Abstract
Ganglioside synthases are glycosyltransferases involved in the biosynthesis of glycoconjugates. A number of ganglioside synthase genes have been cloned and characterized. They are classified into different families of glycosyltransferases based on similarities of their amino acid sequences. Tissue-specific expression of these genes has been analyzed by hybridization using cDNA fragments. Enzymatic characterization with the expressed recombinant enzymes showed these enzymes differ in their donor and acceptor substrate specificities and other biochemical parameters. In vitro enzymatic analysis also showed that one linkage can be synthesized by multiple enzymes and one enzyme may be responsible for synthesis of multiple gangliosides. Following the cloning of the ganglioside synthase genes, the promoters of the key synthase genes in the ganglioside biosynthetic pathway have been cloned and analyzed. All of the promoters are TATA-less, lacking a CCAAT box but containing GC-rich boxes, characteristic of the house-keeping genes, although transcription of ganglioside synthase genes is subject to complex developmental and tissue-specific regulation. A set of cis-acting elements and transcription factors, including Sp1, AP2, and CREB, function in the proximal promoters. Negative-regulatory regions have also been defined in most of the promoters. We present here an overview of these genes and their transcriptional regulation.
Keywords: glycosyltransferase, ganglioside, ganglioside synthase, gene cloning, transcription regulation, transcription factors
Cloning of the Ganglioside Synthase (Glycosyltransferase) Genes
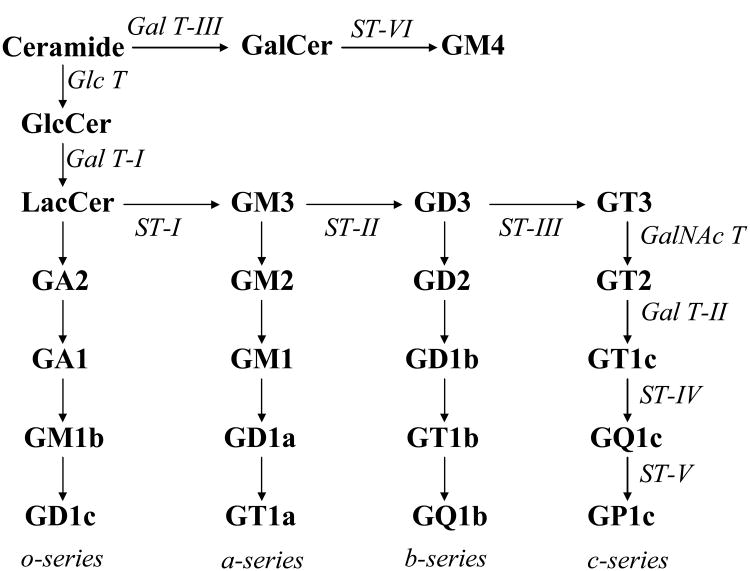
Ganglioside synthases are glycosyltransferases (GTs) that transfer sugar residues from an activated donor substrate, usually a nucleotide-sugar, to a specific acceptor, which may be a lipid, protein, or growing oligosaccharide. GTs are typically grouped into families based on the type of sugar they transfer (i.e., galactosyltransferases, sialyltransferases, and like). Many GT genes have been identified by molecular cloning or homology search of the available genomic databases and have been classified into families by amino-acid-sequence similarities [1, 2] (available at http://afmb.crns-mrs.fr/CAZY and http://glycob.oupjournals.org). Despite the fact that many GTs recognize identical donor or acceptor substrates, surprisingly limited sequence homology exists among different families [2]. However, common structural features are found among mammalian transferases: they are almost all classified as type II integral membrane proteins, with a short amino-terminal cytoplasmic tail, a membrane-anchoring domain, a short proteolytically sensitive stem region, and a large catalytic domain that includes the carboxyl terminus [3]. In addition, analysis of crystal structures of GTs shows that structural folds appear to be more conserved than sequence similarities since only a few topologies exists for most of the GT families although there is a large variability in the acceptor-binding domain [4]. The cloned GT genes (Table 1) involved in ganglioside biosynthetic pathways (Figure 1) are reviewed here.
Table 1. Cloning of the Ganglioside Synthase Genes.
| Glycosyltransferases | Other names in ganglioside biosynthesis | Products | GenBank accession number (References)* |
|---|---|---|---|
| ST3Gal I (EC 2.4.99.4) | GM1b/GD1a/GT1b/GQ1c-synthase, ST-IV | GM1b, GD1a, GT1b, GQ1c | Mmu X73523 (10); Has L29555 (11) |
| ST3Gal V (EC 2.4.99.9) | GM3-synthase, ST-I | GM3 | Has AB018356 (12); Mmu Y15003 (13); Rno AB018049 |
| ST8Sia I (EC 2.4.99.8) | GD3-synthase, ST-II | GD3 (GD1c, GT1a, GQ1b, GT3?) | Has D26360 (15) Mmu X84235; Rno U53883 (19) |
| ST8Sia V | GD1c/GT1a/GQ1b/GQ1c-synthase, ST-V | GD1c, GT1a, GQ1b, GP1c, (GT3?) | Mmu X98014 (24); Has U91641 (25) |
| ST6GalNAc III (EC 2.4.99.7) | GD1α, GM1b | Rno L29554 (29); Y11342 (30) | |
| ST6GalNAc VI | GD1α, GM1b | Mmu AB030836 (31) | |
| UDP-Gal β1,4Gal T | LacCer-synthase, Gal T-I | LacCer | Rno AF048687 (39); Has AF097159 (40) |
| UDP-Gal β1,3Gal T | GA1/GM1/GD1b/GT1c-synthses, Gal T-II | GA1, GM1, GD1b, GT1c | Rno AB003478 (42); Has Y15061 (43); Mmu AF082504 (44) |
| Ceramide UDP-Gal T (EC 2.4.1.45) | GalCer-synthase, Gal T-III | GalCer | Rno L21698 (47); Mmu X92122 (48); Has U62899 (50) |
| β1,4GalNAc T (EC 2.4.1.92) | GA2/GM2/GD2/GT2-synthase, GalNAc T | GA2, GM2, GD2, GT2 | Has M83651 (58); Rno D17809 (59); Mmu L25885 (60) |
| β1,1Glc-T (EC 2.4.1.80) | GlcCer-synthase, Glc T | GlcCer | Has D50840 (66); Mmu D89866 (67); Rno AF047707 (68) |
Sequences obtained by homolog search of the available databases are not present. Mmu, Mus musculus; Rno, Rattus norvegicus; Hsa, Homo sapiens.
Figure 1.
Pathways of ganglioside biosynthesis.
