Abstract
HspB1 is a small heat shock protein implicated in neuronal survival and neurite growth; mutations in HspB1 have been identified in hereditary motor neuronopathies and Charcot Marie Tooth Type 2 neuropathies. In cortical neurons we found that expression of HspB1 decreased RhoA activity and RhoA-GTP protein, and reversed the inhibition of neurite extension induced by NogoA. HspB1 decreased PDZ-RhoGEF, a RhoA specific guanine nucleotide exchange factor, while other regulators of RhoA activity were unchanged. The decrease in PDZ-RhoGEF was independent of proteasomal or lysosomal degradation pathways and was not associated with changes in PDZ-RhoGEF mRNA. We sequenced the 3’UTR of rat PDZ-RhoGEF and found binding sites for miRNAs miR-20a, miR-128 and miR-132. Expression of these microRNAs was substantially increased in cortical neurons transfected with HspB1. Co-transfection of HspB1 with specific inhibitors of miR-20a or miR-128 prevented the decrease in PDZ-RhoGEF and blocked the neurite growth promoting effects of HspB1. Using the 3'UTR of PDZ-RhoGEF mRNA in a luciferase reporter construct we observed that HspB1, miR-20a and miR-128 each inhibited luciferase expression. We conclude that HspB1 regulates RhoA activity through modulation of PDZ-RhoGEF levels achieved by translational control through enhanced expression of specific miRNAs (miR-20a and miR-128). Regulation of RhoA activity by translational silencing of PDZ-RhoGEF may be the mechanism through which HspB1 is involved in regulation of neurite growth. As RhoA-GTPase plays a regulatory role in the organization and stability of cytoskeletal networks through its downstream effectors, the results suggest a possible mechanism linking HspB1 mutations and axonal cytoskeletal pathology.
Keywords: heat shock protein, guanine nucleotide exchange factor (GEF), microRNA, neurite outgrowth, RhoA
Introduction
Heat shock protein B1 (HspB1) belongs to a family of ten small heat shock proteins that share an α-crystallin domain and function as molecular chaperones to maintain proteins in a foldingcompetent state (Kappe et al., 2003; Mymrikov et al., 2011). HspB1 has antiapoptotic and cytoprotective properties and acts as a key regulator of intermediate filament and microtubule networks (Hino et al., 2000; Perng et al., 1999).
HspB1 is found in most neurons of the central and peripheral nervous system. In the setting of metabolic stress or physical injury, expression of HspB1 is required for neuronal survival (Benn et al., 2002; Kalmar et al., 2002; Wagstaff et al., 1999). As a regulator of the cell cytoskeleton HspB1 exerts complex interactions. In its monomeric form, HspB1 may act as a capping protein to prevent actin polymerization, while the phosphorylated dimmers and oligomeric forms stabilize the actin filament network (Doshi et al., 2010; Miron et al., 1991; Mounier and Arrigo, 2002; Perng et al., 1999). In neurons, HspB1 also interacts with microtubules and intermediate filaments (Almeida-Souza et al., 2013). The importance of these interactions in humans is reflected by the observation that mutations in HspB1 cause several forms of hereditary motor neuronopathies and Charcot Marie Tooth type 2 neuropathy (Benedetti et al., 2010; Houlden et al., 2008), that transgenic animals expressing the human mutations develop neuropathy (d'Ydewalle et al., 2011; Srivastava et al., 2012), and expression of mutant forms of HspB1 result in enhanced phosphorylation and aggregation of neurofilament proteins or enhanced microtubule stability (Ackerley et al., 2006; Almeida-Souza et al., 2011; Evgrafov et al., 2004; Holmgren et al., 2013; Zhai et al., 2007).
HspB1 enhances neurite growth and axonal regeneration in the peripheral nervous system where overexpression of HspB1 promotes neurite extension and branching in sensory neurons and enhances axonal regeneration following sciatic nerve injury however the mechanism by which it enhances growth has not been established (Ma et al., 2011; Williams et al., 2006).
Regulation of neurite growth requires signaling through RhoGTPases that act as molecular switches to alter cytoskeleton networks and cell morphology. The dynamic between the inactive Rho guanosine diphosphate (GDP) and active Rho guanosine triphosphate (GTP) states is tightly regulated by guanine nucleotide exchange factors (GEFs), GTPase activating proteins (GAPs) and guanine nucleotide dissociation inhibitors (GDIs) in response to extracellular cues (Nikolic, 2002). Extracellular guidance molecules or receptor ligand interactions that activate RhoA cause growth cone collapse and neurite growth inhibition while growth factor activated receptors promote neurite growth and inhibit RhoA (Geraldo and Gordon-Weeks, 2009; Samuel and Hynds, 2010; Vitriol and Zheng, 2012). Although HspB1 has been suggested as a regulator of RhoA in cell adhesion and smooth muscle contraction (Garcia et al., 2009; Wang and Bitar, 1998), little is known about the interaction of HspB1 with RhoGTPases in neurons.
Our studies were designed to explore the mechanism by which HspB1 enhances neurite growth using cortical neurons in vitro. The results demonstrate a novel function for HspB1 that by upregulating a set of miRNAs (miR-20a, miR-128) causes translational silencing of PDZ-RhoGEF (Arhgef11) to reduce RhoA activation in neurons and thus promote neurite growth.
EXPERIMENTAL PROCEDURES
Vector vHspB1 construction and purification
The full length of HspB1 cDNA was generated from mouse brain RNA using RT-PCR with the following primers: forward GGATCCGCCACCATGACCGAGCGCCGCGTGCCCTTCG and reverse GAATTCATGGCTTCTACTTGGCTCCAGACTGTTCAGACTTCCCAGC. The HspB1 fragment amplified by PCR was ligated into PGEM-T vector using PGEM-T vector system I Kit (Promega). SphI-SalI (AflIII) (124485–126412) and BglII (XhoI)-SmaI (Sty I)-BglII (XhoI) (131934–133520) of herpes simplex type I virus (HSV) was cloned into plasmid SP72 (BglII-SphI) (Promega). This plasmid, designated SASB3 is completed deleted for HSV ICP4 gene sequences. An 648 bp BglII fragment of HCMV-BamHI-ECORI-polyA was inserted into SASB3, and a plasmid in which the human cytomegalovirus (HCMV) promoter was ligated with SmaI site and the polyA was inserted with SalI selected; the resulting plasmid was designated HCMV-polyA/SASB3-16. The entire open reading frame of HspB1 was cut out from PGEM-THspB1 plasmid by Bam HI and ECORI, and cloned into Bam HI-ECORI-cut HCMV-polyA/SASB3-16. The HCMV-polyA/SASB3-16 plasmid with correct HspB1 insertion was selected for making HspB1 vector. Three hundred thousand 7B cells were seeded in each well of a six well plate, and the next day infected with 1000 pfu of the replication defective HSV vector UL41E1G6. After incubation at 37°C for 1 hr, the cells were co-transfected with 1µg of HCMV-HspB1-polyA/SASB3-16 plasmid using Lipofectamine™ 2000 (Invitrogen) and 4 days later the virus harvested and sonicated. 10 µL of virus solution was used to identify white plaques (double deleted HCMV-EGFP), and a single white virus then isolated through three rounds on 96-well plate. Each round of purification was confirmed by Southern Blot. Expression of HspB1 transgene from the final UL41E1G6-HspB1 vector (vHspB1) was examined by infection of primary DRG neurons using Western-blot. vHspB1 was grown to high titer, purified on Nicodenz (Sigma) gradients, and employed in the studies described below. The control vector (vC) was similar to vHspB1 but contained LacZ or EGFP in place of HspB1.
