while taking all images.
Quantitation of ECFC fluorescence/tube length
Network analysis of ECFCs associated with tube forming endothelial cells was performed in an automated fashion using customized visual basic macros developed within Image-Pro Plus (v6.2, Media Cybernetics, Silver Spring, MD). Briefly, phase-contrast and corresponding fluorescence images (ECFCs labeled with Qdot®-655 nm cell tracking-nanocystals) were imported into Image-Pro in batch mode. Each phase-contrast image was then “flattened” to normalize uneven background illumination and then morphologically “closed” to equalize intensity of the tube network. These steps enabled application of a fixed threshold to segment the cell network (will be referred to as the “tube mask”) that was subsequently “skeletonized’ to create continuous single pixel-width medial lines along the entire tube network. Unfortunately, during the skeletonization process, multiple branches entering a single node were often incorrectly terminated, erroneously producing multiple nodes. Therefore, an additional algorithm was applied to cluster nodes that were within a given distance (pre-determined value confirmed visually). These nodes were then classified as 3-, 4- or 5+ branch nodes and summed for each category for output to Excel. In addition to incorrect node classification, the skeletonization process can also produce spurious branches. To eliminate these, a “pruning” filter was applied to remove branches of a predefined length connected to a single node. The total number of branches was then summed and exported to Excel. To determine mean node and branch thickness, a Euclidean distance map was generated from the tube mask and “multiplied” by the node and skeletal branch masks respectively. Thickness values were calculated by summing the resulting pixels values in each of these images, multiplying these values by a factor of 2 and then by the pixel resolution, and lastly dividing by the total number of node pixels or skeletal branch pixels. For a visual representation of thickness values across the tube network, a pseudo-colored “bluered” look-up table was applied to EDM image of the tube mask multiplied by the skeleton of the entire network. The resulting image was then superimposed onto the original tube image where “red” represents the maximum thickness value (branch or node) present in the network. For quantitation of fluorescence overlap, a fixed threshold was applied to the fluorescence channel to segment positive pixels. This fluorescence mask was then “multiplied” by either the tube mask or the tube mask skeleton to determine overlap by tube area or tube length, respectively.
Statistical Methods
All experimental results are summarized as the mean ± SEM and are reported with a 95% confidence interval, unless otherwise specified. Continuous variables within the baseline patient demographics are summarized as mean or median with standard deviation. The Student’s t test was used to compare the means between two groups when a Gaussian distribution was considered appropriate. To compare serum creatinine values, the Mann-Whitney U nonparametric test was used because of the inherent non-Gaussian distribution of creatinine values in patients. For contingency analysis of the demographic comparisons between patient groups, the Fisher’s exact test was used. A p-value of < 0.05 was deemed to be statistically significant in all analyses.
Results
Increased circulating CD34+ CD45− ECFCs in patients with proliferative diabetic retinopathy
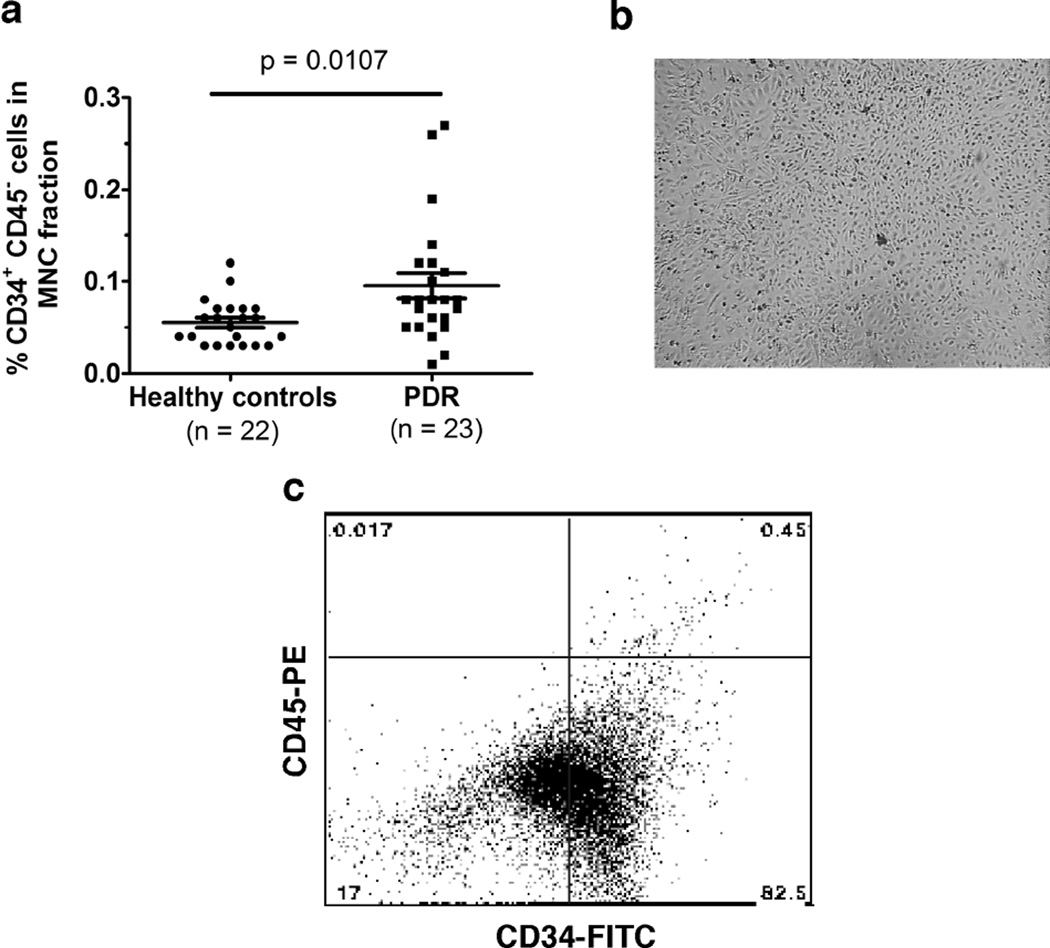
To determine whether there is a difference in the number of circulating ECFCs in healthy versus PDR patients, we analyzed the peripheral blood mononuclear cell (PBMC) layer isolated by density gradient centrifugation of whole blood. Using fluorescence-activated cell sorting (FACS) analysis, we found increased levels of CD34+ CD45− cells in patients with PDR (Figure 1a), which correspond to ECFCs as described previously(Ingram et al., 2004; Yoder et al., 2007) and confirmed here by flow cytometry (Figure 1c). ECFC colonies appeared around 14–21 days after plating (as previously described in the literature), and a colony was marked only if the cells exhibited cobblestone morphology typical of “late outgrowth EPCs” (Figure 1b). Cultured ECFCs were evaluated by FACS for CD34+ CD45− markers (Fig. 1c) following gating with control IgG markers (data not shown). Observation of the initial MNC culture to harvest ECFC colonies continued up until 35 days; if no colonies appeared at this time, the culture was discarded. Interestingly, we found that patients who had PDR were more likely to grow ECFC colonies compared with those derived from healthy patients when their mononuclear cells were plated at the same density (3.0×106 cells/cm2) (Data not shown). In our patient population studied, the mean age and age distribution for both groups of patients were comparable (Table 1). About 41% of the PDR patients recruited were type 1 diabetics (Table 1), and almost all patients (~95%) being seen in the eye clinics for PDR had been previously treated with panretinal photocoagulation (PRP) for retinal neovascularization (data not shown). Patients with PDR exhibited supra-normal mean levels of hemoglobin A1c (HbA1c) at the time blood was drawn (Table 1). Higher median serum creatinine levels were noted in PDR patients at the time of the blood collection (1.4 mg/dl vs 0.8 mg/dl), which would be expected of patients with multiple end-organ damage from diabetic sequelae. However, no difference in statin medication use was observed between patient groups. None of the patients recruited to the study were being treated with exogenous erythropoietin for end-stage renal disease, and no patients were in renal failure when enrolled in the study.