Sialyltransferase Families (EC 2.4.99.y)
The sialyltransferases (STs) are classified as ST3Gal, ST6Gal, ST6GalNAc, and ST8Sia families based on the linkage in which the sialic acid is transferred as well as the acceptor saccharide-specificity. Amino acid sequences of all STs share four peptide-conserved sialylmotifs: L (large), S (small) [5, 6], motif III [7], and VS (very small) [7-9]. Six STs from three ST families are involved in the biosynthetic pathway of major gangliosides (Figure 1).
The ST3Gal family
The ST3Gal family consists of ST3Gal I, II, III, IV, V, and VI, which fall into two main branches (subfamilies) by phylogenetic analysis: ST3Gal I/II and III/IV/V/VI [2]. All of these enzymes transfer Neu5Ac residues in the α2,3-linkage to terminal galactose residues found in glycoproteins or glycolipids but only the ST3Gal I and V use a glycolipid as an acceptor substrate. The cDNA for ST3Gal I (EC 2.4.99.4) was cloned from mouse [10] and human [11]. The mouse gene consists of 6 exons while the human counterpart consists of 9 exons. The transiently expressed product of the mouse ST3Gal I cDNA in COS-7 cells catalyzes the formation of GM1b, GD1a, and GT1b [10], and thus this enzyme is an ST-IV (Figure 1). The cDNA for the human, mouse, and rat ST3Gal V (EC 2.4.99.9) has been cloned [9, 12, 13]. Assays for the expressed enzyme activity showed that ST3Gal V exclusively uses lactosylceramide (Galβ1-4Glcβ1-Cer) as an acceptor substrate to catalyze the synthesis of the ganglioside GM3, and thus is a GM3-synthase (ST-I) [14]. The ST3Gal V gene consists of 7 exons (exon 1 codes for a non-translational region), different from other members of the ST3Gal family [2]. This unique genomic organization likely coincides with its substrate specificity in that ST3Gal V (GM3-synthase,) is the only ganglioside synthase to use Galβ1-4Glcβ1-1′Cer (lactosylceramide) to synthesize GM3.
The ST8Sia family
The ST8Sia family consists of six members (ST8Sia I, II, III, IV, V and VI) in two main branches in the phylogenetic tree (ST8Sia I/V/VI and ST8Sia II/III/IV), based on the amino-acid-sequence similarities [2]. ST8Sia I is involved in the synthesis of gangliosides. The ST8Sia I gene has been cloned from human [15, 16, 17], mouse [18], and rat [19] and consists of 5 exons that show high similarity among the species [2]. Analysis of all cloned enzymes showed that ST8Sia I catalyzes the formation of GD3 [14, 20, 21] and LD1 [20] and thus is a GD3 synthase (ST-II). However, Nara et al. [22] reported that this enzyme utilizes gangliosides GM1b, GD1a, and GT1b as exogenous substrates, in addition to GM3, indicating this enzyme catalyzes the formation of not only GD3, but also GD1c, GT1a, and GQ1b in vitro. In addition, a cloned human ST8Sia I can also efficiently synthesize GT3 in vitro using GD3 as an acceptor [23]. However, it is unlikely that ST8Sia I is actually responsible for the synthesis of GT3 in cells because many cell lines expressing high levels of GD3 do not necessarily synthesize GT3. Therefore, the receptor specificity of ST8Sia I requires further experiments. Another ST in this family catalyzing the synthesis of gangliosides is ST8Sia V. The cDNA for mouse [24] and human [25] ST8Sia V has been cloned. Enzymatic assays in vitro showed ST8Sia V to be involved in the synthesis of several gangliosides including GD1c, GT1a, GQ1b, as well as GT3 [24], and can be considered as an ST-V. However, the ST8Sia I (GD3-synthase) may also synthesize GD1c, GT1a, and GQ1b [22]. Therefore, which, or both, of the ST8Sia I and V is responsible for the synthesis of GD1c/GT1a/GQ1b remains unclear. Besides, the cDNA for mouse [26], rat [27], and human [28] ST8Sia III have been cloned, and the common product was initially suggested to be GT3-synthase. However, analysis of the expressed products of the ST8Sia III cDNA showed an extremely high catalytic activity of transferring a sialic acid residue through α2,8-linkage to glycoproteins, and this enzyme is much more specific to N-linked oligosaccharides of glycolproteins than glycolipids. It is not clear whether ST8Sia III enzyme is indeed GT3-synthase or a ganglioside synthase that utilizes only glycolipids, such as GM3, GD3, and GT3, as acceptors in vivo.
The ST6GalNAc family
A number of the ST6GalNAc genes have been cloned [29-35]. The ST6GalNAc family consists of ST6GalNAc I, II, III, IV, V, and VI, which catalyze the transfer of Neu5Ac residues in an α2–6 linkage to GalNAc residues found in O-glycosylproteins or glycolipids. These six members fall into two main subfamilies by phylogenetic analysis based on sequence similarities: ST6GalNAc I/II and ST6GalNAc III/IV/V/VI [2]. Interestingly, these two subfamilies are in agreement with their substrate specificities as found for the ST3Gal V (GM3-synthase). ST6GalNAc I and II of the first subfamily exhibit broad substrate specificity and catalyze the transfer of Neu5Ac onto Galβ1-3GalNAc peptides [32-34], whereas ST6GalNAc III/IV/V/VI of the second subfamily exhibit more restricted substrate specificity, only utilizing sialylated acceptor substrates (Neu5Acα2-3Galβ1–3GalNAc-R) found either in glycoproteins or glycolipids [29-31, 35]. Such coincidence may indicate that sequence similarities confer functional structures with respect to substrate specificity. As far as can be determined by in vitro assays of the expressed recombinant ST6GalNAc, only two members of the family, ST6GalNAc III [29, 30] and VI [31], can act on the synthesis of glycolipids GD1α and GM1b. Therefore, at least four expressed recombinant sialyltransferases, including ST8Sia V, ST3Gal I, ST6GalNAc III and ST6GalNAc VI, were found to catalyze the formation of GM1b in vitro. However, it is unclear whether GM1b is eventually synthesized from glucosylceramide by four GTs (o-series; Figure 1) under physiological conditions.
Galactosyltransferases (EC 2.4.1.x)
There are three galactosyltransferses (Gal T-I, II and III) [14, 36] involved in the biosynthetic pathway of gangliosides (Figure 1). A conserved putative N-linked glycosylation site is found in all galactosyltransferase genes.