Plasmid constructs
cDNA of HspB1 obtained from vHspB1 transfected cortical neurons by RT-PCR was inserted into pIRES2-AcGFP1 (Clontech) using In-Fusion HD EcoDry Cloning System (Clontech) and the following primers: forward GGACTCAGATCTCGAGATGACCGAGCGCCGCGTGCCCTTC and reverse GTCGACTGCAGAATTCCTACTTGGCTCCAGACTGTTCAGA. The resulting plasmid was designated pHspB1. The 3’UTR of PDZ-RhoGEF mRNA was amplified from cortical neurons by RT-PCR and cloned into TOPO TA cloning vector (Invitrogen) for sequencing. Forward primer AAGACAGTGCCACTGACACAGCTGTGTCACCAGGACCATAG and oligo d(T)16 as reverse primer were used. 3’ UTR of PDZ-RhoGEF mRNA shown in Figure 4 was cloned into pmirGlo luciferase miRNA target expression vector (Promega) using In-Fusion HD EcoDry Cloning System (Clontech) and the following primers: forward GCTCGCTAGCCTCGAGCATCCAGACAACCAGAGTCTGGCC and reverse CGACTCTAGACTCGAGCTGAATATTTAACTTTTCTTTAAA. All plasmid constructs were verified by DNA sequencing. The following expression plasmids were purchased: miR-control, miR-128, miR-20a and miR-132 (ORIGEN) and miArrest™ MicroRNA Inhibitors for miR-20a, miR-128 and miR-132 (GeneCopoeia). Lentiviral vectors expressing microRNA inhibitors for miR-20a (lenti-20a-I) and miR-128 (lenti-128-I) or control vector (lenti-con) were obtained from GeneCopoeia. All lentiviral vectors carry the mCherry reporter gene.
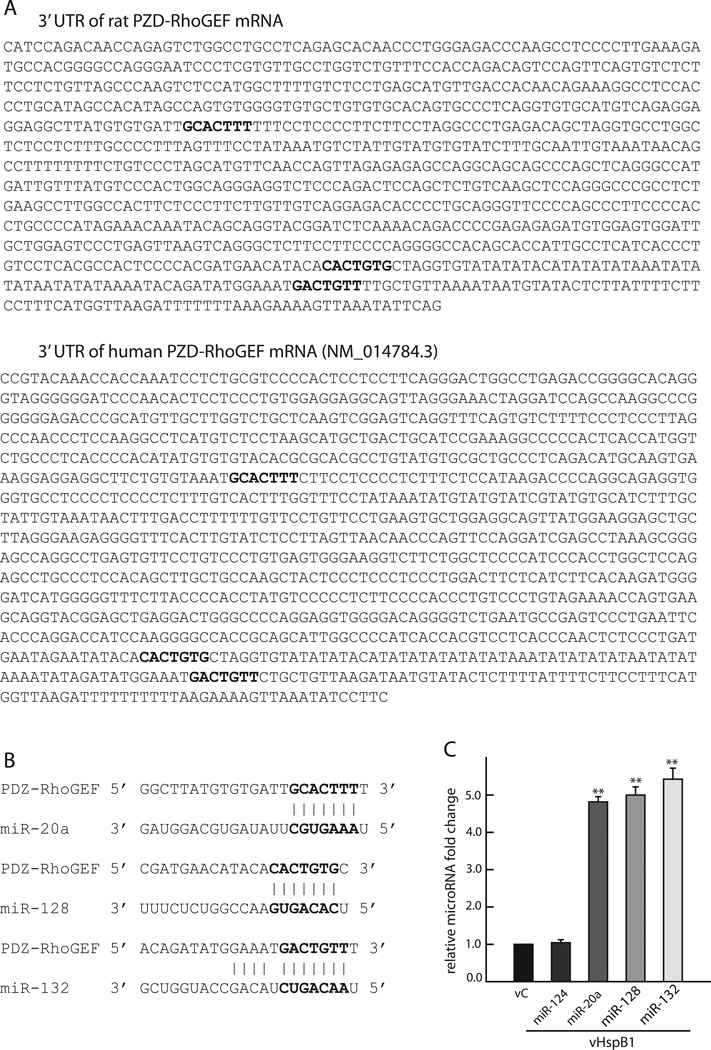
FIGURE 4. Expression of HspB1 in cortical neurons increases miR-20a, miR-128 and miR-132.
A. 3’ UTR sequence of rat (top) and human (bottom) PDZ-RhoGEF mRNA showing conserved binding sites for miR-20a, miR-128 and miR-132 (bold). B. Target sites of miR-20a, miR-128 and miR-132 in 3’ UTR rat sequence. C. Cortical neurons transfected with vHspB1 for 48 h have an increase in miR-20a, miR-128 and miR-132. The relative fold change of each microRNA was calculated using the 2-^ΔΔCt method using U6 as internal control. (n=3; **P < 0.01).
Cell culture and transfection
The study was reviewed and approved by our institutional animal studies committee. Cortical neurons were isolated from E17 rat pups produced by timed pregnant Sprague-Dawley female rats (Charles River). The cells were plated on poly-D-lysine (100 µg/ml, Sigma) coated 12 well plates with or without glass coverslip in defined Neurobasal medium containing B27, Glutamax I, Albumax I, and penicillin/streptomycin (Invitrogen). Twenty µl solution of fluoro-2-deoxyuridine (0.308 µg/µl) and uridine (0.308 µg/µl) was added to each well to inhibit non-neuronal cell proliferation (Sigma). The cells were transfected with vHspB1 and vC at indicated day in vitro (DIV) at a multiplicity of infection of 2 for 2 h and harvested 48 h later for analysis. For protein degradation analysis, 24 h after transfection at DIV7 proteasome inhibitors MG132 (10 µM, Millipore) or lactacystin (10 µM, Sigma); calpain and cathepsin B inhibitor MDL28170 (20 µM, Tocris); or lysosome inhibitor chloroquine (20 µM, Sigma) were added to culture medium and 24 h later, cortical neurons were harvested for analysis.
Neuro-2a cells (ATCC) were maintained in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal calf serum. For transient transfection, Lipofectamine 2000 transfect reagent (Invitrogen) was used according to the manufacture’s protocol and 48 h later the cells were harvested for analysis.
Western blot
Cultured cortical neurons (DIV9) or Neuro-2a cells were collected in lysis buffer containing 62.5 mM Tris–HCl, pH 6.8; 2% SDS; 10% glycerol, 1× protease inhibitor cocktail (Roche), 1× phosphatase inhibitor (Thermo Scientific). Protein concentration was determined by using Bio-Rad Dc protein assay (Bio-Rad). DTT and bromophenol blue were added to each measured sample to 200 mM and 0.002% respectively. Proteins were analyzed by SDS–polyacrylamide gel electrophoresis (Invitrogen) followed by Western blotting using the primary antibodies from Santa Cruz: goat anti-HspB1 (1:1000), rabbit anti-PDZ-RhoGEF (1:500), rabbit anti-p190RhoGAP (1:2000), rabbit anti-LARG (1:500) and from Sigma: mouse anti-RhoA (1:1000), mouse anti-GDIα (1:3000), mouse-anti-GAPDH (1:3000) and mouse anti-β-actin (1:3000) and corresponding secondary antibodies coupled to horseradish peroxidase (Jackson Immunoresearch Laboratories). Protein bands were visualized using the SuperSignal Western Dura Extended Duration Substrate (Thermo Scientific). The intensity of each band was quantitated using the chemiluminescence detector ChemiDoc (Bio-Rad). GAPDH and β-actin determination were used as internal control for each sample.
Determination of RhoA activity
Cortical neurons (DIV7) were transfected with vHspB1 or vC and harvested 48h later harvested. RhoA activity was determined by rhotekin-RBD (Rhobinding domain) bead pull-down assay and G-LISA Rho activation assay using the manufacture’s protocols (Cytoskeleton).
For rhotekin-RBD bead pull-down assay neurons were harvested in lysis buffer and samples were equalized for total protein concentrations. Equal volumes of supernatants were incubated with 50µg rhotekin-RBD beads for 1 h at 4 °C, followed by two washes in lysis buffer and three washes in the supplied wash buffer. Bound RhoA-GTP was eluted in 2% SDS sample buffer. RhoA-GTP and total RhoA were examined by western blot analysis using anti-RhoA antibody.
For G-LISA RhoA activation assay neurons were harvested in lysis buffer. After measurement of the protein concentration using Bio-Rad Dc protein assay reagents. Equal volumes of supernatants were incubated in RhoA-GTP affinity plates. The amount of bound RhoA-GTP was determined using primary anti-RhoA antibody and second HRP-labeled antibody and detected at 490 nm using a DTX880 microplate spectrophotometer (Beckman). The amount of GAPDH determined using a KDalert GAPDH assay kit (Ambion) was used as an internal control for G-LISA assay.
microRNA targeted to PDZ-RhoGEF prediction
Using the web sites www.microrna.org, www.mirbase.org, and www.targetscan.org several microRNAs were predicted from their sequences to target the 3’ UTR of PDZ-RhoGEF mRNA. From those, we selected miR-20a, miR-128, miR-132, miR-93, miR-106b, miR-212, miR-582 and miR-134 for primary screening by microRNA RT-PCR using the same parameters as for qRT-PCR (denature 15 sec at 94°C, annealing and extension 30 sec at 60 °C for 40 cycles). After 1% agarose DNA gel detection of PCR products, clear single bands were obtained from primers for miR-20a, miR-128, and miR-132 and were selected in our experiment, We used miR-124 as negative control because does not target miR-124 PDZ-RhoGEF.