Figure 1. Circulating CD34+ CD45− ECFCs in healthy controls and patients with proliferative diabetic retinopathy.
(a) CD34+CD45− ECFCs in peripheral blood mononuclear cell fractions were analyzed by FACS. (b) Representative photomicrograph of expanded ECFC culture at passage 3 showing a cobblestone morphology of cells. Scale bar: 100 µm. (c) Representative CD45 PE versus CD34 FITC dot plot shows the phenotype of cells following expansion in vitro. Quadrants were defined based on isotype matched control staining.
Table 1.
Baseline Patient Characteristics
| Healthy controls | PDR | p-value | |
|---|---|---|---|
| Total number of patients | 22 | 23 | 1.0 |
| Average age in years (S.D.) | 54.0 (10.9) | 59.2 (13.8) | 0.17 |
| # male patients : # female patients | 4:18 | 11:12 | 0.0546 |
| Average HbA1c (S.D.) | n/a | 8.2 (2.0) | - |
| # Type 1 diabetic patients | 0 | 9 | - |
| # Type 2 diabetic patients | 0 | 13 | - |
| # Patients taking a lipid-lowering statin | 9 | 13 | 0.24 |
| Median serum creatinine in mg/dl (S.D.) | 0.8 (0.1) | 1.4 (2.6) | < 0.0001 |
| # Patients taking Erythropoietin (EPO) for end-stage renal disease | 0 | 0 | 1.0 |
Increased plasma levels of SDF-1 in patients with PDR
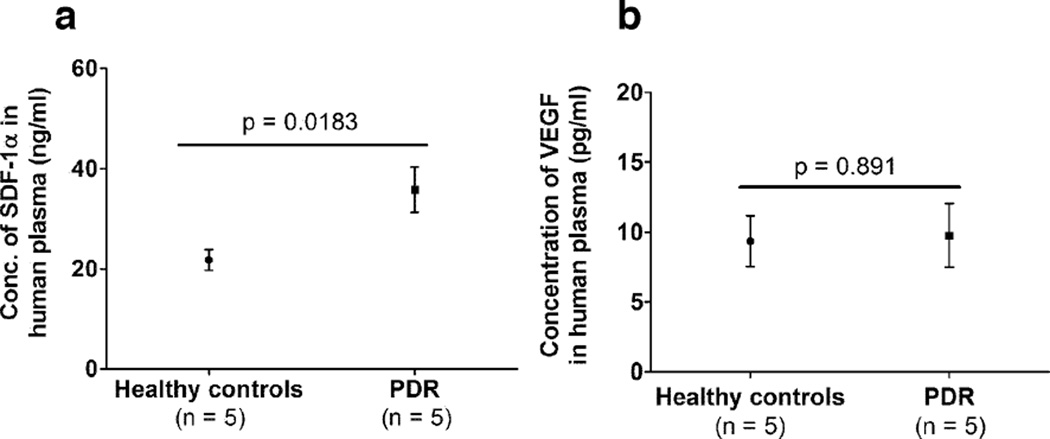
Stromal cell-derived factor (SDF) in the vitreous has been recently hypothesized to promote retinopathy in an oxygen-induced retinopathy model by recruiting CD34+ hematopoietic stem cell-derived endothelial progenitor cells (EPCs)(Butler et al., 2005). Since we detected higher levels of circulating CD34+ CD45− ECFCs in patients with PDR, we hypothesized that there might be an increase in ECFC recruitment factors. Plasma from healthy controls and PDR patients who grew ECFC colonies were analyzed for levels of VEGF and SDF-1. While SDF-1 levels in the plasma of PDR patients were higher compared to healthy controls (Figure 2a), VEGF levels were not significantly different (Figure 2b).
Figure 2. Serum cytokine levels in patients with proliferative diabetic retinopathy.
(a) SDF-1 and (b) VEGF levels were measured in the serum of controls and patients from whom the corresponding ECFCs were isolated.