Gal T-I
Gal T-I (UDP-galactose:glucosylceramide β1,4-galactosyltransferase) catalyzes the synthesis of LacCer by transferring galactose from UDP-Gal to glucosylceramide (GlcCer) [14, 37, 38], generating a β1,4-glycosidic linkage. The cDNA for the enzyme catalyzing this reaction has been cloned from rat [39] and human [40]. The gene consists of 10 exons. The deduced amino-acid sequence of the rat Gal T-I shows 39% homology with mouse β1,4-galactosyltransferase (EC 2.4.1.38), which catalyzes the transfer of Gal to β1,4-GlcNAc in glycoproteins. However, the Gal T-I prefers GlcCer as a substrate for glycolipid synthesis, indicating that this cloned Gal T-I is different from the glycopeptide β1,4-Gal T (EC 2.4.1.38) [39].
Gal T-II
Gal T-II (UDP-galactose:GD2 β1,3-galactosyltransferase) catalyzes the synthesis of GD1b, GM1, or GA1 by transferring galactose from UDP-Gal to β-GalNAc in the receptor substrates, generating a β1,3-glycosidic linkage [14, 36, 41]. The cDNA for Gal T-II has been cloned from rat [42], human [43], and mouse brains [44]. The Gal T-II gene has no significant homology with other galactosyltransferase genes and consists of a single exon, which is different from other GT genes. In addition, Gal T-II, as well as Gal T-III, contains a very hydrophobic putative stem region differing significantly from other animal GTs [43].
Gal T-III
Gal T-III (ceramide UDP-galactosyltransferase, EC2.4.1.45) catalyzes the synthesis of GalCer, the precursor of ganglioside GM4, in embryonic chicken brain [45] and in mouse brain [46]. The Gal T-III cDNA was first cloned from rat [47], and then from mouse [48, 49] and human [50, 51]. The Gal T-III gene in the three species consists of 6 exons. Analysis of the deduced amino-acid sequences showed common structural features of Gal T-III in three species, such as the putative N-glycosylation sites, the C-terminal hydrophobic part of the protein, the terminal signal peptide, and the KKVK endoplasmic reticulum retention signal [48].
GalNAc T (EC 2.4.1.92)
GalNAc T (β1,4 N-acetylgalactosaminyltransferase) catalyzes the transfer of N-acetylgalactosamine onto the receptor substrates to form a β1,4 linkage [14, 36, 52-57]. The cDNA for GalNAc T has been cloned from human [58], rat [59], and mouse [60, 61]. The gene consists of 11 exons. The deduced amino-acid sequences of the cDNA indicate that GalNAc T is a typical type II membrane protein, similar to other GTs, and consists of a short N-terminal residue, a transmembrane region, and a long C-terminal residue, including the catalytic domain. GalNAc T shows no significant homology with other GalNAc transferases but shows 39% identity of the amino-acid sequence with another β1,4-N-acetylgalactosaminyltransferase, which is responsible for the biosynthesis of the blood group Sda/Cad antigen [62, 63]. In vitro assays of the expressed recombinant GalNac T showed that it catalyzes not only the formation of GM2 and GD2 from GM3 and GD3, respectively, but also GA2 from LacCer, and thus this enzyme is designated as a GA2/GM2/GD2/GT2-synthase. This enzyme lacks activity to transfer GalNAc to glycoproteins. However, another purified GalNAc T can transfer GalNAc to both glycolipids and oligosaccharides [64]. It is unclear whether these two GalNAc Ts are the same or different GalNAc transferases.
Glc T (EC 2.4.1.80)
Glc T (glucosylceramide synthase, UDP-glucose:N-acylsphingosine D-glucosyltransferase, EC 2.4.1.80) transfers glucose from UDP-Glc to ceramide in brain tissues [14, 65], which is the first step for biosynthesis of the major gangliosides except GM4 (Figure 1). The cDNA of Glc T has been cloned from human [66], mouse [67], and rat [68]. The structure of the Glc T gene contains 9 exons. The deduced amino-acid sequence shows that Glc T has the type III structure, that is, a short N-terminal segment to the exoplasmic side, a single transmembrane segment, and a long cytosolic tail [69]. Moreover, the catalytic domain of Glc T is in the cytosolic face of the Golgi membrane [66, 68]. These two features of Glc T are different from most of the cloned glycosyltransferases. This enzyme is involved only in glycolipid synthesis, as suggested by the fact that no β-glucosyl residues have been found in either glycoproteins or the lipid precursors.
Transcriptional Regulation of Ganglioside Synthase Genes
Ganglioside biosynthesis is strictly regulated by the activities of GTs and is necessarily controlled at the levels of gene transcription and posttranslational modification. Cells can switch between expressing simple and complex gangliosides or between different series of gangliosides during differentiation. Although post-translational modifications of GTs, as well as the compartmentation of GTs and substrates play important regulatory roles in ganglioside biosynthesis, the expression of GTs themselves are tightly regulated at the transcriptional level. Following the cloning of the GT genes for ganglioside synthase, the genomic regions (promoters) of the GT genes for those key enzymes in ganglioside biosynthesis have been isolated and functionally analyzed [70].
Promoter of the ST3Gal V (GM3-synthase) Gene
ST3Gal V (GM3-synthase) catalyzes the synthesis of GM3, and its expression is regulated in a tissue-specific manner [13, 71]. There are four isoforms of ST3Gal V mRNA in human fetal brain, differing in the 5′-untranslated region, but only one of these isoforms can be detected in adult brain [72]. These data suggest that the regulation of ST3Gal V expression is developmentally regulated, and alterative splicing or transcriptional initiation of the human ST3Gal V RNA may occur in a tissue-specific manner. The promoters of the human [73, 74] and mouse [75] ST3Gal V genes were cloned and analyzed. The mouse ST3Gal V gene uses multiple transcriptional initiation sites, including several downstream initiation sites [75], while the human ST3Gal V gene uses at least two initiation sites [76], consistent with the fact that there are multiple isoforms of transcripts from the ST3Gal V genes [72]. Minimum promoter regions of 177 bp and 254 bp lie upstream of the initiation site of the human and mouse promoters, respectively, and display the highest level of promoter activity [73-75], while longer promoter fragments from the proximal regions to 1,600 bp, do not significantly contribute to transcriptional activity [73-75]. These proximal regions contain no TATA or CCAAT boxes but have multiple Sp1 and AP2 sites.
Transcriptional regulation of the ST3Gal V gene has been further studied recently [75, 76]. A juxtaposed Sp1/AP2-binding site and one of the six AP2-binding sites in the proximal region are essential to maintain a high level of promoter activity [75]. Chromatin immunoprecipitation confirmed the binding of the transcription factors Sp1 and AP2 to the above two sites inside cells [75]. DNA pull-down experiments revealed that 7 factors (CREB, PPAR, Pax-5, Smad3/4, Stat6, MEF-1, and MEF-2) bound to both of the human and mouse promoter fragments [74, 75]. The CREB-binding site was further demonstrated to be critical in transcription of the human ST3Gal V gene in HL-60 cells when activated by PMA (phorbol 12-myristate 13-acetate) [76]. These results indicate that multiple factors, including Sp1, AP2, and CREB, contribute to activation of ST3Gal V gene transcription, and the expression of the human and mouse ST3Gal V genes may be under a similar regulatory mechanism. Future studies may focus on how these factors and regulatory elements function coordinately in vivo in development-dependent or cell-type specific expression of the gene.