RT-PCR and Quantitative RT-PCR (qRT-PCR)
Total RNA from cortical neurons (DIV 9) was extracted by using RNeasy kit (Qiagen) followed the first strand cDNA synthesis by using SuperScript first strand synthesis system (Invitrogen). qRT-PCR was run at iQ™ optical system using iQ™ SYBR® Green Supermix (Bio-Rad). The primers were used as follows: HspB1 forward primer TGCACACAGCCGCCTCTTCG, reverse primer TCCGCTGACTGCGTGACTGC; GAPDH forward primer CGGCCGAGGGCCCACTAAAG, reverse primer GAGCAATGCCAGCCCCAGCA; PDZ-RhoGEF forward primer AAGCAGCTGGCTGCCCTTGG, reverse primer AGACGGATCCCAGCGTGGCT. microRNAs were isolated by using mirVana™ miRNA isolation kit (Ambion) and followed the first strand synthesis by using NCode™ EXPRESS SYBR GreenER™ miRNA qRT-PCR Kit (Invitrogen). miroRNA qRT-PCR was run at iQ™ optical system. Primers were used as follows U6 forward primer CCTCAGAGGAACTGACAAGCA, reverse primer AAACGTGAGTGAGCCTCCAAT; miR-20a forward primer TAAAGTGCTTATAGTGCAGGTAG;miR-132 forward primer TAACAGTCTACAGCCATGGTCG;miR-128 forward primer TCACAGTGAACCGGTCTCTTT; miR-124 forward primer TAAGGCACGCGGTGAATGCC. Reverse primer for all microRNAs was provided in the kit. PCR conditions were 40 cycles of 15 sec at 94°C, 30 sec at 60°C. The relative expression of each mRNA or microRNA was calculated using 2−ΔΔCt method (Livak and Schmittgen, 2001).
Luciferase activity assay
Neuro-2a cells were double transfected by pmirGlo luciferase plasmid 3’ UTR of PDZ-RhoGEF with either pIRES2-AcGFP1 (pC), pAcGFP1-HspB1 (pHspB1) or with the microRNA plasmids and 48h after transfection cells were lysed according to protocol and luciferase activity was measured using Firefly luciferase assay kit (Thermo Scientific). Samples were equalized for total protein concentrations and equal volumes of supernatants incubated with the working solution. The luminescent signals were detected immediately using Multimode Detector DTX880 (Beckman).
Neurite outgrowth assay and Immunofluorescence
Cortical neurons were plated on acid washed glass coverslips (15 mm diameter, Fisher Scientific) coated with nitrocelluose (Sigma) followed by poly-D-lysine and washed in sterile distilled water. 100 ul of 4 ng/µl solution of Nogo-A-Fc corresponding to the Nogo66 sequence (R&D Systems) was dropped and dried on each coverslip. Cortical neurons (3×104) were plated in each well and transfected for 2h at DIV1 with either vHspB1 or vC alone, or co-transfected with vHspB1 and lentiviral vectors (lenti-con or lenti-20a-I or lenti-128-I), then fixed 48h later with 4% paraformaldehyde and immunostained with antibodies against neuron-specific βIII tubulin (Tuj1, Covance), HspB1 (Santa Cruz), MAP2 (Millipore) and rhodamine phalloidin (Invitrogen). All coverslips were mounted using Prolong Gold with DAPI medium (Invitrogen) and sealed with nail polish. The length of neurites was determined using Meta Imaging software (Molecular Devices). More than 100 randomly selected individual neurons were analyzed per condition in each experiment and the experiment was repeated three times.
Data analysis
All quantitative data were analyzed by student two tail t-test. Each experiment was performed at least three independent times and the data expressed as mean ± SEM. P values of < 0.05 were considered significant.
RESULTS
Expression of HspB1 from vHspB1 in cortical neurons
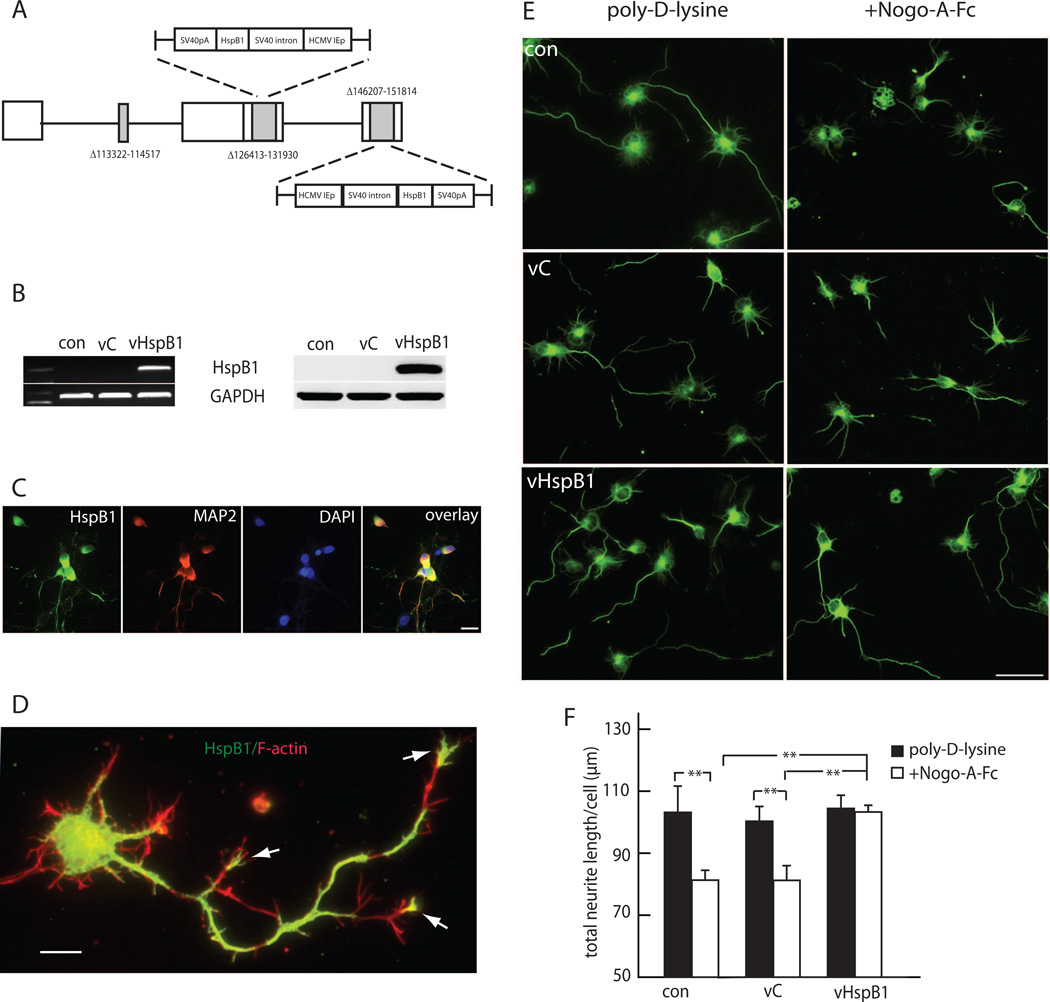
We chose to use embryonic cortical neurons for these studies, because during development the basal expression of HspB1 in these cells is very low so that the effects of HspB1 expression from vHspB1 could readily be examined. The genomic HSV vector vHspB1 we constructed is deleted for four essential early genes that render the viral construct replication incompetent, and carries two copies of HspB1 cDNA, under the control of HCMV IEp in the ICP4 locus (Figure 1A). Cortical neurons in vitro infected with vHspB1 expressed HspB1 mRNA and protein (Figure 1B). HspB1 was not detected in control or vC-infected neurons. Transfection with the vector was quite efficient, with most of the neurons in the vHspB1 transfected cultures expressing HspB1 by immunocytochemistry (Figure 1C). The transgene protein was found in neurites extending to the growth cones of the longest neurites. where HspB1 co-localized with F-actin as determined by phalloidin binding staining (Figure 1D, arrows).