ECFCs from patients with PDR show reduced migration
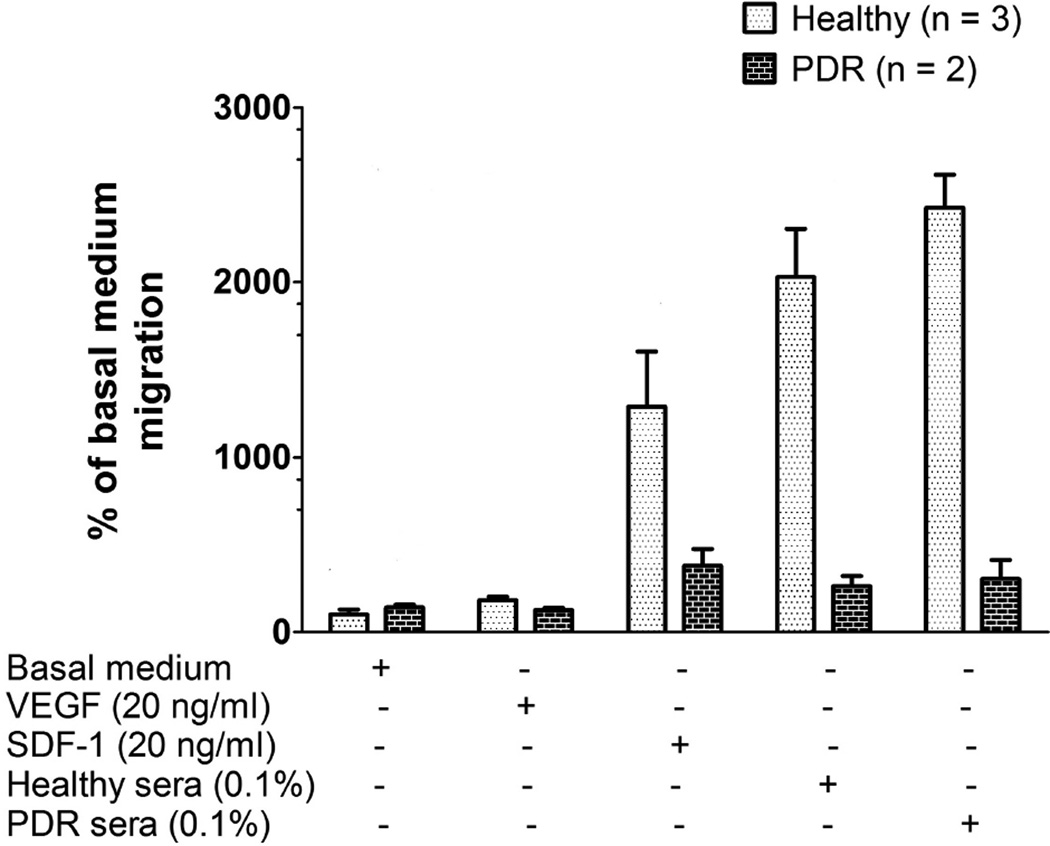
In an effort to determine if ECFCs from patients with PDR could respond to migratory factors, we employed a standard Boyden transwell migration assay and examined the ability of ECFCs to migrate towards SDF-1, VEGF or human sera. We found that circulating ECFCs from patients with PDR were impaired in their ability to migrate towards SDF-1 as well as human sera (Figure 3). We analyzed the chemotactic response of ECFCs to serum from both control and PDR patients. Using sera diluted to 0.1% in EBM-2 basal medium, from three controls and two PDR patients, we found that healthy ECFCs migrate effectively in response to SDF-1 and sera from healthy patients. However, ECFCs in patients with PDR are unable to migrate towards either healthy or PDR sera, suggesting an inherent cellular migratory dysfunction.
Figure 3. Decreased migration of PDR-ECFCs towards chemokines.
Comparison of the migration of ECFCs from healthy controls and patients with PDR towards VEGF, SDF-1, healthy sera and sera from PDR patients. Individual PDR patients’ ECFCs were used in each migration assay (i.e. ECFCs were unpooled) and analyzed as the percent migration of the average migratory response seen in the ECFCs expanded from control patients.
Inefficient incorporation of ECFCs from patients with PDR into vascular tubes in vitro
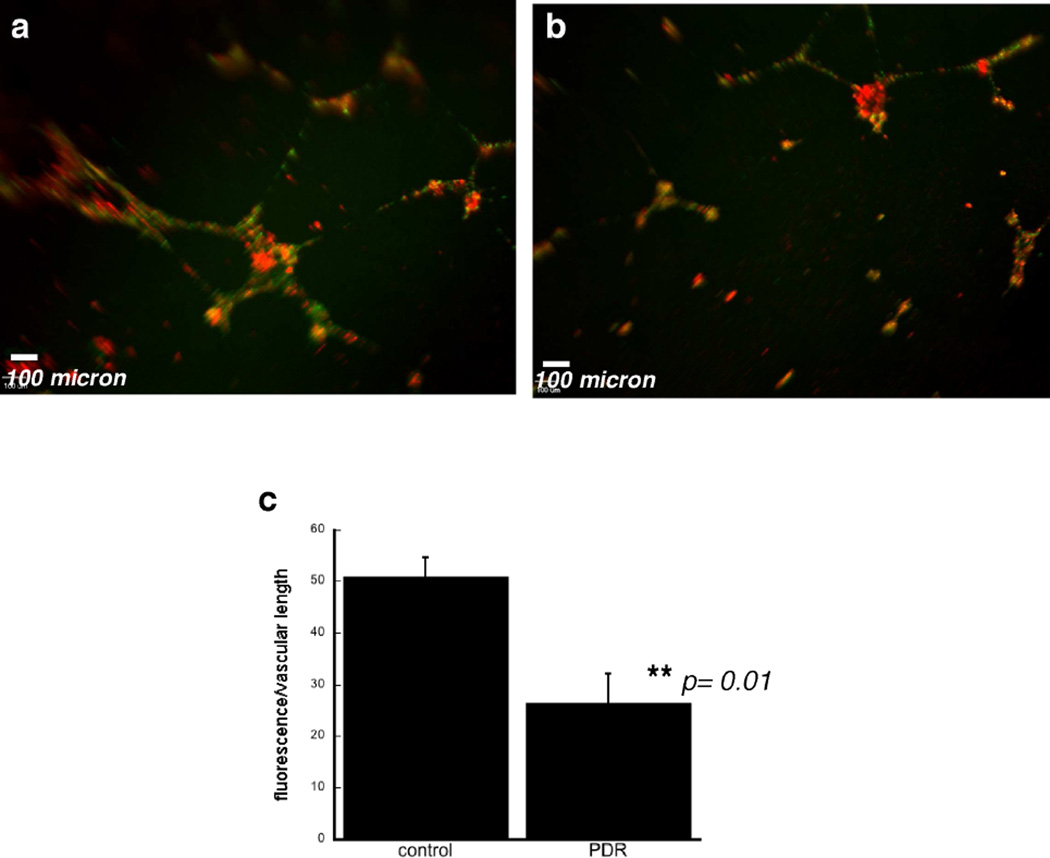
We examined the ability of ECFCs to incorporate into vascular tubes with human retinal endothelial cells (HRECs) in an in vitro angiogenesis matrix model. HRECs and ECFCs were tracked using Qdot nanocrystals with fluorescence at 525 nm and 655 nm, respectively. Vascular tubes that formed after 18–20 h in a 1:1 mixture of normal HRECs and ECFCs from healthy patients were extensive and exhibited normal branching patterns (Figure 4a). However, a 1:1 mixture of normal HRECs and ECFCs from PDR patients led to reduced incorporation of ECFCs into the vascular tubes (Figure 4b). The results shown in Figure 4a and b are representative photographs of an experiment examining vascular tube patterns from ECFCs from 3 PDR patients and 3 healthy controls. These data provide additional in vitro evidence for primary ECFC vasculogenic dysfunction in PDR patients.