Promoter of the ST8Sia I (GD3-synthase) Gene
ST8Sia I (GD3-synthase) is a key enzyme controlling the synthesis of the b- and c-series gangliosides (Figure 1). Because the expression of GD3 and the b-series gangliosides is strictly regulated during brain development [77] and malignancy [78, 79], the transcriptional regulation of the ST8Sia I gene has attracted much more attention than other promoters of the ganglioside synthase genes. The promoter of this gene has been cloned from rat and is the first cloned promoter of a sialyltransferase gene involved in ganglioside biosynthesis [80]. Its mouse and human counterparts were cloned later [81, 82]. The rat promoter is 93% homologous to the mouse promoter, but there is no significant similarity between the rodent and human promoters. However, all of the three promoters contain a negative control region upstream of the proximal promoter regions and a GT/CG repeat sequence with only slight differences in the repeat lengths among the species [80-82]. These unique GT/CG repeats present a structure of Z-DNA and are located in the negative control regions. Whether the GT/CG repeat sequence contributes to the suppressive activity in the transcriptional regulation of the ST8Sia I gene remains to be elucidated.
In spite of the sequence differences in the rodent and human promoters, the promoter sequences of all three species exhibit similar properties. They are TATA-less and contain GC-rich binding sites. The rat and mouse promoters have a single transcription start site [80, 81], whereas the human promoter possesses multiple initiation sites [82]. The proximal promoter region was defined within 500 or 700 bp upstream of the ATG codon in rat and mouse or human promoter, respectively. Although there are no common binding sequences among the species, all the promoters have several Sp1-binding sites present in the proximal promoter region, and deletion of the Sp1 sites results in a dramatic loss of the proximal promoter activities of the mouse [81] and rat (our unpublished data), suggesting that Sp1 plays a significant role in the transcriptional regulation of this gene. Our recent data from the DNA pull-down experiments showed that at least six transcription factors, Sp1, AP2, GATA, Ets-1, AP1, and MRE, bound to their consensus sites on the proximal promoter fragment of the rat ST8Sia I gene (unpublished data). Further analysis showed that the activity of the proximal ST8Sia I promoter is controlled by these regulatory elements (our unpublished data). We also identified an unknown mutation close to the 3′-end of the rat promoter that decreases up to 50% of the proximal promoter activity, suggesting a novel cis-regulatory element in the basal regulation of rat ST8Sia I gene expression (our unpublished data). Expression of the ST8Sia I gene is highly activated in melanoma and many types of brain tumors, and it would be very interesting to investigate how the expression of the ST8Sia I gene is regulated in a cell-type-specific manner.
Promoter of the GalNAc T (GA2/GM2/GD2/GT2-synthase) Gene
The GalNac T is a key enzyme controlling the expression of more complex gangliosides (Figure 1). The GalNac T gene is highly expressed in tumor cell lines such as neuroblastoma and melanoma cell lines [83, 84], and in mouse brain at the late stage of development [18, 59]. The 5′-flanking fragment of the human GalNac T gene was the first cloned promoter for a ganglioside synthase gene [85]. Studies on the promoter and genomic organization showed that this gene has three transcription start sites and three alternative exons (exon 1a, exon 1b, and exon 1c). Three promoters (P1, P2, and P3) in this gene have been defined for the individual transcription start sites, respectively [85]. The third promoter is located in the first intron. There are consensus binding sites for the transcription factors EGR-1, HNF-5, Sp-1, and PEA3 in the 5′-flanking region of exon 1a, three binding sites for Sp-1 and one for AP-2 and PEA3 in the 5′-flanking region of exon 1b, and one site for AP-2 and E2F and S1 HS sites in the 5′-flanking region of exon 1c. The activities of the promoters P1 and P2 were strongly enhanced by a sequence (enhancer) located in exon 1. Furukawa et al. [85] also proposed a suppressive sequence (silencer) of 42 bp located upstream of exon 1c, which suppressed the transcriptional activity of the P3 promoter in a melanoma cell line where no promoter activity was detected. These results indicate that cell type-specific expression of the GalNac T gene may be regulated by alternative promoters or the enhancer or silencer. It is not surprising that the mechanism for transcriptional regulation of the GalNac T gene is complex and tissue-specific, because the enzyme is one of the key enzymes in ganglioside biosynthesis.
Promoter of the Glc T (Glucosylceramide synthase) Gene
Glc T (glucosylceramide synthase) catalyzes the first committed step in glycosphingolipid synthesis, the transfer of glucose from UDP-glucose to ceramide. The product, glucosylceramide, serves as a core structure for more than 300 species of glycosphingolipids. The enzyme is a key regulatory factor controlling intracellular levels of ceramide and glycosphingolipids. The promoter of the mouse Glc T gene has been cloned [86]. Not much information is available for this promoter except that the promoter exhibits the common feature of the ganglioside synthase promoters; it is TATA-less and contains no CCAAT box, but has GC-rich boxes. The proximal promoter of 578 bp contains four Sp1-binding sites located between 558 to 439 bp upstream of the ATG codon. Deletion of a 41-bp fragment (from -538 to -578) dramatically decreases the activity of the proximal promoter. This 41-bp fragment contains one Sp1-binding site, indicating an essential role of this site in maintaining a high level of proximal promoter activity [86]. In addition to these general transcription factor binding sites, the motifs for AhR, NFκB, AP-2, and GATA-1 binding sites are also present but their roles remain to be characterized.
Promoter of the Gal T-II (GA1/GM1/GD1b/GT1c-synthase Gene
Gal T-II is responsible for the synthesis of GA1, GM1, GD1b, and GT1c in the ganglioside biosynthetic pathway (Figure 1). GM1 is one of the most widely investigated gangliosides and plays important roles in the development and functions of the neural system [87]. A 1,448 bp 5′-flanking fragment of the mouse Gal T-II gene has been cloned [88]. This promoter is again TATA-less, and has no CCAAT box. However, there are several features different from other ganglioside synthase promoters as discussed above. The proximal 550-bp fragment that shows the highest level of promoter activity contains no Sp1-binding site, but a number of transcriptional factors including TFIID, NFκB, AP2, AP1, Ets-1, Ets-2, GATA, and NF-1. The Gal T-II gene has a single exon and a single transcription start site. A 350-bp fragment beyond the proximal region strongly suppresses the promoter activity. Twenty-seven transcription factors have been characterized as binding to their consensus sites in the promoter region and four other factors without consensus binding sites were likely recruited from transcription complexes. These data may indicate a quite different mechanism for regulation of Gal T-II gene expression. How Gal T-II gene expression is regulated, particularly during neuronal differentiation, remains to be elucidated.