FIGURE 1. HspB1 reverses the inhibitory effect of Nogo-A on neurite growth.
A. vHspB1 construct. B. Cortical neurons transfected with vHspB1 express HspB1 mRNA (left) and protein (right). C. HspB1 is expressed in the majority of neurons in the culture after transfection with vHspB1. D. Transgene mediated HspB1 (green) was found in neurites and growth cones (arrows) where HspB1 co-localized with phalloidin binding F-actin (red). Scale bar = 10 µm. E. Transfection with vHspB1 prevented inhibition of neurite extension by NogoA-Fc (right panels). Immunostained with anti-βIII tubulin antibody; scale bar = 20µm. F. Quantitative analysis of neurite length. (n=3; **P < 0.01).
HspB1 enhances neurite growth response
To assess the effects of HspB1 expression on neurite growth we took advantage of the known inhibitory effects of Nogo-A (Fournier et al., 2001; GrandPre et al., 2000; Peng et al., 2010). The extracellular Nogo66 loop of Nogo-A interacts with its cognate receptor NogoR1 to induce growth cone collapse and inhibit neurite growth extension (Niederost et al., 2002). Cortical neurons grown on a substrate of Nogo-A-Fc fusion protein containing the Nogo66 sequence (aa 1026–1090) over poly-D-lysine showed a marked reduction of neurite growth length when compared to neurons growing on a similar substrate without Nogo-A-Fc. In contrast, neurons infected with vHspB1 and grown on Nogo-AFc substrate had neurite length similar to non-infected neurons or to neurons transfected with vC cultured in the absence of Nogo-A-Fc substrate (Figure 1E and F), suggesting that HspB1 expression protected the neurons against the inhibitory effects of Nogo-A and restored intrinsic neurite growth capacity.
HspB1 reduces RhoA activity
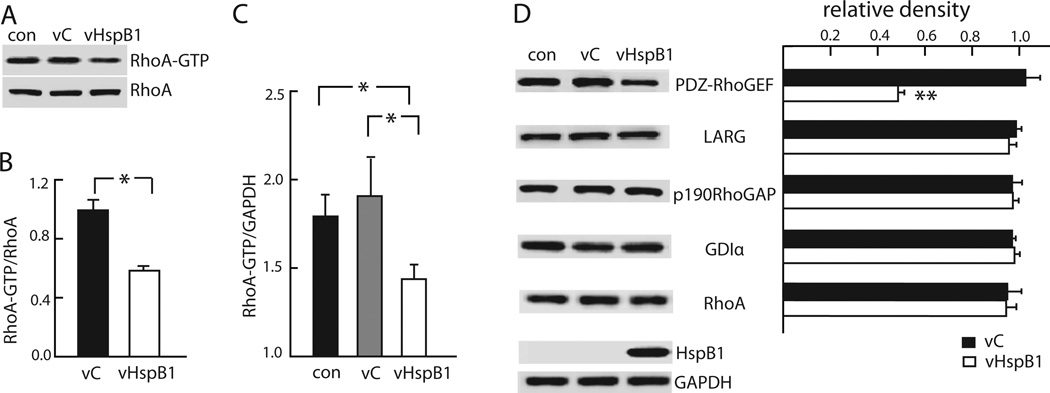
Neurite growth inhibition caused by Nogo-A is mediated through activation of Rho A and its downstream signaling pathways that regulates actin cytoskeleton and actomyosin contractility in developing axons and growth cones (Niederost et al., 2002). RhoA functions as molecular switch, shuttling between an inactive GDP bound conformation and an active GTP bound conformation. In order to assess whether the effects of HspB1 in enhancing neurite growth is mediated by the regulation of RhoA activity we used two well-characterized assays. Using the GST-RBD pull down assay, in which GTP-bound RhoA is isolated using a Rho binding domain (RBD) of rhotekin fused to gluthathione-S-transferase (GST), we analyzed cortical neurons infected with vHspB1, vC or uninfected control and determined total RhoA and RhoA-GTP. A marked decrease in RhoA-GTP was found in cortical neurons expressing HspB1 when compared to control conditions (Figure 2A and B). Using an ELISA based Rho activation assay (G-LISA) we found that HspB1 expression decreased the levels of RhoA-GTP trapped by the antibody (Figure 2C). Taken together these assays indicate that HspB1 markedly reduces RhoA activity without affecting total RhoA protein levels in neurons.
FIGURE 2. HspB1 inhibits RhoA activity and reduces PDZ-RhoGEF protein levels.
A. Transfection of cortical neurons with vHspB1 reduced active RhoA (RhoA-GTP) determined by Rhotekin-RBD bead pull down assay. B. Relative abundance of RhoA-GTP compared to RhoA was determined and the ratio normalized to control (*P<0.05). C. Cortical neurons transfected with vHspB1 have reduced RhoA activity determined by G-LISA; normalized to GAPDH. (*p = <0.05). D. Transfection with vHspB1 reduced PDZ-RhoGEF but not p190RhoGAP, LARG, Rho GDP dissociation inhibitor alpha (GDIα) or RhoA protein levels. The relative density of each band normalized to GAPDH compared to control. (n=3; **P < 0.01).
HspB1 decreases PDZ-RhoGEF in neurons
Rho GTPase activity is regulated by the opposing effects of GEFs and GAPs and GDIs. GEFs enhance the exchange of GDP for GTP thus activating Rho, GAPs inhibit Rho by potentiating intrinsic GTPase activity, while GDIs bind to Rho-GDP to maintain Rho in the inactive state in the cytosol. Inhibition of neurite growth by Nogo-A results from binding of Nogo-A to the NogoR1-p75NTR receptor complex, increasing the association of p75NTR with Rho-GDIα, freeing Rho-GDP to be converted to Rho-GTP by the actions of GEFs (Yamashita and Tohyama, 2003). In order to assess whether the HspB1 inhibitory effects on RhoA are mediated through a Rho regulatory molecule we examined the effect of HspB1 on PDZ-RhoGEF (Arhgef11) and leukemia associated RhoGEF (LARG; Arhgef12) as well as the RhoA inhibitors p190RhoGAP and RhoGDIα. Expression of HspB1 in cortical neurons resulted in a marked decrease in PDZ-RhoGEF protein levels while the levels of LARG, p190RhoGAP and RhoGDIα where not changed by HspB1 expression when compared to neurons infected with vC and uninfected neurons (Figure 2D). This data indicates that the HspB1-mediated regulation of RhoA activity occurs through regulation of PDZ-RhoGEF, an alternative pathway to that engaged by Nogo-A.
HspB1 does not alter PDZ-RhoGEF protein degradation or transcription levels
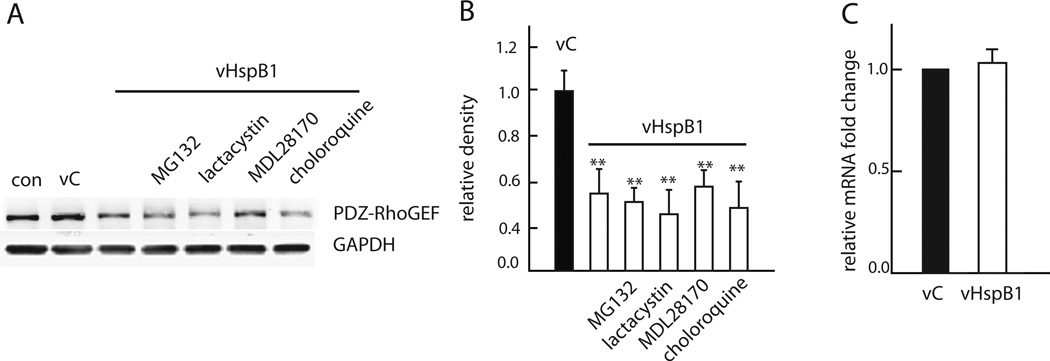
The decrease in PDZ-RhoGEF in cortical neurons suggested the possibility of enhanced degradation by an HspB1-mediated mechanism. We treated neurons expressing HspB1 with proteasome inhibitors MG132 and lactacystin, with the calpain and cathepsin B inhibitor MDL28170 or with the lysosome inhibitor chloroquine applied independently for 24 hr. The reduction in PDZRhoGEF induced by HspB1 was unchanged by these inhibitors as compared to untreated neurons (Figure 3A and 3B), indicating that the effects of HspB1 expression on PDZ-RhoGEF did not result from increased protein degradation (Figure 3A and B).