Figure 4. Co-culture of human retinal endothelial cells and ECFCs in an in vitro tube-forming assay.
Representative photomicrographs of human retinal endothelial cells labeled with Qdot®-525 (green) nm cell-tracking nanocrystals and co-cultured with ECFCs labeled with Qdot®-655 (red) nm cell-tracking nanocrystals from healthy controls (a) and patient with PDR (b). Scale bar (white) = 100 µm. A total of three PDR patients’ and three healthy control patients’ ECFCs were analyzed. c) quantitation of % ECFC fluorescence/vascular length was compared using ECFCs from patients with and without PDR.
Increased expression of angiogenesis inhibitors in ECFCs from patients with PDR
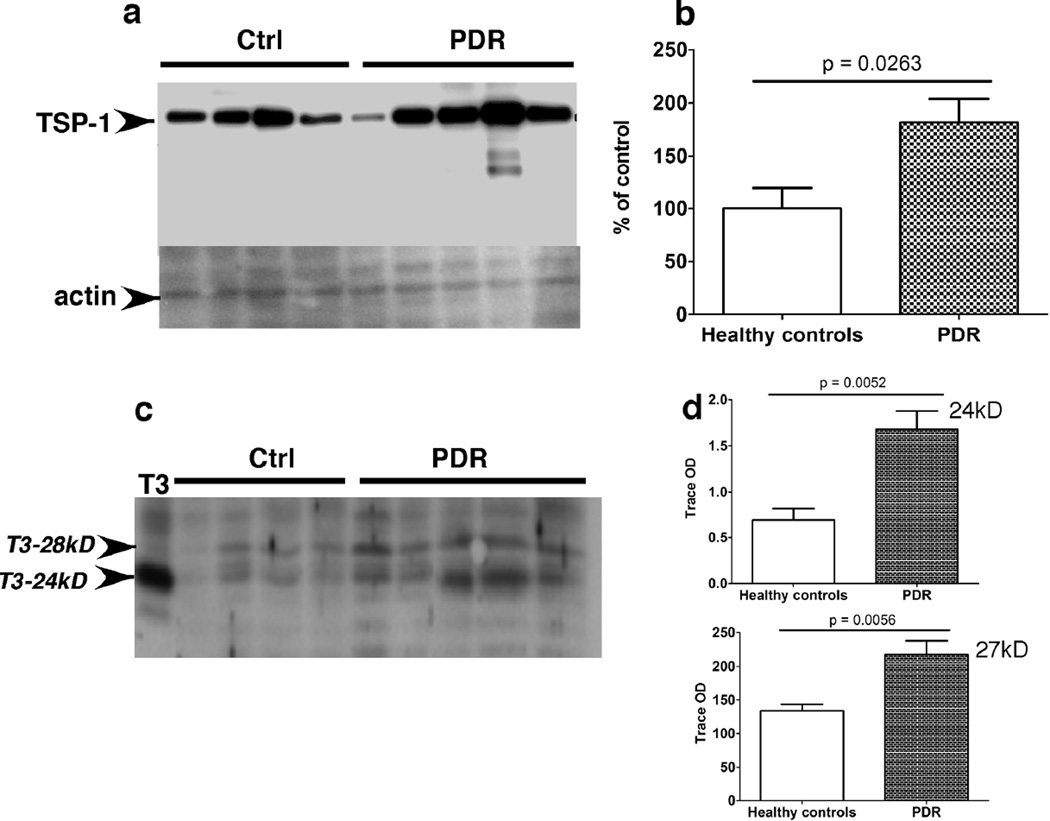
Based on our findings described above, we hypothesized that the inability of ECFCs to repair vascular damage in patients susceptible to developing PDR could be attributed to a dysfunction of ECFCs themselves and not due to alterations in chemokine gradients in peripheral blood. In an attempt to identify altered gene expression profiles in ECFCs from patients with PDR, an Affymetrix microarray analysis was performed on ECFCs from two patients with PDR and compared with ECFCs from two healthy controls. Before analysis, all parameters of the microarray processing and handling were evaluated to rule out irregularities. The spiked bacterial controls, scaling factors and background signals for all chips were within accepted values and similar between chips. Using GCA-RMA quantification on GeneSpring GX 9.0 software, we conducted four binary comparisons of all transcript signals: Healthy control #1 vs PDR #1, Healthy control #1 vs PDR #2, Healthy control #2 vs PDR #1, and Healthy control #2 vs PDR #2. We found a number of genes to be upregulated in ECFCs of PDR patients, including two known angiogenesis inhibitors, thrombospondin-1 and tissue inhibitor of matrix metalloproteinases-3 (TIMP-3) (Table 2). The complete list of credible gene changes and the fold-changes associated with each binary comparison can be viewed in the Supplemental Appendix. Subsequent confirmatory experiments were done using Western blotting to ascertain whether the changes in gene expression from the microarray analysis mirrored changes in protein expression as well. Thrombospondin-1, a protein with anti-angiogenic properties, was increased in ECFC lysates from PDR patients (Figure 5 a,b). The protein levels of both unglycosylated (24 kD) and glycosylated (27 kD) forms of TIMP-3 protein, a potent angiogenesis inhibitor, was also increased in ECFC lysates, derived from patients with PDR compared to healthy controls (Figure 5 c,d). These data suggest that there may be an inherent defect in the ability of ECFCs of patients susceptible to developing PDR to repair damage to the vasculature.
Microarray Analysis: Credible Gene Changes
| Gene name | Fold Change: PDR arrays vs Healthy arrays |
Function |
|---|---|---|
| SPARCL-1 | (↑) | Extracellular matrix |
| Versican | (↑) | Extracellular matrix |
| TIMP-3 | (↑) | Extracellular matrix, angiogenesis inhibitor |
| β-arrestin | (↓) | Signal transduction |
| Discoidin domain receptor-2 | (↓) | Signal transduction, cell adhesion |
| Inhibin, beta A | (↑) | TGF-β family |
| Thrombospondin-1 | (↑) | TGF-β family, angiogenesis inhibitor |
| TGF-β2 | (↑) | TGF-β family |
| Latent TGF-β binding protein-2 | (↑) | TGF-β family |
| Lymphatic vessel hyaluronan receptor-1 | (↓) | Vasculogenesis |
Figure 5. Increased expression of angiogenesis inhibitors TSP-1 and TIMP-3 in PDR-ECFCs.