Promoter of the Gal T-III (Galactosylceramide synthase) Gene
Galactosylceramide synthase is a key enzyme in the biosynthesis of galactocerebroside, the most abundant glycosphingolipid in the myelin sheath. Galactosylceramide serves as the precursor for the biosynthesis of GM4, the simplest ganglioside present, among other tissues, in myelin of the central nervous system [89]. The promoter of the human Gal T-III gene has been cloned and analyzed [90, 91]. This promoter is also TATA-less and has no CCAAT box, but contains GC-rich boxes and multiple putative regulatory elements. The human Gal T-III gene is transcribed from a single initiation site 322 bp upstream of the ATG codon. The cis-acting regulatory elements were found in two small regions at -292/-256 and -747/-688. The region at -292/-256 contains putative binding sites for the transcription factors Ets and Sp1, and the region at -747/-688 exhibits binding sites for ERE, NF-1, TGGCA-BP, and CRE [90]. A third cis-acting region, distally located at -1325/-1083, consists of multiple binding sites for TCF-1, TGGCA-BP, NF-IL6, CF1, bHLH, NF-1, GATA, and Q-IRE. A negative regulatory domain localized in a far region at -1594/-1326 was also identified [90]. Further analysis of the two small regulatory regions demonstrated that the functional elements are an Sp1-binding site at -292/-256 and a CRE-binding site at -747/-688 [91]. The cell type-specific activities of those promoters have been observed between the human oligodendroglioma (HOG) and human neuroblastoma (LAN-5) cell lines, and the factor CREB may account for the different levels of Gal T-III promoter activity in these cell lines [91].
Concluding remarks
All genes coding for GTs involved in the ganglioside biosynthetic pathway (Figure 1) have been cloned with the exception of GT3-synthase (ST-III) gene, which was reported to be the GD3-synthase (ST-II) gene [23]. However, this has been proven to be an artifact since a GD3-synthase over-expression experiment clearly indicates that there is expression of both GD3 and GT3 [92]. Although ST8Sia III [26-28] was initially suggested to be GT3-synthase, it is actually very active in glycoprotein synthesis in vitro. It remains unclear whether ST8Sia III utilizes only glycolipids, such as GM3, GD3, and GT3, as acceptors [26, 28]. Whether a unique cDNA exists coding only for GT3-synthase is still an open question. A particular enzyme is likely responsible for the synthesis of GT3 in vivo since many cells and tissues synthesize GD3 and/or the b-series gangliosides but exhibit no GT3 and/or the c-series gangliosides.
Some of the enzymes, such as GM3-synthase and GlcCer-synthase, are highly specific in both donor and receptor substrates. They exclusively catalyze the reactions for synthesis of gangliosides, such as GM3 and GlcCer, respectively, while others show broad substrate specificities and are responsible for the synthesis of more than one ganglioside or are even involved in the synthesis of both glycoproteins and glycolipids.
Cloning of these genes has allowed determination of biological functions of gangliosides by analysis of loss-of-function or gain-of-function as performed in our laboratory [92-94] or using knockout mice [95]. The availability of the ganglioside synthase sequences has also classified these genes into GT families by amino-acid sequence similarities and helped to identify the sequence motifs. This sequence-based classification is assumed to integrate both structural and mechanistic features within each family [1]. However, prediction of the donor and acceptor specificity of a GT based on sequence homology can be problematic because there are many examples of closely related sequences, such as sequences of blood group A and B transferases [96] having different catalytic activity. As discussed above, the donor and acceptor substrate specificities for some of the ganglioside synthase genes remain unclear [97]. Determination of the X-ray crystal structures of these transferases will provide a structural basis accounting for these specificities, as well as for the catalytic reactions. Over 100 crystal structures have been described for proteins corresponding to 23 different GTs, from prokaryotes and eukaryotes [4]. The 3-dimensional GT database, containing structural information for 17 distinct GT families is available at http://www.cermav.cnrs.fr/glyco3d. Unfortunately, due to difficulties with high-level expression, purification, and crystallization, no crystalline structure for ganglioside synthases have been determined so far. This is clearly an area that is worthy of earnest pursuit.
Temporal and spatial expression of ganglioside synthase genes is subject to complex developmental and tissue-specific regulation. Attempts have been made recently to elucidate the molecular mechanisms involved in expression of these genes. All of the promoters of the ganglioside synthase genes cloned so far lack TATA and CCAAT boxes but do contain GC-rich boxes. A number of cis-acting elements important for regulating expression of these genes have been identified, and a set of basal cis-acting factors, including Sp1, AP2, and CREB, is likely bound to the proximal promoters. In addition, negative regulatory sequences usually located upstream of the proximal regions have been found in many promoters. However, whether these elements and transcription factors are important for expression of these genes in vivo is still subject to investigation. Further analysis of the promoter regions and the functional elements and factors will be a main goal toward the understanding of the regulatory mechanism of their expression in physiological and pathological conditions.
Acknowledgments
The work reported in our lab has been supported by USPHS grant NS11853.