FIGURE 3. Reduction in PDZ-RhoGEF by HspB1 is independent of protein degradation or transcriptional regulation.
A. The effect of vHspB1 on PDZ-RhoGEF was not affected by addition of the proteasome inhibitors MG132 (10 µM) or lactacystin (10 µM), the calpain and cathepsin B inhibitor MDL28170 (20 µM), or the lysosome inhibitor chloroquine (20 µM) B. Quantitative analysis of PDZ-RhoGEF, normalized to GAPDH compared to control. (n=3; **P < 0.01). C. qPCR of PDZ-RhoGEF mRNA levels calculated by 2-^ΔΔCt method using GAPDH as an internal control.
The decrease in PDZ-RhoGEF protein levels caused by HspB1 in cortical neurons was not a consequence of transcription regulation as qPCR determination of PDZ-RhoGEF mRNA levels were unchanged in neurons by HspB1 expression when compared to control (Figure 3C). Together the results suggest an alternative mechanism used by HspB1 for the regulation of PDZRhoGEF that is independent of transcription and protein degradation.
HspB1 enhances the expression of miR-20a, miR-128 and miR-132
A group of small noncoding RNAs (miRNAs) are effective post-transcriptional gene expression regulators that bind to the 3’UTR of target mRNAs by sequence complementarity to diminish efficiency of translation (van Rooij, 2011) and a high number of unique miRNAs have been isolated from the nervous system. In order to explore the possibility that HspB1 downregulation of PDZ-RhoGEF occurs by post-translational silencing. We sequenced the 3’UTR of rat PDZ-RhoGEF mRNA and found several sequences as potential miRNA binding sites. Following the initial screen we selected miR-20a, miR-128 and miR-132 as the most likely candidates for regulation (Fig 4A top and B). While the 3’UTR of human PDZ-RhoGEF sequence is different from the rat sequence, we found that the binding sites for miR-20a, miR-128 and miR-132 are conserved between rat and human (Figure 4A bottom). In cortical neurons transfected with vHspB1 we observed a marked increase in the expression of miR-20a, miR-128 and miR-132 as compared with neurons infected with vC, while expression of miR-124 used as sequence unmatched control was not changed. U6 was used as internal control for the quantitative microRNA RT-PCR determinations (Figure 4C). The results point to the enhanced expression of a small group of target miRNAs by HspB1 may control PDZ-RhoGEF levels by transcriptional silencing.
Inhibitors of miR-20a and miR-128 reverse PDZ-RhoGEF translation inhibition caused by HspB1
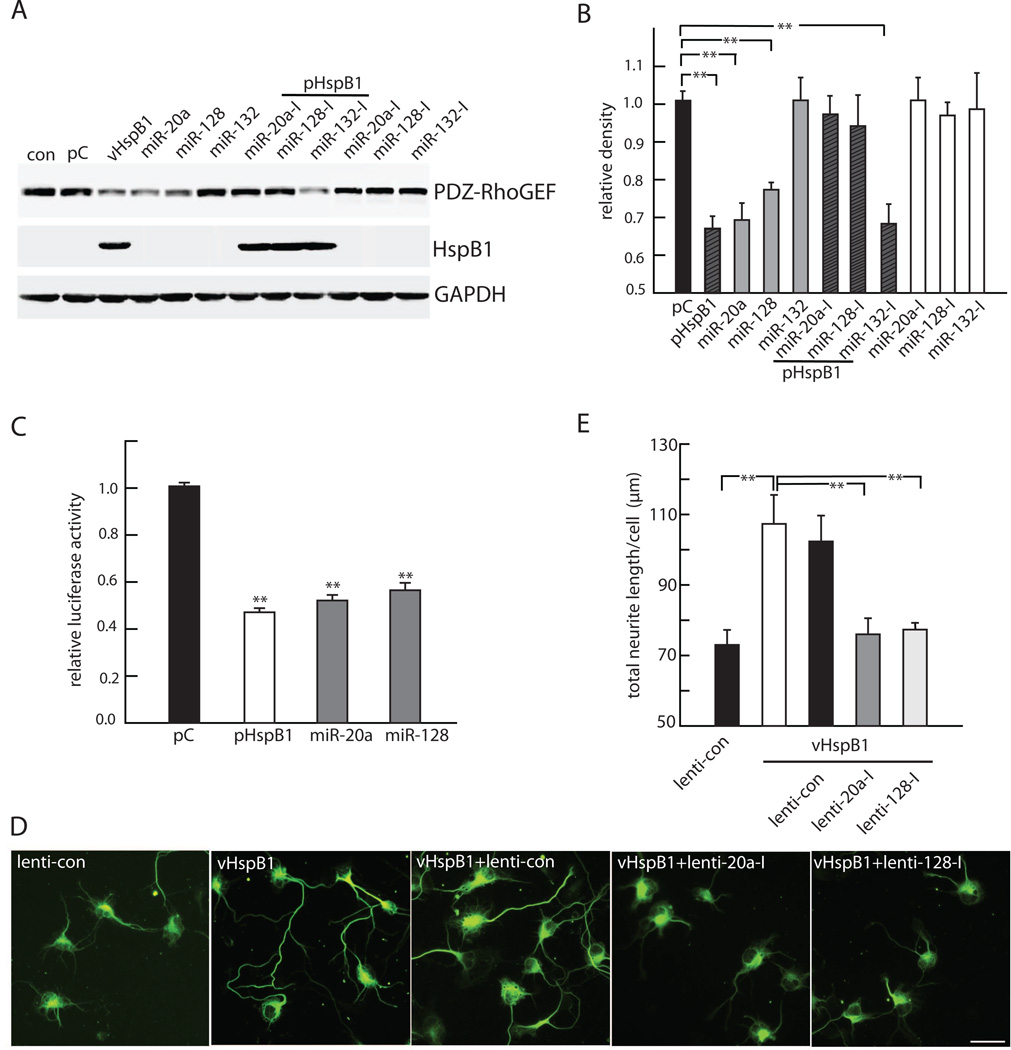
In order to characterize the translation regulation of PDZ-RhoGEF by miRNAs downstream of HspB1 we used commercially available plasmids containing either the cDNA sequence of the miR-20a, miR-128 and miR-132 or their specific antagomirs, complementary oligonucleotide sequences engineered to specifically capture and silence the target miRNAs respectively. We used the neuronal cell line Neuro-2a to efficiently deliver the target miRNAs and their specific antagomir plasmids in cells transfected with either pHspB1 or control plasmid. We achieved similar expression levels of HspB1 protein in transfected Neuro-2a cells as we had observed in primary cortical neurons and there was a similar decrease in PDZ-RhoGEF protein in response to HspB1 expression (Fig 5A and B). The effect on PDZ-RhoGEF level caused by expression of HspB1 was mimicked by miR-20a and miR-128, while transfection of miR-132 had no significant effect on PDZ-RhoGEF level as compared to control (Figure 5A and B). Cotransfection of pHspB1 with the antagomirs miR-20a-I and miR-128-I prevented the silencing effect of HspB1 on PDZ-RhoGEF translation without altering levels of HspB1 protein while cotransfection of the antagomir of miR-132-I had no effect on the reduction of PDZ-RhoGEF protein caused by HspB1 expression. Transfection with specific antagomirs in the absence of overexpression of HspB1 had no effect on PDZ-RhoGEF expression levels (Figure 5A and B), suggesting that in control cells where the levels of the relevant miRNAs is low, there is a “floor” effect because the levels cannot be driven detectably lower. Together these results indicate that miR-20a and miR-128 are translational repressors of PDZ-RhoGEF downstream of HspB1.
FIGURE 5. HspB1 decreases PDZ-RhoGEF through regulation of miR-20a and miR-128.