Expression of TSP-1 (a) and TIMP-3 (b) protein in ECFCs from controls and patients with PDR was analyzed by western blot analysis (a,b), quantified by densitometry and expressed as arbitrary optical density (OD) units (b, d).
Discussion
PDR is characterized by abnormal neovascularization and leakage of retinal vessels in response to retinal ischemia. While a reduction in the number of circulating endothelial progenitor cells (EPCs) as well as their dysfunction has been reported in both type 1 and type 2 diabetic patients(Caballero et al., 2007; Fadini et al., 2005; Fadini et al., 2007; Fadini et al., 2006b; Hill et al., 2003; Loomans et al., 2005; Tepper et al., 2002), there is a dearth of experimental work in the literature exploring the role of EPCs in diabetics with advanced retinal disease. It has been recently reported that neurotropic factors may activate and mobilize EPCs to regulate pathologic neovasculrization in diabetes(Liu et al.). Furthermore, no studies have examined the role of CD34+ CD45− ECFCs in diabetic patients, and very little work has been done to uncover the mechanistic cellular derangements underlying progenitor cell dysfunction. A landmark study initially demonstrated that adult hematopoietic stem cells in the circulation could provide functional hemangioblast activity during retinal neovascularization(Grant et al., 2002). A more recent albeit small clinical study determined that CD34+ and c-kit+ mononuclear cells were increased in the peripheral blood of patients with non-proliferative diabetic retinopathy (NPDR) and PDR(Lee et al., 2006), although cells with positivity to these markers alone may simply represent the general pool of EPCs without regard to subtype. Many now agree that there are clonally distinct subpopulations of peripheral blood-derived EPCs with CD34 positivity. CFU-ECs, which have been extensively used as a predictive marker for vascular disease(Fadini et al., 2006b; Hill et al., 2003), are bone marrow-derived and positive for CD45 with little ability to form vessels in vivo; however, ECFCs (late outgrowth EPCs), which have robust proliferative potential and more closely resemble an endothelial cell phenotype, are CD45− and demonstrate functional incorporation into chimeric vessels in mice(Smadja et al., 2007; Yoder et al., 2007). In the present study, we have identified a significant increase in CD34+ CD45− ECFCs in the circulation of patients with PDR. Although we were trying to quantify circulating ECFCs, it is likely that there were other populations of cells including VEGFR2+ hematopoietic progenitor cells and circulating endothelial cells which were captured in the CD34/CD45 flow cytometric analysis. However, the predominant cell types in this sorted population are angiogenic in nature.
Patients who have PDR typically manifest other late-stage vasculopathic changes as a consequence of diabetes, including complications from peripheral artery disease (PAD) and cardiovascular disease (CVD). Although one study by Fadini and colleagues determined that diabetic patients with PAD had decreased levels of circulating EPCs(Fadini et al., 2006a), we observed an approximately two-fold increase in levels of highly proliferative circulating ECFCs in PDR patients despite a higher prevalence of renal impairment (Table 1) and peripheral vascular disease (47% vs 8%, data not shown). Fadini and colleagues also showed in another study (regarding the “diabetic paradox”) that patients with diabetic retinopathy (severe NPDR or PDR) without PAD had significantly higher levels of circulating KDR+ EPCs than diabetic retinopathy patients with PAD(Fadini et al., 2006b). In light of these data, the subset of EPCs we have isolated may represent an additional cellular contributor to vasculopathic abnormalities seen in diabetic patients. There is also recent evidence supporting our findings from work done in coronary atherosclerosis suggesting that shifts in subsets of circulating progenitor cell populations may be a mechanism for coronary endothelial dysfunction(Boilson et al., 2008). One limitation of our study is the sole comparison between cells from patients with PDR and those from non-diabetics. Ideally, a comparison including cells from diabetics without retinopathy would allow us to definitively conclude whether these functional abnormalities are a consequence of diabetes or occur exclusively in diabetics with a propensity for progressing to PDR. It is also unclear whether diabetics early in their disease or diabetic patients without end-organ damage manifest similar increases in this particular progenitor cell subset. These are questions that warrant further investigation. Our results are unable to delineate between (nor do they explicitly suggest differences in) the characteristics of type 1 and type 2 diabetic ECFCs. It is well known that genetic differences between type 1 and type 2 diabetic patients are myriad. However, our work has shown that there are measurable quantitative and functionally significant changes in the circulating EPC population in diabetics with severe end-organ damage as a generalized group of individuals.
It is becoming evident that mobilization and homing of EPCs to their target sites requires chemotactic gradients. VEGF, SDF-1, and recently erythropoietin (EPO) have all been implicated as important pro-angiogenic signals in proliferative diabetic retinopathy of the eye(Butler et al., 2005; Pe'er et al., 1996; Watanabe et al., 2005). We observed that levels of SDF-1 but not VEGF were higher in the plasma of PDR patients who grew ECFC colonies compared to the healthy patients who grew colonies. SDF-1 is a chemokine that has been shown to be important in EPC migration and proliferation(Shao et al., 2008; Yamaguchi et al., 2003). Intravitreal SDF-1 levels have been found to be elevated in patients with PDR(Abu El-Asrar et al., 2006; Brooks et al., 2004; Butler et al., 2005). From migration assays, however, it appears that ECFCs from PDR patients are impaired in their ability to mobilize in response to either SDF-1 or serum from non-diabetic or diabetic patients. One surprising finding was the inability of ECFCs from both normal and PDR patients to generate a robust migratory response towards VEGF. It is also important to note that in our experimental design, we did not to pool our ECFCs from PDR patients into one chamber experiment in order to preserve replicates for individual patient comparisons to the average control patient’s ECFC migratory potential. Again, the purpose of our study was not intended to distinguish between the migratory responses of type 1 diabetic versus type 2 diabetic ECFCs; interestingly, though, both subgroups exhibited similar migratory dysfunction toward VEGF and SDF-1. From our results, it appears that both types of diabetic ECFCs are impaired in migrating toward angiogenic and stimulatory chemokines.