References
- 1.Coutinho PM, Deleury E, Davies GJ, Henrissat B. J Mol Biol. 2003;328:307–317. doi: 10.1016/s0022-2836(03)00307-3. [DOI] [PubMed] [Google Scholar]
- 2.Harduin-Lepers A, Mollicone R, Delannoy P, Oriol R. Glycobiology. 2005;15:805–817. doi: 10.1093/glycob/cwi063. [DOI] [PubMed] [Google Scholar]
- 3.Seto NO, Palcic MM, Compston CA, Li H, Bundle DR, Narang SA. J Biol Chem. 1997;272:14133–14138. doi: 10.1074/jbc.272.22.14133. [DOI] [PubMed] [Google Scholar]
- 4.Breton C, Snajdrova L, Jeanneau C, Koca J, Imberty A. Glycobiology. 2006;16:29R–37R. doi: 10.1093/glycob/cwj016. [DOI] [PubMed] [Google Scholar]
- 5.Drickamer K. Glycobiology. 1993;3:2–3. doi: 10.1093/glycob/3.2.185. [DOI] [PubMed] [Google Scholar]
- 6.Livingston BD, Paulson JC. J Biol Chem. 1993;268:11504–11507. [PubMed] [Google Scholar]
- 7.Jeanneau C, Chazalet V, Auge C, Soumpasis DM, Harduin-Lepers A, Delannoy P, Imberty A, Breton C. J Biol Chem. 2004;279:13461–13468. doi: 10.1074/jbc.M311764200. [DOI] [PubMed] [Google Scholar]
- 8.Geremia RA, Harduin-Lepers A, Delannoy P. Glycobiology. 1997;7:v–vii. doi: 10.1093/glycob/7.2.161. [DOI] [PubMed] [Google Scholar]
- 9.Kapitonov D, Bieberich E, Yu RK. Glycoconj J. 1999;16:337–350. doi: 10.1023/a:1007091926413. [DOI] [PubMed] [Google Scholar]
- 10.Lee YC, Kurosawa N, Hamamoto T, Nakaoka T, Tsuji S. Eur J Biochem. 1993;216:377–385. doi: 10.1111/j.1432-1033.1993.tb18155.x. [DOI] [PubMed] [Google Scholar]
- 11.Kitagawa H, Paulson JC. J Biol Chem. 1994;269:17872–17878. [PubMed] [Google Scholar]
- 12.Ishii A, Ohta M, Watanabe Y, Matsuda K, Ishiyama K, Sakoe K, Nakamura M, Inokuchi J, Sanai Y, Saito M. J Biol Chem. 1998;273:31652–31655. doi: 10.1074/jbc.273.48.31652. [DOI] [PubMed] [Google Scholar]
- 13.Kono M, Takashima S, Liu H, Inoue M, Kojima N, Lee YC, Hamamoto T, Tsuji S. Biochem Biophys Res Commun. 1998;253:170–175. doi: 10.1006/bbrc.1998.9768. [DOI] [PubMed] [Google Scholar]
- 14.Basu S, Basu M, Dastgheib S, Hawes JW. In: Comprehensive Natural Products Chemistry. Barton D, Nakanishi K, Meth-Cohen O, editors; Pinto BM, editor. Vol. 3. Pergamon Press; New York: 1999. pp. 107–128. [Google Scholar]
- 15.Nara K, Watanabe Y, Maruyama K, Kasahara K, Nagai Y, Sanai Y. Proc Natl Acad Sci USA. 1994;91:7952–7956. doi: 10.1073/pnas.91.17.7952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haraguchi M, Yamashiro S, Yamamoto A, Furukawa K, Takamiya K, Lloyd KO, Shiku H, Furukawa K. Proc Natl Acad Sci USA. 1994;91:10455–10459. doi: 10.1073/pnas.91.22.10455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sasaki K, Kurata K, Kojima N, Kurosawa N, Ohta S, Hanai N, Tsuji S, Nishi T. J Biol Chem. 1994;269:15950–15956. [PubMed] [Google Scholar]
- 18.Yamamoto A, Haraguchi M, Yamashiro S, Fukumoto S, Furukawa K, Takamiya K, Atsuta M, Shiku H, Furukawa K. J Neurochem. 1996;66:26–34. doi: 10.1046/j.1471-4159.1996.66010026.x. [DOI] [PubMed] [Google Scholar]
- 19.Zeng G, Gao L, Ariga T, Yu RK. Biochem Biophys Res Commun. 1996;226:319–323. doi: 10.1006/bbrc.1996.1354. [DOI] [PubMed] [Google Scholar]
- 20.Higashi H, Basu M, Basu S. J Biol Chem. 1985;260:824–828. [PubMed] [Google Scholar]
- 21.Kaufman B, Basu S, Roseman S. J Biol Chem. 1968;243:5804–5806. [PubMed] [Google Scholar]
- 22.Nara K, Watanabe Y, Kawashima I, Tai T, Nagai Y, Sanai Y. Eur J Biochem. 1996;238:647–652. doi: 10.1111/j.1432-1033.1996.0647w.x. [DOI] [PubMed] [Google Scholar]
- 23.Nakayama J, Fukuda MN, Hirabayashi Y, Kanamori A, Sasaki K, Nishi T, Fukuda M. J Biol Chem. 1996;271:3684–3691. [PubMed] [Google Scholar]
- 24.Kono M, Yoshida Y, Kojima N, Tsuji S. J Biol Chem. 1996;271:29366–29371. doi: 10.1074/jbc.271.46.29366. [DOI] [PubMed] [Google Scholar]
- 25.Kim YJ, Kim KS, Do S, Kim CH, Kim SK, Lee YC. Biochem Biophys Res Commun. 1997;235:327–330. doi: 10.1006/bbrc.1997.6725. [DOI] [PubMed] [Google Scholar]
- 26.Yoshida Y, Kojima N, Kurosawa N, Hamamoto T, Tsuji S. J Biol Chem. 1995;270:14628–14633. doi: 10.1074/jbc.270.24.14628. [DOI] [PubMed] [Google Scholar]
- 27.Zeng G, Gao L, Yu RK. Gene. 1997;187:131–134. doi: 10.1016/s0378-1119(96)00735-4. [DOI] [PubMed] [Google Scholar]
- 28.Lee YC, Kim YJ, Lee KY, Kim KS, Kim BU, Kim HN, Kim CH, Do SI. Arch Biochem Biophys. 1998;360:41–46. doi: 10.1006/abbi.1998.0909. [DOI] [PubMed] [Google Scholar]
- 29.Sjoberg ER, Kitagawa H, Glushka J, van Halbeek H, Paulson JC. J Biol Chem. 1996;271:7450–7459. doi: 10.1074/jbc.271.13.7450. [DOI] [PubMed] [Google Scholar]
- 30.Lee YC, Kaufmann M, Kitazume-Kawaguchi S, Kono M, Takashima S, Kurosawa N, Liu H, Pircher H, Tsuji S. J Biol Chem. 1999;274:11958–11967. doi: 10.1074/jbc.274.17.11958. [DOI] [PubMed] [Google Scholar]
- 31.Okajima T, Fukumoto S, Ito H, Kiso M, Hirabayashi Y, Urano T, Furukawa K. J Biol Chem. 1999;274:30557–30562. doi: 10.1074/jbc.274.43.30557. [DOI] [PubMed] [Google Scholar]