A. Neuro-2a cells transfected with pHspB1, miR-20a or miR-128 show a reduction in PZD-RhoGEF measured by Western blot. PDZ-RhoGEF level was unchanged by miR-132. The effect of pHspB1 is blocked by the specific inhibitors miR-20a-I and miR-128-I but not by mi-R132-I. The inhibitors alone have no effect on PDZ-RhoGEF levels. B. Quantitative analysis of PDZRhoGEF normalized to GAPDH and compared to control (n=3. **P < 0.01). C. Neuro-2a cells transfected with pmirGlo luciferase plasmid containing the 3’ UTR of PDZ-RhoGEF and: pAcGFP1-HspB1 (pHspB1); pIRES2-AcGFP1(pC); miR-20a; or, miR-128 plasmids and luciferase activity was measured 48h after transfection. Relative luciferase activity was normalized to the control. (n=3; ** P < 0.01). D. Cortical neurons (DIV1) plated on Nogo-A-Fc coated coverslips and transfected with lenti-control, or double transfected with vHspB1 and either lenti-control, lenti-20a-I or lenti-128-I; and 48h later immunostained with anti-βIII tubulin antibody. Scale bar = 20 µm. E. Quantitative analysis of neurite length showing lenti-20a-I and lenti-128-I transfection prevented the enhanced neurite growth effect of HspB1 (n=3; **P < 0.01).
Translational silencing of PDZ-RhoGEF by HspB1, miR-20a and miR-128
To determine the specific targeting of miR-20a and miR-128 to the 3’UTR of PDZ-RhoGEF mRNA, we cloned the 3’UTR sequence into the plasmid pmiRGlo carrying a luciferase reporter gene. Neuro-2a cells were co-transfected with the 3’UTR modified pmirGlo plasmid construct and either HspB1, miR-20a or miR-128 plasmids and analyzed 48h later. Transfection with miR-20a and miR-128 or HspB1 produced a 50% reduction in luciferase activity as compared to unmodified plasmid transduced cells used as control (Figure 5C). The results indicate that miR-20a and miR-128 binding to the PDZ-RhoGEF 3’UTR regulates the expression of the gene and confirm that HspB1 exerts translational silencing activity on PDZ-RhoGEF at least in part by miR-20a and miR-128.
Inhibitors of miR-20a and miR-128 block the neurite growth promoting effects of HspB1
In order to assess if the regulation of PDZ-RhoGEF through translation inhibition is important for the neurite growth effects of HspB1 we used lentiviral vectors expressing either the antagomirs of miR-20a and miR-128 or vector control. The transfection efficiency of the lentiviral vectors in cortical neurons was greater than 90% as determined by expression of mCherry reporter gene (data not shown). In cortical neurons co-transfection of vHspB1 with lenti vectors expressing either miR-20a-I or miR-128-I for 48 h blocked the neurite growth promoting effects of HspB1 in cortical neurons plated on Nogo-A-Fc substrate (Fig 5D and E). The results suggest that miR-20a and miR-128 are key mediators of the neurite growth response of HspB1 most likely through translational silencing of PDZRhoGEF to reduce RhoA activation.
DISCUSSION
The aim of the present study was to delineate the role that HspB1 play in neurite growth and to define the mechanism of action underlying this effect. We found that HspB1 prevents the inhibitory effects of Nogo-A on neurite growth in cortical neurons where HspB1 expression lowers the basal level of RhoA GTP by translational silencing of the guanine nucleotide exchange factor PDZ-RhoGEF, a RhoA specific GEF found in neurons. HspB1 enhances the expression of a group of microRNAs including miR-20a, miR-128 and miR-132; two of these microRNAs, miR-20a and miR-128 bind to the 3’UTR promoter region of PDZ RhoGEF mRNA to inhibit translation. These results provide a previously undescribed link between HspB1 and PDZ-RhoGEF in the regulation of neurite growth and a novel mechanism used by HspB1 to regulate translational activity of specific target molecules.
Prior studies have demonstrated that HspB1 regulates processing and stability of mRNA by acting as a chaperone protein. HspB1 can enhance the rate of RNA decay by forming a complex with cap dependent translation initiation factors and other 3’UTR binding proteins to attract the ribonuclease machinery necessary for degradation of targeted mRNAs (Cuesta et al., 2000; Knapinska et al., 2011; Sinsimer et al., 2008) or modulate the activity of the splicing regulator SRp38 (Marin-Vinader et al., 2006). In association with HspB8, a closely related family member, HspB1 may participate in a complex that binds the DEAD box protein Ddx20 and the survival motor neuron proteins to regulate pre-RNA processing (Sun et al., 2010). In addition, HspB8 has been reported to cause a non-chaperone mediated translational arrest by inducing the phosphorylation of translation initiator factor 2α (Carra et al., 2009).
The novel finding of the current study is the demonstration of an alternative non-chaperone mechanism of translational regulation by HspB1 in neurons through enhanced expression of miRNAs. While increased expression of miR-20a and miR-128 silences translation of PDZ-RhoGEF, expression of miR-132 does not affect PDZ-RhoGEF, but may be regulating additional targets. This would be consistent with reports that miR-132 can induce dendritic maturation and modulate synaptic plasticity in hippocampus and perirhinal cortex (Magill et al., 2010; Scott et al., 2012). Transfection with plasmids expressing either miR-20A or miR-128 alone each had effects similar in magnitude to the effect of HspB1 on PDZ-RhoGEF expression measured by luciferase activity and by protein levels, reflecting a “ceiling” effect that is reached in these overexpression models.
PDZ-RhoGEF (Arhgef11) and LARG (Arhgef12) belong to a subgroup of the Dbl family of GEFs that contain a regulator of G protein signaling domain, and function upstream of Rho GTPases to initiate signaling in response to growth factors, cytokines, cell adhesion molecules or in response to direct cell-to-cell contacts (Zheng, 2001). PDZ-RhoGEF and LARG are specific activators of RhoA, but do not activate other members of the RhoGTPase family (Reuther et al., 2001; Togashi et al., 2000). HspB1 inhibited translation of PDZ-RhoGEF but not of LARG. In contrast to LARG that is found in most cellular elements of the nervous system, PDZ-RhoGEF is predominantly a neuronal protein and localizes to processes in the neuropil (Banerjee and Wedegaertner, 2004; Kuner et al., 2002; Togashi et al., 2000). The observation that HspB1 reduced PDZ-RhoGEF but did not change the levels of LARG or other RhoA regulatory proteins suggests that HspB1 regulation of PDZ-RhoGEF is an important step in establishing the level of RhoA activity in neurites and in protecting the neurons against the effects of neurite growth inhibitors.
In neurons, where specialized cellular domains require local control of protein synthesis, the targeting of ribosomes and miRNAs has been demonstrated in the synaptic terminal (Krichevsky and Kosik, 2001; Lugli et al., 2008) and in growth cones of developing axons (Hengst et al., 2006; Hengst et al., 2009). Following injury local regulation of protein synthesis in axons occurs in the setting of regeneration (Twiss and van Minnen, 2006; Zheng et al., 2001). Of particular interest is that RhoA mRNA and protein are found in neurites and growth cones and a similar localization has been described for HspB1 and PDZ-RhoGEF, suggesting that the interaction between these molecules and their regulation may occur locally (Williams et al., 2005; Wu et al., 2005).
In summary our studies support that HspB1 translational silencing of PDZ-RhoGEF may be important not only during development when Rho-GTPase homeostasis regulates axon growth and guidance but also possibly following injury, where RhoA is activated and inhibition of RhoA-dependent signaling is essential for the cytoskeletal changes required for axonal regeneration.
ACKNOWLEDGMENTS
We acknowledge the excellent technical assistance of Vikram Thakur Singh who propagated the HSV vectors. This work was supported by grants from the Department of Veterans Affairs and from the NIH NS038850.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no conflicts of interest related to this work.