Moreover, ECFCs from PDR patients are unable to form functional vascular tubes with normal human retinal endothelial cells. We have shown that despite higher SDF-1 levels in the plasma and in the vitreous and increased circulating CD34+ CD45− ECFCs, ECFCs from patients with PDR are impaired in their ability to form functional vessels. Although we were limited in the number of samples in each patient group, our results show observable (and reproducible) differences consistent with our findings of impaired cell migration in PDR patients’ ECFCs. The biochemical mechanism underlying this observation is beyond the scope of this study, but it could be hypothesized that down-regulation of SDF-1 receptors on ECFCs contributes to the lack of migratory response even with high levels of SDF-1 in the circulation and vitreous. Downstream signaling from the SDF-1 receptor may also be disrupted in PDR ECFCs for unknown reasons. Despite our limited sample size in our migration experiments, migratory dysfunction and abnormal vascular tube formation are mutually consistent and can be synthesized into a unifying theory of stem cell dysfunction in healthy capillary repair and aberrant angiogenesis in neovascularized retinas of patients with end-stage diabetic eye disease.
These data led us to further investigate whether there were any specific proteins and pathways involved in ECFC dysfunction in PDR patients. Using binary comparisons of microarray results of two healthy patients and two PDR patients, we found that ECFCs from PDR patients had higher thrombospondin-1 (TSP-1) and TIMP-3 transcript expression when compared to ECFCs from healthy controls, among other transcript changes (Table 2). Several mediators of the TGF-β family were highly upregulated in the ECFCs obtained from PDR patients. We decided to evaluate TSP-1 and TIMP-3 because of their well-established roles as antiangiogenic molecules. Western blot analysis demonstrated that both TSP-1 and TIMP-3 protein expression and activity were increased in concordance with the microarray data. Thrombospondin-1 is a cellular matrix protein known to have potent anti-angiogenic potential. Although not clearly understood, TSP-1 has been increasingly implicated in many studies as a causal link between diabetes and vascular complications. One study showed that diabetic EPCs were impaired in re-endothelialization following arterial injury and express increased TSP-1(Ii et al., 2006). These EPCs also had deficient adhesion and migration properties. Another study demonstrated that neovascular cells (adjacent to the areas of nonperfusion) expressed higher levels of TSP-1 in an ischemia-induced retinal neovascularization mouse model(Suzuma et al., 1999). We also found that TIMP-3 protein levels and activity were higher in ECFCs from patients with PDR. TIMP-3 has been well established as an MMP inhibitor that ultimately blocks angiogenesis(Anand-Apte et al., 1997; Qi et al., 2003). These data suggest that peripheral blood ECFCs in PDR patients may be impaired in their normal reparative ability due to changes in TIMP-3 and TSP-1 expression.
Taken together, our data indicate that despite upregulation of SDF-1 level, patients with PDR are unable to utilize their increased ECFC pools to form functional vessels. In light of our results and from work already done in the EPC field, the “diabetic paradox” might actually be explained by two synergistic vascular derangements: impairment of EPC-mediated vessel repair and aberrant vasculogenesis induced by functionally modified EPCs. This finding suggests that the microvascular and macrovascular changes seen in diabetic patients may be attributed to functionally altered ECFCs both in normal vessel repair and in abnormal neovascularization. Ex vivo cultured ECFCs from healthy patients may therefore eventually serve as a viable means of autologous or heterologous therapeutic transplantation in the amelioration or even prevention of global diabetic vascular complications.
Acknowledgements
This work was supported in part by US National Institute of Health EY016490, CA106415, EY015638, Foundation Fighting Blindness Center Grant, Research to Prevent Blindness (RPB) Challenge Grant and Ohio BRTT 05-29 and RPB Lew Wasserman award to BA-A. We thank members of the laboratory of David Ingram at the Indiana university school of medicine for technical advice on culturing ECFCs. The authors gratefully acknowledge the support of the retina faculty, Dr. Kaiser, Dr. Schachat, Dr. Waheed, Dr. Sears and Dr. Singh at the Cole Eye institute and Dr. Hoogwerf at the endocrinology clinic at the Cleveland Clinic in the identification of patients for this study. In addition, the authors would like to thank the volunteers and patients who participated in this study. We wish to extend a sincere apology to colleagues whose work was not cited because of space limitations.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abu El-Asrar AM, Struyf S, Kangave D, Geboes K, Van Damme J. Chemokines in proliferative diabetic retinopathy and proliferative vitreoretinopathy. Eur Cytokine Netw. 2006;17:155–165. [PubMed] [Google Scholar]
- Alessandri G, Girelli M, Taccagni G, Colombo A, Nicosia R, Caruso A, Baronio M, Pagano S, Cova L, Parati E. Human vasculogenesis ex vivo: embryonal aorta as a tool for isolation of endothelial cell progenitors. Lab Invest. 2001;81:875–885. doi: 10.1038/labinvest.3780296. [DOI] [PubMed] [Google Scholar]
- Anand-Apte B, Pepper MS, Voest E, Montesano R, Olsen B, Murphy G, Apte SS, Zetter B. Inhibition of Angiogenesis by Tissue Inhibitor of Metalloproteinase-3. Invest. Ophthal. Vis. Sci. 1997;38:817–823. [PubMed] [Google Scholar]
- Arjamaa O, Nikinmaa M. Oxygen-dependent diseases in the retina: role of hypoxia-inducible factors. Exp Eye Res. 2006;83:473–483. doi: 10.1016/j.exer.2006.01.016. [DOI] [PubMed] [Google Scholar]
- Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- Boilson BA, Kiernan TJ, Harbuzariu A, Nelson RE, Lerman A, Simari RD. Circulating CD34+ cell subsets in patients with coronary endothelial dysfunction. Nat Clin Pract Cardiovasc Med. 2008;5:489–496. doi: 10.1038/ncpcardio1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks HL, Jr, Caballero S, Jr, Newell CK, Steinmetz RL, Watson D, Segal MS, Harrison JK, Scott EW, Grant MB. Vitreous levels of vascular endothelial growth factor and stromal-derived factor 1 in patients with diabetic retinopathy and cystoid macular edema before and after intraocular injection of triamcinolone. Arch Ophthalmol. 2004;122:1801–1807. doi: 10.1001/archopht.122.12.1801. [DOI] [PubMed] [Google Scholar]
- Butler JM, Guthrie SM, Koc M, Afzal A, Caballero S, Brooks HL, Mames RN, Segal MS, Grant MB, Scott EW. SDF-1 is both necessary and sufficient to promote proliferative retinopathy. J Clin Invest. 2005;115:86–93. doi: 10.1172/JCI22869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caballero S, Sengupta N, Afzal A, Chang KH, Li Calzi S, Guberski DL, Kern TS, Grant MB. Ischemic vascular damage can be repaired by healthy, but not diabetic, endothelial progenitor cells. Diabetes. 2007;56:960–967. doi: 10.2337/db06-1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadini GP. An underlying principle for the study of circulating progenitor cells in diabetes and its complications. Diabetologia. 2008;51:1091–1094. doi: 10.1007/s00125-008-1021-0. [DOI] [PubMed] [Google Scholar]
- Fadini GP, Agostini C, Avogaro A. Endothelial progenitor cells and vascular biology in diabetes mellitus: current knowledge and future perspectives. Curr Diabetes Rev. 2005;1:41–58. doi: 10.2174/1573399052952640. [DOI] [PubMed] [Google Scholar]
- Fadini GP, Sartore S, Agostini C, Avogaro A. Significance of endothelial progenitor cells in subjects with diabetes. Diabetes Care. 2007;30:1305–1313. doi: 10.2337/dc06-2305. [DOI] [PubMed] [Google Scholar]
- Fadini GP, Sartore S, Albiero M, Baesso I, Murphy E, Menegolo M, Grego F, Vigili de Kreutzenberg S, Tiengo A, Agostini C, Avogaro A. Number and function of endothelial progenitor cells as a marker of severity for diabetic vasculopathy. Arterioscler Thromb Vasc Biol. 2006a;26:2140–2146. doi: 10.1161/01.ATV.0000237750.44469.88. [DOI] [PubMed] [Google Scholar]
- Fadini GP, Sartore S, Baesso I, Lenzi M, Agostini C, Tiengo A, Avogaro A. Endothelial progenitor cells and the diabetic paradox. Diabetes Care. 2006b;29:714–716. doi: 10.2337/diacare.29.03.06.dc05-1834. [DOI] [PubMed] [Google Scholar]
- Fadini GP, Sartore S, Schiavon M, Albiero M, Baesso I, Cabrelle A, Agostini C, Avogaro A. Diabetes impairs progenitor cell mobilisation after hindlimb ischaemia-reperfusion injury in rats. Diabetologia. 2006c;49:3075–3084. doi: 10.1007/s00125-006-0401-6. [DOI] [PubMed] [Google Scholar]
- Funatsu H, Yamashita H, Shimizu E, Kojima R, Hori S. Relationship between vascular endothelial growth factor and interleukin-6 in diabetic retinopathy. Retina. 2001;21:469–477. doi: 10.1097/00006982-200110000-00009. [DOI] [PubMed] [Google Scholar]
- Grant MB, Caballero S, Brown GA, Guthrie SM, Mames RN, Vaught T, Scott EW. The contribution of adult hematopoietic stem cells to retinal neovascularization. Adv Exp Med Biol. 2003;522:37–45. doi: 10.1007/978-1-4615-0169-5_5. [DOI] [PubMed] [Google Scholar]
- Grant MB, May WS, Caballero S, Brown GA, Guthrie SM, Mames RN, Byrne BJ, Vaught T, Spoerri PE, Peck AB, Scott EW. Adult hematopoietic stem cells provide functional hemangioblast activity during retinal neovascularization. Nat Med. 2002;8:607–612. doi: 10.1038/nm0602-607. [DOI] [PubMed] [Google Scholar]
- Group. T.D.C.a.C.T.R. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med. 1993;329:977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- Hill JM, Zalos G, Halcox JP, Schenke WH, Waclawiw MA, Quyyumi AA, Finkel T. Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N Engl J Med. 2003;348:593–600. doi: 10.1056/NEJMoa022287. [DOI] [PubMed] [Google Scholar]
- Ii M, Takenaka H, Asai J, Ibusuki K, Mizukami Y, Maruyama K, Yoon YS, Wecker A, Luedemann C, Eaton E, Silver M, Thorne T, Losordo DW. Endothelial progenitor thrombospondin-mediates diabetes-induced delay in reendothelialization following arterial injury. Circ Res. 2006;98:697–704. doi: 10.1161/01.RES.0000209948.50943.ea. [DOI] [PubMed] [Google Scholar]
- Ingram DA, Caplice NM, Yoder MC. Unresolved questions, changing definitions, and novel paradigms for defining endothelial progenitor cells. Blood. 2005a;106:1525–1531. doi: 10.1182/blood-2005-04-1509. [DOI] [PubMed] [Google Scholar]
- Ingram DA, Mead LE, Moore DB, Woodard W, Fenoglio A, Yoder MC. Vessel wall-derived endothelial cells rapidly proliferate because they contain a complete hierarchy of endothelial progenitor cells. Blood. 2005b;105:2783–2786. doi: 10.1182/blood-2004-08-3057. [DOI] [PubMed] [Google Scholar]
- Ingram DA, Mead LE, Tanaka H, Meade V, Fenoglio A, Mortell K, Pollok K, Ferkowicz MJ, Gilley D, Yoder MC. Identification of a novel hierarchy of endothelial progenitor cells using human peripheral and umbilical cord blood. Blood. 2004;104:2752–2760. doi: 10.1182/blood-2004-04-1396. [DOI] [PubMed] [Google Scholar]
- Lee IG, Chae SL, Kim JC. Involvement of circulating endothelial progenitor cells and vasculogenic factors in the pathogenesis of diabetic retinopathy. Eye (Lond) 2006;20:546–552. doi: 10.1038/sj.eye.6701920. [DOI] [PubMed] [Google Scholar]