- 32.Kurosawa N, Kojima N, Inoue M, Hamamoto T, Tsuji S. J Biol Chem. 1994;269:19048–19053. [PubMed] [Google Scholar]
- 33.Kurosawa N, Inoue M, Yoshida Y, Tsuji S. J Biol Chem. 1996;271:15109–15116. doi: 10.1074/jbc.271.25.15109. [DOI] [PubMed] [Google Scholar]
- 34.Samyn-Petit B, Krzewinski-Recchi MA, Steelant WF, Delannoy P, Harduin-Lepers A. Biochim Biophys Acta. 2000;1474:201–211. doi: 10.1016/s0304-4165(00)00020-9. [DOI] [PubMed] [Google Scholar]
- 35.Ikehara Y, Shimizu N, Kono M, Nishihara S, Nakanishi H, Kitamura T, Narimatsu H, Tsuji S, Tatematsu M. FEBS Lett. 1999;463:92–96. doi: 10.1016/s0014-5793(99)01605-1. [DOI] [PubMed] [Google Scholar]
- 36.Van Echten-Deckart G, Sandhoff K. Comprehensive Natural Products Chemistry. Pergamon Press; New York: 1999. pp. 87–106. [Google Scholar]
- 37.Basu S, Kaufman B, Roseman S. J Biol Chem. 1968;243:5802–5804. [PubMed] [Google Scholar]
- 38.Chatterjee S, Ghosh N, Khurana S. J Biol Chem. 1992;267:7148–7153. [PubMed] [Google Scholar]
- 39.Nomura T, Takizawa M, Aoki J, Arai H, Inoue K, Wakisaka E, Yoshizuka N, Imokawa G, Dohmae N, Takio K, Hattori M, Matsuo N. J Biol Chem. 1998;273:13570–13577. doi: 10.1074/jbc.273.22.13570. [DOI] [PubMed] [Google Scholar]
- 40.Takizawa M, Nomura T, Wakisaka E, Yoshizuka N, Aoki J, Arai H, Inoue K, Hattori M, Matsuo N. Biochim Biophys Acta. 1999;1438:301–304. doi: 10.1016/s1388-1981(99)00051-7. [DOI] [PubMed] [Google Scholar]
- 41.Basu SS, Dastgheib S, Basu M, Kelly P, Ghosh S, Basu S. Acta Biochim Pol. 1998;45:451–467. [PubMed] [Google Scholar]
- 42.Miyazaki H, Fukumoto S, Okada M, Hasegawa T, Furukawa K. J Biol Chem. 1997;272:24794–24799. doi: 10.1074/jbc.272.40.24794. [DOI] [PubMed] [Google Scholar]
- 43.Amado M, Almeida R, Carneiro F, Levery SB, Holmes EH, Nomoto M, Hollingsworth MA, Hassan H, Schwientek T, Nielsen PA, Bennett EP, Clausen H. J Biol Chem. 1998;273:12770–12778. doi: 10.1074/jbc.273.21.12770. [DOI] [PubMed] [Google Scholar]
- 44.Daniotti JL, Martina JA, Zurita AR, Maccioni HJ. J Neurosci Res. 1999;58:318–327. [PubMed] [Google Scholar]
- 45.Basu S, Shultz A, Basu M, Roseman S. J Biol Chem. 1971;243:4272–4279. [PubMed] [Google Scholar]
- 46.Morell P, Radin NS. Biochemistry. 1969;8:506–514. doi: 10.1021/bi00830a008. [DOI] [PubMed] [Google Scholar]
- 47.Schulte S, Stoffel W. Proc Natl Acad Sci USA. 1993;90:10265–10269. doi: 10.1073/pnas.90.21.10265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bosio A, Binczek E, Stoffel W. Genomics. 1996;35:223–226. doi: 10.1006/geno.1996.0342. [DOI] [PubMed] [Google Scholar]
- 49.Coetzee T, Li X, Fujita N, Marcus J, Suzuki K, Francke U, Popko B. Genomics. 1996;35:215–222. doi: 10.1006/geno.1996.0341. [DOI] [PubMed] [Google Scholar]
- 50.Kapitonov D, Yu RK. Biochem Biophys Res Commun. 1997;232:449–453. doi: 10.1006/bbrc.1997.6240. [DOI] [PubMed] [Google Scholar]
- 51.Bosio A, Binczek E, Le Beau MM, Fernald AA, Stoffel W. Genomics. 1996;34:69–75. doi: 10.1006/geno.1996.0242. [DOI] [PubMed] [Google Scholar]
- 52.Kaufman B, Basu S, Roseman S. In: Inborn errors of sphingolipid metabolism. Aronso SM, Volk BW, editors. New York: Pergamon Press; 1967. pp. 193–213. [Google Scholar]
- 53.Chien JL, Williams T, Basu S. J Biol Chem. 1973;248:1778–1785. [PubMed] [Google Scholar]
- 54.Steigerwald J, Basu S, Kaufman B, Roseman S. J Biol Chem. 1975;250:6727–6734. [PubMed] [Google Scholar]
- 55.Taniguchi N, Makita A. J Biol Chem. 1984;259:5637–5642. [PubMed] [Google Scholar]
- 56.Schaeper RJ, Das KK, Li Z, Basu S. Carbohydrate Res. 1992;236:227–244. doi: 10.1016/0008-6215(92)85018-u. [DOI] [PubMed] [Google Scholar]
- 57.Basu S. The serendipity of ganglioside biosynthesis: pathway to CARS and HY-CARS glycosyltransferases. Glycobiology. 1991;1:469–475. doi: 10.1093/glycob/1.5.469. [DOI] [PubMed] [Google Scholar]
- 58.Nagata Y, Yamashiro S, Yodoi J, Lloyd KO, Shiku H, Furukawa K. J Biol Chem. 1992;267:12082–12089. [PubMed] [Google Scholar]
- 59.Hidari JK, Ichikawa S, Furukawa K, Yamasaki M, Hirabayashi Y. Biochem J. 1994;303:957–965. doi: 10.1042/bj3030957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Takamiya K, Yamamoto A, Yamashiro S, Furukawa K, Haraguchi M, Okada M, Ikeda T, Shiku H, Furukawa K. FEBS Lett. 1995;358:79–83. doi: 10.1016/0014-5793(94)01395-h. [DOI] [PubMed] [Google Scholar]
- 61.Sango K, Johnson ON, Kozak CA, Proia RL. Genomics. 1995;27:362–365. doi: 10.1006/geno.1995.1058. [DOI] [PubMed] [Google Scholar]
- 62.Lowe AM, Lambert PA, Smith AW. Infect Immun. 1995;63:703–706. doi: 10.1128/iai.63.2.703-706.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lo Presti L, Cabuy E, Chiricolo M, Chiricolo M, Dall'Olio F. J Biochem (Tokyo) 2003;134:675–682. doi: 10.1093/jb/mvg192. [DOI] [PubMed] [Google Scholar]