REFERENCES
- Ackerley S, James PA, Kalli A, French S, Davies KE, Talbot K. A mutation in the small heat-shock protein HSPB1 leading to distal hereditary motor neuronopathy disrupts neurofilament assembly and the axonal transport of specific cellular cargoes. Hum Mol Genet. 2006;15:347–354. doi: 10.1093/hmg/ddi452. [DOI] [PubMed] [Google Scholar]
- Almeida-Souza L, Asselbergh B, d'Ydewalle C, Moonens K, Goethals S, de Winter V, Azmi A, Irobi J, Timmermans JP, Gevaert K, Remaut H, Van Den Bosch L, Timmerman V, Janssens S. Small heat-shock protein HSPB1 mutants stabilize microtubules in Charcot-Marie-Tooth neuropathy. J Neurosci. 2011;31:15320–15328. doi: 10.1523/JNEUROSCI.3266-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida-Souza L, Asselbergh B, De Winter V, Goethals S, Timmerman V, Janssens S. HSPB1 Facilitates the Formation of Non-Centrosomal Microtubules. PLoS One. 2013;8:e66541. doi: 10.1371/journal.pone.0066541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee J, Wedegaertner PB. Identification of a novel sequence in PDZ-RhoGEF that mediates interaction with the actin cytoskeleton. Mol Biol Cell. 2004;15:1760–1775. doi: 10.1091/mbc.E03-07-0527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedetti S, Previtali SC, Coviello S, Scarlato M, Cerri F, Di Pierri E, Piantoni L, Spiga I, Fazio R, Riva N, Natali Sora MG, Dacci P, Malaguti MC, Munerati E, Grimaldi LM, Marrosu MG, De Pellegrin M, Ferrari M, Comi G, Quattrini A, Bolino A. Analyzing histopathological features of rare charcot-marie-tooth neuropathies to unravel their pathogenesis. Arch Neurol. 2010;67:1498–1505. doi: 10.1001/archneurol.2010.303. [DOI] [PubMed] [Google Scholar]
- Benn SC, Perrelet D, Kato AC, Scholz J, Decosterd I, Mannion RJ, Bakowska JC, Woolf CJ. Hsp27 upregulation and phosphorylation is required for injured sensory and motor neuron survival. Neuron. 2002;36:45–56. doi: 10.1016/s0896-6273(02)00941-8. [DOI] [PubMed] [Google Scholar]
- Carra S, Brunsting JF, Lambert H, Landry J, Kampinga HH. HspB8 participates in protein quality control by a non-chaperone-like mechanism that requires eIF2{alpha} phosphorylation. J Biol Chem. 2009;284:5523–5532. doi: 10.1074/jbc.M807440200. [DOI] [PubMed] [Google Scholar]
- Cuesta R, Laroia G, Schneider RJ. Chaperone hsp27 inhibits translation during heat shock by binding eIF4G and facilitating dissociation of cap-initiation complexes. Genes Dev. 2000;14:1460–1470. [PMC free article] [PubMed] [Google Scholar]
- d'Ydewalle C, Krishnan J, Chiheb DM, Van Damme P, Irobi J, Kozikowski AP, Vanden Berghe P, Timmerman V, Robberecht W, Van Den Bosch L. HDAC6 inhibitors reverse axonal loss in a mouse model of mutant HSPB1-induced Charcot-Marie-Tooth disease. Nat Med. 2011;17:968–974. doi: 10.1038/nm.2396. [DOI] [PubMed] [Google Scholar]
- Doshi BM, Hightower LE, Lee J. HSPB1, actin filament dynamics, and aging cells. Ann N Y Acad Sci. 2010;1197:76–84. doi: 10.1111/j.1749-6632.2010.05191.x. [DOI] [PubMed] [Google Scholar]
- Evgrafov OV, Mersiyanova I, Irobi J, Van Den Bosch L, Dierick I, Leung CL, Schagina O, Verpoorten N, Van Impe K, Fedotov V, Dadali E, Auer-Grumbach M, Windpassinger C, Wagner K, Mitrovic Z, Hilton-Jones D, Talbot K, Martin JJ, Vasserman N, Tverskaya S, Polyakov A, Liem RK, Gettemans J, Robberecht W, De Jonghe P, Timmerman V. Mutant small heat-shock protein 27 causes axonal Charcot-Marie-Tooth disease and distal hereditary motor neuropathy. Nat Genet. 2004;36:602–606. doi: 10.1038/ng1354. [DOI] [PubMed] [Google Scholar]
- Fournier AE, GrandPre T, Strittmatter SM. Identification of a receptor mediating Nogo-66 inhibition of axonal regeneration. Nature. 2001;409:341–346. doi: 10.1038/35053072. [DOI] [PubMed] [Google Scholar]
- Garcia MC, Ray DM, Lackford B, Rubino M, Olden K, Roberts JD. Arachidonic acid stimulates cell adhesion through a novel p38 MAPK-RhoA signaling pathway that involves heat shock protein 27. J Biol Chem. 2009;284:20936–20945. doi: 10.1074/jbc.M109.020271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geraldo S, Gordon-Weeks PR. Cytoskeletal dynamics in growth-cone steering. J Cell Sci. 2009;122:3595–3604. doi: 10.1242/jcs.042309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GrandPre T, Nakamura F, Vartanian T, Strittmatter SM. Identification of the Nogo inhibitor of axon regeneration as a Reticulon protein. Nature. 2000;403:439–444. doi: 10.1038/35000226. [DOI] [PubMed] [Google Scholar]
- Hengst U, Cox LJ, Macosko EZ, Jaffrey SR. Functional and selective RNA interference in developing axons and growth cones. J Neurosci. 2006;26:5727–5732. doi: 10.1523/JNEUROSCI.5229-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hengst U, Deglincerti A, Kim HJ, Jeon NL, Jaffrey SR. Axonal elongation triggered by stimulus-induced local translation of a polarity complex protein. Nat Cell Biol. 2009;11:1024–1030. doi: 10.1038/ncb1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hino M, Kurogi K, Okubo MA, Murata-Hori M, Hosoya H. Small heat shock protein 27 (HSP27) associates with tubulin/microtubules in HeLa cells. Biochem Biophys Res Commun. 2000;271:164–169. doi: 10.1006/bbrc.2000.2553. [DOI] [PubMed] [Google Scholar]
- Holmgren A, Bouhy D, De Winter V, Asselbergh B, Timmermans JP, Irobi J, Timmerman V. Charcot-Marie-Tooth causing HSPB1 mutations increase Cdk5-mediated phosphorylation of neurofilaments. Acta Neuropathol. 2013;126:93–108. doi: 10.1007/s00401-013-1133-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houlden H, Laura M, Wavrant-De Vrieze F, Blake J, Wood N, Reilly MM. Mutations in the HSP27 (HSPB1) gene cause dominant, recessive, and sporadic distal HMN/CMT type 2. Neurology. 2008;71:1660–1668. doi: 10.1212/01.wnl.0000319696.14225.67. [DOI] [PubMed] [Google Scholar]
- Kalmar B, Burnstock G, Vrbova G, Greensmith L. The effect of neonatal nerve injury on the expression of heat shock proteins in developing rat motoneurones. J Neurotrauma. 2002;19:667–679. doi: 10.1089/089771502753754127. [DOI] [PubMed] [Google Scholar]
- Kappe G, Franck E, Verschuure P, Boelens WC, Leunissen JA, de Jong WW. The human genome encodes 10 alpha-crystallin-related small heat shock proteins: HspB1-10. Cell Stress Chaperones. 2003;8:53–61. doi: 10.1379/1466-1268(2003)8<53:thgecs>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapinska AM, Gratacos FM, Krause CD, Hernandez K, Jensen AG, Bradley JJ, Wu X, Pestka S, Brewer G. Chaperone Hsp27 modulates AUF1 proteolysis and AU-rich element-mediated mRNA degradation. Mol Cell Biol. 2011;31:1419–1431. doi: 10.1128/MCB.00907-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krichevsky AM, Kosik KS. Neuronal RNA granules: a link between RNA localization and stimulation-dependent translation. Neuron. 2001;32:683–696. doi: 10.1016/s0896-6273(01)00508-6. [DOI] [PubMed] [Google Scholar]