- Liu X, Li Y, Liu Y, Luo Y, Wang D, Annex BH, Goldschmidt-Clermont PJ. Endothelial Progenitor Cells (EPCs) Mobilized and Activated by Neurotrophic Factors May Contribute to Pathologic Neovascularization in Diabetic Retinopathy. Am J Pathol. 2010;176:504–515. doi: 10.2353/ajpath.2010.081152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loomans CJ, De Koning EJ, Staal FJ, Rabelink TJ, Zonneveld AJ. Endothelial progenitor cell dysfunction in type 1 diabetes: another consequence of oxidative stress? Antioxid Redox Signal. 2005;7:1468–1475. doi: 10.1089/ars.2005.7.1468. [DOI] [PubMed] [Google Scholar]
- Loomans CJ, de Koning EJ, Staal FJ, Rookmaaker MB, Verseyden C, de Boer HC, Verhaar MC, Braam B, Rabelink TJ, van Zonneveld AJ. Endothelial progenitor cell dysfunction: a novel concept in the pathogenesis of vascular complications of type 1 diabetes. Diabetes. 2004;53:195–199. doi: 10.2337/diabetes.53.1.195. [DOI] [PubMed] [Google Scholar]
- Otani A, Kinder K, Ewalt K, Otero FJ, Schimmel P, Friedlander M. Bone marrow-derived stem cells target retinal astrocytes and can promote or inhibit retinal angiogenesis. Nat Med. 2002;8:1004–1010. doi: 10.1038/nm744. [DOI] [PubMed] [Google Scholar]
- Pe'er J, Folberg R, Itin A, Gnessin H, Hemo I, Keshet E. Upregulated expression of vascular endothelial growth factor in proliferative diabetic retinopathy. Br J Ophthalmol. 1996;80:241–245. doi: 10.1136/bjo.80.3.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi JH, Ebrahem Q, Moore N, Murphy G, Claesson-Welsh L, Bond M, Baker A, Anand-Apte B. A novel function for tissue inhibitor of metalloproteinases-3 (TIMP3): inhibition of angiogenesis by blockage of VEGF binding to VEGF receptor-2. Nat Med. 2003;9:407–415. doi: 10.1038/nm846. [DOI] [PubMed] [Google Scholar]
- Rafii S, Lyden D. Therapeutic stem and progenitor cell transplantation for organ vascularization and regeneration. Nat Med. 2003;9:702–712. doi: 10.1038/nm0603-702. [DOI] [PubMed] [Google Scholar]
- Shao H, Tan Y, Eton D, Yang Z, Uberti MG, Li S, Schulick A, Yu H. Statin and stromal cell-derived factor-1 additively promote angiogenesis by enhancement of progenitor cells incorporation into new vessels. Stem Cells. 2008;26:1376–1384. doi: 10.1634/stemcells.2007-0785. [DOI] [PubMed] [Google Scholar]
- Shi Q, Rafii S, Wu MH, Wijelath ES, Yu C, Ishida A, Fujita Y, Kothari S, Mohle R, Sauvage LR, Moore MA, Storb RF, Hammond WP. Evidence for circulating bone marrow-derived endothelial cells. Blood. 1998;92:362–367. [PubMed] [Google Scholar]
- Smadja DM, Bieche I, Helley D, Laurendeau I, Simonin G, Muller L, Aiach M, Gaussem P. Increased VEGFR2 expression during human late endothelial progenitor cells expansion enhances in vitro angiogenesis with up-regulation of integrin alpha(6) J Cell Mol Med. 2007;11:1149–1161. doi: 10.1111/j.1582-4934.2007.00090.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stratton IM, Adler AI, Neil HA, Matthews DR, Manley SE, Cull CA, Hadden D, Turner RC, Holman RR. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. Bmj. 2000;321:405–412. doi: 10.1136/bmj.321.7258.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuma K, Takagi H, Otani A, Oh H, Honda Y. Expression of thrombospondin-1 in ischemia-induced retinal neovascularization. Am J Pathol. 1999;154:343–354. doi: 10.1016/S0002-9440(10)65281-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T, Kalka C, Masuda H, Chen D, Silver M, Kearney M, Magner M, Isner JM, Asahara T. Ischemia- and cytokine-induced mobilization of bone marrow-derived endothelial progenitor cells for neovascularization. Nat Med. 1999;5:434–438. doi: 10.1038/7434. [DOI] [PubMed] [Google Scholar]
- Tepper OM, Galiano RD, Capla JM, Kalka C, Gagne PJ, Jacobowitz GR, Levine JP, Gurtner GC. Human endothelial progenitor cells from type II diabetics exhibit impaired proliferation, adhesion, and incorporation into vascular structures. Circulation. 2002;106:2781–2786. doi: 10.1161/01.cir.0000039526.42991.93. [DOI] [PubMed] [Google Scholar]
- Thill M, Strunnikova NV, Berna MJ, Gordiyenko N, Schmid K, Cousins SW, Thompson DJ, Csaky KG. Late outgrowth endothelial progenitor cells in patients with age-related macular degeneration. Invest Ophthalmol Vis Sci. 2008;49:2696–2708. doi: 10.1167/iovs.07-0955. [DOI] [PubMed] [Google Scholar]
- Watanabe D, Suzuma K, Matsui S, Kurimoto M, Kiryu J, Kita M, Suzuma I, Ohashi H, Ojima T, Murakami T, Kobayashi T, Masuda S, Nagao M, Yoshimura N, Takagi H. Erythropoietin as a retinal angiogenic factor in proliferative diabetic retinopathy. N Engl J Med. 2005;353:782–792. doi: 10.1056/NEJMoa041773. [DOI] [PubMed] [Google Scholar]
- Yamaguchi J, Kusano KF, Masuo O, Kawamoto A, Silver M, Murasawa S, Bosch-Marce M, Masuda H, Losordo DW, Isner JM, Asahara T. Stromal cell-derived factor-1 effects on ex vivo expanded endothelial progenitor cell recruitment for ischemic neovascularization. Circulation. 2003;107:1322–1328. doi: 10.1161/01.cir.0000055313.77510.22. [DOI] [PubMed] [Google Scholar]
- Yoder MC. Defining human endothelial progenitor cells. J Thromb Haemost. 2009;7(Suppl 1):49–52. doi: 10.1111/j.1538-7836.2009.03407.x. [DOI] [PubMed] [Google Scholar]
- Yoder MC, Ingram DA. Endothelial progenitor cell: ongoing controversy for defining these cells and their role in neoangiogenesis in the murine system. Curr Opin Hematol. 2009a;16:269–273. doi: 10.1097/MOH.0b013e32832bbcab. [DOI] [PubMed] [Google Scholar]
- Yoder MC, Ingram DA. The definition of EPCs and other bone marrow cells contributing to neoangiogenesis and tumor growth: is there common ground for understanding the roles of numerous marrow-derived cells in the neoangiogenic process? Biochim Biophys Acta. 2009b;1796:50–54. doi: 10.1016/j.bbcan.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoder MC, Mead LE, Prater D, Krier TR, Mroueh KN, Li F, Krasich R, Temm CJ, Prchal JT, Ingram DA. Redefining endothelial progenitor cells via clonal analysis and hematopoietic stem/progenitor cell principals. Blood. 2007;109:1801–1809. doi: 10.1182/blood-2006-08-043471. [DOI] [PMC free article] [PubMed] [Google Scholar]