- 64.Yanagisawa K, Taniguchi N, Makita A. Biochim Biophys Acta. 1987;919:213–220. doi: 10.1016/0005-2760(87)90260-8. [DOI] [PubMed] [Google Scholar]
- 65.Basu S, Kaufman B, Roseman S. J Biol Chem. 1973;248:1388–1394. [PubMed] [Google Scholar]
- 66.Ichikawa S, Sakiyama H, Suzuki G, Hidari KI, Hirabayashi Y. Proc Nati Acad Sci USA. 1996;93:12654. doi: 10.1073/pnas.93.22.12654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ichikawa S, Ozawa K, Hirabayashi Y. Biochem Mol Biol Int. 1998;44:1193–1202. doi: 10.1080/15216549800202282. [DOI] [PubMed] [Google Scholar]
- 68.Wu K, Marks DL, Watanabe R, Paul P, Rajan N, Pagano RE. Biochem J. 1999;341:395–400. [PMC free article] [PubMed] [Google Scholar]
- 69.Spiess M. FEBS Lett. 1995;369:76–79. doi: 10.1016/0014-5793(95)00551-j. [DOI] [PubMed] [Google Scholar]
- 70.Yu RK, Bieberich E, Xia T, Zeng G. J Lipid Res. 2004;45:783–793. doi: 10.1194/jlr.R300020-JLR200. [DOI] [PubMed] [Google Scholar]
- 71.Fukumoto S, Miyazaki H, Goto G, Urano T, Furukawa K, Furukawa K. J Biol Chem. 1999;274:9271–9276. doi: 10.1074/jbc.274.14.9271. [DOI] [PubMed] [Google Scholar]
- 72.Kim KW, Kim SW, Min KS, Kim CH, Lee YC. Gene. 2001;273:163–171. doi: 10.1016/s0378-1119(01)00595-9. [DOI] [PubMed] [Google Scholar]
- 73.Kim SW, Lee SH, Kim KS, Kim CH, Choo YK, Lee YC. Biochim Biophys Acta. 2002;1578:84–89. doi: 10.1016/s0167-4781(02)00505-5. [DOI] [PubMed] [Google Scholar]
- 74.Zeng G, Gao L, Xia T, Tencomnao T, Yu RK. Biochim Biophys Acta. 2003;1625:30–35. doi: 10.1016/s0167-4781(02)00573-0. [DOI] [PubMed] [Google Scholar]
- 75.Xia T, Zeng G, Gao L, Yu RK. Gene. 2005;351:109–118. doi: 10.1016/j.gene.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 76.Chung TW, Choi HJ, Lee YC, Kim CH. Glycobiology. 2005;15:233–244. doi: 10.1093/glycob/cwh156. [DOI] [PubMed] [Google Scholar]
- 77.Yu RK. Prog Brain Res. 1994;101:31–44. doi: 10.1016/s0079-6123(08)61938-x. [DOI] [PubMed] [Google Scholar]
- 78.Hakomori S. Cancer Res. 1996;56:5309–5318. [PubMed] [Google Scholar]
- 79.Hakomori S. Proc Natl Acad Sci USA. 2002;99:10231–10233. doi: 10.1073/pnas.172380699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zeng G, Gao L, Yu RK. Biochim Biophys Acta. 1998;1397:126–130. doi: 10.1016/s0167-4781(98)00030-x. [DOI] [PubMed] [Google Scholar]
- 81.Takashima S, Kono M, Kurosawa N, Yoshida Y, Tachida Y, Inoue M, Kanematsu T, Tsuji S. J Biochem (Tokyo) 2000;128:1033–1043. doi: 10.1093/oxfordjournals.jbchem.a022831. [DOI] [PubMed] [Google Scholar]
- 82.Furukawa K, Horie M, Okutomi K, Sugano S, Furukawa K. Biochim Biophys Acta. 2003;1627:71–78. doi: 10.1016/s0167-4781(03)00076-9. [DOI] [PubMed] [Google Scholar]
- 83.Cheung NK, Saarinen UM, Neely JE, Landmeier B, Donovan D, Coccia PF. Cancer Res. 1985;45:2642–2649. [PubMed] [Google Scholar]
- 84.Yamashiro S, Ruan S, Furukawa K, Tai T, Lloyd KO, Shiku H, Furukawa K. Cancer Res. 1993;53:5395–5400. [PubMed] [Google Scholar]
- 85.Furukawa K, Soejima H, Niikawa N, Shiku H. J Biol Chem. 1996;271:20836–20844. doi: 10.1074/jbc.271.34.20836. [DOI] [PubMed] [Google Scholar]
- 86.Ichikawa S, Ozawa K, Hirabayashi Y. Biochem Biophys Res Commun. 1998;253:707–711. doi: 10.1006/bbrc.1998.9855. [DOI] [PubMed] [Google Scholar]
- 87.Ledeen RW, Wu G, Lu ZH, Kozireski-Chuback D, Fang Y. Ann N Y Acad Sci. 1998;845:161–175. doi: 10.1111/j.1749-6632.1998.tb09669.x. [DOI] [PubMed] [Google Scholar]
- 88.Xia T, Gao L, Yu RK, Zeng G. Gene. 2003;309:117–123. doi: 10.1016/s0378-1119(03)00496-7. [DOI] [PubMed] [Google Scholar]
- 89.Yu RK, Lee SH. J Biol Chem. 1976;251:198–203. [PubMed] [Google Scholar]
- 90.Tencomnao T, Yu RK, Kapitonov D. Biochim Biophys Acta. 2001;1517:416–423. doi: 10.1016/s0167-4781(00)00283-9. [DOI] [PubMed] [Google Scholar]
- 91.Tencomnao T, Kapitonov D, Bieberich E, Yu RK. Glycoconj J. 2004;20:339–351. doi: 10.1023/B:GLYC.0000033630.58533.16. [DOI] [PubMed] [Google Scholar]
- 92.Yanagisawa M, Liour SS, Yu RK. J Neurochem. 2004;91:804–812. doi: 10.1111/j.1471-4159.2004.02750.x. [DOI] [PubMed] [Google Scholar]
- 93.Zeng G, Ariga T, Gu XB, Yu RK. Proc Natl Acad Sci USA. 1995;92:8670–8674. doi: 10.1073/pnas.92.19.8670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zeng G, Li DD, Gao L, Birkle S, Bieberich E, Tokuda A, Yu RK. Biochemistry. 1999;38:8762–8769. doi: 10.1021/bi9906726. [DOI] [PubMed] [Google Scholar]
- 95.Furukawa K, Tokuda N, Okuda T, Tajima O, Furukawa K. Semin Cell Dev Biol. 2004;15:389–396. doi: 10.1016/j.semcdb.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 96.Yamamoto F, Clausen H, White T, Marken J, Hakomori S. Nature. 1990;345:229–233. doi: 10.1038/345229a0. [DOI] [PubMed] [Google Scholar]
- 97.Basu S, Basu M. In: Chemistry and Biology-A comprehensive Handbood. Ernst E, Sinay P, Hart G, editors. Wiley-VCH Verlag GmbH; Germany: 2000. pp. 329–347. [Google Scholar]