- Kuner R, Swiercz JM, Zywietz A, Tappe A, Offermanns S. Characterization of the expression of PDZ-RhoGEF, LARG and G(alpha)12/G(alpha)13 proteins in the murine nervous system. Eur J Neurosci. 2002;16:2333–2341. doi: 10.1046/j.1460-9568.2002.02402.x. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lugli G, Torvik VI, Larson J, Smalheiser NR. Expression of microRNAs and their precursors in synaptic fractions of adult mouse forebrain. J Neurochem. 2008;106:650–661. doi: 10.1111/j.1471-4159.2008.05413.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma CH, Omura T, Cobos EJ, Latremoliere A, Ghasemlou N, Brenner GJ, van Veen E, Barrett L, Sawada T, Gao F, Coppola G, Gertler F, Costigan M, Geschwind D, Woolf CJ. Accelerating axonal growth promotes motor recovery after peripheral nerve injury in mice. J Clin Invest. 2011;121:4332–4347. doi: 10.1172/JCI58675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magill ST, Cambronne XA, Luikart BW, Lioy DT, Leighton BH, Westbrook GL, Mandel G, Goodman RH. microRNA-132 regulates dendritic growth and arborization of newborn neurons in the adult hippocampus. Proc Natl Acad Sci U S A. 2010;107:20382–20387. doi: 10.1073/pnas.1015691107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin-Vinader L, Shin C, Onnekink C, Manley JL, Lubsen NH. Hsp27 enhances recovery of splicing as well as rephosphorylation of SRp38 after heat shock. Mol Biol Cell. 2006;17:886–894. doi: 10.1091/mbc.E05-07-0596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miron T, Vancompernolle K, Vandekerckhove J, Wilchek M, Geiger B. A 25-kD inhibitor of actin polymerization is a low molecular mass heat shock protein. J Cell Biol. 1991;114:255–261. doi: 10.1083/jcb.114.2.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mounier N, Arrigo AP. Actin cytoskeleton and small heat shock proteins: how do they interact? Cell Stress Chaperones. 2002;7:167–176. doi: 10.1379/1466-1268(2002)007<0167:acashs>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mymrikov EV, Seit-Nebi AS, Gusev NB. Large potentials of small heat shock proteins. Physiol Rev. 2011;91:1123–1159. doi: 10.1152/physrev.00023.2010. [DOI] [PubMed] [Google Scholar]
- Niederost B, Oertle T, Fritsche J, McKinney RA, Bandtlow CE. Nogo-A and myelin-associated glycoprotein mediate neurite growth inhibition by antagonistic regulation of RhoA and Rac1. J Neurosci. 2002;22:10368–10376. doi: 10.1523/JNEUROSCI.22-23-10368.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolic M. The role of Rho GTPases and associated kinases in regulating neurite outgrowth. Int J Biochem Cell Biol. 2002;34:731–745. doi: 10.1016/s1357-2725(01)00167-4. [DOI] [PubMed] [Google Scholar]
- Peng X, Zhou Z, Hu J, Fink DJ, Mata M. Soluble Nogo receptor down-regulates expression of neuronal Nogo-A to enhance axonal regeneration. J Biol Chem. 2010;285:2783–2795. doi: 10.1074/jbc.M109.046425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perng MD, Cairns L, van den IP, Prescott A, Hutcheson AM, Quinlan RA. Intermediate filament interactions can be altered by HSP27 and alphaB-crystallin. J Cell Sci. 1999;112(Pt 13):2099–2112. doi: 10.1242/jcs.112.13.2099. [DOI] [PubMed] [Google Scholar]
- Reuther GW, Lambert QT, Booden MA, Wennerberg K, Becknell B, Marcucci G, Sondek J, Caligiuri MA, Der CJ. Leukemia-associated Rho guanine nucleotide exchange factor, a Dbl family protein found mutated in leukemia, causes transformation by activation of RhoA. J Biol Chem. 2001;276:27145–27151. doi: 10.1074/jbc.M103565200. [DOI] [PubMed] [Google Scholar]
- Samuel F, Hynds DL. RHO GTPase signaling for axon extension: is prenylation important? Mol Neurobiol. 2010;42:133–142. doi: 10.1007/s12035-010-8144-2. [DOI] [PubMed] [Google Scholar]
- Scott HL, Tamagnini F, Narduzzo KE, Howarth JL, Lee YB, Wong LF, Brown MW, Warburton EC, Bashir ZI, Uney JB. MicroRNA-132 regulates recognition memory and synaptic plasticity in the perirhinal cortex. Eur J Neurosci. 2012;36:2941–2948. doi: 10.1111/j.1460-9568.2012.08220.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinsimer KS, Gratacos FM, Knapinska AM, Lu J, Krause CD, Wierzbowski AV, Maher LR, Scrudato S, Rivera YM, Gupta S, Turrin DK, De La Cruz MP, Pestka S, Brewer G. Chaperone Hsp27, a novel subunit of AUF1 protein complexes, functions in AU-rich element-mediated mRNA decay. Mol Cell Biol. 2008;28:5223–5237. doi: 10.1128/MCB.00431-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava AK, Renusch SR, Naiman NE, Gu S, Sneh A, Arnold WD, Sahenk Z, Kolb SJ. Mutant HSPB1 overexpression in neurons is sufficient to cause age-related motor neuronopathy in mice. Neurobiol Dis. 2012;47:163–173. doi: 10.1016/j.nbd.2012.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X, Fontaine JM, Hoppe AD, Carra S, DeGuzman C, Martin JL, Simon S, Vicart P, Welsh MJ, Landry J, Benndorf R. Abnormal interaction of motor neuropathy-associated mutant HspB8 (Hsp22) forms with the RNA helicase Ddx20 (gemin3) Cell Stress Chaperones. 2010;15:567–582. doi: 10.1007/s12192-010-0169-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Togashi H, Nagata K, Takagishi M, Saitoh N, Inagaki M. Functions of a rho-specific guanine nucleotide exchange factor in neurite retraction. Possible role of a proline-rich motif of KIAA0380 in localization. J Biol Chem. 2000;275:29570–29578. doi: 10.1074/jbc.M003726200. [DOI] [PubMed] [Google Scholar]
- Twiss JL, van Minnen J. New insights into neuronal regeneration: the role of axonal protein synthesis in pathfinding and axonal extension. J Neurotrauma. 2006;23:295–308. doi: 10.1089/neu.2006.23.295. [DOI] [PubMed] [Google Scholar]
- van Rooij E. The art of microRNA research. Circ Res. 2011;108:219–234. doi: 10.1161/CIRCRESAHA.110.227496. [DOI] [PubMed] [Google Scholar]
- Vitriol EA, Zheng JQ. Growth cone travel in space and time: the cellular ensemble of cytoskeleton, adhesion, and membrane. Neuron. 2012;73:1068–1081. doi: 10.1016/j.neuron.2012.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagstaff MJ, Collaco-Moraes Y, Smith J, de Belleroche JS, Coffin RS, Latchman DS. Protection of neuronal cells from apoptosis by Hsp27 delivered with a herpes simplex virus-based vector. J Biol Chem. 1999;274:5061–5069. doi: 10.1074/jbc.274.8.5061. [DOI] [PubMed] [Google Scholar]
- Wang P, Bitar KN. Rho A regulates sustained smooth muscle contraction through cytoskeletal reorganization of HSP27. Am J Physiol. 1998;275:G1454–G1462. doi: 10.1152/ajpgi.1998.275.6.G1454. [DOI] [PubMed] [Google Scholar]
- Williams KL, Rahimtula M, Mearow KM. Hsp27 and axonal growth in adult sensory neurons in vitro. BMC Neurosci. 2005;6:24. doi: 10.1186/1471-2202-6-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams KL, Rahimtula M, Mearow KM. Heat shock protein 27 is involved in neurite extension and branching of dorsal root ganglion neurons in vitro. J Neurosci Res. 2006;84:716–723. doi: 10.1002/jnr.20983. [DOI] [PubMed] [Google Scholar]
- Wu KY, Hengst U, Cox LJ, Macosko EZ, Jeromin A, Urquhart ER, Jaffrey SR. Local translation of RhoA regulates growth cone collapse. Nature. 2005;436:1020–1024. doi: 10.1038/nature03885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita T, Tohyama M. The p75 receptor acts as a displacement factor that releases Rho from Rho-GDI. Nat Neurosci. 2003;6:461–467. doi: 10.1038/nn1045. [DOI] [PubMed] [Google Scholar]
- Zhai J, Lin H, Julien JP, Schlaepfer WW. Disruption of neurofilament network with aggregation of light neurofilament protein: a common pathway leading to motor neuron degeneration due to Charcot-Marie-Tooth disease-linked mutations in NFL and HSPB1. Hum Mol Genet. 2007;16:3103–3116. doi: 10.1093/hmg/ddm272. [DOI] [PubMed] [Google Scholar]
- Zheng JQ, Kelly TK, Chang B, Ryazantsev S, Rajasekaran AK, Martin KC, Twiss JL. A functional role for intra-axonal protein synthesis during axonal regeneration from adult sensory neurons. J Neurosci. 2001;21:9291–9303. doi: 10.1523/JNEUROSCI.21-23-09291.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y. Dbl family guanine nucleotide exchange factors. Trends Biochem Sci. 2001;26:724–732. doi: 10.1016/s0968-0004(01)01973-9. [DOI] [PubMed] [Google Scholar]